Abstract
How any complex trait has evolved is a fascinating question, yet the evolution of parasitism among the nematodes is arguably one of the most arresting. How did free-living nematodes cross that seemingly insurmountable evolutionary chasm between soil dwelling and survival inside another organism? Which of the many finely honed responses to the varied and harsh environments of free-living nematodes provided the material upon which natural selection could act? Although several complementary theories explain this phenomenon, I will focus on the dauer hypothesis. The dauer hypothesis posits that the arrested third-stage dauer larvae of free-living nematodes such as Caenorhabditis elegans are, due to their many physiological similarities with infective third-stage larvae of parasitic nematodes, a pre-adaptation to parasitism. If so, then a logical extension of this hypothesis is that the molecular pathways which control entry into and recovery from dauer formation by free-living nematodes in response to environmental cues have been co-opted to control the processes of infective larval arrest and activation in parasitic nematodes. The molecular machinery that controls dauer entry and exit is present in a wide range of parasitic nematodes. However, the developmental outputs of the different pathways are both conserved and divergent, not only between populations of C. elegans or between C. elegans and parasitic nematodes but also between different species of parasitic nematodes. Thus the picture that emerges is more nuanced than originally predicted and may provide insights into the evolution of such an interesting and complex trait.
Keywords: Dauer hypothesis, Evolution, Co-option, TGF-β, Insulin signaling
1. Introduction
A remarkable range of hosts are parasitized by nematodes, to the extent that it is likely that every possible host species has been colonized. Nematode body size varies massively across the phylum and, although the basic nematode body plan is retained, many parasitic nematodes have specialized adaptations to allow the invasion and colonisation of their host(s). Prospective animal parasitic nematodes need to adapt to hostile conditions inside and outside the host, including high temperature, low oxygen potential, gut pH and enzymes, and the host immune response, with a different set of equally hostile conditions facing plant parasitic nematodes. However, phylogenetic analysis by rRNA sequencing shows that parasitism, be it of plants, insects or animals, has evolved from free-living ancestors at least five times (Blaxter et al., 1998). Thus not only is parasitism a successful strategy but the obstacles to becoming a parasitic nematode have been overcome multiple times. This then posits the following questions: how has parasitism evolved in nematodes and is the mechanism conserved across multiple occurrences of free-living to parasitic evolution?
These questions can be considered from two points of view, which are essentially different sides of the same coin; the evolutionary ecology of nematodes and the pre-adaptation to parasitism. From the evolutionary ecology viewpoint, the evolution towards parasitism proceeded via an intermediate phoretic association with another organism, such as that seen with multiple Caenorhabditis spp. (Baird, 1999; Barriere and Felix, 2005), towards endophoresy, necromeny (Hong and Sommer, 2006), facultative parasitism and finally obligate parasitism. However, it is most likely that the ecological changes towards parasitism and the role of existing pre-adaptations favouring parasitism are inseparable as each is unlikely to have occurred without the other. There is an expanding body of work on the ecological changes that may have lead towards parasitism, such as excellent work on host-nematode interactions by the Sommer laboratory (Hong et al., 2008).
From the pre-adaptation viewpoint, the physiological similarities between arrested dauer L3s of free-living nematodes and arrested infective L3s (iL3) of parasitic nematodes represent an underlying pre-adaptation to parasitism (Rogers and Sommerville, 1963; Hawdon and Schad, 1991; Hotez et al., 1993). Although the ability to develop directly to adulthood without entering larval arrest is known to occur in a limited number of parasitic nematode species, such as Parastrongyloides trichosuri (Grant et al., 2006) and Strongyloides spp. (Yamada et al., 1991; Viney, 1996), and has most likely been lost from obligate parasites, infective larvae of all parasitic and entomopathogenic nematodes must precisely control their activation upon infecting a suitable host. This review will focus on the pre-adaptation to parasitism perspective within the framework of the dauer hypothesis.
2. The dauer hypothesis
Dauers of free-living nematodes are arrested, non-feeding L3s that are resistant to a wide range of environmental insults and act as a dispersal stage to colonise distant high nutrient microenvironments. In Caenorhabditis elegans, dauer larvae are formed under conditions of low food availability, high con-specific population density sensed via a constitutively produced pheromone and high temperature (Riddle and Albert, 1997). In parasitic nematodes, infective larvae are also arrested, primarily L3s, non-feeding and able to survive under harsh conditions. Their role is also to act as a dispersal stage, in this case between hosts. For obligate parasitic nematodes such as Haemonchus contortus, formation of infective larvae is constitutive. For parasitic nematodes with free-living generations, infective larvae formation is influenced by host immune status and environmental temperature, e.g. in Strongyloides ratti (Harvey et al., 2000), or a secreted compound analogous to dauer pheromone, e.g. in P. trichosuri (Grant et al., 2006; Stasiuk et al., 2012). In addition, both dauer and infective larvae share a radial constriction and filariform, as opposed to rhabditiform, pharynx. These similarities in physiology and role between dauer larvae of free-living nematodes and infective larvae of parasitic nematodes have long been commented upon (Rogers and Sommerville, 1963; Hawdon and Schad, 1991) and were canonised as the dauer hypothesis; the prediction that dauer larvae are a pre-adaptation to parasitism and that the mechanisms used to control dauer developmental decisions have been co-opted to control infective larval development and reactivation (Hotez et al., 1993).
