Abstract
The mammalian heart is responsible for not only pumping blood throughout the body but also adjusting this pumping activity quickly depending upon sudden changes in the metabolic demands of the body. For the most part, the human heart is capable of performing its duties without complications; however, throughout many decades of use, at some point this system encounters problems. Research into the heart’s activities during healthy states and during adverse impacts that occur in disease states is necessary in order to strategize novel treatment options to ultimately prolong and improve patients’ lives. Animal models are an important aspect of cardiac research where a variety of cardiac processes and therapeutic targets can be studied. However, there are differences between the heart of a human being and an animal and depending on the specific animal, these differences can become more pronounced and in certain cases limiting. There is no ideal animal model available for cardiac research, the use of each animal model is accompanied with its own set of advantages and disadvantages. In this review, we will discuss these advantages and disadvantages of commonly used laboratory animals including mouse, rat, rabbit, canine, swine, and sheep. Since the goal of cardiac research is to enhance our understanding of human health and disease and help improve clinical outcomes, we will also discuss the role of human cardiac tissue in cardiac research. This review will focus on the cardiac ventricular contractile and relaxation kinetics of humans and animal models in order to illustrate these differences.
Keywords: Species, Cardiac Contractility, Heart Failure, Cardiac Kinetics, Heart Rate
1. Introduction
According to a preliminary report by the National Vital and Statistics Reports, heart disease remains the number one cause of mortality in the United States, accounting for almost 600,000, or about a quarter of all, deaths in 2010 (Murphy, Xu, & Kochanek, 2012). Our understanding of the cardiovascular system has greatly advanced in the past decades, however, further research is required in order to expand our knowledge and provide novel therapeutic avenues. Donated human hearts, either non-transplantable (ranging from healthy to diseased) or end-stage failing hearts typically obtained at time of transplantation, are a great tool for addressing this issue. These samples are however available in very limited quantities, and exhibit great variability due to differences in factors such as genetics, medications, diets, social habits, and diseases. There thus exists a need to have a suitable animal model where cardiovascular physiology and disease can be studied efficiently and reliably with potential translational applicability to humans.
The basic principles of cardiac excitation and contraction in all of the species discussed in this review are relatively conserved. The cardiac cycle sequence starts with the sino-atrial node depolarization which spreads to the atria causing their activation. After a brief pause at the atrio-ventricular node, the depolarization current spreads to the Bundle of His, purkinje fiber system, and ultimately results in stimulation of ventricular myocardium. On a cellular level, depolarization along the cardiomyocyte T-tubules activates the voltage gated calcium ion channels, and this influx of calcium induces release of calcium from the sarcoplasmic reticulum. Calcium binding to troponin activates the myofilaments and permits cross-bridge cycling and cardiomyocyte shortening, resulting in pumping of blood from the ventricles into the pulmonary and systematic circulations. Relaxation process involves de-activation of the myofilaments and removal of cytoplasmic calcium mainly by the sarcoplasmic-reticulum calcium ATPase (SERCA) and sodium calcium exchanger (NCX) systems. This sequence is very rapid and occurs, depending on the species and heart rate, within tens of milliseconds in mice to a couple of hundred milliseconds in humans. This rapid and cyclic nature presents a significant obstacle in cardiac research. Membrane potential, ion concentrations, mechanical force, and modulation factors are never in equilibrium. Moreover, each of these factors constantly modulate each other. As a result, modulation of one factor typically impacts all other factors, and thus it is hard, if not impossible, to assess the specific impact of an intervention on one of these factors alone.
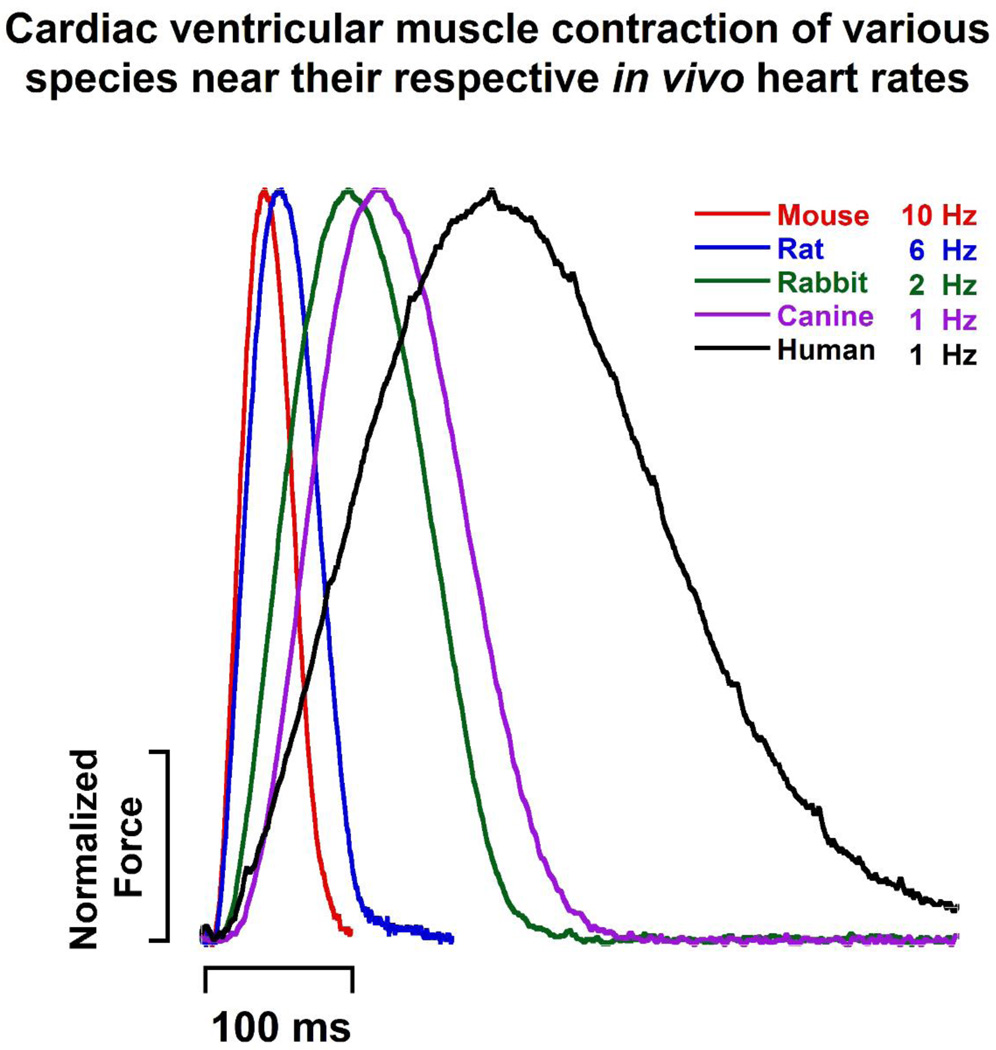
What is the single best animal model in the cardiac field that can be used in lieu of human hearts? One major concern in addressing this question is that the cardiovascular system of each animal has evolved differently in order to meet the demands of that species. The heart of a small animal such as mouse can easily beat up to 800 times per minute (Ostergaard, Hansen, & Ottesen, 2010), an elephant has a heart rate of only 35 bpm (Bartlett, et al., 2009). Table 1 shows the inverse relationship between body weight and heart rate while blood pressure remains relatively constant across various laboratory animals and humans. In fact, the relationship between body weight and various cardiovascular parameters can be described with allometric equations such as heart weight (HW (g)= 6.0 × BM0.98) and P-R interval (PR (ms) = 53 × BM0.24) where BM is body mass in kg (Noujaim, et al., 2004; Prothero, 1979). Hearts of smaller species need to contract and relax more rapidly than larger species in order to maintain cardiac output at high heart rates. This difference in cardiac contractile kinetics is highlighted in Figure 1; ventricular muscles of various species were stimulated ex vivo at or slightly below their in vivo resting heart rates. The time it takes for contraction and relaxation vary between species due to differences such as excitation, calcium handling, and myofilament protein isoforms(Janssen & Periasamy, 2007). As a simple rule, the closer the heart or body weight of the animal model to human; the more similar are the hearts. Depending on the cardiovascular process being studied, the choice of animal model needs to be considered carefully since it affects experimental outcomes and whether findings of the study can be reasonably translated to humans.
Table 1.
Comparison of cardiovascular parameters of human and animal models. Lowest and highest values from multiple sources as summarized in (Ostergaard, et al., 2010)
| Species | Body Weight (kg) |
Heart Rate (bpm) |
Systolic Pressure (mmHg) |
Diastolic Pressure (mmHg) |
|---|---|---|---|---|
| Mouse | 0.02–0.063 | 310–840 | 113–160 | 81–110 |
| Rat | 0.225–0.52 | 250–493 | 84–184 | 58–145 |
| Rabbit | 1–6 | 130–300 | 90–130 | 60–90 |
| Canine | 7–16 | 70–160 | 95–136 | 43–66 |
| Sheep | 20–160 | 60–120 | 91–116 | 102 |
| Pig | 200–300 | 50–116 | 135–150 | - |
| Human | 50–86 | 72 | 120 | 80 |
Figure 1.
Right ventricle muscles were stimulated ex vivo near the species’ resting heart rates as indicated. For clarity purposes, only a single twitch of each species is shown. Temperature is 37 °C in all traces. Sources of tracings are as follows, mouse: C57BL/10 strain adapted from (J.A. Rafael-Fortney, et al., 2011), rat: male LBNF-1 strain adapted from (Monasky, et al., 2007), rabbit: unpublished data, male New Zealand White rabbit, canine: mixed-breed canine adapted from (Billman, et al., 2010), Human: unpublished data, donor heart with abnormal ECG, borderline concentric left ventricular hypertrophy, mitral valve regurgitation, and ejection fraction of 55%
As will be discussed later in detail, there is no perfect animal model of the human heart; each model has its own set of advantages and disadvantages. The purpose of this review is to provide an overview of the strengths and weaknesses of commonly used animal models including mouse, rat, rabbit, canine, pig, and sheep in cardiovascular research.
2. Small Rodent Models (Mouse and Rat)
Advantages
The small rodent animal models, mouse and rat, have unique properties that make them valuable and indispensable to cardiac research. Although, as discussed below, there are very significant differences between small rodents and humans; the use of rodent animal models has provided a wealth of valuable insight into the human cardiac physiology and disease.
Small rodents are easier to handle and house, have a shorter gestation time, and have lower maintenance cost than larger animal models. These characteristics make small rodent models logistically and economically the most used model for cardiac physiology and disease, genetics, pharmacology, and long-term survival studies. Technological advances have allowed various in vivo cardiac parameters to be measured in small rodents which can complement molecular, in vitro, and ex vivo functional studies. These techniques include but not limited to echocardiography (Elnakish, Hassanain, & Janssen, 2012; Pleger, et al., 2007), cardiovascular magnetic resonance imaging (Moon, et al., 2012; J. A. Rafael-Fortney, et al., 2011), electrocardiography (Elnakish, et al., 2012; Fischer, et al., 2007), pressure-volume loops (Georgakopoulos & Kass, 2001; Joho, et al., 2007; Lieber, et al., 2008; Murphy, et al., 2012), and blood pressure (Elnakish, et al., 2012; Fischer, et al., 2007). For comprehensive reviews of some of these techniques please see (Cingolani & Kass, 2011; Hartley, et al., 2011; Ram, Mickelsen, Theodoropoulos, & Blaxall, 2011).
One of the most advantageous aspects of utilizing mice is the ability to make genetic models. Although such models can be produced in larger species as well, mouse models can be developed in a shorter period of time due to their short gestation age of ~18–21 days (Ostergaard, et al., 2010) and with substantially lower cost. Genetic mouse models have been developed that target a variety of cardiovascular processes ranging from excitation to metabolism. Such models provide a wealth of knowledge ranging from protein function to mechanism and progression of cardiovascular disease. Cardiac excitation, contraction, and relaxation of small rodents and humans share many similarities and both groups express proteins with similar functions and roles. Therefore, genetically modified mouse models can be utilized in order to probe the function of various genes in cardiac physiology and disease which can be utilized to address vital questions. However, there are also important differences between mouse and humans myocardium which can hamper translation of such mouse studies to humans. The use of genetically modified mouse models allows for rapid establishment of proof-of-principle that can later be extended into larger animal models (which better represent the human myocardium) and eventually into humans.
Despite their widespread use, of all the most commonly used animal models, mice are most remote from human contractile function, mainly due to their small size and short lifespan. Thus, translational aspects and value of genetic mouse models must be interpreted with caution. Although they may recapitulate some of the characteristics of the human cardiac phenotype of a disease, they typically do not recapitulate all aspects of human cardiovascular disease. For instance, patients suffering from Duchenne Muscular Dystrophy (DMD), an X-linked recessive disorder resulting from lack of dystrophin protein (Bulfield, Siller, Wight, & Moore, 1984; Hoffman, Brown, & Kunkel, 1987), have a life expectancy of mid-20s with about 90% having some degree of cardiac involvement and 10–20% developing heart failure (Finsterer & Stollberger, 2003). The mdx (dystrophin deficient) mouse model has a rather mild cardiomyopathy, mild limb-skeletal muscle dysfunction, and a normal life span, in contrast to human counterparts (Bulfield, et al., 1984; Delfin, et al., 2011). This is partially due to the compensatory effects of utrophin, a dystrophin homolog, which can offset the loss of dystrophin in mice. Indeed it has been shown that a mouse model lacking both dystrophin and utrophin shows a much more severe phenotype, and a reduced lifespan (Deconinck, et al., 1997; Delfin, et al., 2011; Janssen, Hiranandani, Mays, & Rafael-Fortney, 2005). Another notable example of how murine models may differ in phenotype resulting from functional absence of the same protein can have opposing effects in mouse and humans. The phospholamban (PLB) knock-out mouse has enhanced cardiac contractility, without affecting life span (Slack, et al., 2001). In contrast, a nonsense T116G gene mutation in humans that causes a deficiency of phospholamban results in severe dilated cardiomyopathy in individuals homozygous for the mutation, requiring cardiac transplantation (Haghighi, et al., 2003). These cases illustrate the differential response of mice and humans to genetic alterations and the limitations of genetic mouse models in studying human cardiovascular disease.
Genetic manipulation of myocardial tissue via viral vectors is easier to perform in small rodents than larger animal models. This is mainly due to the cost associated with production of virus: typically, the absolute amount of virus needed is roughly proportional to body weight. Various different types of viral vectors have been used for myocardial transduction of both animal models and humans. The adeno-associated virus serotypes AAV-1 (Jessup, et al., 2011; Palomeque, et al., 2007), AAV-6 (Palomeque, et al., 2007; Pleger, et al., 2007), AAV-8 (Palomeque, et al., 2007; Z. Wang, et al., 2005), and AAV-9 (Bish, Morine, et al., 2008; Fang, et al., 2012; Goehringer, et al., 2009; Inagaki, et al., 2006) have emerged as suitable vectors in cardiovascular research due to their ability to transduce cardiac tissue efficiently. Targeting myocardium of large species with such viral vectors is not simple; in one study intravenous delivery of AAV-9 failed to transduce cardiomyocytes in dogs (Yue, et al., 2008). Successful cardiac transduction in large species requires surgical and catheter-based approaches that can preferentially target the heart (Pepe, et al., 2010; Pleger, et al., 2011). Conversely, mouse myocardium can be successfully transduced via intraperitoneal (Z. Wang, et al., 2005) and intravenous tail-vein injections (Inagaki, et al., 2006) which are technically less challenging and financially less costly. It must be mentioned that methods such as intracoronary delivery that preferentially target the heart can also be performed in mouse (Fang, et al., 2012) and rats (Pleger, et al., 2007) as well. AAV-9 has been shown to have superior cardiotropism than other serotypes in small rodents (Bish, Morine, et al., 2008; Fang, et al., 2012; Inagaki, et al., 2006); however, the cardiac tropism of this serotype is dependent on species. For example, in one study in canines, self-complimentary (sc) AAV-6 was superior to both scAAV-8 and scAAV-9 serotypes when delivered via percutaneous endomyocardial injections (Bish, Sleeper, et al., 2008). Furthermore, different laboratory animal models have different levels of neutralizing antibodies against various AAV serotypes which can limit the efficiency of transduction (Rapti, et al., 2012). Therefore, it is necessary to account for these variables if studies are extended from small rodents into larger species.
