Abstract
Previously we have shown that stimulation of inhibitory A1 adenosine receptors located in the nucleus tractus solitarii (NTS) attenuates cardiopulmonary chemoreflex (CCR) evoked inhibition of renal, adrenal and lumbar sympathetic nerve activity and reflex decreases in arterial pressure and heart rate. Activation of facilitatory A2a adenosine receptors, which dominate over A1 receptors in the NTS, contrastingly alters baseline activity of regional sympathetic outputs: it decreases renal, increases adrenal and does not change lumbar nerve activity. Considering that NTS A2a receptors may facilitate release of inhibitory transmitters we hypothesized that A2a receptors will act in concert with A1 receptors differentially inhibiting regional sympathetic CCR responses (adrenal>lumbar>renal). In urethane/chloralose anesthetized rats (n=38) we compared regional sympathetic responses evoked by stimulation of the CCR with right atrial injections of serotonin 5HT3 receptor agonist, phenylbiguanide, (1–8 µg/kg) before and after selective stimulation, blockade or combined blockade and stimulation of NTS A2a adenosine receptors (microinjections into the NTS of CGS-21680 0.2–20 pmol/50 nl, ZM-241385 40 pmol/100 nl or ZM-241385+CGS-21680, respectively). We found that stimulation of A2a adenosine receptors uniformly inhibited the regional sympathetic and hemodynamic reflex responses and this effect was abolished by the selective blockade of NTS A2a receptors. This indicates that A2a receptor triggered inhibition of CCR responses and the contrasting shifts in baseline sympathetic activity are mediated via different mechanisms. These data implicate that stimulation of NTS A2a receptors triggers unknown inhibitory mechanism(s) which in turn inhibit transmission in the CCR pathway when adenosine is released into the NTS during severe hypotension.
Keywords: nucleus of the solitary tract, purinergic receptors, renal nerve, adrenal nerve, lumbar nerve
1. Introduction
Adenosine operating via A1 and A2a receptors is a powerful central modulator of cardiovascular reflexes primarily integrated in the nucleus of the solitary tract (NTS) (Abdel-Rahman and Tao, 1996;Mosqueda-Garcia et al., 1989;Thomas et al., 2000;Scislo et al., 2001;Scislo and O'Leary, 2005) and its importance is underscored by the observation that the NTS contains the greatest density of adenosine uptake sites in the entire central nervous system (Bisserbe et al., 1985). Adenosine is released into the NTS during life threatening situations. During the stress/hypothalamic defense response the source of endogenous adenosine is extracellular ATP released as a neurotransmitter or co-transmitter from descending hypothalamic terminals or activated glial cells and catabolized to adenosine by ectonucleotidases (St Lambert et al., 1997;St Lambert et al., 1994;St Lambert et al., 1996;Dale et al., 2002;Zimmermann, 1996). In contrast, during severe hemodynamic imbalance (ischemia, hypoxia or severe hemorrhage) intracellular ATP is catabolized to adenosine inside hypoxic neurons and glial cells and then adenosine is released into the extracellular space and envelopes all neurons in the area (Yan et al., 1995;Van Wylen et al., 1988;Winn et al., 1979;Phillis et al., 1987). Despite this global release, adenosine evokes specific and contrasting effects on regional sympathetic outputs and blood pressure control via two antagonistic A1 and A2a receptor subtypes which inhibit and activate neurotransmitter release and central neurons, respectively (Ralevic and Burnstock, 1998). In the central nervous system A1 receptors usually dominate over A2a receptors (Ribeiro et al., 2002;Burnstock, 2007). In contrast, in the NTS the facilitatory A2a receptors functionally prevail over A1 receptors. Cardiovascular responses to exogenous adenosine (decreases in MAP and HR) are mimicked by agonists to A2a receptors whereas exogenous A1 receptor agonists evoke pressor responses (Barraco et al., 1991;Barraco and Phillis, 1991;Abdel-Rahman and Tao, 1996;Mosqueda-Garcia et al., 1989).
Stimulation of NTS A1 adenosine receptors uniformly inhibits reflex decreases in renal (RSNA), preganglionic adrenal (pre-ASNA) and lumbar (LSNA) sympathetic nerve activity evoked by stimulation of cardiopulmonary chemoreflex (CCR) afferents, although this reflex (also known as the Bezold Jarisch reflex) evoked differential regional sympathoinhibition (RSNA>pre-ASNA>LSNA) (Ichinose et al., 2012). The Bezold-Jarisch reflex is a powerful depressor, cardiac slowing and sympathoinhibitory reflex activated by polymodal mechano- and chemoreceptors which transmit information from cardiopulmonary area to the NTS via unmyelinated vagal C fibers in the rat (Coleridge et al., 1973;Paintal, 1977;Paintal, 1973;Thoren, 1977;Thoren et al., 1979a;Thoren et al., 1979b). Powerful activation of this reflex may contribute to fainting and is even blamed for some episodes of sudden cardiac death in young athletes (Benton and Maginot, 2007;Mark, 1983;Greenberg, 1984). Since severe hypotension triggers release of adenosine into the NTS (Van Wylen et al., 1988;Yan et al., 1995;Winn et al., 1979;Phillis et al., 1987) we speculated that adenosine may serve as a negative feedback regulator for the CCR by attenuating the potentially deadly consequences of severe reflex hypotension. This hypothesis is supported by our recent preliminary data which showed that during severe hemorrhage, when adenosine is released into the NTS (Yan et al., 1995;Scislo and O'Leary, 2006), CCR control of regional sympathetic outputs is inhibited and nonselective blockade of NTS adenosine receptors removes this inhibition (Minic, Li et al., 2013). These findings are consistent with inhibition of the CCR by NTS A1 adenosine receptors. However, since inhibitory A1 adenosine receptors play minor role in the NTS compared with the dominating role of facilitatory A2a receptors, whether facilitatory A2a adenosine receptors oppose A1 receptor - mediated inhibition of the CCR in the NTS or both receptor subtypes collaborate in mediating adenosinergic inhibition of CCR control of regional sympathetic outputs is unknown. This last possibility is likely because activation of A2a adenosine receptors may facilitate release of inhibitory neurotransmitters via various mechanisms (Fuxe et al., 2007;Dayne et al., 1996;Cunha-Reis et al., 2008;Edwards and Robertson, 1999;Baptista and Varanda, 2005;Pimentel et al., 2003).