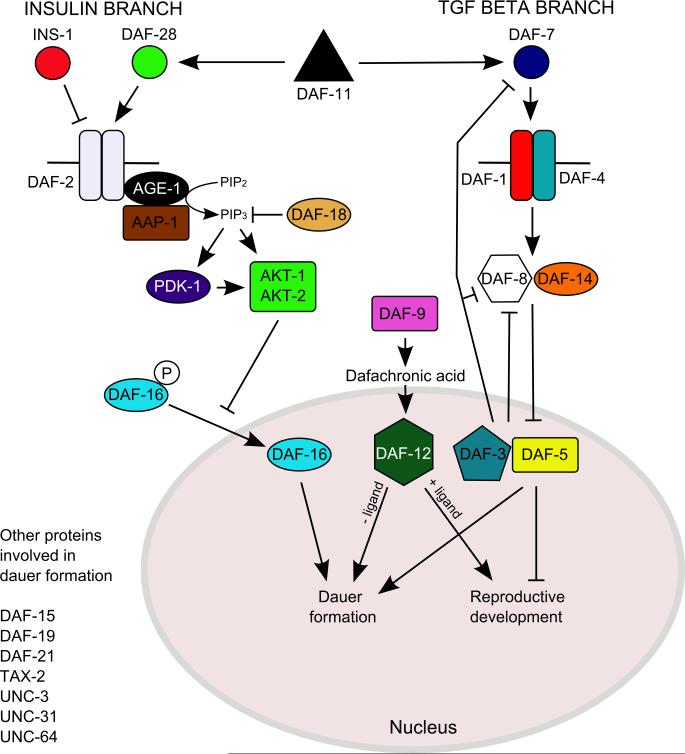
3. The control of entry into C. elegans dauer development
Entry into and exit from dauer formation in C. elegans is precisely controlled in response to temperature, food and population density, sensed as a constitutively produced ascaroside pheromone complex (Riddle and Albert, 1997; Jeong et al., 2005; Butcher et al., 2007). These environmental stimuli are collated by a defined set of sensory neurons, the ASI, ASG, ASJ and ADF plus, to a lesser extent, ASK, amphidial neurons (Bargmann and Horvitz, 1991; Schackwitz et al., 1996), and four molecular pathways, cGMP, insulin and TGF-β pathways, and dafachronic acid signaling via the nuclear hormone receptor, DAF-12 (Riddle and Albert, 1997; Motola et al., 2006) (Fig. 1.; see Stoltzfus et al. (2012b) for additional pathway components). Laser ablation of ASI, ASG and, to a lesser extent, ADF neurons results in constitutive dauer formation (Bargmann and Horvitz, 1991; Schackwitz et al., 1996), whereas laser ablation of ASJ neurons prevents recovery from dauer arrest (Bargmann and Horvitz, 1991). Although the amphidial neuron controlling entry into dauer development at high temperatures has not been described, ALD is a likely candidate, given its role in thermotaxis (Mori and Ohshima, 1995).
Fig. 1.
The molecular pathways that control entry into and exit from dauer development. Adapted from Von Stetina et al. (2007) and modified using information from Park et al. (2010). For a more in-depth graphical representation of these molecular pathways, readers are referred to Stoltzfus et al. (2012b).
The four molecular pathways that control entry into dauer development have been similarly well characterised. Dauer pheromone, a complex of C3, C6, C7 and C9 side chain ascarosides (Jeong et al., 2005; Butcher et al., 2007; Butcher et al., 2008) is sensed in the ASK neuron with different affinities by at least four G-protein coupled receptors, SRBC-64, SRBC-66, SRG-36 and SRG-37 (Kim et al., 2009; McGrath et al., 2011). G-proteins GPA-2 and GPA-3 (Zwaal et al., 1997) transduce this signal to the guanylyl cyclase receptor DAF-11 and HSP-90 protein DAF-21 (Birnby et al., 2000), which then act via cyclic nucleotide-gated channel subunits TAX-2 and TAX-4 (Ailion and Thomas, 2000) to suppress daf-7 and daf-28 expression in response to pheromone.
A large family of insulin ligands, with agonists (e.g. daf-28 (Li et al., 2003)) and antagonists (e.g. ins-18 (Matsunaga et al., 2012) and ins-1 (Pierce et al., 2001)), binds to the DAF-2 insulin receptor orthologue (Kimura et al., 1997), the summation of which determines the output of DAF-2. When activated, DAF-2 in turn activates the AGE-1 phosphoinositide 3-kinase (PI3K) and downstream protein kinases, PDK-1, AKT-1 and AKT-2 (Paradis and Ruvkun, 1998). AKT-1 then phosphorylates DAF-16, a FOXO orthologue, resulting in the cytoplasmic localisation of DAF-16 (Paradis and Ruvkun, 1998; Lee et al., 2001) and reproductive development. Under low food and high pheromone conditions, a lack of DAF-2 activation results in the nuclear localisation of DAF-16 and entry into dauer development (Paradis and Ruvkun, 1998).
DAF-7 is expressed in ASI neurons under low pheromone and high food conditions (Ren et al., 1996; Schackwitz et al., 1996; Crook et al., 2010). DAF-7 binding to the DAF-1/DAF-4 heterodimeric receptor results in phosphorylation of the R-SMADs DAF-8 and DAF-14 (Georgi et al., 1990; Estevez et al., 1993; Inoue and Thomas, 2000), which in turn inhibit Sno-like DAF-5 and SMAD DAF-3 binding to the promoters of genes involved in dauer development (Patterson et al., 1997; da Graca et al., 2004). DAF-3 also acts as part of a feedback loop by repressing DAF-7 and DAF-8 expression under dauer inducing conditions (Park et al., 2010). Mutations in pro-reproductive development genes, such as daf-7 and daf-1 (Estevez et al., 1993; Ren et al., 1996), result in constitutive dauer formation (daf-c), even under favourable conditions, whereas mutations in pro-dauer genes, such as daf-3, result in defective dauer formation, even under dauer inducing conditions (daf-d) (Patterson et al., 1997). In addition, daf-7 expression is switched on when pheromone induced dauer larvae are transferred to freshly seeded plates without pheromone, which suggests a role for daf-7 signaling during exit from the dauer stage (Ren et al., 1996).
Finally, outputs of these three pathways are collated by a nuclear hormone receptor, DAF-12, which is considered the master regulator for entry into dauer development (Inoue and Thomas, 2000; Snow and Larsen, 2000), for which the ligands are dafachronic acids, sterols produced by cytochrome p450 DAF-9 (Jia et al., 2002; Motola et al., 2006). Therefore under the dauer hypothesis, one would expect both the molecular and cellular control of dauer entry to be conserved in parasitic nematodes. I will first cover the conservation of the cellular control of dauer development, then the three pathways that control dauer development.