Cardiovascular pharmacology can be investigated in rodents readily, efficiently, and with relatively high through-put when compared to larger animal models. The low housing cost of rodents means that multiple combinations of medication regiments can be studied simultaneously short-term as well as long-term with relatively low financial cost. It must be cautioned that certain drugs can have variable effects in humans vs. rodents. For example, diazoxide, an ATP sensitive potassium (KATP) channel opener, affects action potential duration (APD) of atria and ventricles of mouse and humans differently. The same group of investigators using similar optical mapping technique showed that the same concentration of diazoxide decreased mouse atria APD while it had no effect on human atria APD. The opposite was observed in the ventricles; diazoxide did not affect mouse ventricle APD while it slightly decreased human ventricle APD (Fedorov, et al., 2011; Glukhov, Fedorov, et al., 2010). These studies highlight the limitations of directly translating pharmacological efficacy and toxicity of compounds from murines to humans, which is in addition to known differences in pharmacokinetics.
There are a variety of mouse and rat cardiac dysfunction and heart failure models available, each with its own advantages and disadvantages. Briefly, two of the most commonly utilized techniques for inducing left-sided heart failure in mouse and rats are aortic constriction induced pressure overload (Chen, et al., 2012; del Monte, et al., 2001; Respress, et al., 2012) and coronary ligation induced myocardial infarction (Y. H. Liu, et al., 1997; Most, et al., 2006; Ontkean, Gay, & Greenberg, 1991; Vercauteren, et al., 2006). The advantages of using these models are that they present significantly lower cost than larger animals, and the ability to investigate roles of various proteins and signaling pathways in progression of heart disease by combining these intervention models and genetically modified mouse models. Right-sided hypertrophy (Hessel, Steendijk, den Adel, Schutte, & van der Laarse, 2006; Kogler, et al., 2003) and heart failure (Hessel, et al., 2006; Redout, et al., 2007; Seyfarth, et al., 2000) can be induced in rats with relative ease with use of monocrotaline, a plant alkaloid which causes pulmonary hypertension without affecting systemic blood pressure (Seyfarth, et al., 2000). This model is particularly attractive as it can be established via a single injection without the need of surgical procedures. However, results are not always highly consistent as not all animals respond similarly; in a single study half of the animals developed heart failure while the other half merely developed hypertrophy despite receiving the same dose (Seyfarth, et al., 2000). Another comprehensive study showed that lower doses of this compound result in right ventricular hypertrophy while higher dosage is required in order to induce right-sided heart failure (Hessel, et al., 2006).
Small rodents are effective animal models with distinct advantages over larger animal models; however, as will be discussed next in detail, they have various limitations and disadvantages.
Disadvantages
There are prominent differences between rodent and human hearts that must be taken into consideration when small rodents are being utilized as cardiac animal models. Rodent hearts, unlike humans, have adapted to function at very high heart rates where rapid systolic contraction and diastolic filling are necessary for maintaining cardiac output. The ventricular APD in small rodents (review; (Nerbonne, 2004), mouse: (Glukhov, Flagg, Fedorov, Efimov, & Nichols, 2010) is much shorter than humans (Fedorov, et al., 2011; Glukhov, Fedorov, et al., 2010). Mouse action potentials have a rapid repolarization and lack a prominent plateau phase which is found in human cardiomyocytes (Review, (Nerbonne, 2004)). Differences between small animal models and human cardiomyocytes are also present at the level of the myofilaments. Small rodent ventricular cardiomyocytes predominately express fast α-MHC (>94–100%) (Alpert, et al., 2002; Hamilton & Ianuzzo, 1991; Krenz, et al., 2003; Lemon, Papst, Joly, Plato, & McKinsey, 2011; J. Wang, et al., 2002) which has faster kinetics (Locher, et al., 2009; Milani-Nejad, Xu, Davis, Campbell, & Janssen, 2013; Rundell, Manaves, Martin, & de Tombe, 2005) but also higher tension cost and lower economy (Locher, et al., 2009; Rundell, et al., 2005) than the slow β-MHC (>90–95%) found in human ventricles (Miyata, Minobe, Bristow, & Leinwand, 2000; Reiser, Portman, Ning, & Schomisch Moravec, 2001). Human ventricles express both compliant N2BA and stiff N2B titin isoforms with a relative N2BA:N2B ratio of ~0.4–1.2 (Cazorla, et al., 2000; Makarenko, et al., 2004; Nagueh, et al., 2004). Mouse and rat predominately express N2B titin and considerably lower N2BA titin isoform with N2BA:N2B ratio ranges of ~0.05–0.25 in mouse and ~0.06–0.1 in rat (Cazorla, et al., 2000; Neagoe, Opitz, Makarenko, & Linke, 2003; Opitz & Linke, 2005). The mentioned ratio in rats does not take into account the study by Cazorla et al., which did not detect a quantifiable N2BA titin isoform band (Cazorla, et al., 2000). In addition to isoform variations; differences can also be encountered at the level of post-translational modifications. Mouse cardiac troponin I (cTnI) is phosphorylated to a greater degree (only ~20–24% un-phosphorylated) than its human counterpart (~44–50% un-phosphorylated) (Ayaz-Guner, Zhang, Li, Walker, & Ge, 2009; Taglieri, et al., 2011; van der Velden, et al., 2003; Zhang, et al., 2011). We must caution this comparative analysis by citing other studies which have shown a much higher extent of human cTnI phosphorylation (with un-phosphorylated group accounting for as low as ~2–10%) (Kooij, et al., 2010; van Dijk, et al., 2011; Wijnker, et al., 2013). At least one of these reports acknowledged that the use of inotropic agents affected the level of phosphorylation in the samples (Kooij et al. 2010). The elevated levels of mouse cTnI phosphorylation might be necessary at high heart rates for accelerating relaxation by decreasing myofilament calcium sensitivity at physiological temperature (Janssen, Stull, & Marban, 2002; Yasuda, Coutu, Sadayappan, Robbins, & Metzger, 2007). Furthermore, myofilament calcium sensitivity is often reported to be increased in several forms of heart failure (Marston & de Tombe, 2008); the magnitude of this shift is less in mouse and rat than humans and it has been suggested that the higher basal phosphorylation level of mouse cTnI might be responsible (Hamdani, et al., 2008). Calcium is necessary for activation of myofilaments and removal of activating calcium is essential for cardiac relaxation and proper diastolic filling; SERCA is responsible for resequestration of 90–92% (Bassani, Bassani, & Bers, 1994; L. Li, Chu, Kranias, & Bers, 1998) of this calcium in rodents while in humans it accounts for 76% (Piacentino, et al., 2003) with NCX accounting for majority of the rest in both groups. In one study it was shown that the relative contributions of SERCA and NCX in humans (as assessed by paired rapid cooling contractions) can vary with stimulation frequency (Pieske, Maier, Bers, & Hasenfuss, 1999) while a similar phenomenon was not observed at sub-physiological frequencies (0.25–3 Hz) in rats in another study (Maier, Bers, & Pieske, 2000).
It needs to be emphasized that the heart is a heterogeneous organ with molecular and functional differences between not only the atria and ventricles (Cazorla, et al., 2000; Glukhov, Fedorov, et al., 2010; Locher, Razumova, Stelzer, Norman, & Moss, 2011) and left and right ventricles (Neagoe, et al., 2003; G. Y. Wang, et al., 2006); but also apico-basally (Neagoe, et al., 2003; Szentadrassy, et al., 2005) and transmurally from sub-epicardial to sub-endocardial regions within the same ventricle (Bell, et al., 2000; Bouvagnet, Mairhofer, Leger, Puech, & Leger, 1989; Cazorla, et al., 2000; Glukhov, Fedorov, et al., 2010; Laurita, Katra, Wible, Wan, & Koo, 2003; D. W. Liu, Gintant, & Antzelevitch, 1993; Stelzer, Norman, Chen, Patel, & Moss, 2008). Therefore, slight variations in function can be observed when different regions of the heart, even within the same ventricle, are studied and compared across species.
Differences at the cardiomyocyte level have profound impact on cardiac contraction. Table 2 shows contractile parameters of mouse, rat, and human myocardium ex vivo (isometric contraction of right ventricular trabeculae within or slightly below resting heart rate) and in vivo (left ventricular pressure-volume catheterization). The kinetics of contraction (ex vivo: TTP, normalized (dF/dtmax)/F and in vivo: dP/dtmax) and relaxation (ex vivo: RT50 and normalized (dF/dtmin)/F and in vivo: τ and dP/dtmin) are considerably accelerated in small rodents. Note that the dF/dtmax and dF/dtmin of experiments on trabeculae were normalized to the developed forces (yielding (dF/dtmax)/F and (dF/dtmin)/F, respectively) in order to account for the differences in force production, i.e. the resulting parameter is purely kinetic (s−1). Temperature is a very powerful modulator of contractile kinetics in in vitro experiments, (Hiranandani, Varian, Monasky, & Janssen, 2006; Janssen, et al., 2002; Little, et al., 2012). However, temperature is not a factor in this species-dependent “acceleration”, since experiments of all three species were performed at 37 °C and catheterizations in animals/subjects are also performed at physiological body temperature, which is very much similar across these species. The cardiomyocyte differences outlined above such as action potential properties and myosin heavy chain isoforms are responsible for faster contractile and relaxation kinetics in small rodents. The frequency of contraction is a contributing factor; an increase in this rate results in acceleration of contraction and relaxation both in vivo and ex vivo within the physiological heart rate range (Lujan, Janbaih, Feng, Jin, & DiCarlo, 2012; Monasky, Varian, & Janssen, 2007; Slabaugh, Brunello, Gyorke, & Janssen, 2012). Differences in stimulation frequencies are only partially and to a minor extent responsible for faster kinetics in smaller species. To address the differences in stimulation frequencies, consider contractile parameters if the same group of mouse trabeculae are stimulated at 2 Hz (far outside of the lower physiological range of mouse). In such a scenario the TTP, RT50, dF/dtmax/F and dF/dtmin/F slow down to 46.5 ms, 26.6 ms, 36.2 s−1, −27.7 s−1 respectively (Stull, et al., 2006). The contractile kinetics of the mouse myocardium are still considerably faster (approximately five times) than human myocardium under these “sub-physiological” conditions, albeit at a lower magnitude. This analysis underscores the limitations of using small rodents for investigating cardiac kinetics and translating such studies to humans; larger animal species will generally be a better model in such situations. It must also be noted that anesthetic agents such as isoflurane can depress cardiac contractility and kinetic parameters (Joho, et al., 2007); the in vivo data for animal models in Table 2 were conducted when animals were in conscious state.
Table 2.
Selected ex vivo and in vivo cardiac parameters in small rodents and humans. Right ventricular trabeculae from mouse (male C57-BL/6, FVBN, and SV129 strains), rat (male LBN-F1 strain), and human (2 females, 3 male) were stimulated at 10 Hz, 6 Hz, and 0.5 Hz, respectively. Temperature of 37 °C in all groups. Calcium concentrations (in mM) were 1.5 (mouse and rat) and 1.75 (human). TTP (time to peak: time from stimulation to peak force), dF/dtmax (maximal velocity of contraction), and (dF/dtmax)/F (maximal velocity of contraction divided by developed force) represent contraction kinetics. RT50 (Relaxation time 50%: time from peak force to 50% relaxation), dF/dtmin (maximal velocity of relaxation), and (dF/dtmin)/F (maximal velocity of relaxation divided by developed force) represent relaxation kinetics. dF/dtmax and dF/dtmin values were divided by developed force yielding (dF/dtmax)/F and (dF/dtmin)/F, respectively. Cardiac catheterization measurements performed in control humans (4 females/8 males) and conscious mouse (male C57-BL/6) and conscious rat (F344×BN). LVSP: left ventricular systolic pressure. LV τ: left ventricular isovolumic relaxation time constant. LV dP/dtmax: left ventricular maximal rate of pressure rise. LV dP/dtmin: left ventricular maximal rate of pressure decline. Isovolumic relaxation time constant of mouse and rat calculated with Weiss method.
| Parameter | Mousea,b | Ratc,d | Humane,f | Ratio |
|
|---|---|---|---|---|---|
| Mouse/Human | Rat/Human | ||||
| Ex Vivo Right Ventricular Trabeculae | |||||
| Force (mN/mm2) | 40.0 | 27.8 | 16.7 | 2.40 | 1.66 |
| TTP (ms) | 36.3 | 54.2 | 235 | 0.15 | 0.23 |
| RT50 (ms) | 20.4 | 29.4 | 153 | 0.13 | 0.19 |
| dF/dtmax (mN/mm2/s) | 1,899.5 | 842.9 | 129.0 | 14.72 | 6.53 |
| dF/dtmin (mN/mm2/s) | −1,579.8 | −768.7 | −98.3 | 16.07 | 7.82 |
| (dF/dtmax)/F (s−1) | 47.5 | 30.3 | 7.7 | 6.17 | 3.94 |
| (dF/dtmin)/F (s−1) | −39.5 | −27.7 | −5.9 | 6.69 | 4.69 |
| In Vivo Catheterization | |||||
| LVSP (mmHg) | 110 | 106 | 115 | 0.96 | 0.92 |
| LV τ(ms) | 4.4 | 6.3 | 43 | 0.10 | 0.15 |
| LV dP/dtmax (mmHg/s) | 18,560 | 11,106 | 1,491 | 12.45 | 7.45 |
| LV dP/dtmin (mmHg/s) | −17,616 | −7,133 | −1,869 | 9.43 | 3.82 |
The differences outlined above can also result in differential response of small rodents and humans to cardiac diseases. For example, the expression of a variety of cardiac proteins is altered in heart failure; one such alteration is down-regulation of fast α-MHC and up-regulation of slow β-MHC at the protein level in both human heart failure and experimental animal heart failure models. In rats, α-MHC protein can decrease from 93–97% to 22–71% while β-MHC protein can increase from 3–6% to 29–78% during heart failure (Machackova, Sanganalmath, Elimban, & Dhalla, 2009; J. Wang, Guo, & Dhalla, 2004; J. Wang, et al., 2002) and this alteration can have profound impact on slowing of cross-bridge cycling kinetics. Krenz et al. made a transgenic β-MHC mouse line of which heterozygous mice expressed ~40% β-MHC (Krenz, et al., 2003). This 40% alteration, which is close to the values observed in experimental small rodent heart failure, resulted in decreases in unloaded shortening velocity, maximal shortening velocity, and power output. Therefore, MHC isoform switching is a major contributing factor to the decline in cross-bridge kinetics observed in experimental rodent heart failure models. However, the magnitude of this isoform switching in small rodents is much higher than the 5–10% α-MHC which is normally expressed in non-failing human myocardium (Miyata, et al., 2000; Reiser, et al., 2001). Cross-bridge cycling kinetics are also slowed in failing human myocardium (Hajjar & Gwathmey, 1992; Ruf, et al., 1998) and α-MHC has been shown to be downregulated (Miyata, et al., 2000; Reiser, et al., 2001); however, the extent of this isoform switching is much lower than small rodents. Even if 100% isoform switching takes place in human heart failure it would results in a mere 5–10% reduction of expression of α-MHC. One group of investigators addressed whether this nominal isoform switching can impact cross-bridge cycling kinetics by developing two transgenic rabbit lines (expressing 15% and 40% α-MHC). The rabbit myocardium is more similar to the human myocardium than mice and rats (see next section). Cross-bridge cycling kinetics, as assessed by sinusoidal length perturbation, was increased in the 40% line but unchanged in the 15% over-expression line. Although, it must be noted that the 15% α-MHC line was capable of producing 20% more overall power than control (Suzuki, et al., 2009). Such experiments question whether the 5–10% decrease in α-MHC has important functional effects in human heart failure as compared to the dramatic decline seen in small rodent heart failure. This notion has been pointed out by others as well (Jweied, deTombe, & Buttrick, 2007). We must also cite other works which have shown that minimal changes in MHC isoform content can have profound impacts on cardiac contraction. A mouse transgenic line expressing only 12% β-MHC had a 23% reduction in ATPase activity and 15% reduction in dP/dtmax as assessed using a Langendorff preparation (Tardiff, et al., 2000). Similarly, in another study using rats expressing β-MHC (hypothyroidism induced), it was shown that 12% α-MHC can dramatically increase power output by 52% (Herron & McDonald, 2002). Model predictions in porcine myocardium suggest that a 10% increase in β-MHC can accelerate simulated twitch kinetics (Locher, et al., 2011). The porcine sub-endocardium expresses ~10% less α-MHC than the sub-epicardium; interestingly the sub-endocardial porcine fibers had proportionately reduced rate of tension redevelopment (ktr) and stretch activation kinetics (Stelzer, et al., 2008). Further studies are needed to address these discrepancies but nonetheless the magnitude of myosin heavy chain isoform switching is much greater in experimental small rodent heart failure models than in human heart failure, and the impact of this switch is more dramatic in the former group. Similar lines of arguments can be made for other processes that are altered in heart failure such as calcium handling where small rodents rely more upon SR calcium (as apposed to L-type calcium channel / NCX cycling) than humans (Jweied, et al., 2007). Therefore, caution should be observed when relating and translating studies and mechanisms of disease from small rodents to humans.