Taking into consideration that stimulation of NTS A2a receptors evokes regionally specific contrasting shifts in baseline sympathetic nerve activity (decreases in RSNA, increases in pre-ASNA and no changes in LSNA) (Scislo and O'Leary, 1998b;Scislo and O'Leary, 1998a), we hypothesized that activation of these receptors will inhibit CCR control of regional sympathetic outputs in a differential manner. CCR responses of pre-ASNA may be attenuated the most, as activation of A2a adenosine receptors and CCR afferents exerts the opposite effects on this sympathetic output. In contrast, the smallest attenuation of reflex responses may be observed in RSNA as activation of A2a adenosine receptors and the CCR exert synergistic effects on this sympathetic output. We expected that LSNA will show intermediate level of inhibition of the reflex responses, as activation of NTS A2a receptors do not alter baseline LSNA. Therefore in the present study we compared the regional sympathetic responses evoked by gradual activation of CCR afferents before and after stimulation of NTS A2a adenosine receptors with increasing doses of the selective agonist CGS-21680. We also investigated the effect of inhibition of these receptors with the selective antagonist ZM-241385 to test whether A2a receptors are tonically active in the CCR pathway. To our surprise, we observed that activation of NTS A2a receptors, which triggers reciprocal regional sympathetic responses, evoked uniform inhibition of CCR mediated regional sympathoinhibition. This suggested that A2a-mediated shifts of baseline sympathetic activity and inhibition of CCR control of the regional sympathetic outputs occur via two different mechanisms.
2. Methods and materials
2.1. Ethical considerations
All protocols and surgical procedures employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals endorsed by the American Physiological Society and published by the National Institutes of Health.
2.2. Design
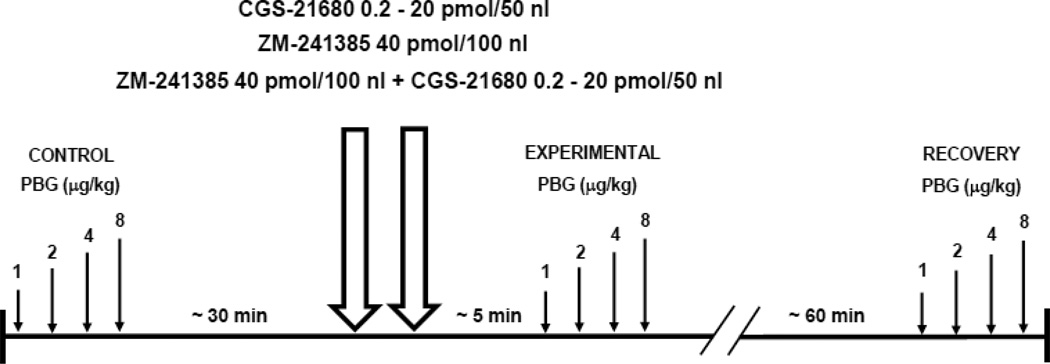
We compared regional sympathetic (renal, adrenal, and lumbar) and hemodynamic (heart rate, HR, and mean arterial pressure, MAP) responses evoked by graded stimulation of the cardiopulmonary chemoreceptors before and after selective activation or inhibition of NTS A2a adenosine receptors in a total of 44 male Sprague-Dawley rats, weight = 370.0 ± 5.8 g. In 29 rats, the comparisons were made between CCR stimulus response function curves obtained under control conditions and approximately 5 min after microinjections into the NTS of three different doses of the selective A2a adenosine receptor agonist, CGS-21680, (0.2, 2.0, and 20 pmol in 50 nl; numbers of animals in each group were 10, 10 and 9, respectively). In an additional 9 rats the CCR function curves were compared before and ~ 5 min after inhibition of NTS A2a adenosine receptors with a water soluble, selective A2a receptor antagonist; ZM-214385 (40 pmol in 100 nl). The CCR reactivity was also assessed approximately one hour after the pharmacological manipulations in the NTS (recovery) in 36 rats; in two of them the evaluation of the recovery reflex curves was impossible for technical reasons. Finally, in 6 additional animals we tested weather the effects of the highest dose of A2a receptor agonist, CGS 21680 (20 pmol/50 nl) on CCR reactivity could be blocked by pretreatment with the selective receptor antagonist ZM-214385 (40 pmol in 100 nl performed ~ 5 minutes before bilateral agonist microinjections). The timeline of the protocol is presented in Figure 1.
Figure 1.
Timeline of experiments. Small arrows indicate bolus injections of phenylbiguanide (PBG) in increasing doses (1–8 µg/kg) into the right atrium in 2.5 min intervals. Double large arrows represent bilateral microinjections into the nucleus tractus solitarii (NTS) of: A2a adenosine receptor agonist CGS-21680 (0.2, 2 and 20 pmol in 50 nl), and selective A2a adenosine receptor antagonist, ZM-241385 (40 pmol in 100 nl).
2.3. Instrumentation and measurements
All procedures were described in detail previously (Barraco et al., 1996;Ichinose et al., 2012;Scislo and O'Leary, 1998b;Scislo and DiCarlo, 1994;Scislo and O'Leary, 2000). Briefly, male Sprague-Dawley rats (Charles River) were anesthetized with a mixture of α-chloralose (80 mg·kg−1) and urethane (500 mg·kg−1 i.p.), tracheotomized, connected to a small-animal respirator (SAR-830, CWE, Ardmore, PA), and artificially ventilated with 40% oxygen, 60% nitrogen mixture. Arterial blood gases were tested occasionally (Radiometer, ABL500, OSM3), and ventilation was adjusted to maintain Po2, Pco2, and pH within normal ranges. Average values measured at the end of each experiment were: Po2 = 151.8 ± 7.3 Torr, Pco2 = 33.4 ± 1.1 Torr and pH = 7.4 ± 0.0. The left femoral artery and vein were catheterized to monitor arterial blood pressure; infuse drugs and to supplement anesthesia (12–21 mgkg−1h−1 of α-chloralose and 76–133 mgkg−1h−1 of urethane dissolved in 2.4–4.2 ml·kg−1·h−1 saline), respectively. An additional catheter was inserted into the right atrium via right jugular vein for intra atrial PBG injections. The appropriate position of the catheter in the right atrium was confirmed post mortem. Sinoaortic denervation was performed and tested similarly as in the previous studies from our laboratory; special attention was given to preserve the vagus nerve intact (Ichinose et al., 2012;Scislo and O'Leary, 2002;Scislo and O'Leary, 1998b;Scislo and DiCarlo, 1994).