4. Cellular conservation of the control of arrested development
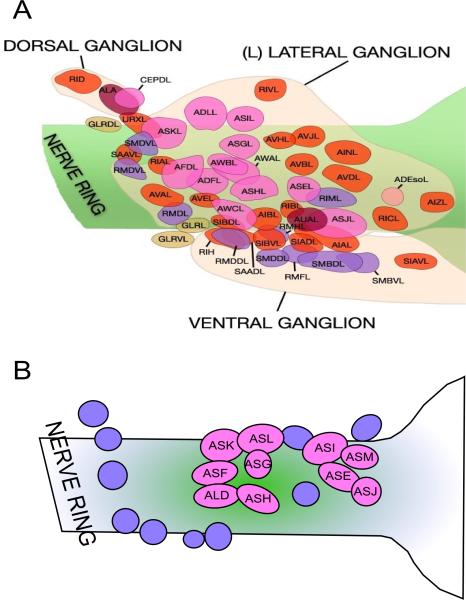
The identity of sensory neurons in parasitic nematodes has been determined by positional homology for Strongyloides stercoralis, H. contortus and P. trichosuri (Ashton et al., 1995; Li et al., 2000; Li et al., 2001; Zhu et al., 2011) (Fig. 2). To demonstrate functional homology, laser ablation of ASI and ADF (ASF) neurons in S. stercoralis L1s resulted in direct development into the dauer-analogous iL3 stage as opposed to development into free-living adults, showing that the role of ASI and ADF (ASF) neurons in controlling entry into arrested development is conserved between distantly related free-living and parasitic nematodes (Ashton et al., 1998). Further conservation of neuron function in developmental arrest was demonstrated by laser ablation of the ASJ cell pair in S. stercoralis and Heterorhabditis bacteriophora, which resulted in partial blocking of the resumption of iL3 development under appropriate conditions (Ashton et al., 2007; Hallem et al., 2007). Similarly, the functional homology of the ALD thermotaxis neuron was demonstrated in S. stercoralis by the failure to migrate along a thermal gradient and inability to form iL3s at high temperatures in larvae in which this neuron pair was ablated at the L1 stage (Lopez et al., 2000; Nolan et al., 2004). Thus the chemo- and thermosensory apparatus used by free-living nematodes and parasitic nematodes to control entry into and exit from L3 arrest is broadly conserved. Expansion of the analysis of the conservation of neuron function into other free-living and parasitic nematodes such as P. trichosuri (Zhu et al., 2011) would increase our understanding of the role of conservation of cellular control of larval arrest in the evolution of parasitism.
Fig. 2.
Conservation of sensory neuroanatomy between (A) Caenorhabditis elegans and (B) Stronglyoides stercoralis. Pale pink circles labeled Axx represent amphidial neurons in S. stercoralis that have been identified by positional homology. Adapted from www.wormatlas.org (Altun and Hall, 2008) and Ashton et al. (1998).
5. Co-option of molecular pathways
5.1. cGMP signaling
cGMP signaling has been investigated in only four parasitic nematodes, with conflicting results. A membrane permeable cGMP analogue, 8-bromo-cyclic GMP, is able to activate feeding in Ancylostoma caninum iL3s and exsheathment of H. bacteriophora infective juveniles, indicating that cGMP plays a role in the resumption of development (Hawdon and Datu, 2003; Hallem et al., 2007). However, in Nippostrongylus brasiliensis, where iL3 activation is dependent solely on a shift in host temperature, 8-bromo-cyclic GMP had no effect on activation of feeding (Huang et al., 2010). Although these species represent only three data points from which to draw conclusions, the response to 8-bromo-cyclic GMP does fit with the observation that host cues are needed for resumption of development in A. caninum and H. bacteriophora, a situation analogous to C. elegans dauer recovery, whereas N. brasiliensis relies only on host temperature cues. In addition, orthologues of Ce-gpa-2 and Ce-gpa-3 were cloned from S. stercoralis (Massey Jr et al., 2001) and other members of the cGMP signaling pathway in S. stercoralis were identified by RNAseq analysis, with a consistent expression peak in infective larvae (Stoltzfus et al., 2012b), suggesting a role for cGMP signaling in the maintenance of larval arrest.
5.2. Insulin signaling
In contrast to the guanylyl cyclase pathway, there has been considerably more research on the role of insulin signaling in parasitic nematode development. Early research using muscarinic agonists hinted at a conserved role for insulin signaling in recovery from dauer and iL3 arrest in C. elegans and A. caninum (Tissenbaum et al., 2000; Hawdon and Datu, 2003), which was subsequently confirmed by the inhibition of hookworm and N. brasiliensis infective larvae activation by a PI3-kinase inhibitor, LY294002 (Brand and Hawdon, 2004; Huang et al., 2010).
Subsequent research focused on daf-16, identifying homologues from S. stercoralis (Massey Jr et al., 2003), A. caninum (Gao et al., 2009), H. contortus (Hu et al., 2010) and the phoretic nematode Pristionchus pacificus (Ogawa et al., 2011). Conservation of function was demonstrated by complementation rescue of C. elegans daf-16 mutants (Massey et al., 2006; Hu et al., 2010; Gelmedin et al., 2011) by DNA binding assays (Gao et al., 2009; Gao et al., 2010) and by AKT phosphorylation-dependent binding to the A. caninum 14-3-3 orthologue, FTT-2 (Kiss et al., 2009). The use of purified recombinant Ac-daf-16 DNA binding domain as bait was especially interesting as it identified 24 Ac-daf-16 binding sites, eight of which corresponded to genes differentially expressed on infective larvae activation (Gao et al., 2010).
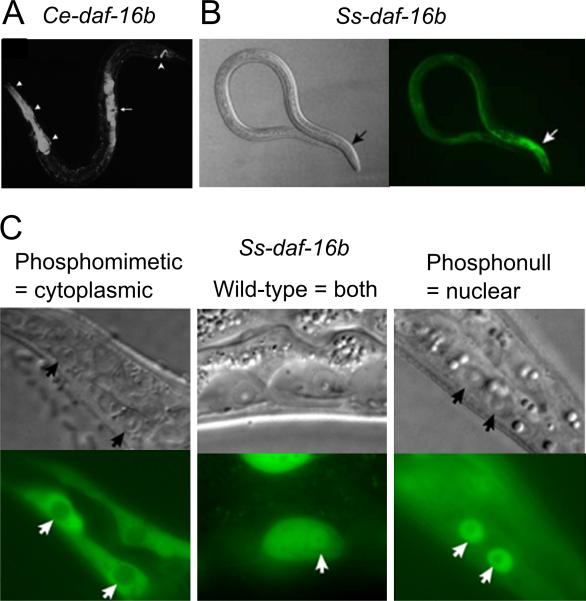
However, conclusive evidence for the conservation of insulin signaling in parasitic nematodes was provided by the use of a dominant negative form of DAF-16 (Castelletto et al., 2009). Not only was the Ss-daf-16b expression pattern in S. stercoralis analogous to that of Ce-daf-16b in C. elegans, but modification of the phosphorylation sites to create phosphonull and phosphomimetic Ss-daf-16b transgenes reproduced the nuclear and cytoplasmic localisation, respectively, seen in C. elegans (Lee et al., 2001; Castelletto et al., 2009) (Fig. 3). Finally, Ss-daf-16b transgenes containing the DNA binding domains but lacking C-terminal transactivating domains produced a dominant negative phenotype, whereby L3s either transiently arrested and proceeded to an L4 stage or incompletely entered iL3 development (Castelletto et al., 2009). This is the first known reported case of affecting gene function in a parasitic nematode using a gene knock-in approach and holds huge potential.