In addition, the cardiac response of small rodents and humans to exercise is different. The average human can dramatically increase their heart rate from ~65–79 bpm during rest to ~173–188 bpm during exercise (Flamm, et al., 1990; Stratton, Levy, Cerqueira, Schwartz, & Abrass, 1994), an increase of approximately 140–170%. Although this maximal heart rate is age dependent (Stratton, et al., 1994) and can be predicted via formula such as HRmax = 220 – age or HRmax = 208 – 0.7 × age (Tanaka, Monahan, & Seals, 2001). Rats can increase their heart rates from approximately 350–370 bpm to 500–550 bpm (~40–50% increase) (Bolter & Atkinson, 1988; Lujan, et al., 2012) and mice can increase their hear rates from 500–600 bpm to 700–800 bpm (~30–40% increase) (Desai, et al., 1997; Fewell, et al., 1997; Lujan, et al., 2012). The force-frequency relationship (cardiac contractile force as a function of stimulation frequency) near these in vivo heart rates is severely blunted in small rodents as compared to humans. Contractile force (assessed ex vivo by isometric contraction of cardiac trabeculae) of human myocardium increases 81–168% from 0.5 Hz to 2.5 Hz (Chaudhary, et al., 2004; Pieske, et al., 1996; Rossman, et al., 2004). There is an increase of roughly 30–40% from 4 Hz to 8 Hz in rat myocardium (Modified from (Monasky, et al., 2007)) whereas there is a nearly flat relationship from 8 to 12 Hz in mouse myocardium (Modified from (Stull, et al., 2006)). Because cardiac output (CO) is the product of heart rate (HR) and stroke volume (SV), humans have a much greater ability than rodents to increase their cardiac output during exercise by both heart rate and stroke volume mediated mechanisms. In other words, the cardiac reserve of humans is much greater than rodents. Where a mouse can increase its cardiac output by typically no more than one-third, humans can increase cardiac output as high as 10-fold in highly trained individuals. These differences can affect outcomes of studies and experiments that involve exercise training.
Genetic and pharmaceutical means can be employed to circumvent some of these differences. For example, hypothyroidism (either by administration of propylthiouracil or thyroidectomy) in rats results in down-regulation of α-MHC and up-regulation of β-MHC (Rundell, et al., 2005; Tang, et al., 2005). Although, this procedure allows for studying β-MHC (which is predominately expressed in human ventricles) in rats, there are also reports that indicate hypothyroidism also induces changes in calcium handling (Cernohorsky, Kolar, Pelouch, Korecky, & Vetter, 1998; Kiss, Jakab, Kranias, & Edes, 1994; Shenoy, Klein, & Ojamaa, 2001), titin and collagen expression (Wu, Peng, Campbell, Labeit, & Granzier, 2007), and phosphorylation of some proteins (Ojamaa, Kenessey, & Klein, 2000; Suarez, Scott, Suarez-Ramirez, Chavira, & Dillmann, 2010; Tang, et al., 2005).
The hearts of mouse and rat share many characteristics; however, there are differences even between these two species. As discussed earlier, rats have a greater ability to increase their heart rate during exercise, a more positive FFR, and slightly slower kinetics of contraction and relaxation as compared to mice. Besides these variations in kinetics and heart rate ranges, other differences also exist. For example, phenylephrine (a nonselective α1-adrenerecptor agonist) induces a triphasic response in both mouse and rat right ventricular myocardium. However, in mice the overall effect of this drug is a net negative inotropic response while in rats it is a net positive inotropic response (McCloskey, et al., 2002).
Mice and rats are essential animal models in cardiac research allowing for relatively rapid, high through-put, and cost effective means of studying cardiac physiology, disease and novel therapeutic targets. Cardiac excitation and contractility in these small rodents are inherently different from human hearts and whether these differences can limit translation of rodent studies to humans depends on the particular cardiac process being investigated.
3. Rabbit Models
Advantages
Rabbits have several advantages that make them valuable to cardiac research. Although, other large species such as canine and sheep more closely resemble the human heart; the cost of acquiring and housing rabbits is still significantly much lower which makes them an attractive alternative to larger animal models in cardiac research from an economic/logistic perspective. Assessing myocardial function in intact animals is not a limitation for this species, as various in vivo quantitative and imaging techniques such as echocardiography (Pogwizd, Qi, Yuan, Samarel, & Bers, 1999), hemodynamic cardiac catheterization (Mahaffey, Raya, Pennock, Morkin, & Goldman, 1995; A. S. Shah, et al., 2000), electrocardiography (Pogwizd, et al., 1999), and cardiac magnetic resonance imaging (Jung, et al., 2012) are well established and are routinely performed in rabbits.
Many aspects of rabbit myocardium have greater similarity to humans than small rodents, making rabbits a model generally more closely representative of the human heart. Rabbits have an in vivo heart rate range of 155–360 bpm (Gaustad, Rolim, & Wisloff, 2010; Jover, McGrath, & Ludbrook, 1987) which is still higher than humans (Flamm, et al., 1990; Stratton, et al., 1994) but significantly closer than mouse and rats (see previous section for heart rates). Both rabbit and human ventricles exhibit robust positive force-frequency responses. Increasing the stimulation frequency in rabbit (from 1 Hz to 4 Hz) and human in vitro muscle preparations (from 0.5 Hz to 2.5 Hz) results increase in contractile force of about 120–200% and 81–168%, respectively (Chaudhary, et al., 2004; Monasky & Janssen, 2009; Pieske, et al., 1996; Rossman, et al., 2004; Varian & Janssen, 2007; Varian, et al., 2009). Rabbit ventricles predominately express β-MHC (88% to >95%) (Hamilton & Ianuzzo, 1991; James, et al., 2005; Suzuki, et al., 2009) similar to humans (>90–95%) (Miyata, et al., 2000; Reiser, et al., 2001). Rabbit ventricular SERCA and NCX systems are responsible for removing 70–74% and 23–28% of calcium, respectively (Bassani, et al., 1994; Puglisi, Bassani, Bassani, Amin, & Bers, 1996). These values are again similar to the relative activities of SERCA (76%) and NCX (24%) in human ventricles (Piacentino, et al., 2003). On the other hand, besides the relative contributions of these two systems to calcium removal, one report showed that rabbit SERCA by itself resequesters calcium faster than human SERCA in isolated myocytes. The time constant of SERCA dependent calcium decline alone (when NCX activity is blocked) is about 50 percent slower in humans than rabbits (approximately 600 ms in humans vs. 400 ms in rabbits) (Z. Su, et al., 2003). Therefore, slight differences in calcium handling exist when comparing rabbit and human cardiomyocytes.
The net effects of these similarities can be observed by comparing and contrasting cardiac contraction of rabbit and human myocardium ex vivo and in vivo (Table 3). The kinetics of rabbit cardiac contraction and relaxation are approximately 2–3 times faster than humans yet more similar than smaller rodents where kinetics are as much as 4–12 times faster (See Table 2). Note that stimulation frequency affects contractile and relaxation kinetics to some degree (See “Small Rodent Models” section for discussion) which is partially responsible for faster kinetics observed in rabbits. These similarities also result in the response of rabbit myocardium to stress and disease to better reflect and represent the human situation than smaller rodent models. Various methods for generating rabbit heart failure models are available including pacing-induced failure (D. Liu, Gao, Roy, Cornish, & Zucker, 2006), combined aortic insufficiency induced volume overload and aortic constriction induced pressure overload (Pogwizd, et al., 1999), and coronary ligation (Maurice, et al., 1999; D. C. White, et al., 2000). Due to the characteristics outlined above, using rabbits removes the concerns that are present with small rodent models such as differential responses of MHC isoform switching and relative changes in the contribution of SERCA and NCX to relaxation. Rabbits can also be used to study hypertrophy induced by pressure overload: ten weeks of pulmonary artery banding (PAB) is sufficient to induce hypertrophy in rabbits without signs and symptoms of heart failure in the right ventricle (Varian, et al., 2009) and with altered right atrial calcium handling (Gupta, et al., 2009).
Table 3.
Selected ex vivo and in vivo cardiac parameters in rabbits and humans. Right ventricular trabeculae from rabbit (female White New Zealand) and human (2 females, 3 male) were stimulated at 1 Hz and 0.5 Hz, respectively. Temperature of 37 °C and calcium concentration of 1.75 mM in both groups. TTP (time to peak: time from stimulation to peak force) and (dF/dtmax)/F (maximal velocity of contraction divided by developed force) represent contraction kinetics. RT50 (Relaxation time 50%: time from peak force to 50% relaxation) and (dF/dtmin)/F (maximal velocity of relaxation divided by developed force) represent relaxation kinetics. dF/dtmax and dF/dtmin (not shown) values were divided by the developed force yielding (dF/dtmax)/F and (dF/dtmin)/F, respectively. Cardiac catheterization measurements performed in control humans (4 females/8 males) and conscious rabbits (male New Zealand White, sham operated). LVSP: left ventricular systolic pressure. LV τ: left ventricular isovolumic relaxation time constant. LV dP/dtmax left ventricular maximal rate of pressure rise. LV dP/dtmin left ventricular maximal rate of pressure decline.
| Parameter | Rabbita,b | Humanc,d | Ratio |
|---|---|---|---|
| Rabbit/Human | |||
| Ex Vivo Right Ventricular Trabeculae | |||
| Force (mN/mm2) | 7.98 | 16.7 | 0.48 |
| TTP (ms) | 117 | 235 | 0.50 |
| RT50 (ms) | 61 | 153 | 0.40 |
| (dF/dtmax)/F (s −1) | 15.2 | 7.7 | 1.97 |
| (dF/dtmin)/F (s −1) | −12.5 | −5.9 | 2.12 |
| In Vivo Catheterization | |||
| LVSP (mmHg) | 112 | 115 | 0.97 |
| LV τ (ms) | 13.7 | 43 | 0.32 |
| LV dP/dtmax (mmHg/s) | 5,367 | 1,491 | 3.60 |
| LV dP/dtmin (mmHg/s) | −3,679 | −1,869 | 1.97 |
References:
Modified from (Janssen, et al., 2000),
Genetic models targeting a variety of cardiac excitation, contraction, and regulatory processes have been developed in rabbits. As discussed earlier, rabbit myocardium shares more similarities with the human myocardium than small rodents; therefore, rabbit genetic models, albeit more expensive, can be used as a stepping stone to determine whether a particular study can be extended to humans and larger animal models. Some rabbit models have actually shown divergent effects of a particular manipulation as compared to mouse models (Nishizawa, et al., 2006). Furthermore, it has been argued and shown that rabbits harboring human hypertrophic cardiomyopathy mutations often better recapitulate this category of human diseases than their murine model equivalents (Marian, et al., 1999; Sanbe, et al., 2005).
Disadvantages
One of the major disadvantages of using rabbits is the cost associated with acquiring and housing them as compared to mice and rats. This becomes a particular concern when long-term studies and genetic models are conducted in this species. However, as discussed earlier, rabbits are still cheaper than larger animal models including canine, pig, and sheep.
Despite a closer similarity to humans than mice/rats, differences between rabbit and human myocardium remain, which can result in a particular study or therapeutic intervention to have differential effects in rabbits and humans. Some of these differences include atrial transient outward current (Fermini, Wang, Duan, & Nattel, 1992; Z. Wang, et al., 1999), ventricular expression levels of some potassium ion channel subunits (Zicha, et al., 2003), relative expression levels of cardiac titin N2B and N2BA isoforms (Cazorla, et al., 2000; Makarenko, et al., 2004; Nagueh, et al., 2004; Neagoe, et al., 2003), and left-ventricular wall motion (Jung, et al., 2012). When comparing in vivo and ex vivo cardiac contractile parameters of rabbit and human hearts, the former has faster kinetics (Table 3). Therefore, depending on the particular cardiovascular process being studied, caution needs to be taken when trying to extrapolate studies from rabbits to humans.
Some experimental phenomena in rabbit and human hearts appear to be species-dependent; one such example is post-rest potentiation behavior of isolated cardiac muscles. In this type of experiment, electrical stimulation is paused for a given amount of time and the first twitch tension is measured once stimulation is resumed. Following periods of rest after steady pacing, rabbits exhibit a post-rest decay where the first twitch tension is decreased while humans and rats show post-rest potentiation where the first twitch tension is increased as the rest periods are prolonged (Janssen, Lehnart, Prestle, & Hasenfuss, 1999; Maier, Bers, et al., 2000). This has largely been attributed to changes in sarcoplasmic reticulum calcium content during rest periods where rabbits’ and humans’ are decreased and increased, respectively (Maier, Bers, et al., 2000; Pieske, et al., 1999). Interestingly, the rat myocardium similar to humans has post-rest potentiation (Maier, Barckhausen, et al., 2000). Therefore, even though rabbit and human calcium handling mechanisms are more closely related than those of rat, this particular phenomenon in humans phenotypically more closely resembles that of the rat.