2.4. Neural recordings
In each experiment, simultaneous recordings from three (n=35) or two sympathetic outputs (n=3) were performed. Renal and adrenal nerves were exposed retroperitoneally form a flank incision, whereas lumbar sympathetic trunk was exposed from midabdominal incision. Neural recordings were accomplished as described previously (Ichinose et al., 2012;Scislo and O'Leary, 1998b;Scislo and DiCarlo, 1994). Neural signals were initially amplified (2,000–20,000×) with bandwidth set at 100–1,000 Hz, digitized, rectified, and averaged in 1-s intervals. Background noise was determined after the animal was euthanized yet artificially ventilated to leave unaltered any potential movement artifacts. Resting level of the nerve activity was normalized to 100% before each stimulation of the cardiopulmonary receptors.
The ratio between preganglionic and total nerve activity was initially tested with an intravenous bolus injection of the short-lasting (1–2 min) ganglionic blocker, Arfonad (trimethaphan, 2 mg·kg−1; Hoffmann-La Roche), and finally evaluated at the end of each experiment with hexamethonium (20 mgkg−1 i.v.). RSNA was almost completely postganglionic; 8.2± 2.2% (n = 44) of the activity persisted after the ganglionic blockade; LSNA was predominantly postganglionic; 39.8 ± 4.8 % (n = 40) of preganglionic activity remained after the blockade. Of total of 41 adrenal nerves recorded 25 were predominantly preganglionic pre-ASNA (>75% of activity remained after the ganglionic blockade), 4 predominantly postganglionic post-ASNA (<50% of activity remained after the ganglionic blockade) and 12 had intermediate composition, int-ASNA (50%–75% of activity remained after the ganglionic blockade). Average levels of preganglionic, intermediate, and postganglionic adrenal nerve activity after ganglionic blockade were: 102.9 ± 7.5, 64.2 ± 2.8, 23.3 ± 5.9 %, respectively. Similarly as in our previous study predominantly postganglionic ASNA was excluded from further calculations whereas pre-ASNA (n=22) and int-ASNA (n=10) were combined as predominantly preganglionic ASNA (>50% of activity remained after the ganglionic blockade) (Ichinose et al., 2012). Nevertheless, a separate comparison of the effects of adenosine A2a receptor agonist and antagonist on all components of the adrenal nerve was also performed (n=36). The arterial pressure and neural signals were digitized and recorded with a Hemodynamic and Neural Data Analyzer (Biotech Products, Greenwood, IN), averaged over 1-s intervals, and stored on the hard disk for subsequent analysis.
2.5. Microinjections into the NTS
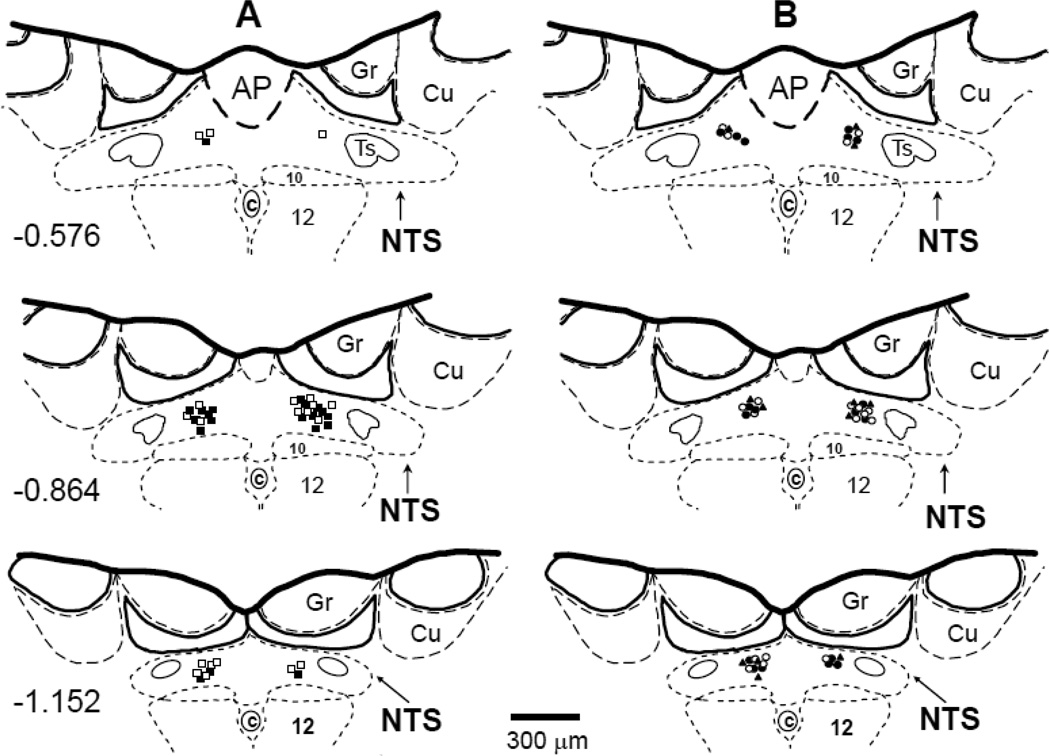
Bilateral microinjections of three different doses of the selective A2a receptor agonist, CGS-21680 (0.2, 2 and 20 pmol in 50 nl of ACF), and the antagonist, ZM-214385 (40 pmol in 100 nl of ACF), were made with multibarrel glass micropipettes into the medial region of the caudal NTS as described previously (Barraco et al., 1996;Ichinose et al., 2012;Scislo and O'Leary, 1998b). The doses and volumes of the agonist and antagonist were the same as used in our previous studies (Ichinose et al., 2009;Ichinose et al., 2012;Scislo et al., 2008;Scislo and O'Leary, 2006). The drugs were dissolved in ACF with pH adjusted to 7.2; the selective A2a adenosine receptor agonist CGS-21680 often precipitates in a pH >7.2. All microinjection sites were marked with Dil lypophilic dye (Molecular Probes, CA) and verified histologically as described previously (Barraco et al., 1996;Ichinose et al., 2012;Scislo and O'Leary, 1998b). The microinjection sites are presented in Figure. 2 using diagrams based on the atlas of the rat subpostremal NTS by Barraco et al. (Barraco et al., 1992).