Fig. 3.
Conservation of expression patterns for daf-16b from Caenorhabditis elegans (Ce) and Strongyloides stercoralis (Ss). (A) Ce-daf-16b expression in the pharynx, somatic gonad and tail neurons. (B) Ss-daf-16b expression, predominantly in the pharynx. (C) The localisation of phosphomimetic (i.e. constitutive insulin signaling) and phosphonull (no insulin signaling) forms of Ss-DAF-16b mimic that of Ce-DAF-16b in the presence and absence of insulin signaling. Adapted from Lee et al. (2001) (A) and Castelletto et al. (2009) (B and C).
Subsequent work by the same group has identified an orthologue of daf-2, the C. elegans insulin receptor, and age-1, part of the downstream kinase cascade that inhibits DAF-16 (Stoltzfus et al., 2012a; Massey et al., 2013), as well as confirming the requirement for PI3-kinase activity in the resumption of development (Stoltzfus et al., 2012a). Interestingly, the two Ss-daf-2 splice isoforms are differentially expressed, with Ss-daf-2a expressed throughout the life-cycle and Ss-daf-2b expression peaks in infective and post-iL3, perhaps reflecting a role for this splice isoform in the control of the resumption of development (Massey et al., 2013). The flexible gene architecture of two key members of the insulin signaling pathway, with the demonstrably different roles and temporal expression patterns of their splice isoforms (Lee et al., 2001; Massey et al., 2006; Massey et al., 2013), suggests that this may be the material on which the co-option of this pathway in the evolution of parasitism occurred.
In addition to insulin signaling, three recent papers have explored the role of daf-12 and its ligands, Δ4-dafachronic (Δ4-DA) and Δ7-dafachronic acid (Δ7-DA), in parasitic nematode development. Wang et al. (2009) succeeded in cloning full or partial daf-12 sequences from A. caninum, Ancylostoma ceylanicum, S. stercoralis and Necator americanus, each with significant sequence identity in their ligand binding domains. Each DAF-12 orthologue, including that of C. elegans, demonstrated Δ4-DA and Δ7-DA binding in cell reporter assays, which was abolished when conserved DA binding pocket residues were altered (Wang et al., 2009). Incubation of iL3s with either Δ4-DA or Δ7-DA resulted in resumption of feeding and, for A. caninum, the production of secreted proteins produced on infection of a host. Further supporting a role for daf-12 signaling and dafachronic acids in the control of development in parasitic nematodes, Δ7-DA but not Δ4-DA prevented entry into iL3 development for S. stercoralis larvae. The ability of Δ7-DA to prevent entry into arrested larval development and promote recovery from arrest was also seen for Strongyloides papillosus, a parasite of ruminants, and P. pacificus (Ogawa et al., 2009). Another piece of evidence corroborating the role of Δ7-DA and Δ4-DA in mediating the resumption of development was their ability to overcome the inhibition of DAF-9 P450, which synthesizes Δ7-DA and Δ4-DA from 3-keto-sterols (Motola et al., 2006), by ketoconazole, demonstrating the role of DAF-9 in transducing environmental signals via Δ7-DA and Δ4-DA and daf-12 (Wang et al., 2009). Inhibition of DAF-9 by ketoconazole also blocked the resumption of feeding and production of secreted proteins in N. brasiliensis (Huang et al., 2010). A review by Sommer and Ogawa (2011) elegantly summarises the current view on the role of hormone signaling in the evolution of novel traits in nematodes.
Thus, not only are the insulin signaling pathway and daf-12 present in free-living and parasitic nematodes but their roles appear to be deeply conserved. This deep conservation in the role of two key parts of the molecular machinery in the control of the development of both dauer larvae and infective larvae lends considerable support to the dauer hypothesis.
5.3. daf-7 signaling
Given the clear conservation of the roles of insulin signaling and daf-12 activation from free-living to parasitic nematodes, it was of considerable interest to determine whether the same degree of conservation existed for TGF-β signaling. The first daf-7 orthologue discovered was tgh-2 in Brugia malayi, where its expression fluctuated with the molting cycle (Gomez-Escobar et al., 2000). Subsequently, daf-7 orthologues were characterized from S. ratti, S. stercoralis and P. trichosuri, as well as from the more distantly related Heligomosomoides polygyrus, Teladorsagia circumcincta, A. caninum, N. brasiliensis and H. contortus which are from the same phylogenetic clade as C. elegans (Brand et al., 2005; Crook et al., 2005; Freitas and Arasu, 2005; Massey et al., 2005; McSorley et al., 2010). With the exception of H. polygyrus and T. circumcincta, where daf-7 expression is maximal in adult stages (McSorley et al., 2010), and B. malayi mentioned above, parasite daf-7 expression peaks in the arrested L3. This observation lead to the hypothesis that, contrary to its role in controlling entry into the arrested state in C. elegans, daf-7 signaling in parasitic nematodes acts to maintain the arrested state until a suitable host is found (Viney et al., 2005). A role for daf-7 in the maintenance of infective larval arrest would predict that daf-7 expression would drop in larvae that had infected a suitable host and resumed development. Indeed, for H. contortus, N. brasiliensis, P. trichosuri and S. ratti daf-7 expression was low to barely detectable in post-infective L3s and L4s recovered from the host, and significantly lower in exsheathed H. contortus iL3s and S. ratti iL3s that had penetrated through host skin (Crook et al., 2005; McSorley et al., 2010). These results suggest that the role of daf-7 signaling, to suppress dauer entry and promote dauer exit, has been reversed in a wide variety of parasitic nematodes.