Exercise studies suggest that rabbits can increase their heart rates from 155–211 bpm to 328–360 bpm (71–112% increase) during peak exercise (Gaustad, et al., 2010; Jover, et al., 1987). Two notes in regards to the range of this heart rate. First, the resting heart rate of a rabbit is considerably higher than humans; rabbit resting heart rate is near the upper limit of human heart rate even during exercise conditions. Secondly, although this heart rate reserve (and consequently cardiac output reserve) is more than reserves of mice (~30–40%) and rats (~40–50%), it is still less than the reserve (~140–170%) available in humans (Flamm, et al., 1990; Stratton, et al., 1994). Additionally, one report suggests that rabbits are better adapted to run intermittently at higher speeds than continuously at lower speeds. This observation was attributed to the running behavior of rabbits in the wild (Meng & Pierce, 1990). Therefore, rabbits might not serve as the best animal model for studying the effects of exercise on the cardiovascular system. Large animal models, such as canines, will serve as better animal models in such cases.
Overall, rabbits are an attractive model in cardiac research, balancing the increased cost when compared to mice and rats with a generally closer biological resemblance to human. The rabbit heart shares many similarities to that of human although some variations exist. Despite the noted differences, rabbits can serve as a good cardiovascular animal model in lieu of larger animal models.
4. Canine Models
Advantages
The canine and human hearts share many characteristics at both the organ and cellular levels. Canine heart rate, body weight and heart weight are more comparable to humans than mice, rats, and rabbits (Table 1). Furthermore, the human population is heterogeneous with slight genetic variations; a property which is not reflected in the inbred laboratory mouse, rat, or rabbit strains. In that regard, it is suggested that mixed canine breeds such as mongrels are better suited for addressing this issue (Billman, 2005). This is supported by reports that various cardiac functions, parameters, properties, and response to stress vary depending on the mouse strains (Barnabei, Palpant, & Metzger, 2010; Hoit, et al., 2002; Howden, et al., 2008; A. P. Shah, et al., 2010; Stull, et al., 2006; van den Borne, et al., 2009). Due to their size, pretty much all in vivo techniques used to assess contractility of human hearts can be utilized in canines. Beyond these characteristics, the myocardium of canines and humans share many properties and similarities. The action potential duration of canine is only a little bit shorter than that of humans, much more comparable than the animal models discussed previously (canine APD90: ~ 219 ms, human APD90: ~290 ms both measured in right ventricular papillary muscles at cycle length of 1000 ms and 37 °C) (Szel et al. 2011). Additionally, expression patterns of various ion channels both apico-basally (Szentadrassy, et al., 2005) and transmurally (Szabo, et al., 2005; Zicha, et al., 2004) with some exceptions, are very similar between these two species. Furthermore, there is a high degree of resemblance between human and canine sino-atrial (SA) node activity and structure (Review: (Fedorov, Glukhov, & Chang, 2012)), purkinje fiber distribution, and activation sequence (Allison, et al., 2007; Hamlin, 2007). Three separate surveys of the pharmaceutical industry revealed that canine was the most commonly used animal model for evaluation and determination of QT interval prolongation of novel compounds (Friedrichs, Patmore, & Bass, 2005; Hammond, et al., 2001; Lindgren, et al., 2008). For thoroughness purposes, we must point out that it is proposed that the “effective size” of the heart (third root of heart weight divided by spiral wave rotation period) determines ventricular fibrillation pattern. Based upon this analysis, it is argued that rabbits have a closer effective size to humans and hence more similar ventricular fibrillation patterns than either canines or pigs (Panfilov, 2006). Nevertheless, the similarities of human and canine excitation processes and structure make canine a good animal model for studying cardiac electrophysiology as it relates to human health and disease.
The contractile machineries of canine and human cardiomyocytes share many similarities. The canine ventricular myocardium predominately expresses slow β-MHC (>80–100%) similar to humans (>90–95%) (Fuller, Bicer, Hamlin, Yamaguchi, & Reiser, 2007; Hamilton & Ianuzzo, 1991; Miyata, et al., 2000; Reiser, et al., 2001). Both compliant N2BA and stiff N2B isoforms are expressed in canines (N2BA:N2B ratio of ~ 0.6–1) and humans (N2BA:N2B ratio of ~0.4–1.2) (Bell, et al., 2000; Cazorla, et al., 2000; Jaber, et al., 2008; Makarenko, et al., 2004; Nagueh, et al., 2004). Calcium handling mechanisms are also similar between these two species: the relative contributions of SERCA and NCX to calcium removal in canine myocardium are 73% and 27% (Bers, 2001) and in human myocardium are estimated to be 76% and 24% (Piacentino, et al., 2003), respectively. However, one report noted that when NCX is blocked, SERCA is faster at removing calcium by itself in canines than humans (Z. Su, et al., 2003). The same report also showed that the canine SERCA activity is most comparable to humans, followed by rabbits and then mice. Overall, despite the slight differences, the excitation-contraction coupling of canine myocardium is very similar to that of human. These similarities become evident by comparing the contractile parameters of ex vivo cardiac trabeculae and in vivo catheterization studies from canine and human hearts. Contractile and relaxation kinetics of canines are much more comparable to humans than mice and rats (Table 4).
Table 4.
Selected ex vivo and in vivo cardiac parameters in canines and humans. Right ventricular trabeculae from mixed-breed canines and human (2 females, 3 male) were stimulated at 1 Hz and 0.5 Hz, respectively. Temperature of 37 °C in both groups. Calcium concentrations (in mM) were 2 (canine) and 1.75 (human). TTP (time to peak: time from stimulation to peak force) and (dF/dtmax)/F (maximal velocity of contraction divided by developed force) represent contraction kinetics. RT50 (Relaxation time 50%: time from peak force to 50% relaxation) and (dF/dtmin)/F (maximal velocity of relaxation divided by developed force) represent relaxation kinetics. dF/dtmax and dF/dtmin (not shown) values were divided by the developed force yielding (dF/dtmax)/F and (dF/dtmin)/F, respectively. Cardiac catheterization measurements performed in control humans (4 females/8 males) and conscious standing canines (mongrel). LVSP: left ventricular systolic pressure. LV τ: left ventricular isovolumic relaxation time constant. LV dP/dtmax left ventricular maximal rate of pressure rise. LV dP/dtmin left ventricular maximal rate of pressure decline.
| Parameter | Caninea,b | Humanc,d | Ratio |
|---|---|---|---|
| Canine/Human | |||
| Ex Vivo Right Ventricular Trabeculae | |||
| Force (mN/mm2) | 15.8 | 16.7 | 0.95 |
| TTP (ms) | 110.7 | 235 | 0.47 |
| RT50 (ms) | 61.4 | 153 | 0.40 |
| dF/dtmax/F (s −1) | 17.6 | 7.7 | 2.29 |
| dF/dtmin/F (s −1) | −11.9 | −5.9 | 2.02 |
| In Vivo Catheterization | |||
| LVSP (mmHg) | 120 | 115 | 1.04 |
| LV τ(ms) | 28 | 43 | 0.65 |
| LV dP/dtmax (mmHg/s) | 2,948 | 1,491 | 1.98 |
| LV dP/dtmin (mmHg/s) | −2,454 | −1,869 | 1.31 |
References:
Modified from subset of data reported in (Billman, et al., 2010),
The similarities between canine and human myocardium have the consequence that canine models inherently represent human physiology and disease better than small rodent models. Take Duchenne Muscular Dystrophy (DMD) as an example that was previously discussed in the “Small Rodent Models” section. The loss of dystrophin gene product has a profound impact on human hearts while mouse myocardium is only minimally affected. Spontaneous occurring mutations in various canines resulting in lack of dystrophin have been identified and canine muscular dystrophy lines are established. One of these lines is the Golden Retriever Muscular Dystrophy (Cooper, et al., 1988) which develops cardiomyopathy with features that closely resemble those of affected humans (Moise, et al., 1991; Valentine, Cooper, de Lahunta, O'Quinn, & Blue, 1988; Valentine, Cummings, & Cooper, 1989) and is considered to better represent cardiac involvement in human DMD than their mouse mdx counterparts (Nakamura & Takeda, 2010). This case highlights the advantage of utilizing canine genetic models as compared to small rodents in cardiovascular studies.
Canines are commonly utilized as animal models for exercise studies in the cardiac field. The typical canine can dramatically increase its heart rate from 118–125 bpm during rest to 241–279 bpm during maximal exercise (Haidet, Musch, Friedman, & Ordway, 1989; Juneau, Calderone, & Rouleau, 1992; Musch, et al., 1987). This reflects an increase of approximately 96–136% which is close-lower than the 140–170% increase in heart rate observed in humans (Flamm, et al., 1990; Stratton, et al., 1994). Note that canine’s heart rate is faster than human’s during both rest and exercise conditions but closer than the previous species discussed. Furthermore, canine myocardium, similar to healthy human myocardium, exhibit a positive force-frequency relationship as determined by isometrically contracting muscles ex vivo where tension increases 185% from 1 Hz to 3.4 Hz (Taylor, Parilak, LeWinter, & Knot, 2004). We must point out that this particular study was performed at sub-physiological temperature of 30 °C. In fact, the shape of the force-frequency relationship of canines is the closest to humans when compared to mice, rats, and rabbits (Billman, et al., 2010; Janssen & Periasamy, 2007; Monasky & Janssen, 2009; Varian, et al., 2009). These characteristics make canine and human myocardium behave similarly in response to exercise, making the canine generally a good model for exercise studies in cardiovascular research. Indeed, besides force-frequency relationship and alterations in heart rates, changes in other hemodynamic parameters are comparable between canines and humans as well. Some of these parameters include cardiac output (canine: ~2.3, human: ~1.8 fold increase), O2 consumption (canine: ~7 fold increase, human: ~5.7 fold increase), systolic blood pressure (canine: ~0.4, human: ~0.6 fold increase), diastolic blood pressure (canine: 0.16, human: 0.07 fold decrease), and mean arterial pressure (canine: ~0.2, human: ~0.2 fold increase) (Flamm, et al., 1990; Haidet, et al., 1989; Musch, et al., 1987; Stratton, et al., 1994).
Different types of heart disease can be induced in canines by a variety of methods. Some of these models include sudden cardiac death (review: (Billman, 2006)), rapid pacing induced heart failure (Recchia, et al., 1998; Sabbah, et al., 1991), repeated microembolization induced chronic heart failure (Sabbah, et al., 1991), gradual aortic constriction induced left-ventricular pressure-overload (Koide, et al., 1997). Each model has its own set of advantages and disadvantages but a detailed discussion is beyond the scope of this review; however, some of models have been discussed in detail elsewhere (Dixon & Spinale, 2009).
Disadvantages
The housing and maintenance of canine models is considerably more expensive than small rodents and rabbits; therefore, this issue is often to be taken into consideration when long-term studies and disease models are being considered. This is also a downside associated with development of genetic canine models; although as discussed earlier these genetic models have a valid role in cardiovascular research and can in most cases significantly better resemble human disease than small rodents. The rationale for pursuing these studies in canine becomes more convincing if prior studies in small rodents have already established the role of a particular gene, process, or phenomenon in cardiovascular health and disease. One disadvantage unique to canines as compared to other large animal models is obtaining the necessary approval for performing experiments in this species. Although, IACUC (Institutional Animal Care and Use Committee) guidelines vary depending on institutions, it is generally difficult to obtain such approval for using canines as an animal model. Furthermore, even if the use of canines is justified and the appropriate approval is obtained, the number of animals that can be used can be limited. This issue should be taken into account by reviewing the specific institutional policies before designing the study and considering canines as an animal model.
Despite the high degree of similarity between human and canine myocardium, some differences have been reported between these two species. Such differences include resting and exercise heart rates (see above), kinetics of Ito current inactivation, its recovery, and molecular size of its corresponding ion channels, sensitivity to flecanide (Akar, et al., 2004), and transmural epicardial:endocardial expression levels of HERG ion channel (Szabo, et al., 2005).
Introduction of genetic materials with use of viral vectors to canine myocardium is not as straightforward as it is in the case of small rodents. As discussed earlier, mouse myocardial tissue can be successfully transduced with use of AAV (in particular AAV-9 which has a high degree of cardiotropism in mouse) via intraperitoneal (IP) or intravenous (IV) injections. Canine hearts are typically transduced via methods that specifically target the myocardium such as direct intramyocardial (Pepe, et al., 2010) and percutaneous transendocardial injections (Bish, Sleeper, et al., 2008) which are technically more challenging than simple IP and IV methods. Successfully transducing canine myocardium via intravenous injections is not as straightforward and effortless as it is in small rodents. In one study by Yue et al. jugular vein injected AAV-9 in neonatal dogs while successfully transduced skeletal tissue, it failed to do so with cardiac muscle (Yue, et al., 2008). Although, at least two reports suggest that transduction of canine myocardial tissue can be achieved intravenously. Gregorevic et al. were able to transduce canine myocardium by injecting recombinant AAV-6 intravenously in immune-suppressed animals (Gregorevic, et al., 2009). In another recent report, recombinant AAV-8 was able to transduce canine myocardium up to 12 months post-injection when it was injected at high doses intravenously in neonatal canines. (Pan, et al., 2013). These published studies suggest that intravenous introduction of AAV vectors is possible under certain conditions in canine myocardium but how common practice and efficient as compared to its use in mouse models it becomes needs to be further determined.
Overall, the canine myocardium, with its many similarities and relatively few and minor differences, serves as a very good model of the human heart. However, a particular disadvantage is the cost associated with using this model that can hamper performing extensive and widespread studies in this model. The high cost-high risk aspect can be partially alleviated by establishing proof-of-principle studies in smaller models (to reduce likelihood of failure) and later complementing those studies by extending them to canines.
5. Swine Models
Advantages
The swine with its large heart/body weight and similarities to the human cardiovascular system is a valuable pre-clinical animal model. The swine and human myocardium share many characteristics in excitation-contraction coupling. Swine ventricles predominately express β-MHC (~100%, 87% sub-epicardium, 97% sub-endocardium) similar to humans’ (>90–95%) (Hamilton & Ianuzzo, 1991; Locher, et al., 2011; Miyata, et al., 2000; Reiser, et al., 2001; Stelzer, et al., 2008). Both stiff N2B and compliant N2BA titin isoforms are expressed at considerable levels in swine myocardium (Cazorla, et al., 2000; Chung, et al., 2011; Opitz & Linke, 2005). However, differences in the relative amounts exist when compared to humans (See “Disadvantages” section below). Swine hearts have in vivo contractile and relaxation kinetics that are slightly faster than humans but much more similar than either small rodents or rabbits (Table 5).
Table 5.
Selected in vivo cardiac catheterization parameters in swine and humans. Cardiac catheterization measurements performed in control humans (4 females/8 males) and conscious swine (Landacre × Yorkshire pigs). LVSP: left ventricular systolic pressure. LV τ: left ventricular isovolumic relaxation time constant. LV dP/dtmax: left ventricular maximal rate of pressure rise. LV dP/dtmin: left ventricular maximal rate of pressure decline.
Swine have a large heart rate reserve as they can increase their heart rates from approximately 89–117 bpm at rest to 259–306 bpm during exercise (Armstrong, Delp, Goljan, & Laughlin, 1987; Delp, et al., 2001; Krombach, et al., 1998; Roth, et al., 1990). This gain of 128–219% in heart rate is very similar to the 140–170% available in humans (Flamm, et al., 1990; Stratton, et al., 1994). Note that while the resting heart rates are just slightly higher than humans; the exercise heart rates are moderately higher than achievable in humans. Nonetheless, swine similar to humans have a substantial heart rate reserve that can be recruited during exercise. Furthermore, swine also have a positive force-frequency relationship as assessed by in vivo studies. Increasing pacing frequencies in swine results in increases in various indices of contractility such as dP/dtmax and myocardial acceleration during isovolumic contraction (IVA) (Vogel, et al., 2003; Vogel, et al., 2002).