Figure 2.
The microinjection sites for all experiments were located within subpostremal nucleus tractus solitarii (NTS) as shown in schematic diagrams of transverse sections of the medulla oblongata from a rat brain. AP, area postrema; c, central canal; 10, dorsal motor nucleus of vagus nerve; 12, nucleus of hypoglossal nerve; Ts, tractus solitarius; Gr, gracile nucleus; Cu, cuneate nucleus. Scale is shown at the bottom; number on the left of schematic diagram denotes the rostrocaudal position in millimeters of the section relative to the obex according to the atlas of the rat subpostremal NTS by Barraco et al. (1992). Bilateral microinjection sites for different doses of A2a receptor agonist, CGS-21680 and A2a receptor antagonist, ZM-241385 were marked with fluorescent dye. A: ■ 0.2 pmol CGS-21680; □, 2 pmol CGS-21680; B: #, 20 pmol CGS-21680, ○, 40 pmol ZM-241385, ▲, ZM-241385 + CGS-21680.
2.6. Cardiopulmonary chemoreflex stimulus-response curves
The CCR stimulus-response curves were generated as described in our previous studies (Ichinose et al., 2012;Scislo and DiCarlo, 1994;Scislo, DiCarlo et al., 1993). Briefly, the cardiopulmonary receptors were stimulated using bolus injections into the right atrium of increasing doses (1–8 µg/kg) of serotonin 5HT3 receptor agonist, phenylbiguanide (PBG, Sigma, 20 µg/1ml solution in 0.9% NaCl) in 2.5 min intervals between the injections. Total time to complete the stimulus-response curve was ~ 8 min. The curves were obtained under control conditions; 30 min later, approximately 5 min after bilateral microinjections of drugs (CGS-21680 or ZM-214385) into the caudal NTS, and again 60 min after the microinjections, recovery (Figure 1). This approach allowed assessment of the CCR reactivity during the most effective action of the NTS adenosine A2a receptor activation which occurs ~ 5–15 min after the microinjection of the agonist (Scislo and O'Leary, 2000;Scislo and O'Leary, 1998b).
2.7. Data analysis
The cardiopulmonary reflex stimulus-response curves constructed for MAP, HR and the regional sympathetic outputs under control conditions were compared with those generated after bilateral stimulation/inhibition of NTS A2a adenosine receptors with microinjections of the selective agonist and antagonist, CGS-21680 and ZM-214385, respectively. Control reflex stimulus-response curves obtained before microinjections of CGS-21680 or ZM-214385 were also compared with the functions generated ~60 min after the microinjections (recovery). In addition, the effects of the A2a receptor agonist (CGS 21680) on CCR reactivity was also analyzed after bilateral pretreatment of the NTS with the selective antagonist (ZM-214385). The maximal reflex responses in all measured variables were compared between the experimental conditions. The effects exerted by different levels of activation of NTS A2a receptors (three doses of CGS-21680, 0.2, 2.0, and 20 pmol) on the reflex responses were calculated according to the equation: [(control response - response after the microinjection into the NTS)/control response] x 100%. 100% inhibition reflects complete abolition of the response, whereas negative values represent facilitation of the reflex responses. The same equation was used to calculate the effect of inhibition of NTS A2a receptors (ZM-214385) on the reflex responses.
The data were analyzed using the statistical package SYSTAT version 11 (SYSTAT Software Inc., Richmond, CA, USA). Two-way ANOVA for repeated measures was used to compare the reflex responses of each variable across the experimental conditions (control vs. microinjection and control vs. recovery) and across four levels of activation of the CCR (four doses of PBG, 1–8 µg/kg). Two-way ANOVA for independent measures was used to compare control renal (n=44), preganglionic adrenal (n=37) and lumbar (n=40) sympathetic responses to four doses of PBG. The effects of three doses of CGS-21680 (0.2–20 pmol) on the reflex responses of the three sympathetic outputs (RSNA vs. pre-ASNA vs. LSNA) at each level of activation of the CCR (comparisons of reflex response curves obtained after gradual activation of NTS A2a adenosine receptors and for changes of the reflex responses vs. control conditions, A%) were also evaluated using two-way ANOVA for independent measures. If significant interactions were found (nerve × dose), the differences between the means were calculated with a modified Bonferroni t-test. One-way ANOVA for independent measures followed by modified Bonferroni t-test was used for comparison of HR and MAP reflex response curves obtained after microinjections of the three doses of adenosine receptor agonist, CGS-21680. The changes in baseline levels of MAP, HR, RSNA, pre-ASNA and LSNA evoked by bilateral microinjections of CGS-21680 and ZM-214385 were calculated as a difference between baseline values averaged during the last 30 s preceding the microinjections and the last 30 s of the 5 min period of the response to microinjections measured before generating the second cardiopulmonary chemoreflex function-curve. An α-level of P< 0.05 was used to determine statistical significance.
3. Results
3.1. Baseline values
Average resting MAP and HR values measured before the control CCR stimulus-response curves (n=44) were: 83± 1 mmHg and 411 ± 5 bpm, respectively. Basal parameters measured just before NTS microinjections of A2a adenosine receptor agonist, CGS-21680, or antagonist, ZM-241385 or combined antagonist and agonist, returned to normal resting values: 83± 2 mmHg and 405± 6 bpm for MAP and HR, respectively. Also, in those animals in which the third reflex curve was generated ~60 min after the microinjections (recovery, n=36), baseline MAP and HR were similar to those measured at the beginning of experiment (82 ± 1 mmHg and 398 ± 6 bpm, respectively). There were no differences between these three baseline levels of MAP and HR measured during three stages of the experiments: control, stimulation or blockade of NTS A2a adenosine receptors, and recovery (P>0.05 for all comparisons).
3.2. The effect of NTS A2a adenosine receptors on baseline values
The effects of bilateral microinjections of the selective A2a adenosine receptor agonist, CGS-21680 (0.2, 2, and 20 pmol in 50 nl, n = 10, 10, and 9, respectively), and the selective A2a receptor antagonist, ZM-241385 (40 pmol in 100 nl, n = 9) on resting hemodynamic and neural variables are presented in Table 1. The effects of the smallest dose of CGS-21680 (0.2 pmol/ 50 nl) on baseline values were similar to the effects of bilateral microinjections of 50 nl of ACF (volume control) reported previously (Ichinose, Minic, Li, O'Leary, and Scislo, 2012). Graded levels of A2a receptor activation (CGS-21680; 2, 20 pmol/ 50 nl) evoked similar patterns of changes in baseline levels of hemodynamic and regional sympathetic variables as observed previously: decreases in MAP, HR, RSNA and post-ASNA, increases in pre-ASNA and much smaller changes in LSNA (Scislo and O'Leary, 2000;Scislo and O'Leary, 1998b;Scislo and O'Leary, 1998a). Selective blockade of the A2a adenosine receptors with microinjections of ZM-241385, did not alter baseline levels of any variables (P>0.05).