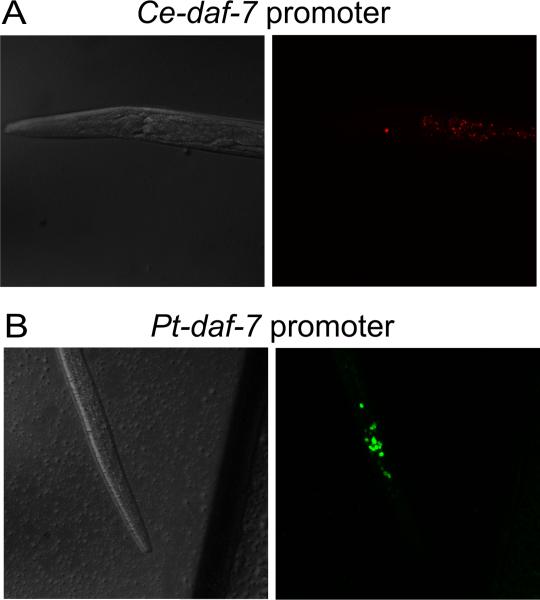
Evolution via changes in the control of developmental genes is a central tenet of EvoDevo (Carroll, 2000, 2005), into which the apparent changes in control of daf-7 expression in parasitic nematodes fit. To investigate this further Crook et al. (2010) tested the function of the Pt-daf-7 promoter in C. elegans, driving both a GFP reporter and a copy of Ce-daf-7.. They found that, while the Pt-daf-7 promoter was functional in C. elegans, GFP expression was both spatially and temporally expanded compared with that of Ce-daf-7, with expression predominantly in dauer larvae (Fig. 4). This expression pattern suggests that the Ptdaf-7 promoter contains some as yet unidentified arrested larva-specific regulatory element(s) and that the acquisition of these elements may be the mechanism by which the role of daf-7 changed in some parasitic nematodes. In addition, the Cedaf-7 coding region under control of the Pt-daf-7 promoter was unable to rescue a daf-7 mutant, as expected given the differences in expression, further highlighting the divergence in the role of daf-7 in P. trichosuri and C. elegans (Crook et al., 2010). Unfortunately, as with Ss-age-1 (Stoltzfus et al., 2012a), the Pt-daf-7 coding region, even just the ligand domain, was efficiently silenced in C. elegans so it was not possible to confirm its function (Crook et al., 2010).
Fig. 4.
Divergent control of spatial and temporal expression by (A) Caenorhabditis elegans and (B) Pt-daf-7 promoters in C. elegans. DsRed under the control of the Ce-daf-7 promoter is restricted to the ASI neuron pair only and is visible only in L1s and L2s (L2 pictured). In contrast, GFP under the control of the Pt-daf-7 promoter is expressed in a large number of cells in the head, including amphidial neurons, predominantly in dauer larvae (pictured). This expression pattern from the Pt-daf-7 promoter correlates with its peak expression in Parastrongyloides trichosuri infective larvae (Crook et al., 2005). Adapted from Crook et al. (2010).
What is the role of daf-7 signaling in parasitic nematodes? Expression studies suggest a role in the maintenance of larval arrest, although this is by no means consistent across the phylum, and the activity of the Pt-daf-7 promoter in C. elegans dauer larvae implies that this role change has occurred via changes in gene regulation. However, a definitive role in the maintenance of larval arrest requires modification of daf-7 signaling levels. Lower levels of daf-7 signaling would be predicted to result in transient larval arrest or inappropriate resumption of development; higher levels of daf-7 signaling would be predicted to result in an inability to resume development. The use of dominant negative daf-7 transgenes, as with daf-16 and insulin signaling (Castelletto et al., 2009), offers one possible approach, although recent work in C. elegans suggests that this may not be straightforward (Crook and Grant, 2013). Another tantalizing approach would be the use of random insertional mutagenesis using the piggyBac transposon pioneered in S. stercoralis (Shao et al., 2012). Given that any daf-7 mutants thus generated may be incapable of forming infective larvae, this approach would need to be undertaken in a parasitic nematode, such as P. trichosuri, that can undergo multiple free-living generations (Grant et al., 2006).
6. Advances from whole genome sequencing and microarray studies
The targeted approach of searching for parasitic nematode homologues of C. elegans dauer development has clearly been a productive one. However, an equally successful approach has been to harness the ever increasing power of expressed sequence tag (EST) libraries, microarrays and now deep sequencing to perform unbiased searches for parasite-specific genes. Two Strongyloides spp., S. ratti and S. stercoralis, are especially well suited to this approach due to their free-living and parasitic generations, which enables the comparison of transcriptional profiles from iL3 versus free-living L3 (Mitreva et al., 2004; Thompson et al., 2006; Ramanathan et al., 2011), parasitic versus free-living adults (Thompson et al., 2005) and even parasitic adults from naïve versus immune hosts (Thompson et al., 2008; O'Meara et al., 2010). The stage-specific expression of candidate parasite-specific genes from S. ratti was further characterized by real-time PCR and western blotting, with one protein being detected in the excretory/secretory products of the parasitic adult, demonstrating the power of this unbiased approach (Spinner et al., 2012).
In addition, deep sequencing has allowed a large-scale analysis of dauer developmental control homologues in S. stercoralis (Stoltzfus et al., 2012b). Of particular interest, this study discovered a paucity of insulin-like ligands and an expansion of DAF-7-like TGF-β ligands in S. stercoralis, with distinct expression patterns, which suggest a far more nuanced role for insulin and daf-7 signaling in the control of S. stercoralis infective larval formation and activation (Stoltzfus et al., 2012b). Clearly the increasing availability of parasitic nematode whole genome sequences and the decreasing cost of next generation sequencing technologies will lead to a greater understanding of what it means to be a parasite.
7. Conclusion
The original dauer hypothesis stated that the life-history and physiological traits of dauer larvae of free-living nematodes confer features that are ideally suited to infectivity (Rogers and Sommerville, 1963; Hawdon and Schad, 1991; Hotez et al., 1993) and, by extension, these features may constitute a pre-adaptation to the evolution of parasitism. Therefore the simplest hypothesis describing how this evolutionary event may have occurred would be that the mechanisms which control dauer entry and exit are used to control entry into and exit from infective larval development plus, where this life-style option exists, between infective larval development and free-living development.