Heart failure can be induced in swine by a variety of methods such as rapid pacing (Lacroix, et al., 2002) and myocardial infarction (Pleger, et al., 2011). Myocardial gene deliveries have been performed with the aid of AAVs with methods that deliver the vectors preferentially to the myocardium such as myocardial injections (H. Su, et al., 2008), direct intracoronary (Kaspar, et al., 2005), and selective retroinfusion coupled with coronary artery occlusion (Pleger, et al., 2011; Raake, et al., 2008). As discussed for other large animal models, these techniques are not as simple as the intraperitoneal and intravenous techniques available in small rodents.
Disadvantages
Swine, typical of large animal models, have a relatively high cost of associated with housing and maintenance. This is a particular disadvantage when you compare the cost of performing experiments in this model to small species especially mice and rats. The time and cost associated with development of genetic swine models is a disadvantage when compared to genetic mice models which can be developed more rapidly and with lower cost.
Despite the many similarities between porcine and human myocardium, differences between these two species persist. For example, as discussed earlier, swine hearts similar to humans express significant amounts of both N2BA and N2B titin isoforms. Based on reports where expression levels were quantified, the swine expresses more N2BA isoform (N2BA:N2B ratio of 1–1.5) than humans (N2BA:N2B ratio of 0.4–1.2) (Cazorla, et al., 2000; Chung, et al., 2011; Makarenko, et al., 2004; Nagueh, et al., 2004). Furthermore, one of these reports (Chung, et al., 2011) concluded that not only swine expressed more N2BA than humans, but additionally the N2BA:N2B ratio of this animal model was more similar to dilated human cardiomyopathy, a condition were N2BA expression is upregulated (Makarenko, et al., 2004; Nagueh, et al., 2004). Other differences include small or lack of transient outward current (Ito) in swine (Lacroix, et al., 2002; G. R. Li, et al., 2003; Schultz, et al., 2007) and purkinje fiber distribution (Hamlin, 2007).
Overall, the high degree of similarities with some variations between swine and human myocardium makes this animal a good model for cardiovascular research.
6. Sheep Models
Advantages
The sheep myocardium, similar to other large animal models discussed previously, shares many similarities to humans. The predominate myosin heavy chain isoform is the slow β-MHC in sheep (~100%) similar to humans (>90–95%) (Hamilton & Ianuzzo, 1991; Miyata, et al., 2000; Reiser, et al., 2001). Sheep and human left ventricles express both major titin isoforms in considerable amounts with N2BA:N2B ratios of ~0.35–0.43 and ~0.4–1.2, respectively (Cazorla, et al., 2000; Kruger & Linke, 2006; Makarenko, et al., 2004; Nagueh, et al., 2004; Neagoe, et al., 2003). One study estimated that SERCA contributes to approximately 81.5% of calcium removal during relaxation in sheep (Dibb, Rueckschloss, Eisner, Isenberg, & Trafford, 2004) which is close to the 76% responsibility in humans (Piacentino, et al., 2003). Typical, of large animal models, sheep contractile and relaxation kinetics are similar-slightly faster than humans (Table 6).
Table 6.
Selected in vivo cardiac catheterization parameters in sheep and humans. Cardiac catheterization measurements performed in control humans (4 females/8 males) and conscious sheep (female). LVSP: left ventricular systolic pressure. LV τ: left ventricular isovolumic relaxation time constant. LV dP/dtmax: left ventricular maximal rate of pressure rise. LV dP/dtmin left ventricular maximal rate of pressure decline.
Sheep have a resting heart rate which is slightly higher than humans (See Table 1). Furthermore, both sheep and human hearts have a positive force-frequency relationship. As discussed earlier, the force of isolated human cardiac trabeculae increases ~81–168% as the stimulation frequency is increased from 0.5 Hz to 2.5 Hz (Chaudhary, et al., 2004; Pieske, et al., 1996; Rossman, et al., 2004). Increasing stimulation frequency in sheep trabeculae from 0.5 Hz to 2 Hz resulted in a 139–171% increase in force production (Schotola, et al., 2011; Sossalla, et al., 2010). Although, further increasing stimulation frequency to 3 Hz resulted in force decline. Note that the trabeculae from Sossalla et al. 2010 were in presence of KN-92, an inactive analogue of the CaMKII inhibitor KN-93.
Sheep will serve as a good pre-clinical model for studying cardiovascular diseases. Various disease types can be developed in sheep, including, but not limited to myocardial infarction (Kelley, et al., 1999; Kramer, et al., 1993), gradual aortic constriction (Aoyagi, et al., 1993; Moorjani, et al., 2003), and tachypacing induced heart failure (Byrne, et al., 2002). Due to the greater resemblance of the hearts of sheep and other large animal models discussed previously to humans; disease models in these large animals will generally better recapitulate changes in humans and efficacy of novel therapeutic avenues than small animal models. Transduction of sheep myocardium with AAV vectors (such as AAV-6 and AAV-2/1) have been successfully performed with techniques such as coronary infusion coupled with brief coronary and venous blockage (Beeri, et al., 2010) and re-circulating systems (Byrne, et al., 2008; J. D. White, et al., 2011) which target the myocardium directly and increase exposure time.
Disadvantages
The disadvantages of using sheep are typical of those described for other large animal models. Most notably, sheep are much more expensive to purchase and maintain in animal facilities than small animal models. Throughout this manuscript, this disadvantage was described for other large animal models as well. Just the daily housing fees of sheep, pigs, canines, and rabbits are approximately 80, 60, 90, and 30 times more expensive than mice, respectively (values based upon 2013 fees charged by The Ohio State University ULAR and assuming 5 mice per cage). This becomes a hindrance in utilizing sheep and other large animal models on a large scale especially for long-term studies. Another limitation is the need for techniques for transduction of myocardial tissue with AAV vectors (see above) as compared to intraperitoneal and intravenous injections which are feasible in small rodents.
Overall, sheep as a large animal model with its similarities to the human heart will serve as a good pre-clinical animal model for cardiovascular research.
7. Verification Using Human Tissue
Each of the cardiovascular animal models discussed above has its own set of advantages and disadvantages. Therefore, no animal model can solely be used to address all of the questions in the cardiovascular field. Use of multiple, complementary animal models will address this issue by taking advantage of the strengths of each model. Small rodents can be used to perform proof-of-principle experiments rapidly and cost-efficiently. However, as discussed previously, there are notable differences between small rodent and human hearts. Experiments in larger species can shed light whether results and findings obtained with smaller species are reproducible in models that more closely reflect the human situation. The choice of the large animal model (either canine, swine, or sheep) should be dependent on the particular cardiac process being investigated. For example, if the focus of the study is on sino-atrial node or purkinje fibers, it is recommended that canines be used due to the high degree of similarity that they share in these aspects with humans. On the other hand, if the objective of the study is to understand alterations in contractile and relaxation kinetics in heart failure, any of the large animal models will be appropriate, and the choice may be more dependent on economic or logistical factors. Depending on the particular institution, it might be favorable to use swine and sheep as opposed to canines for this type of study due to the often greater difficulty associated with obtaining approval for performing experiments on canines. Nonetheless, even the hearts of these larger species and humans are inherently different. Since the ultimate goal of biomedical research is to address human health and disease, experimental verification of potential treatment strategies or quantitative verification of novel pharmacological compounds in actual human tissue would further aid in moving treatments to clinical trials with minimal risk to the patient.
Human myocardium suitable for ex vivo analysis can be typically obtained from 1) rejected donor hearts that were not suitable for cardiac transplantation or 2) end-stage failing hearts that were donated after the patient received cardiac transplantation. Even human samples have limitations and disadvantages that must be taken into consideration. Various compounding factors such as disease severity and extent, genetics, diet, exercise, medications, and social habits cannot be regulated in the human population as one could in a laboratory animal study. Furthermore, the left-ventricular assist devices (LVAD) are now widely used for bridge-therapy for cardiac transplantation. These devices can induce some changes and reverse-remodeling in the myocardium (Ambardekar & Buttrick, 2011) which can further complicate analysis of results obtained from failing human samples. Typically, heart failure patients who are eligible for transplantation undergo a battery of tests and regular physician appointments where information ranging from ejection fraction to medication compliance is recorded. With the proper donor consent and Institutional Review Board (IRB) approval, such information can be de-identified and can aid in addressing the influence of the compounding factors. On the other hand, such information is much more limited and in some cases unavailable if the sample was from a donor without significant medical problems whose heart was not used for transplantation. In most cases, such donor hearts have some underlying anatomical and/or acquired abnormalities (such as coronary artery disease, low ejection fraction, and/or advanced age) that excluded them for cardiac transplantation. This has important consequences on defining what is a “control” human sample. Whether these donor samples can be used as control is dependent on the objective of the study. It is recommended that the reason(s) for which these hearts were excluded for transplantation should be noted at the time of publication. Furthermore, in some cases inotropic agents are used in order to maintain proper organ perfusion during the organ procurement process, and the use of such agents can cause alterations in post-translational modifications of cardiomyocyte proteins, or when given for extended periods of time, modify protein expression levels. Unfortunately, documentation of the use of such agents is not always readily available to researchers, and/or omitted at the time of publication. One concern, common to both rejected donor and end-stage failing hearts, is that ischemia can happen during excision of the heart from the thoracic cavity. Such ischemia can affect the myocardial tissue and should be minimized by rapidly flushing the coronary arteries with cold cardioplegic solution as soon as possible after removal of the heart. Another issue is the amount of time it takes for removal of the heart from the body to processing of samples. Heart samples from animal models can be quickly (if necessary within seconds) be flash frozen. However, in most settings such quick freezing is not practical for donated human hearts and in most cases it might take several minutes or even hours to freeze tissues or at least place on ice. Such delays will become problematic if the goal of the study is to determine the post-translational modifications of proteins, as several of these process can occur, and reverse, in minutes. Some of these concerns in regards to utilizing human samples have been discussed by others as well (Jweied, et al., 2007; Marston & de Tombe, 2008). Still, despite these difficulties, in vitro assessment of contraction in human cardiac tissue can be an important tool to study drugs or physiological processes (Hermann, et al., 2002; Janssen, et al., 1999; Janssen, et al., 2000; Pieske, et al., 1995; Rossman, et al., 2004).
One common limitation of both groups is that these human samples are available in limited quantities. This issue can be addressed by collaboration between multiple laboratories in order to use these samples efficiently. Currently in our institution, human hearts are shared among several laboratories which study processes ranging from contraction at the myofilament level to cardiac excitation at the tissue level. We believe that an approach that uses multiple animal models with verification using human myocardium is essential in advancing research in the cardiovascular field.
8. Conclusion
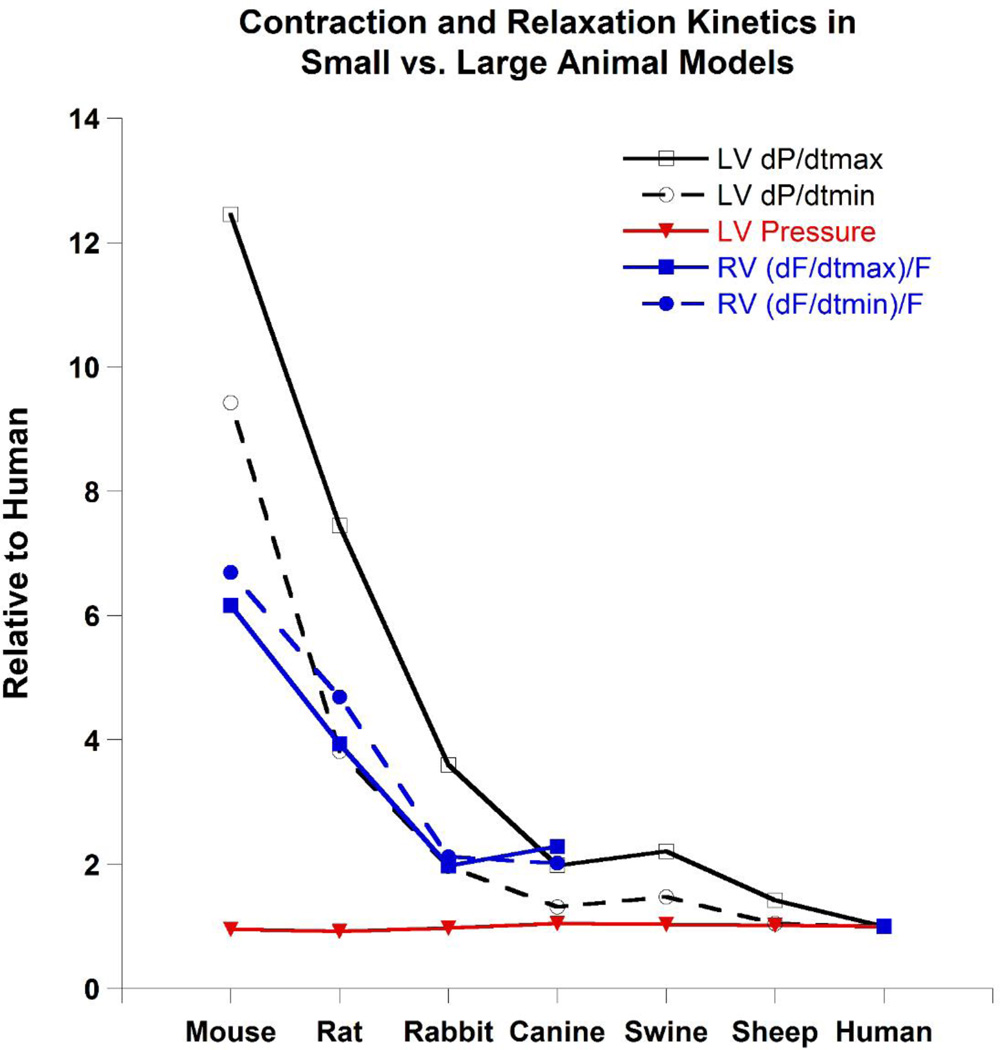
Despite the preservation and conservation of many aspect of the cardiovascular system during evolution of humans and commonly used laboratory animal models, various differences exist that must be considered. These differences result in variations in properties and characteristics of the hearts of humans vs. laboratory animal models. One of these differences is cardiac kinetics and will be used here to summarize and illustrate this point. Figure 2 shows various ex vivo (only mouse, rat, rabbit, canine, and human available) and in vivo (all species) contractile and relaxation parameters normalized to human values. Note that while left ventricular pressure remains constant, contractile and relaxation kinetics scale with body weight and these kinetics approach those of humans as animal size increases. Therefore, the differences between laboratory animals and humans become less as the body/heart weight of the model approaches that of humans.
Figure 2.
In vivo and ex vivo kinetics of contraction and relaxation of large animal models are more similar to humans than small animal models while pressure generated by the left ventricle remains relatively constant across species. Data was obtained from animal/human ratios indicated in Tables 2–6. Note that no ex vivo right ventricular trabeculae data is presented for swine and sheep. LV dP/dtmax: left ventricular maximal rate of pressure rise. LV dP/dtmin: left ventricular maximal rate of pressure decline. RV (dF/dtmax)/F: maximal velocity of contraction divided by developed force in right ventricular trabeculae. RV (dF/dtmin)/F: maximal velocity of relaxation divided by developed force in right ventricular trabeculae. See Tables 2–6 for references and values.