Table 1.
Changes in baseline values of MAP, HR, RSNA, pre-ASNA, and LSNA following bilateral NTS microinjections of selective A2a adenosine receptor agonist, CGS-21680, and antagonist ZM-241385
| CGS-21680 pmol/ 50 nl |
ZM-241385 40 pmol/ 100 nl |
|||
|---|---|---|---|---|
| 0.2 | 2 | 20 | ||
| MAP, Δ mmHg | −6 ± 4 | −6 ± 3 | −9 ± 4# | −2 ± 4 |
| HR, Δ bpm | −31 ± 5.# | −30 ± 80# | −55 ± 11# | −11 ± 10 |
| RSNA, Δ% | −15.3 ± 4.1 # | −17.7 ± 4.9‡# | −33.3 ± 6.5*‡# | −3.4 ± 7.2 |
| pre-ASNA, Δ% | 2.6 ± 4.1†# | 21.1 ± 6.0*†‡# | 14.6 ± 8.3†‡# | −6.0 ± 4.1 |
| LSNA, Δ% | −6.1 ± 3.0† | −8.6 ± 3.3†# | −15.4 ± 4.3†# | −3.5 ± 3.3 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity; pre-ASNA, preganglionic adrenal sympathetic nerve activity; and LSNA, lumbar sympathetic nerve activity; CGS-21680 (0.2–20 pmol/50 nl); ZM-241385 (40 pmol/100 nl).
P < 0.05 vs. 0.2 pmol CGS;
P<0.05 vs. RSNA;
P<0.05 ASNA vs. LSNA;
vs. 0
3.3. Activation of NTS A2a adenosine receptors inhibits cardiopulmonary chemoreflex responses
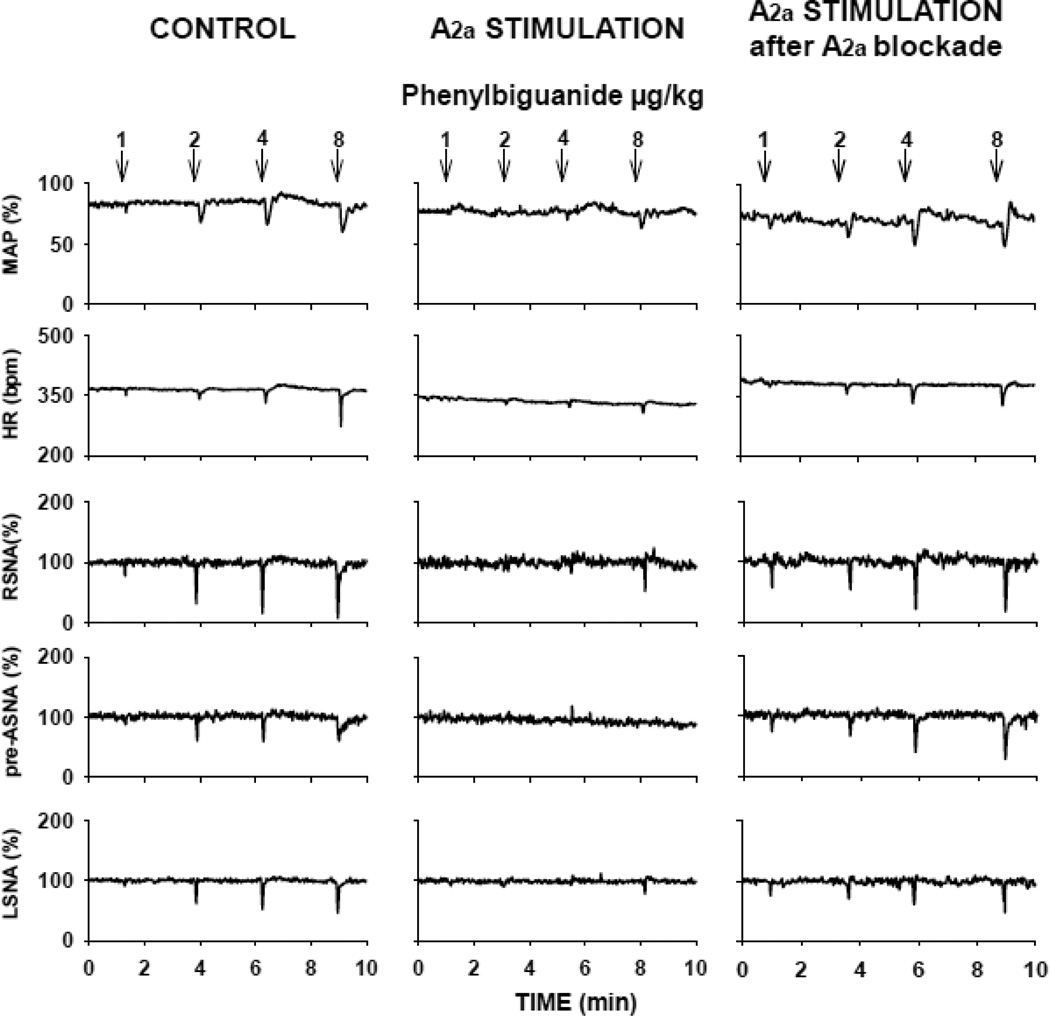
An example of original recordings of hemodynamic and neural responses to activation of the CCR under control and experimental conditions is presented in Figure 3 (left and right panels, respectively). Under control conditions gradual activation of the cardiopulmonary receptors by increasing doses of PBG (1–8 µg/kg) evoked dose-dependent decreases in MAP and HR and differential decreases in regional sympathetic nerve activity (RSNA > pre-ASNA > LSNA) (nerve effect P<0.0001; nerve vs. dose interaction P=0.011). These differential neural responses were similar to those observed previously (Ichinose, Minic, Li, O'Leary, and Scislo, 2012). The hemodynamic and neural reflex responses were powerfully inhibited following bilateral activation of NTS A2a adenosine receptors with the maximal dose of CGS-21680 (20 pmol/50 nl) (Figure 3, middle panels) and this inhibition was removed by bilateral blockade of NTS A2a adenosine receptors (Figure 3, right panels). The inhibitory effect was dose dependent as shown in Figure 4. The lowest level of A2a receptor activation (CGS-21680, 0.2 pmol/50 nl) did not alter the control reflex responses (control vs. experimental condition effect P>0.05 for all variables). Therefore this group may be considered as a volume control containing a physiologically insignificant amount of the agonist. Increasing activation of A2a receptors (CGS-21680 2 and 20 pmol) gradually shifted all neural and hemodynamic reflex response curves upward and flattened them. At the highest dose of the agonist the inhibitory effect was highly significant (control vs. experimental condition effect, P<0.0001 for MAP and all the neural outputs and P=0.012 for HR). The slope of all reflex response function curves was significantly alerted vs. control conditions (experimental effect vs. PBG dose interactions, P<0.02 for all variables).