Despite the simplicity of this hypothesis, the picture that has emerged over the last three decades is far more nuanced than anyone would have expected. It is clear that the sensory neuroanatomy used to control entry into and exit from arrested larval development is conserved between free-living and parasitic nematodes. It is also clear that the four molecular pathways used to control entry into dauer development are present in a wide range of parasitic nematodes. However, what was a surprise is that the role of these pathways appears both conserved (insulin signaling, daf-12 and dafachronic acid) and divergent (daf-7 signaling). It is especially interesting to note that even the divergence of daf-7 signaling is not conserved across all parasitic nematodes. In addition, work with the facultative parasitic nematode P. trichosuri and multiple isogenic C. elegans lines suggests that phenotypic plasticity in dauer formation may be one trait upon which selective pressure has acted in the evolution of parasitism (Harvey et al., 2008; Stasiuk et al., 2012).
The work over the last 20 years has painted a compelling picture not only of how parasitic nematodes control their entry into and exit from developmental arrest, but also how the conservation and divergence of those control mechanisms may have facilitated the colonization of a new environment and the evolution of parasitism. However, many questions still remain: what is the role of daf-7 signaling in parasitic nematodes, how are the multiple signals (host entry, temperature, pH) collated into a developmental output, what pathways lie downstream of the daf-7 and insulin pathways, what role do these pathways have in parasitic nematodes with multiple hosts or facultative free-living generations? One hopes that new tools currently being developed make the next 20 years as fruitful as the last.
Highlights.
Parasitism has evolved multiple times across the phylum Nematoda.
Similarities between dauer larvae and infective larvae suggest that dauer larvae are a pre-adaptation for parasitism.
The dauer hypothesis: “the mechanisms used to control dauer development are conserved in parasitic nematodes”.
The neurobiology of the control of larval arrest and exit is conserved between free-living and parasitic nematodes.
However, these pathways in infective larvae are both conserved (insulin signaling) and divergent (TGF-β signaling).
Acknowledgements
I would like to thank Wendy Hanna-Rose for her constructive criticism of the manuscript and Warwick Grant for the inspiration to write it. MC was supported during the preparation of this manuscript by National Institutes of Health, USA, grant R01GM086786 awarded to WHR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun ZF, Hall DH. Handbook of C. elegans anatomy.In: Wormatlas. 2008 http://www.wormatlas.org/hermaphrodite/hermaphroditehomepage.htm.
- Ashton FT, Bhopale VM, Fine AE, Schad GA. Sensory neuroanatomy of a skin-penetrating nematode parasite: Strongyloides stercoralis. I. Amphidial neurons. J Comp Neurol. 1995;357:281–295. doi: 10.1002/cne.903570208. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J Parasitol. 1998;84:691–695. [PubMed] [Google Scholar]
- Ashton FT, Zhu XD, Lok JB, Schad GA. Strongyloides stercoralis: Amphidial neuron pair ASJ triggers significant resumption of development by infective larvae under host-mimicking in vitro conditions. Exp Parasitol. 2007;115:92–97. doi: 10.1016/j.exppara.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SE. Natural and experimental associations of Caenorhabditis remanei with Trachelipus rathkii and other terrestrial isopods. Nematology. 1999;1:471–475. [Google Scholar]
- Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Barriere A, Felix MA. Natural variation and population genetics of Caenorhabditis elegans. [8th July 2013];WormBook. 2005 doi: 10.1895/wormbook.1.43.1. doi/10.1895/wormbook.1.43.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Birnby DA, Link EM, Vowels JJ, Tian H, Colacurcio PL, Thomas JH. A transmembrane guanylyl cyclase (DAF-11) and Hsp-90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics. 2000;155:85–104. doi: 10.1093/genetics/155.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter M, De Ley P, Garey J, Liu L, Scheldeman P, Vierstraete A, Vanfleteren J, Mackey L, Dorris M, Frisse L. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- Brand A, Hawdon JM. Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol. 2004;34:909–914. doi: 10.1016/j.ijpara.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Brand AM, Varghese G, Majewski W, Hawdon JM. Identification of a DAF-7 ortholog from the hookworm Ancylostoma caninum. Int J Parasitol. 2005;35:1489–1498. doi: 10.1016/j.ijpara.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- Butcher RA, Ragains JR, Kim E, Clardy J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc Natl Acad Sci U S A. 2008;105:14288–14292. doi: 10.1073/pnas.0806676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Endless forms: The evolution of gene regulation and morphological diversity. Cell. 2000;101:577–580. doi: 10.1016/s0092-8674(00)80868-5. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Evolution at two levels: on genes and form. PLoS Biol. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelletto ML, Massey HC, Jr., Lok JB. Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathog. 2009;5:e1000370. doi: 10.1371/journal.ppat.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook M, Thompson FJ, Grant WN, Viney ME. daf-7 and the development of Strongyloides ratti and Parastrongyloides trichosuri. Mol Biochem Parasitol. 2005;139:213–223. doi: 10.1016/j.molbiopara.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Crook M, Grant K, Grant WN. Failure of Parastrongyloides trichosuri daf-7 to complement a Caenorhabditis elegans daf-7 (e1372) mutant: Implications for the evolution of parasitism. Int J Parasitol. 2010;40:1675–1683. doi: 10.1016/j.ijpara.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Crook M, Grant WN. Dominant negative mutations of Caenorhabditis elegans daf-7 confer a novel developmental phenotype. Dev Dynam. 2013;242:654–664. doi: 10.1002/dvdy.23963. [DOI] [PubMed] [Google Scholar]