The choice of the animal model is an important determinant of the relevance of the disease model to the human situation. Additionally, it should be considered not only how the end phenotype manifests, but also how the progression towards the pathological phenotype is achieved. Typically, for reducing complexity and accelerating studies, cardiac diseases in animals are by necessity induced by means that do not fully recapitulate human diseases. For example, consider aortic constriction that is used to induce hypertrophy and heart failure in both small and large animal models. Typically, such pressure-overload in animal models is induced suddenly with placing of a band over some part of the aorta. How similar are such surgical interventions to aortic constriction in humans? The most common cause of non-congenital aortic constriction is calcification of aortic valve which is a gradual progressive and not sudden process resulting in progressive cardiac hypertrophy and heart failure (Dweck, Boon, & Newby, 2012; Nishimura, 2002). This might not be detrimental if the objective is to induce heart disease (in this case hypertrophy/heart failure) in an animal model. However, it can be a limitation, if the purpose of the study is to recapitulate human diseases (in this case aortic constriction) and study their progression and underlying patho-physiologies. Similar caution should be considered when trying to extrapolate data from disease models such as myocardial infarction and tachy-pacing induced cardiomyopathy to humans. The type of disease model along with the animal model itself should be considered carefully when using disease models in animals.
No “ideal” animal model of the human cardiovascular system exists. Therefore, studies in cardiovascular system should not rely on a single animal model to address all questions. The choice of the model should depend on various factors, most importantly including but not limited to 1) What process(es) are being investigated? and 2) How far along is the study? (i.e. preliminary vs. preclinical). Both small and large animal models have specific strengths and weaknesses, and all of the animal models discussed in this manuscript have an important role in cardiovascular research. Furthermore, the use of human tissues, if available, can shed further light onto translational applications for human patients.
Acknowledgements
This study was in part supported by RC1 HL099538 and R01 HL113084 from the National Institutes of Health to P.M.L. Janssen and American Heart Association Great Rivers Affiliate Predoctoral Fellowship Award 1148008 to N. Milani-Nejad.
Abbreviations
- AAV
Adeno-associated virus
- APD
Action potential duration
- dF/dtmax
Maximal velocity of contraction in cardiac trabeculae
- (dF/dtmax)/F
Maximal velocity of contraction divided by developed force in cardiac trabeculae
- dF/dtmin
Maximal velocity of relaxation in cardiac trabeculae
- (dF/dtmin)/F
Maximal velocity of relaxation divided by developed force in cardiac trabeculae
- LV dP/dtmax
Left ventricular maximal rate of pressure rise
- LV dP/dtmin
Left ventricular maximal rate of pressure decline
- LVSP
Left ventricular systolic pressure
- LV τ
Left ventricular isovolumic relaxation time constant
- MHC
Myosin heavy chain
- NCX
Sodium calcium exchanger
- RT50
Relaxation time 50%: Time from peak force to 50% relaxation in cardiac trabeculae
- SERCA
Sarcoplasmic-reticulum calcium ATPase
- TTP
Time to Peak: time from stimulation to peak force in cardiac trabeculae
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V, 3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286:H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- Allison JS, Qin H, Dosdall DJ, Huang J, Newton JC, Allred JD, Smith WM, Ideker RE. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18:1306–1312. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- Alpert NR, Brosseau C, Federico A, Krenz M, Robbins J, Warshaw DM. Molecular mechanics of mouse cardiac myosin isoforms. Am J Physiol Heart Circ Physiol. 2002;283:H1446–H1454. doi: 10.1152/ajpheart.00274.2002. [DOI] [PubMed] [Google Scholar]
- Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T, Fujii AM, Flanagan MF, Arnold LW, Brathwaite KW, Colan SD, Mirsky I. Transition from compensated hypertrophy to intrinsic myocardial dysfunction during development of left ventricular pressure-overload hypertrophy in conscious sheep. Systolic dysfunction precedes diastolic dysfunction. Circulation. 1993;88:2415–2425. doi: 10.1161/01.cir.88.5.2415. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Progressive elevations in muscle blood flow during prolonged exercise in swine. J Appl Physiol. 1987;63:285–291. doi: 10.1152/jappl.1987.63.1.285. [DOI] [PubMed] [Google Scholar]
- Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In vivo phosphorylation site mapping in mouse cardiac troponin I by high resolution topdown electron capture dissociation mass spectrometry: Ser22/23 are the only sites basally phosphorylated. Biochemistry. 2009;48:8161–8170. doi: 10.1021/bi900739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genomics. 2010;42A:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett SL, Abou-Madi N, Kraus MS, Wiedner EB, Starkey SR, Kollias GV. Electrocardiography of the Asian elephant (Elephas maximus) J Zoo Wildl Med. 2009;40:466–473. doi: 10.1638/2008-0140.1. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri R, Chaput M, Guerrero JL, Kawase Y, Yosefy C, Abedat S, Karakikes I, Morel C, Tisosky A, Sullivan S, Handschumacher MD, Gilon D, Vlahakes GJ, Hajjar RJ, Levine RA. Gene delivery of sarcoplasmic reticulum calcium ATPase inhibits ventricular remodeling in ischemic mitral regurgitation. Circ Heart Fail. 2010;3:627–634. doi: 10.1161/CIRCHEARTFAILURE.109.891184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circ Res. 2000;87:235–240. doi: 10.1161/01.res.87.3.235. [DOI] [PubMed] [Google Scholar]
- Bers D. Excitation-Contraction Coupling and Cardiac Contractile Force. Second ed. Dordrecht, Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- Billman GE. In-Vivo Models of Arrhythmias: A Canine Model of Studden Cardiac Death. In: Dhein S, Mohr FW, Delmar M, editors. Practical Methods in Cardiovascular Research. Berlin Heidelberg: Springer; 2005. pp. 111–128. [Google Scholar]
- Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future antiarrhythmic drug development. Pharmacol Ther. 2006;111:808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, Carnes CA, Janssen PML. Effects of Dietary Omega-3 Fatty Acids on Ventricular Function in Dogs with Healed Myocardial Infarctions: In Vivo and In Vitro Studies. Am J Physiol Heart Circ Physiol. 2010;298:H1219–H1228. doi: 10.1152/ajpheart.01065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney HL. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19:1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bish LT, Sleeper MM, Brainard B, Cole S, Russell N, Withnall E, Arndt J, Reynolds C, Davison E, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney HL. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther. 2008;16:1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- Bolter CP, Atkinson KJ. Maximum heart rate responses to exercise and isoproterenol in the trained rat. Am J Physiol. 1988;254:R834–R839. doi: 10.1152/ajpregu.1988.254.5.R834. [DOI] [PubMed] [Google Scholar]
- Bouvagnet P, Mairhofer H, Leger JO, Puech P, Leger JJ. Distribution pattern of alpha and beta myosin in normal and diseased human ventricular myocardium. Basic Res Cardiol. 1989;84:91–102. doi: 10.1007/BF01907006. [DOI] [PubMed] [Google Scholar]
- Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- Byrne MJ, Raman JS, Alferness CA, Esler MD, Kaye DM, Power JM. An ovine model of tachycardia-induced degenerative dilated cardiomyopathy and heart failure with prolonged onset. J Card Fail. 2002;8:108–115. doi: 10.1054/jcaf.2002.32323. [DOI] [PubMed] [Google Scholar]
- Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res. 2000;86:59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- Cernohorsky J, Kolar F, Pelouch V, Korecky B, Vetter R. Thyroid control of sarcolemmal Na+/Ca2+ exchanger and SR Ca2+-ATPase in developing rat heart. Am J Physiol. 1998;275:H264–H273. doi: 10.1152/ajpheart.1998.275.1.H264. [DOI] [PubMed] [Google Scholar]
- Chaudhary KW, Rossman EI, 3rd, Piacentino V, Kenessey A, Weber C, Gaughan JP, Ojamaa K, Klein I, Bers DM, Houser SR, Margulies KB. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol. 2004;44:837–845. doi: 10.1016/j.jacc.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y, Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension. 2012;59:1170–1178. doi: 10.1161/HYPERTENSIONAHA.111.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CP, Igarashi Y, Little WC. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res. 1992;70:9–19. doi: 10.1161/01.res.70.1.9. [DOI] [PubMed] [Google Scholar]
- Chung CS, Bogomolovas J, Gasch A, Hidalgo CG, Labeit S, Granzier HL. Titin-actin interaction: PEVK-actin-based viscosity in a large animal. J Biomed Biotechnol. 2011;2011:310791. doi: 10.1155/2011/310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani OH, Kass DA. Pressure-volume relation analysis of mouse ventricular function. Am J Physiol Heart Circ Physiol. 2011;301:H2198–H2206. doi: 10.1152/ajpheart.00781.2011. [DOI] [PubMed] [Google Scholar]
- Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott MO, Fischbeck KH, Kornegay JN, Avery RJ, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophindystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–1429. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfin DA, Zang KE, Schill KE, Patel NT, Janssen PM, Raman SV, Rafael-Fortney JA. Cardiomyopathy in the dystrophin/utrophin-deficient mouse model of severe muscular dystrophy is characterized by dysregulation of matrix metalloproteinases. Neuromuscul Disord. 2011;22:1006–1014. doi: 10.1016/j.nmd.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol. 2001;533:849–859. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- Dibb KM, Rueckschloss U, Eisner DA, Isenberg G, Trafford AW. Mechanisms underlying enhanced cardiac excitation contraction coupling observed in the senescent sheep myocardium. J Mol Cell Cardiol. 2004;37:1171–1181. doi: 10.1016/j.yjmcc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- Elnakish MT, Hassanain HH, Janssen PM. Vascular remodelingassociated hypertension leads to left ventricular hypertrophy and contractile dysfunction in profilin-1 transgenic mice. J Cardiovasc Pharmacol. 2012;60:544–552. doi: 10.1097/FJC.0b013e318271225d. [DOI] [PubMed] [Google Scholar]
- Fang H, Lai NC, Gao MH, Miyanohara A, Roth DM, Tang T, Hammond HK. Comparison of adeno-associated virus serotypes and delivery methods for cardiac gene transfer. Hum Gene Ther Methods. 2012;23:234–241. doi: 10.1089/hgtb.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG, Efimov IR. Effects of KATP channel openers diazoxide and pinacidil in coronary-perfused atria and ventricles from failing and non-failing human hearts. J Mol Cell Cardiol. 2011;51:215–225. doi: 10.1016/j.yjmcc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Chang R. Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2012;302:H1773–H1783. doi: 10.1152/ajpheart.00892.2011. [DOI] [PubMed] [Google Scholar]
- Fermini B, Wang Z, Duan D, Nattel S. Differences in rate dependence of transient outward current in rabbit and human atrium. Am J Physiol. 1992;263:H1747–H1754. doi: 10.1152/ajpheart.1992.263.6.H1747. [DOI] [PubMed] [Google Scholar]
- Fewell JG, Osinska H, Klevitsky R, Ng W, Sfyris G, Bahrehmand F, Robbins J, Vornanen M. A treadmill exercise regimen for identifying cardiovascular phenotypes in transgenic mice. Am J Physiol. 1997;273:H1595–H1605. doi: 10.1152/ajpheart.1997.273.3.H1595. [DOI] [PubMed] [Google Scholar]
- Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, Gratze P, Qadri F, Wellner M, Fiebeler A, Dietz R, Luft FC, Muller DN, Schirdewan A. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- Flamm SD, Taki J, Moore R, Lewis SF, Keech F, Maltais F, Ahmad M, Callahan R, Dragotakes S, Alpert N, et al. Redistribution of regional and organ blood volume and effect on cardiac function in relation to upright exercise intensity in healthy human subjects. Circulation. 1990;81:1550–1559. doi: 10.1161/01.cir.81.5.1550. [DOI] [PubMed] [Google Scholar]
- Friedrichs GS, Patmore L, Bass A. Non-clinical evaluation of ventricular repolarization (ICH S7B): results of an interim survey of international pharmaceutical companies. J Pharmacol Toxicol Methods. 2005;52:6–11. doi: 10.1016/j.vascn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fuller GA, Bicer S, Hamlin RL, Yamaguchi M, Reiser PJ. Increased myosin heavy chain-beta with atrial expression of ventricular light chain-2 in canine cardiomyopathy. J Card Fail. 2007;13:680–686. doi: 10.1016/j.cardfail.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Gaustad SE, Rolim N, Wisloff U. A valid and reproducible protocol for testing maximal oxygen uptake in rabbits. Eur J Cardiovasc Prev Rehabil. 2010;17:83–88. doi: 10.1097/HJR.0b013e32833090c4. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhov AV, Flagg TP, Fedorov VV, Efimov IR, Nichols CG. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2010;48:152–160. doi: 10.1016/j.yjmcc.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehringer C, Rutschow D, Bauer R, Schinkel S, Weichenhan D, Bekeredjian R, Straub V, Kleinschmidt JA, Katus HA, Muller OJ. Prevention of cardiomyopathy in delta-sarcoglycan knockout mice after systemic transfer of targeted adeno-associated viral vectors. Cardiovasc Res. 2009;82:404–410. doi: 10.1093/cvr/cvp061. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Schultz BR, Allen JM, Halldorson JB, Blankinship MJ, Meznarich NA, Kuhr CS, Doremus C, Finn E, Liggitt D, Chamberlain JS. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Varian KD, Bal NC, Abraham JL, Periasamy M, Janssen PML. Pulmonary artery banding alters the expression of Ca2+ transport proteins in the right atrium in rabbits. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Kolokathis F, Pater L, Lynch RA, Asahi M, Gramolini AO, Fan GC, Tsiapras D, Hahn HS, Adamopoulos S, Liggett SB, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest. 2003;111:869–876. doi: 10.1172/JCI17892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet GC, Musch TI, Friedman DB, Ordway GA. Cardiovascular effects of dobutamine during exercise in dogs. Am J Physiol. 1989;257:H954–H960. doi: 10.1152/ajpheart.1989.257.3.H954. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Gwathmey JK. Cross-bridge dynamics in human ventricular myocardium. Regulation of contractility in the failing heart. Circulation. 1992;86:1819–1826. doi: 10.1161/01.cir.86.6.1819. [DOI] [PubMed] [Google Scholar]
- Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, Versteilen A, Lamberts R, Merkus D, Dos Remedios C, Duncker DJ, Borbely A, Papp Z, Paulus W, Stienen GJ, Marston SB, van der Velden J. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Ianuzzo CD. Contractile and calcium regulating capacities of myocardia of different sized mammals scale with resting heart rate. Mol Cell Biochem. 1991;106:133–141. doi: 10.1007/BF00230179. [DOI] [PubMed] [Google Scholar]
- Hamlin RL. Animal models of ventricular arrhythmias. Pharmacol Ther. 2007;113:276–295. doi: 10.1016/j.pharmthera.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hammond TG, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Redfern WS, Sullivan AT, Camm AJ. Methods of collecting and evaluating non-clinical cardiac electrophysiology data in the pharmaceutical industry: results of an international survey. Cardiovasc Res. 2001;49:741–750. doi: 10.1016/s0008-6363(00)00310-2. [DOI] [PubMed] [Google Scholar]