Figure 3.
An example of cardiopulmonary chemoreflex responses in mean arterial pressure (MAP), heart rate (HR), renal (RSNA), pre-adrenal (pre-ASNA) and lumbar (LSNA) sympathetic nerve activity evoked by intra-atrial injections of phenylbiguanide (1–8 µg/kg) under control conditions (left panels), after stimulation of the NTS A2a adenosine receptors (middle panels) with the highest dose of the A2a agonist, CGS-21680 (20 pmol in 50 nl), and after combined blockade and subsequent stimulation of NTS A2a receptors (right panels). Recordings in left and medium panels were performed in one animal whereas recordings in right panel were performed in another animal. Stimulation of the A2a receptors in the NTS virtually abolished the cardiopulmonary chemoreflex responses.
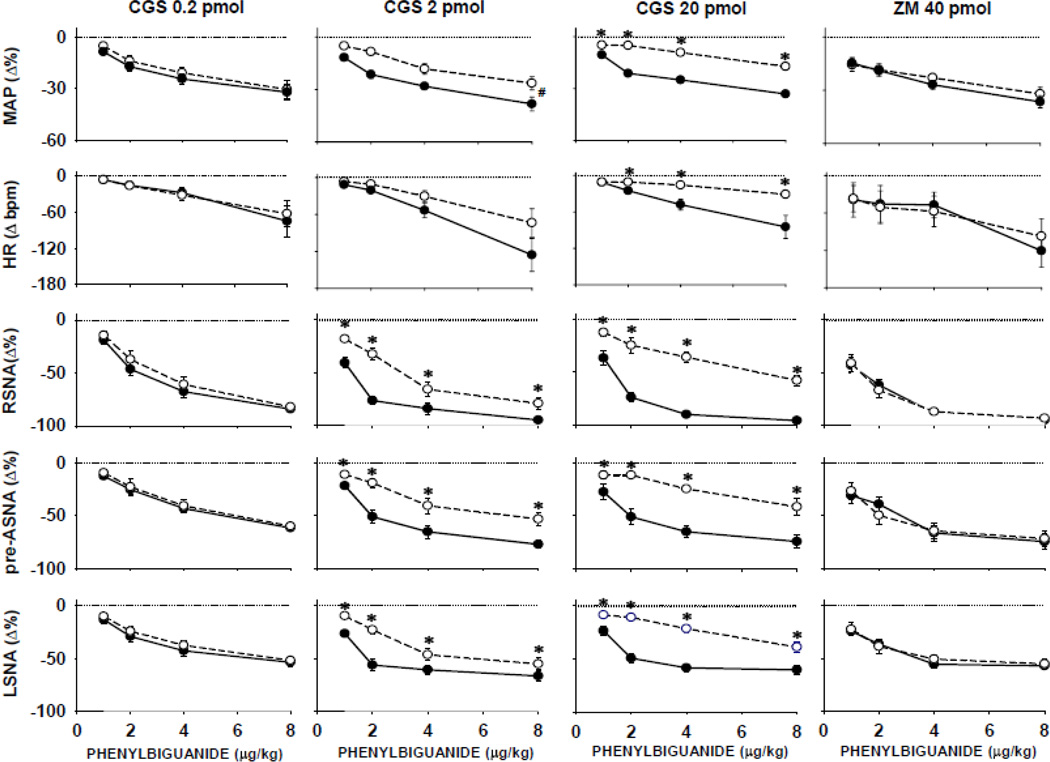
Figure 4.
Comparisons of cardiopulmonary chemoreflex responses obtained under control conditions (●) vs. the responses obtained after microinjections of A2a receptor agonist CGS-21680, in increasing doses, or the selective A2a antagonist, ZM-241385 (○). Data are means ± SE. *, P<0.05 vs. control; #, P<0.05 significant parallel shift of the experimental vs. control curves without significant PBG dose vs. experimental condition interaction. Progressive activation of A2a adenosine receptors gradually shifted the control reflex function upward indicating dose dependent inhibition of the reflex responses. The A2a receptor blockade had no effect on the reflex responses.
Comparisons across the nerves (renal, pre-adrenal, and lumbar) and doses of CGS-21680 (0.2–20 pmol/ 50 nl) showed that the nerves responded differentially at each level of activation of the reflex (nerve effect, P<0.05) and were inhibited in dose-dependent manner by A2a receptor agonist (CGS dose effect, P<0.05). However, no differences were found between the inhibition of the CCR responses in RSNA, vs. pre-ASNA, vs. LSNA; slopes of the curves were similar (no significant nerve x CGS dose interactions were found). This suggests that despite the contrasting shifts in the baseline levels of regional sympathetic nerve activity, the stimulation of NTS A2a adenosine receptors similarly inhibited the reflex responses of all sympathetic outputs. Blockade of A2a adenosine receptors with ZM-241385, did not alter the CCR reactivity of all variables (far right panels of Figure. 4); control vs. blockade condition effect, P>0.05 for all variables.
Interestingly, although stimulation of NTS adenosine A2a receptors reciprocally affects baseline pre-ASNA vs. post-ASNA there were no differences between the inhibition of different components of the adrenal nerve. Comparisons across pre-ASNA, int-ASNA and post-ASNA vs. dose of CGS 21680 (0.2–20 pmol) with two-way ANOVA showed no nerve component effect (P>0.05 at each level of activation of the reflex).