- da Graca LS, Zimmerman KK, Mitchell MC, Kozhan-Gorodetska M, Sekiewicz K, Morales Y, Patterson, Garth I. DAF-5 is a Ski oncoprotein homolog that functions in a neuronal TGF-B pathway to regulate C. elegans dauer development. Development. 2004;131:435–446. doi: 10.1242/dev.00922. [DOI] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana JL, Albert PS, Massague J, Riddle DL. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Freitas TC, Arasu P. Cloning and characterisation of genes encoding two transforming growth factor-beta-like ligands from the hookworm, Ancylostoma caninum. Int J Parasitol. 2005;35:1477–1487. doi: 10.1016/j.ijpara.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gao X, Frank D, Hawdon JM. Molecular cloning and DNA binding characterization of DAF-16 orthologs from Ancylostoma hookworms. Int J Parasitol. 2009;39:407–415. doi: 10.1016/j.ijpara.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wang Z, Martin J, Abubucker S, Zhang X, Mitreva M, Hawdon JM. Identification of hookworm DAF-16/FOXO response elements and direct gene targets. PLoS ONE. 2010;5:e12289. doi: 10.1371/journal.pone.0012289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmedin V, Brodigan T, Gao X, Krause M, Wang Z, Hawdon JM. Transgenic C. elegans dauer larvae expressing hookworm phospho null DAF-16/FoxO exit dauer. PLoS ONE. 2011;6:e25996. doi: 10.1371/journal.pone.0025996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta , expressed in microfilarial and adult stages of Brugia malayi. Infect Immun. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WN, Stasiuk S, Newton-Howes J, Ralston M, Bisset SA, Heath DD, Shoemaker CB. Parastrongyloides trichosuri, a nematode parasite of mammals that is uniquely suited to genetic analysis. Int J Parasitol. 2006;36:453–466. doi: 10.1016/j.ijpara.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Rengarajan M, Ciche, Todd A, Sternberg PW. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol. 2007;17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Harvey S, Shorto A, Viney M. Quantitative genetic analysis of life-history traits of Caenorhabditis elegans in stressful environments. BMC Evol Biol. 2008;8:15. doi: 10.1186/1471-2148-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Gemmill AW, Read AF, Viney ME. The control of morph development in the parasitic nematode Strongyloides ratti. P Roy Soc Lond B Bio. 2000;267:2057–2063. doi: 10.1098/rspb.2000.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon JM, Schad GA. Albumin and a dialyzable serum factor stimulate feeding in vitro by third-stage larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1991;77:587–591. [PubMed] [Google Scholar]
- Hawdon JM, Datu B. The second messenger cyclic GMP mediates activation in Ancylostoma caninum infective larvae. Int J Parasitol. 2003;33:787–793. doi: 10.1016/s0020-7519(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Hong RL, Sommer RJ. Pristionchus pacificus: a well-rounded nematode. Bioessays. 2006;28:651–659. doi: 10.1002/bies.20404. [DOI] [PubMed] [Google Scholar]
- Hong RL, Svatos A, Herrmann M, Sommer RJ. Species-specific recognition of beetle cues by the nematode Pristionchus maupasi. Evol Dev. 2008;10:273–279. doi: 10.1111/j.1525-142X.2008.00236.x. [DOI] [PubMed] [Google Scholar]
- Hotez P, Hawdon J, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans daf-c paradigm. Parasitol Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Hu M, Lok JB, Ranjit N, Massey HC, Jr., Sternberg PW, Gasser RB. Structural and functional characterisation of the fork head transcription factor-encoding gene, Hc-daf-16, from the parasitic nematode Haemonchus contortus (Strongylida). Int J Parasitol. 2010;40:405–415. doi: 10.1016/j.ijpara.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC-C, Chan DTY, Smyth DJ, Ball G, Gounaris K, Selkirk ME. Activation of Nippostrongylus brasiliensis infective larvae is regulated by a pathway distinct from the hookworm Ancylostoma caninum. Int J Parasitol. 2010;40:1619–1628. doi: 10.1016/j.ijpara.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Inoue T, Thomas JH. Suppressors of transforming growth factor-beta pathway mutants in the Caenorhabditis elegans dauer formation pathway. Genetics. 2000;156:1035–1046. doi: 10.1093/genetics/156.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong EM, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kiss J, Gao X, Krepp J, Hawdon J. Interaction of hookworm 14-3-3 with the forkhead transcription factor DAF-16 requires intact Akt phosphorylation sites. Parasit Vectors. 2009;2:21. doi: 10.1186/1756-3305-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RYN, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Li J, Ashton FT, Gamble HR, Schad GA. Sensory neuroanatomy of a passively ingested nematode parasite, Haemonchus contortus: Amphidial neurons of the first stage larva. J Comp Neurol. 2000;417:299–314. [PubMed] [Google Scholar]
- Li J, Zhu X, Ashton FT, Gamble HR, Schad GA. Sensory neuroanatomy of a passively ingested nematode parasite, Haemonchus contortus: Amphidial neurons of the third-stage larva. J Parasitol. 2001;87:65–72. doi: 10.1645/0022-3395(2001)087[0065:SNOAPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Li WQ, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PM, Boston R, Ashton FT, Schad GA. The neurons of class ALD mediate thermotaxis in the parasitic nematode, Strongyloides stercoralis. Int J Parasitol. 2000;30:1115–1121. doi: 10.1016/s0020-7519(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Massey HC, Castelletto ML, Bhopale VM, Schad GA, Lok JB. Sst-tgh-1 from Strongyloides stercoralis encodes a proposed ortholog of daf-7 in Caenorhabditis elegans. Mol Biochem Parasitol. 2005;142:116–120. doi: 10.1016/j.molbiopara.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Massey HC, Jr., Bhopale MK, Li X, Castelletto M, Lok JB. The fork head transcription factor FKTF-1b from Strongyloides stercoralis restores DAF-16 developmental function to mutant Caenorhabditis elegans. Int J Parasitol. 2006;36:347–352. doi: 10.1016/j.ijpara.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey HC, Jr., Ranjit N, Stoltzfus JD, Lok JB. Strongyloides stercoralis daf-2 encodes a divergent ortholog of Caenorhabditis elegans DAF-2. Int J Parasitol. 2013;43:515–520. doi: 10.1016/j.ijpara.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey HC, Jr, Ball CC, Lok JB. PCR amplification of putative gpa-2 and gpa-3 orthologs from the (A+T)-rich genome of Strongyloides stercoralis. Int J Parasitol. 2001;31:377–383. doi: 10.1016/s0020-7519(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Massey HC, Jr, Nishi M, Chaudhary K, Pakpour N, Lok JB. Structure and developmental expression of Strongyloides stercoralis fktf-1, a proposed ortholog of daf-16 in Caenorhabditis elegans. Int J Parasitol. 2003;33:1537–1544. doi: 10.1016/s0020-7519(03)00205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y, Gengyo-Ando K, Mitani S, Iwasaki T, Kawano T. Physiological function, expression pattern, and transcriptional regulation of a Caenorhabditis elegans insulin-like peptide, INS-18. Biochem Biophys Res Comm. 2012;423:478–483. doi: 10.1016/j.bbrc.2012.05.145. [DOI] [PubMed] [Google Scholar]
- McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley HJ, Grainger JR, Harcus Y, Murray J, Nisbet AJ, Knox DP, Maizels RM. daf-7-related TGF-B homologues from Trichostrongyloid nematodes show contrasting life-cycle expression patterns. Parasitology. 2010;137:159–171. doi: 10.1017/S0031182009990321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitreva M, McCarter JP, Martin JF, Dante M, Wylie T, Chiapelli B, Pape D, Clifton SW, Nutman TB, Waterston RH. Comparative genomics of gene expression in the parasitic and free-living nematodes Strongyloides stercoralis and Caenorhabditis elegans. Genome Res. 2004;14:209–220. doi: 10.1101/gr.1524804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, Mangelsdorf DJ. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nolan TJ, Brenes M, Ashton FT, Zhu X, Forbes WM, Boston R, Schad GA. The amphidial neuron pair ALD controls the temperature-sensitive choice of alternative developmental pathways in the parasitic nematode, Strongyloides stercoralis. Parasitology. 2004;129:753–759. doi: 10.1017/s0031182004006092. [DOI] [PubMed] [Google Scholar]
- O'Meara H, Barber R, Mello LV, Sangaralingam A, Viney ME, Paterson S. Response of the Strongyloides ratti transcriptome to host immunological environment. Int J Parasitol. 2010;40:1609–1617. doi: 10.1016/j.ijpara.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Streit A, Antebi A, Sommer RJ. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol. 2009;19:67–71. doi: 10.1016/j.cub.2008.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Bento G, Bartelmes G, Dieterich C, Sommer RJ. Pristionchus pacificus daf-16 is essential for dauer formation but dispensable for mouth form dimorphism. Development. 2011;138:1281–1284. doi: 10.1242/dev.058909. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Estevez A, Riddle DL. Antagonistic Smad transcription factors control the dauer/non-dauer switch in C. elegans. Development. 2010;137:477–485. doi: 10.1242/dev.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Varma S, Ribeiro JMC, Myers TG, Nolan TJ, Abraham D, Lok JB, Nutman TB. Microarray-based analysis of differential gene expression between infective and noninfective larvae of Strongyloides stercoralis. PLoS Negl Trop Dis. 2011;5:e1039. doi: 10.1371/journal.pntd.0001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1997. pp. 739–768. [PubMed] [Google Scholar]
- Rogers WP, Sommerville RI. The infective stage of nematode parasites and its significance in parasitism. Adv Parasitol. 1963;1:109–177. doi: 10.1016/s0065-308x(08)60503-5. [DOI] [PubMed] [Google Scholar]
- Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- Shao H, Li X, Nolan TJ, Massey HC, Jr., Pearce EJ, Lok JB. Transposon-mediated chromosomal integration of transgenes in the parasitic nematode Strongyloides ratti and establishment of stable transgenic lines. PLOS Pathog. 2012;8:e1002871. doi: 10.1371/journal.ppat.1002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow MI, Larsen PL. Structure and expression of daf-12: a nuclear hormone receptor with three isoforms that are involved in development and aging in Caenorhabditis elegans. Biochim Biophys Acta - Gene Struct Express. 2000;1494:104–116. doi: 10.1016/s0167-4781(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Spinner WG, Thompson FJ, Emery DC, Viney ME. Characterization of genes with a putative key role in the parasitic lifestyle of the nematode Strongyloides ratti. Parasitology. 2012;139:1317–1328. doi: 10.1017/S0031182012000637. [DOI] [PubMed] [Google Scholar]
- Stasiuk S, Scott M, Grant W. Developmental plasticity and the evolution of parasitism in an unusual nematode, Parastrongyloides trichosuri. Evol Dev. 2012;3:1. doi: 10.1186/2041-9139-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus JD, Massey HC, Jr., Nolan TJ, Griffith SD, Lok JB. Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PLoS ONE. 2012a;7:e38587. doi: 10.1371/journal.pone.0038587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus JD, Minot S, Berriman M, Nolan TJ, Lok JB. RNAseq analysis of the parasitic nematode Strongyloides stercoralis reveals divergent regulation of canonical dauer pathways. PLoS Negl Trop Dis. 2012b;6:e1854. doi: 10.1371/journal.pntd.0001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson F, Mitreva M, Barker G, Martin J, Waterston R, McCarter J, Viney M. An expressed sequence tag analysis of the life-cycle of the parasitic nematode Strongyloides ratti. Mol Biochem Parasitol. 2005;142:32–46. doi: 10.1016/j.molbiopara.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson FJ, Barker GL, Hughes L, Wilkes CP, Coghill J, Viney ME. A microarray analysis of gene expression in the free-living stages of the parasitic nematode Strongyloides ratti. BMC Genomics. 2006;7:157. doi: 10.1186/1471-2164-7-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Barker GLA, Hughes L, Viney ME. Genes important in the parasitic life of the nematode Strongyloides ratti. Mol Biochem Parasitol. 2008;158:112–119. doi: 10.1016/j.molbiopara.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney ME. Developmental switching in the parasitic nematode Strongyloides ratti. P Roy Soc Lond B Bio. 1996;263:201–208. doi: 10.1098/rspb.1996.0032. [DOI] [PubMed] [Google Scholar]
- Viney ME, Thompson FJ, Crook M. TGF-beta and the evolution of nematode parasitism. Int J Parasitol. 2005;35:1473–1475. doi: 10.1016/j.ijpara.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Von Stetina SE, Watson JD, Fox RM, Olszewski KL, Spencer WC, Roy PJ, Miller DM., 3rd Cell-specific microarray profiling experiments reveal a comprehensive picture of gene expression in the C. elegans nervous system. Genome Biol. 2007;8:R135. doi: 10.1186/gb-2007-8-7-r135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, Conneely A, Ogata C, Sharma KK, Auchus RJ, Lok JB, Hawdon JM, Kliewer SA, Xu HE, Mangelsdorf DJ. Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A. 2009;106:9138–9143. doi: 10.1073/pnas.0904064106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Matsuda S, Nakazawa M, Arizono N. Species-specific differences in heterogonic development of serially transferred free-living generations of Strongyloides planiceps and Strongyloides stercoralis. J Parasitol. 1991;77:592–594. [PubMed] [Google Scholar]
- Zhu H, Li J, Nolan TJ, Schad GA, Lok JB. Sensory neuroanatomy of Parastrongyloides trichosuri, a nematode parasite of mammals: Amphidial neurons of the first-stage larva. J Comp Neurol. 2011;519:2493–2507. doi: 10.1002/cne.22637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]