- Hartley CJ, Reddy AK, Madala S, Entman ML, Michael LH, Taffet GE. Doppler velocity measurements from large and small arteries of mice. Am J Physiol Heart Circ Physiol. 2011;301:H269–H278. doi: 10.1152/ajpheart.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann HP, Zeitz O, Lehnart SE, Keweloh B, Datz N, Hasenfuss G, Janssen PML. Potentiation of beta-adrenergic inotropic response by pyruvate in failing human myocardium. Cardiovasc Res. 2002;53:116–123. doi: 10.1016/s0008-6363(01)00437-0. [DOI] [PubMed] [Google Scholar]
- Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291:H2424–H2430. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- Hiranandani N, Varian KD, Monasky MM, Janssen PML. Frequencydependent contractile response of isolated cardiac trabeculae under hypo-, normo-, and hyperthermic conditions. J Appl Physiol. 2006;100:1727–1732. doi: 10.1152/japplphysiol.01244.2005. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics. 2002;79:679–685. doi: 10.1006/geno.2002.6754. [DOI] [PubMed] [Google Scholar]
- Howden R, Liu E, Miller-DeGraff L, Keener HL, Walker C, Clark JA, Myers PH, Rouse DC, Wiltshire T, Kleeberger SR. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol. 2008;295:H59–H68. doi: 10.1152/ajpheart.00941.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, Nakai H. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber WA, Maniu C, Krysiak J, Shapiro BP, Meyer DM, Linke WA, Redfield MM. Titin isoforms, extracellular matrix, and global chamber remodeling in experimental dilated cardiomyopathy: functional implications and mechanistic insight. Circ Heart Fail. 2008;1:192–199. doi: 10.1161/CIRCHEARTFAILURE.108.768465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J, Martin L, Krenz M, Quatman C, Jones F, Klevitsky R, Gulick J, Robbins J. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation. 2005;111:2339–2346. doi: 10.1161/01.CIR.0000164233.09448.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2005;289:H2373–2378. doi: 10.1152/ajpheart.00448.2005. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Lehnart SE, Prestle J, Hasenfuss G. Preservation of contractile characteristics of human myocardium in multi-day cell culture. J Mol Cell Cardiol. 1999;31:1419–1427. doi: 10.1006/jmcc.1999.0978. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43:523–531. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H499–H507. doi: 10.1152/ajpheart.00595.2001. [DOI] [PubMed] [Google Scholar]
- Janssen PML, Zeitz O, Keweloh B, Siegel U, Maier LS, Barckhausen P, Pieske B, Prestle J, Lehnart SE, Hasenfuss G. Influence of cyclosporine A on contractile function, calcium handling, and energetics in isolated human and rabbit myocardium [In Process Citation] Cardiovasc Res. 2000;47:99–107. doi: 10.1016/s0008-6363(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho S, Ishizaka S, Sievers R, Foster E, Simpson PC, Grossman W. Left ventricular pressure-volume relationship in conscious mice. Am J Physiol Heart Circ Physiol. 2007;292:H369–H377. doi: 10.1152/ajpheart.00704.2006. [DOI] [PubMed] [Google Scholar]
- Jover B, McGrath BP, Ludbrook J. Haemodynamic and metabolic responses of laboratory rabbits to near-maximal treadmill exercise. Clin Exp Pharmacol Physiol. 1987;14:811–823. doi: 10.1111/j.1440-1681.1987.tb02418.x. [DOI] [PubMed] [Google Scholar]
- Juneau C, Calderone A, Rouleau JL. Myocardial beta-adrenergic and mechanical properties in pacing-induced heart failure in dogs. Am J Physiol. 1992;262:H1458–H1467. doi: 10.1152/ajpheart.1992.262.5.H1458. [DOI] [PubMed] [Google Scholar]
- Jung B, Odening KE, Dall'armellina E, Foll D, Menza M, Markl M, Schneider JE. A quantitative comparison of regional myocardial motion in mice, rabbits and humans using in-vivo phase contrast CMR. J Cardiovasc Magn Reson. 2012;14:87. doi: 10.1186/1532-429X-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jweied E, deTombe P, Buttrick PM. The use of human cardiac tissue in biophysical research: the risks of translation. J Mol Cell Cardiol. 2007;42:722–726. doi: 10.1016/j.yjmcc.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschope C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, McKirnan MD, Hammond HK. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med. 2005;7:316–324. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- Kelley ST, Malekan R, Gorman JH, 3rd, Jackson BM, Gorman RC, Suzuki Y, Plappert T, Bogen DK, Sutton MG, Edmunds LH., Jr Restraining infarct expansion preserves left ventricular geometry and function after acute anteroapical infarction. Circulation. 1999;99:135–142. doi: 10.1161/01.cir.99.1.135. [DOI] [PubMed] [Google Scholar]
- Kiss E, Jakab G, Kranias EG, Edes I. Thyroid hormone-induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res. 1994;75:245–251. doi: 10.1161/01.res.75.2.245. [DOI] [PubMed] [Google Scholar]
- Kogler H, Hartmann O, Leineweber K, Nguyen van P, Schott P, Brodde OE, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res. 2003;93:230–237. doi: 10.1161/01.RES.0000085042.89656.C7. Epub 2003 Jul 2003. [DOI] [PubMed] [Google Scholar]
- Koide M, Nagatsu M, Zile MR, Hamawaki M, Swindle MM, Keech G, DeFreyte G, Tagawa H, Cooper Gt, Carabello BA. Premorbid determinants of left ventricular dysfunction in a novel model of gradually induced pressure overload in the adult canine. Circulation. 1997;95:1601–1610. doi: 10.1161/01.cir.95.6.1601. [DOI] [PubMed] [Google Scholar]
- Kooij V, Saes M, Jaquet K, Zaremba R, Foster DB, Murphy AM, Dos Remedios C, van der Velden J, Stienen GJ. Effect of troponin I Ser23/24 phosphorylation on Ca2+-sensitivity in human myocardium depends on the phosphorylation background. J Mol Cell Cardiol. 2010;48:954–963. doi: 10.1016/j.yjmcc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer CM, Lima JA, Reichek N, Ferrari VA, Llaneras MR, Palmon LC, Yeh IT, Tallant B, Axel L. Regional differences in function within noninfarcted myocardium during left ventricular remodeling. Circulation. 1993;88:1279–1288. doi: 10.1161/01.cir.88.3.1279. [DOI] [PubMed] [Google Scholar]
- Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, Alpert NR, Warshaw DM, Perryman MB, Helmke SM, Robbins J. Analysis of myosin heavy chain functionality in the heart. J Biol Chem. 2003;278:17466–17474. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- Krombach RS, Clair MJ, Hendrick JW, Houck WV, Zellner JL, Kribbs SB, Whitebread S, Mukherjee R, de Gasparo M, Spinale FG. Angiotensin converting enzyme inhibition, AT1 receptor inhibition, and combination therapy with pacing induced heart failure: effects on left ventricular performance and regional blood flow patterns. Cardiovasc Res. 1998;38:631–645. doi: 10.1016/s0008-6363(98)00050-9. [DOI] [PubMed] [Google Scholar]
- Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27:435–444. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- Lacroix D, Gluais P, Marquie C, D'Hoinne C, Adamantidis M, Bastide M. Repolarization abnormalities and their arrhythmogenic consequences in porcine tachycardia-induced cardiomyopathy. Cardiovasc Res. 2002;54:42–50. doi: 10.1016/s0008-6363(02)00236-5. [DOI] [PubMed] [Google Scholar]
- Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- Lemon DD, Papst PJ, Joly K, Plato CF, McKinsey TA. A highperformance liquid chromatography assay for quantification of cardiac myosin heavy chain isoform protein expression. Anal Biochem. 2011;408:132–135. doi: 10.1016/j.ab.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Li GR, Du XL, Siow YL, O K, Tse HF, Lau CP. Calcium-activated transient outward chloride current and phase 1 repolarization of swine ventricular action potential. Cardiovasc Res. 2003;58:89–98. doi: 10.1016/s0008-6363(02)00859-3. [DOI] [PubMed] [Google Scholar]
- Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998;274:H1335–1347. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- Lieber SC, Qiu H, Chen L, Shen YT, Hong C, Hunter WC, Aubry N, Vatner SF, Vatner DE. Cardiac dysfunction in aging conscious rats: altered cardiac cytoskeletal proteins as a potential mechanism. Am J Physiol Heart Circ Physiol. 2008;295:H860–H866. doi: 10.1152/ajpheart.00146.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S, Bass AS, Briscoe R, Bruse K, Friedrichs GS, Kallman MJ, Markgraf C, Patmore L, Pugsley MK. Benchmarking safety pharmacology regulatory packages and best practice. J Pharmacol Toxicol Methods. 2008;58:99–109. doi: 10.1016/j.vascn.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Little SC, Biesiadecki BJ, Kilic A, Higgins RS, Janssen PM, Davis JP. The rates of Ca2+ dissociation and cross-bridge detachment from ventricular myofibrils as reported by a fluorescent cardiac Troponin C. J Biol Chem. 2012 doi: 10.1074/jbc.M111.337295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: activation of activator protein 1 and Jun N-terminal kinase. Circ Res. 2006;99:1004–1011. doi: 10.1161/01.RES.0000247066.19878.93. [DOI] [PubMed] [Google Scholar]
- Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circ Res. 1993;72:671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- Liu YH, Yang XP, Nass O, Sabbah HN, Peterson E, Carretero OA. Chronic heart failure induced by coronary artery ligation in Lewis inbred rats. Am J Physiol. 1997;272:H722–H727. doi: 10.1152/ajpheart.1997.272.2.H722. [DOI] [PubMed] [Google Scholar]
- Locher MR, Razumova MV, Stelzer JE, Norman HS, Moss RL. Effects of low-level alpha-myosin heavy chain expression on contractile kinetics in porcine myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H869–H878. doi: 10.1152/ajpheart.00452.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher MR, Razumova MV, Stelzer JE, Norman HS, Patel JR, Moss RL. Determination of rate constants for turnover of myosin isoforms in rat myocardium: implications for in vivo contractile kinetics. Am J Physiol Heart Circ Physiol. 2009;297:H247–H256. doi: 10.1152/ajpheart.00922.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HL, Janbaih H, Feng HZ, Jin JP, DiCarlo SE. Ventricular function during exercise in mice and rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R68–R74. doi: 10.1152/ajpregu.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machackova J, Sanganalmath SK, Elimban V, Dhalla NS. betaadrenergic blockade attenuates cardiac dysfunction and myofibrillar remodelling in congestive heart failure. J Cell Mol Med. 2009;15:545–554. doi: 10.1111/j.1582-4934.2010.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KW, Raya TE, Pennock GD, Morkin E, Goldman S. Left ventricular performance and remodeling in rabbits after myocardial infarction. Effects of a thyroid hormone analogue. Circulation. 1995;91:794–801. doi: 10.1161/01.cir.91.3.794. [DOI] [PubMed] [Google Scholar]
- Maier LS, Barckhausen P, Weisser J, Aleksic I, Baryalei M, Pieske B. Ca(2+) handling in isolated human atrial myocardium. Am J Physiol Heart Circ Physiol. 2000;279:H952–H958. doi: 10.1152/ajpheart.2000.279.3.H952. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM, Pieske B. Differences in Ca(2+)-Handling and Sarcoplasmic Reticulum Ca(2+)-Content in Isolated Rat and Rabbit Myocardium. J Mol Cell Cardiol. 2000;32:2249–2258. doi: 10.1006/jmcc.2000.1252. [DOI] [PubMed] [Google Scholar]
- Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT, Brugada R, DeMayo F, Quinones M, Roberts R. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest. 1999;104:1683–1692. doi: 10.1172/JCI7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca(2+)-sensitivity in heart failure: Increased or decreased? J Mol Cell Cardiol. 2008;45:603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice JP, Shah AS, Kypson AP, Hata JA, White DC, Glower DD, Koch WJ. Molecular beta-adrenergic signaling abnormalities in failing rabbit hearts after infarction. Am J Physiol. 1999;276:H1853–H1860. doi: 10.1152/ajpheart.1999.276.6.H1853. [DOI] [PubMed] [Google Scholar]
- McCloskey DT, Rokosh DG, O'Connell TD, Keung EC, Simpson PC, Baker AJ. Alpha(1)-adrenoceptor subtypes mediate negative inotropy in myocardium from alpha(1A/C)-knockout and wild type mice. J Mol Cell Cardiol. 2002;34:1007–1017. doi: 10.1006/jmcc.2002.2049. [DOI] [PubMed] [Google Scholar]
- Meng H, Pierce GN. Metabolic and physiological response of the rabbit to continuous and intermittent treadmill exercise. Can J Physiol Pharmacol. 1990;68:856–862. doi: 10.1139/y90-130. [DOI] [PubMed] [Google Scholar]
- Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PM. Effect of muscle length on cross-bridge kinetics in intact cardiac trabeculae at body temperature. J Gen Physiol. 2013;141:133–139. doi: 10.1085/jgp.201210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- Moise NS, Valentine BA, Brown CA, Erb HN, Beck KA, Cooper BJ, Gilmour RF. Duchenne's cardiomyopathy in a canine model: electrocardiographic and echocardiographic studies. J Am Coll Cardiol. 1991;17:812–820. doi: 10.1016/s0735-1097(10)80202-5. [DOI] [PubMed] [Google Scholar]
- Monasky MM, Janssen PML. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol B. 2009;179:469–479. doi: 10.1007/s00360-008-0331-3. [DOI] [PubMed] [Google Scholar]
- Monasky MM, Varian KD, Janssen PM. Gender comparison of contractile performance and beta-adrenergic response in isolated rat cardiac trabeculae. J Comp Physiol [B] 2007 doi: 10.1007/s00360-007-0223-y. [DOI] [PubMed] [Google Scholar]
- Moon H, Park HE, Kang J, Lee H, Cheong C, Lim YT, Ihm SH, Seung KB, Jaffer FA, Narula J, Chang K, Hong KS. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation. 2012;125:2603–2612. doi: 10.1161/CIRCULATIONAHA.111.075283. [DOI] [PubMed] [Google Scholar]
- Moorjani N, Catarino P, El-Sayed R, Al-Ahmed S, Meyer B, Al-Mohanna F, Westaby S. A pressure overload model to track the molecular biology of heart failure. Eur J Cardiothorac Surg. 2003;24:920–925. doi: 10.1016/s1010-7940(03)00514-1. [DOI] [PubMed] [Google Scholar]
- Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr, Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114:1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Xu JQ, Kochanek KD. Deaths: preliminary data for 2010. Natl Vital Stat Rep. 2012;60:1–51. [PubMed] [Google Scholar]
- Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63:2269–2277. doi: 10.1152/jappl.1987.63.6.2269. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Takeda S. Mammalian models of Duchenne Muscular Dystrophy: pathological characteristics and therapeutic applications. J Biomed Biotechnol. 2010;2011:184393. doi: 10.1155/2011/184393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil. 2003;24:175–189. doi: 10.1023/a:1026053530766. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM. Studying cardiac arrhythmias in the mouse--a reasonable model for probing mechanisms? Trends Cardiovasc Med. 2004;14:83–93. doi: 10.1016/j.tcm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Nishimura RA. Cardiology patient pages. Aortic valve disease. Circulation. 2002;106:770–772. doi: 10.1161/01.cir.0000027621.26167.5e. [DOI] [PubMed] [Google Scholar]
- Nishizawa T, Vatner SF, Hong C, Shen YT, Hardt SE, Robbins J, Ishikawa Y, Sadoshima J, Vatner DE. Overexpressed cardiac Gsalpha in rabbits. J Mol Cell Cardiol. 2006;41:44–50. doi: 10.1016/j.yjmcc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Noujaim SF, Lucca E, Munoz V, Persaud D, Berenfeld O, Meijler FL, Jalife J. From mouse to whale: a universal scaling relation for the PR Interval of the electrocardiogram of mammals. Circulation. 2004;110:2802–2808. doi: 10.1161/01.CIR.0000146785.15995.67. [DOI] [PubMed] [Google Scholar]