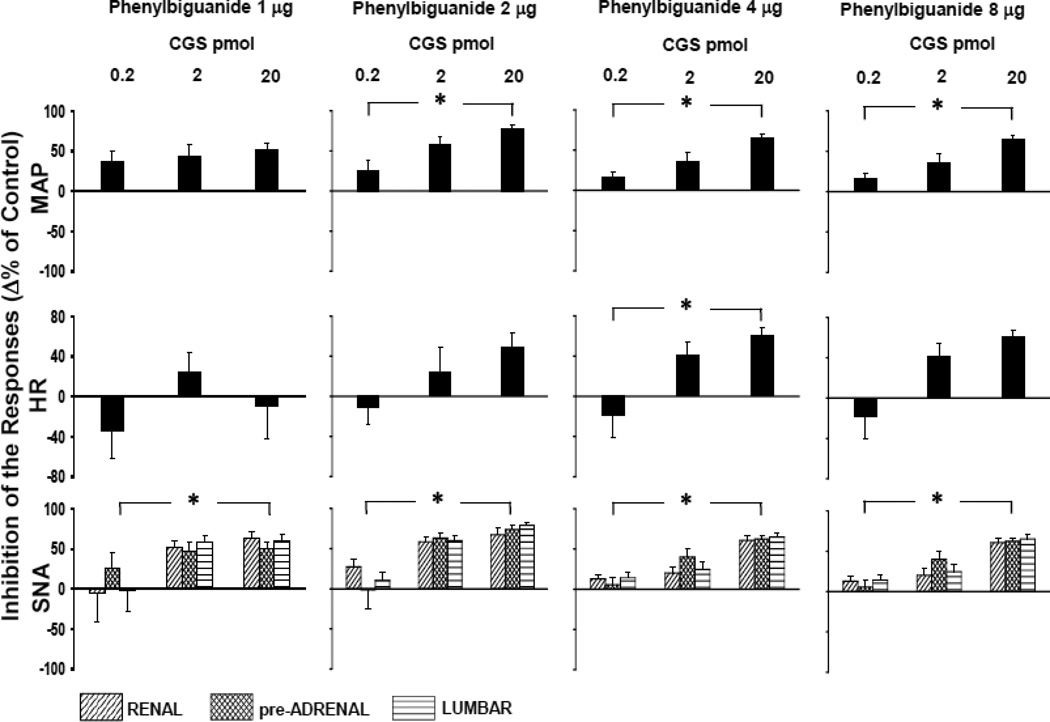
Figure 5 shows to what extent the reflex responses were inhibited after activation of NTS A2a adenosine receptors compared to the control reflex responses (Δ% of inhibition). A 100% inhibition corresponds to completely abolished CCR responses. Downward deflections of some bars (which may represent a tendency to facilitation of the reflex) were not significantly different from zero. The regional sympathetic outputs were inhibited by microinjections of the A2a receptor agonist (CGS-21680) in a dose dependent manner (highly significant dose effect P=0.002 for the lowest level of activation of the CCR and P<0.0001 for all higher levels of the activation); however, no differences between the inhibition of the sympathetic outputs were found (nerve effect, P>0.05 for each level of activation of the CCR). Also the inhibition of all adrenal nerve components (pre-ASNA, int-ASNA and post-ASNA) was uniform (nerve component effect, P>0.05 for each level of activation of the CCR). MAP reflex responses were also inhibited in a dose dependent manner (P<0.02) at all but the lowest level of activation of the CCR. However, the inhibition of the HR responses was more variable reaching significance only for the moderate activation of the CCR (4 µg of PBG).
Figure 5.
The inhibiton of hemodynamic and regional sympathetic control reflex responses evoked by increasing doses of the A2a receptor agonist, CGS-21680 (0.2–20 pmol) at four different levels of activation of the reflex (phenylbiguanide 1–8 µg/kg). Data are means ± SE. SNA - renal, preganglionic adrenal and lumbar sympathetic nerve activity. CGS-21680 exerted significant, dose dependent inhibition of the reflex responses at all levels of activation of the reflex. No differences between the inhibition of the reflex responses in all regional sympathetic outputs were found. The negative deflections of the bars may correspond to potential facilitation of the reflex; however, all the negative bars were not significantly different from zero.
Although the antagonist and the smallest dose of the agonist of A2a adenosine receptors had no significant effect on the CCR responses (Figure 4, the far right and left panels, respectively) the comparison of these two experimental conditions may suggest that the antagonist may show some tendency to facilitate the reflex at the moderate level of its activation (Table 2). However, the drug effect reached significance only for regional sympathetic responses evoked by moderate activation of the CCR (2µg of PBG) without significant drug vs. nerve interaction.
Table 2.
Inhibition of the cardiopulmonary chemoreflex responses, PBG (1–8 µg/kg), after microinjections of selective A2a agonist (CGS-21680) or A2a antagonist (ZM-241385).
| DRUGS | PBG µg/kg | MAP Δ% | HR Δ bpm | RSNA Δ% | pre-ASNA Δ% |
LSNA Δ% |
|---|---|---|---|---|---|---|
| CGS-21680 0.2 pmol/ 50 nl |
1 | 36.8 ± 13.5 | −33.8 ± 28.2 | −4.4 ± 37.4 | 26.4 ± 18.2 | −1.3 ± 26.9 |
| 2 | 26.0 ± 12.8 | −10.9 ± 16.8 | 27.4 ± 10.3 | −1.3 ± 22.9 | 10.4 ± 10.4 | |
| 4 | 16.6 ± 6.8 | −18.9 ± 22.0 | 12.8 ± 5.5 | 5.4 ± 9.7 | 14.2 ± 7.2 | |
| 8 | 9.0 ± 8.2 | 5.6 ± 10.3 | 2.3 ± 3.0 | 1.5 ± 5.7 | 3.0 ± 4.2 | |
| ZM-241385 40 pmol/ 100 nl |
1 | −23.8 ± 36.1 | −43.9 ± 30.2 | 5.6 ± 14.3 | 16.7 ± 10.0 | 9.9 ± 20.3 |
| 2 | −2.7 ± 18.3 | −53.2 ± 26.3 | −9.9 ± 10.2 | −36.6 ± 26.0 | −5.0 ± 12.0 | |
| 4 | 14.0 ± 6.0 | −25.1 ± 23.1 | 1.4 ± 2.6 | 1.0 ± 5.5 | 8.7 ± 3.1 | |
| 8 | 13.0 ± 3.8 | 23.1 ± 10.4 | 1.9 ± 1.6 | 2.6 ± 4.6 | 3.8 ± 4.4 |
Data are expressed as percent inhibition from control responses which are standardized to zero level. Positive values represent inhibition of the reflex while negative numbers suggest facilitation of the responses. Values are means ± SE. PBG, phenylbiguanide; MAP, mean arterial pressure; HR, heart rate; RSNA, renal sympathetic nerve activity; pre-ASNA, preganglionic adrenal sympathetic nerve activity; LSNA, lumbar sympathetic nerve activity. Two-way ANOVA showed a significant drug effect (P=0.022) at moderate activation of the reflex (PBG, 2 µg/kg) but no differences across the nerves (P>0.05 for all doses of PBG).