- Ojamaa K, Kenessey A, Klein I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000;141:2139–2144. doi: 10.1210/endo.141.6.7514. [DOI] [PubMed] [Google Scholar]
- Ontkean M, Gay R, Greenberg B. Diminished endothelium-derived relaxing factor activity in an experimental model of chronic heart failure. Circ Res. 1991;69:1088–1096. doi: 10.1161/01.res.69.4.1088. [DOI] [PubMed] [Google Scholar]
- Opitz CA, Linke WA. Plasticity of cardiac titin/connectin in heart development. J Muscle Res Cell Motil. 2005;26:333–342. doi: 10.1007/s10974-005-9040-7. [DOI] [PubMed] [Google Scholar]
- Ostergaard G, Hansen HN, Ottesen JL. Physiological, Hematological, and Clinical Chemistry Parameters, Including Conversion Factors. In: Hau J, Schapiro SJ, editors. Handbook of laboratory animal science, Volume I: Essential Principles and Practices. 3rd ed. Vol. 1. Boca Raton, FL: CRC Press; 2010. pp. 667–707. [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, Hajjar RJ. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Pan X, Yue Y, Zhang K, Lostal W, Shin JH, Duan D. Long-term robust myocardial transduction of the dog heart from a peripheral vein by adenoassociated virus serotype-8. Hum Gene Ther. 2013 doi: 10.1089/hum.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfilov AV. Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts? Heart Rhythm. 2006;3:862–864. doi: 10.1016/j.hrthm.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S, Ungvari Z, Puligadda U, Moimas S, Xu X, Edwards JG, Hintze TH, Giacca M, Recchia FA. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res. 2010;106:1893–1903. doi: 10.1161/CIRCRESAHA.110.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, Minami K, Just H, Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circ Res. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling. J Clin Invest. 1996;98:764–776. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, Gao E, Dasgupta A, Rengo G, Remppis A, Katus HA, Eckhart AD, Rabinowitz JE, Koch WJ. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007;115:2506–2515. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- Pleger ST, Shan C, Ksienzyk J, Bekeredjian R, Boekstegers P, Hinkel R, Schinkel S, Leuchs B, Ludwig J, Qiu G, Weber C, Raake P, Koch WJ, Katus HA, Muller OJ, Most P. Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med. 2011;3:92ra64. doi: 10.1126/scitranslmed.3002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- Prothero J. Heart weight as a function of body weight in mammals. Growth. 1979;43:139–150. [PubMed] [Google Scholar]
- Puglisi JL, Bassani RA, Bassani JW, Amin JN, Bers DM. Temperature and relative contributions of Ca transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270:H1772–H1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- Raake PW, Hinkel R, Muller S, Delker S, Kreuzpointner R, Kupatt C, Katus HA, Kleinschmidt JA, Boekstegers P, Muller OJ. Cardio-specific long-term gene expression in a porcine model after selective pressure-regulated retroinfusion of adeno-associated viral (AAV) vectors. Gene Ther. 2008;15:12–17. doi: 10.1038/sj.gt.3303035. [DOI] [PubMed] [Google Scholar]
- Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PML, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation. 2011;124:582–588. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram R, Mickelsen DM, Theodoropoulos C, Blaxall BC. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:H1765–H1780. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S, Hajjar RJ, Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog [see comments] Circ Res. 1998;83:969–979. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Portman MA, Ning XH, Schomisch Moravec C. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;280:H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman EI, Petre RE, Chaudhary KW, Piacentino V, 3rd, Janssen PM, Gaughan JP, Houser SR, Margulies KB. Abnormal frequencydependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol. 2004;36:33–42. doi: 10.1016/j.yjmcc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation. 1990;82:1778–1789. doi: 10.1161/01.cir.82.5.1778. [DOI] [PubMed] [Google Scholar]
- Ruf T, Schulte-Baukloh H, Ludemann J, Posival H, Beyersdorf F, Just H, Holubarsch C. Alterations of cross-bridge kinetics in human atrial and ventricular myocardium. Cardiovasc Res. 1998;40:580–590. doi: 10.1016/s0008-6363(98)00164-3. [DOI] [PubMed] [Google Scholar]
- Rundell VL, Manaves V, Martin AF, de Tombe PP. Impact of betamyosin heavy chain isoform expression on cross-bridge cycling kinetics. Am J Physiol Heart Circ Physiol. 2005;288:H896–H903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- Sanbe A, James J, Tuzcu V, Nas S, Martin L, Gulick J, Osinska H, Sakthivel S, Klevitsky R, Ginsburg KS, Bers DM, Zinman B, Lakatta EG, Robbins J. Transgenic rabbit model for human troponin I-based hypertrophic cardiomyopathy. Circulation. 2005;111:2330–2338. doi: 10.1161/01.CIR.0000164234.24957.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotola H, Sossalla S, Rajab TK, Toischer K, Quintel M, Bauer M, Schmitto JD. Influence of mild metabolic acidosis on cardiac contractility and isoprenaline response in isolated ovine myocardium. Artif Organs. 2011;35:1065–1074. doi: 10.1111/j.1525-1594.2011.01390.x. [DOI] [PubMed] [Google Scholar]
- Schultz JH, Volk T, Bassalay P, Hennings JC, Hubner CA, Ehmke H. Molecular and functional characterization of Kv4.2 and KChIP2 expressed in the porcine left ventricle. Pflugers Arch. 2007;454:195–207. doi: 10.1007/s00424-006-0203-1. [DOI] [PubMed] [Google Scholar]
- Seyfarth T, Gerbershagen HP, Giessler C, Leineweber K, Heinroth-Hoffmann I, Ponicke K, Brodde OE. The cardiac beta-adrenoceptor-Gprotein( s)-adenylyl cyclase system in monocrotaline-treated rats. J Mol Cell Cardiol. 2000;32:2315–2326. doi: 10.1006/jmcc.2000.1262. [DOI] [PubMed] [Google Scholar]
- Shah AP, Siedlecka U, Gandhi A, Navaratnarajah M, Al-Saud SA, Yacoub MH, Terracciano CM. Genetic background affects function and intracellular calcium regulation of mouse hearts. Cardiovasc Res. 2010;87:683–693. doi: 10.1093/cvr/cvq111. [DOI] [PubMed] [Google Scholar]
- Shah AS, Lilly RE, Kypson AP, Tai O, Hata JA, Pippen A, Silvestry SC, Lefkowitz RJ, Glower DD, Koch WJ. Intracoronary adenovirusmediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart : prospects for molecular ventricular assistance. Circulation. 2000;101:408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- Shenoy R, Klein I, Ojamaa K. Differential regulation of SR calcium transporters by thyroid hormone in rat atria and ventricles. Am J Physiol Heart Circ Physiol. 2001;281:H1690–H1696. doi: 10.1152/ajpheart.2001.281.4.H1690. [DOI] [PubMed] [Google Scholar]
- Slabaugh JL, Brunello L, Gyorke S, Janssen PM. Contractile parameters and occurrence of alternans in isolated rat myocardium at supraphysiological stimulation frequency. Am J Physiol Heart Circ Physiol. 2012;302:H2267–H2275. doi: 10.1152/ajpheart.01004.2011. [DOI] [PubMed] [Google Scholar]
- Slack JP, Grupp IL, Dash R, Holder D, Schmidt A, Gerst MJ, Tamura T, Tilgmann C, James PF, Johnson R, Gerdes AM, Kranias EG. The enhanced contractility of the phospholamban-deficient mouse heart persists with aging. J Mol Cell Cardiol. 2001;33:1031–1040. doi: 10.1006/jmcc.2001.1370. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- Stelzer JE, Norman HS, Chen PP, Patel JR, Moss RL. Transmural variation in myosin heavy chain isoform expression modulates the timing of myocardial force generation in porcine left ventricle. J Physiol. 2008;586:5203–5214. doi: 10.1113/jphysiol.2008.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton JR, Levy WC, Cerqueira MD, Schwartz RS, Abrass IB. Cardiovascular responses to exercise. Effects of aging and exercise training in healthy men. Circulation. 1994;89:1648–1655. doi: 10.1161/01.cir.89.4.1648. [DOI] [PubMed] [Google Scholar]
- Stubenitsky R, van der Weerd RW, Haitsma DB, Verdouw PD, Duncker DJ. Cardiovascular effects of the novel Ca2+-sensitiser EMD 57033 in pigs at rest and during treadmill exercise. Br J Pharmacol. 1997;122:1257–1270. doi: 10.1038/sj.bjp.0701491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM. Murine strain differences in contractile function are temperature- and frequency-dependent. Pflugers Arch. 2006:1–6. doi: 10.1007/s00424-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Su H, Yeghiazarians Y, Lee A, Huang Y, Arakawa-Hoyt J, Ye J, Orcino G, Grossman W, Kan YW. AAV serotype 1 mediates more efficient gene transfer to pig myocardium than AAV serotype 2 and plasmid. J Gene Med. 2008;10:33–41. doi: 10.1002/jgm.1129. [DOI] [PubMed] [Google Scholar]
- Su Z, Li F, Spitzer KW, Yao A, Ritter M, Barry WH. Comparison of sarcoplasmic reticulum Ca2+-ATPase function in human, dog, rabbit, and mouse ventricular myocytes. J Mol Cell Cardiol. 2003;35:761–767. doi: 10.1016/s0022-2828(03)00119-6. [DOI] [PubMed] [Google Scholar]
- Suarez J, Scott BT, Suarez-Ramirez JA, Chavira CV, Dillmann WH. Thyroid hormone inhibits ERK phosphorylation in pressure overloadinduced hypertrophied mouse hearts through a receptor-mediated mechanism. Am J Physiol Cell Physiol. 2010;299:C1524–C1529. doi: 10.1152/ajpcell.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Palmer BM, James J, Wang Y, Chen Z, VanBuren P, Maughan DW, Robbins J, LeWinter MM. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail. 2009;2:334–341. doi: 10.1161/CIRCHEARTFAILURE.108.802298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Szentandrassy N, Biro T, Toth BI, Czifra G, Magyar J, Banyasz T, Varro A, Kovacs L, Nanasi PP. Asymmetrical distribution of ion channels in canine and human left-ventricular wall: epicardium versus midmyocardium. Pflugers Arch. 2005;450:307–316. doi: 10.1007/s00424-005-1445-z. [DOI] [PubMed] [Google Scholar]
- Szentadrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J, Lazar J, Varro A, Kovacs L, Nanasi PP. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res. 2005;65:851–860. doi: 10.1016/j.cardiores.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, Chernoff J, Wolska BM, Ke Y, Solaro RJ. Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol. 2011;51:988–996. doi: 10.1016/j.yjmcc.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Tang YD, Kuzman JA, Said S, Anderson BE, Wang X, Gerdes AM. Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation. 2005;112:3122–3130. doi: 10.1161/CIRCULATIONAHA.105.572883. [DOI] [PubMed] [Google Scholar]
- Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- Taylor DG, Parilak LD, LeWinter MM, Knot HJ. Quantification of the rat left ventricle force and Ca2+ -frequency relationships: similarities to dog and human. Cardiovasc Res. 2004;61:77–86. doi: 10.1016/j.cardiores.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Valentine BA, Cummings JF, Cooper BJ. Development of Duchennetype cardiomyopathy. Morphologic studies in a canine model. Am J Pathol. 1989;135:671–678. [PMC free article] [PubMed] [Google Scholar]
- van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- van der Velden J, Papp Z, Boontje NM, Zaremba R, de Jong JW, Janssen PM, Hasenfuss G, Stienen GJ. The effect of myosin light chain 2 dephosphorylation on Ca2+ -sensitivity of force is enhanced in failing human hearts. Cardiovasc Res. 2003;57:505–514. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DW, van Slegtenhorst M, Dooijes D, dos Remedios C, ten Cate FJ, Stienen GJ, van der Velden J. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail. 2011;5:36–46. doi: 10.1161/CIRCHEARTFAILURE.111.963702. [DOI] [PubMed] [Google Scholar]
- Varian KD, Janssen PML. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol. 2007;292:H2212–H2219. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- Varian KD, Kijtawornrat A, Gupta SC, Torres CA, Monasky MM, Hiranandani N, Delfin DA, Rafael-Fortney JA, Periasamy M, Hamlin RL, Janssen PML. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization rabbit right ventricular hypertrophy. Circ Heart Fail. 2009;2:472–481. doi: 10.1161/CIRCHEARTFAILURE.109.853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren M, Remy E, Devaux C, Dautreaux B, Henry JP, Bauer F, Mulder P, Hooft van Huijsduijnen R, Bombrun A, Thuillez C, Richard V. Improvement of peripheral endothelial dysfunction by protein tyrosine phosphatase inhibitors in heart failure. Circulation. 2006;114:2498–2507. doi: 10.1161/CIRCULATIONAHA.106.630129. [DOI] [PubMed] [Google Scholar]
- Vogel M, Cheung MM, Li J, Kristiansen SB, Schmidt MR, White PA, Sorensen K, Redington AN. Noninvasive assessment of left ventricular force-frequency relationships using tissue Doppler-derived isovolumic acceleration: validation in an animal model. Circulation. 2003;107:1647–1652. doi: 10.1161/01.CIR.0000058171.62847.90. [DOI] [PubMed] [Google Scholar]
- Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, Redington AN. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002;105:1693–1699. doi: 10.1161/01.cir.0000013773.67850.ba. [DOI] [PubMed] [Google Scholar]
- Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H2013–H2017. doi: 10.1152/ajpheart.00167.2006. [DOI] [PubMed] [Google Scholar]
- Wang J, Guo X, Dhalla NS. Modification of myosin protein and gene expression in failing hearts due to myocardial infarction by enalapril or losartan. Biochim Biophys Acta. 2004;1690:177–184. doi: 10.1016/j.bbadis.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu X, Ren B, Rupp H, Takeda N, Dhalla NS. Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. J Mol Cell Cardiol. 2002;34:847–857. doi: 10.1006/jmcc.2002.2023. [DOI] [PubMed] [Google Scholar]
- Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res. 1999;84:551–561. doi: 10.1161/01.res.84.5.551. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- White DC, Hata JA, Shah AS, Glower DD, Lefkowitz RJ, Koch WJ. Preservation of myocardial beta-adrenergic receptor signaling delays the development of heart failure after myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5428–5433. doi: 10.1073/pnas.090091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JD, Thesier DM, Swain JB, Katz MG, Tomasulo C, Henderson A, Wang L, Yarnall C, Fargnoli A, Sumaroka M, Isidro A, Petrov M, Holt D, Nolen-Walston R, Koch WJ, Stedman HH, Rabinowitz J, Bridges CR. Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo. Gene Ther. 2011;18:546–552. doi: 10.1038/gt.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnker PJ, Foster DB, Tsao AL, Frazier AH, Dos Remedios CG, Murphy AM, Stienen GJ, van der Velden J. Impact of site-specific phosphorylation of protein kinase A sites Ser23 and Ser24 of cardiac troponin I in human cardiomyocytes. Am J Physiol Heart Circ Physiol. 2013;304:H260–H268. doi: 10.1152/ajpheart.00498.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. J Mol Cell Cardiol. 2007;42:186–195. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Coutu P, Sadayappan S, Robbins J, Metzger JM. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101:377–386. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guy MJ, Norman HS, Chen YC, Xu Q, Dong X, Guner H, Wang S, Kohmoto T, Young KH, Moss RL, Ge Y. Top-down quantitative proteomics identified phosphorylation of cardiac troponin I as a candidate biomarker for chronic heart failure. J Proteome Res. 2011;10:4054–4065. doi: 10.1021/pr200258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, Antzelevich C, Nattel S. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol. 2003;285:H1641–H1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]
- Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–748. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]