The A2a-mediated inhibition of the reflex responses was reversible. The CCR responses almost completely recovered ~60 min following NTS microinjections of A2a receptor agonists (Table 3).
Table 3.
Averaged cardiopulmonary chemoreflex responses to PBG (1–8 µg/kg) evoked under control conditions (control) and 1 hour after microinjection of A2a adenosine receptor agonist CGS-21680 (0.2–20 pmol/ 50 nl) (recovery).
| CGS- 21680 pmol/50 nl |
n | PBG µg/kg |
Control |
Recovery |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP Δ% |
HR Δbpm |
RSNA Δ% |
pre- ASNA Δ% |
LSNA Δ% |
MAP Δ% |
HR Δbpm |
RSNA Δ% |
pre- ASNA Δ% |
LSNA Δ% |
|||
| 0.2 | 10 | 1 | −9 ± 2 | −7 ± 2 | −18.9 ± 3.9 | −12.4 ± 1.7 | −13.2 ± 3.3 | −10 ± 2 | −7 ± 2 | −24.1 ± 7.5 | −18.9 ± 5.7 | −14.5 ± 3.3 |
| 2 | −17 ± 3 | −16 ± 3 | −46.5 ± 6.4 | −25.3 ± 5.2 | −29.2 ± 5.5 | −18 ± 3 | −21 ± 8 | −53.2 ± 8.3 | −35.4 ± 8.4 | −33.2 ± 5.5 | ||
| 4 | −25 ± 3 | −29 ± 8 | −67.7 ± 6.2 | −43.1 ± 4.3 | −42.7 ± 5.0 | −23 ± 4 | −31 ± 9 | −73.2 ± 5.7 | −53.7 ± 7.5 | −46.9 ± 4.2 | ||
| 8 | −32 ± 4 | −75 ± 25 | −84.2 ± 2.7 | −61.5 ± 2.8 | −53.6 ± 4.2 | −34 ± 6 | −97 ± 35 | −88.0 ± 2.5 | −64.2 ± 5.2 | −54.2 ± 3.4 | ||
| 2 | 10 | 1 | −12± 2 | −12± 3 | −40.6 ± 4.9 | −21.7 ± 2.7 | −26.5 ± 3.1 | −14 ± 2 | −11 ± 1 | −43.2 ± 6.1 | −24.6 ± 3.6 | −32.4 ± 6.6 |
| 2 | −22± 2 | −21 ± 4 | −76.2 ± 3.4 | −51.0 ± 5.9 | −56.2 ± 5.3 | −17 ± 1* | −20 ± 4 | −75.9 ± 4.6 | −41.5 ± 5.8 | −51.0 ± 5.9 | ||
| 4 | −28 ± 1 | −53 ± 12 | −83.8 ± 5.3 | −65.2 ± 6.3 | −60.7 ± 3.9 | −25 ± 2* | −38 ± 6 | −91.1 ± 2.0 | −60.3 ± 5.8 | −63.1 ± 5.7 | ||
| 8 | −38 ± 4 | −126 ± 27 | −94.7 ± 0.8 | −77.1 ± 3.9 | −66.6 ± 4.5 | −34 ± 3 | −131 ± 33 | −94.0 ± 1.7 | −70.9 ± 5.0 | −69.5 ± 5.1 | ||
| 20 | 9 | 1 | −11 ± 2 | −11 ± 3 | −36.7 ± 7.2 | −27.8 ± 7.2 | −23.4 ± 4.3 | −10 ± 2 | −14 ± 3 | −36.8 ± 6.8 | −31.3 ± 4.7 | −27.1 ± 4.9 |
| 2 | −21 ± 2 | −25 ± 5 | −73.6 ± 4.1 | −51.1 ± 7.6 | −49.2 ± 4.6 | −18 ± 2 | −28 ± 6 | −62.6 ± 3.7* | −42.4 ± 6.8 | −44.0 ± 5.4 | ||
| 4 | −25 ± 2 | −48 ± 9 | −89.8 ± 3.0 | −65.2 ± 5.5 | −55.3 ± 3.8 | −25 ± 2 | −43 ± 8 | −83.9 ± 2.6 | −57.8 ± 6.7* | −60.0 ± 3.9 | ||
| 8 | −32 ± 2 | −85 ± 19 | −95.5 ± 1.4 | −74.4 ± 6.4 | −60.2 ± 4.0 | −30 ± 3* | −85 ± 18 | −86.5 ± 5.5 | −65.4 ± 7.9 | −62.6 ± 4.1 | ||
Values are means ± SE. PBG, phenylbiguanide; HR, heart rate, MAP, mean arterial pressure, RSNA, renal sympathetic nerve activity, pre-ASNA, preganglionic adrenal sympathetic nerve activity; LSNA, lumbar sympathetic nerve activity.
P<0.05 vs. control
Bilateral pretreatment of the NTS with the selective A2a receptor antagonist (ZM-241385) removed the inhibition of the CCR (Figure 3, Table 4) indicating that the inhibitory effects evoked by microinjections of CGS 21680 into the NTS were mediated selectively by NTS A2a adenosine receptors. No significant differences were found between control CCR responses and the responses observed after combined microinjections into the NTS of ZM-241385 followed by the highest, equimolar dose of CGS 21680 (20 pmol) (Table 4, lower panel). In addition, the CCR responses observed after the combined blockade and stimulation of NTS A2a receptors were significantly greater than the responses observed after stimulation of NTS A2a receptors alone, although the control responses were not different between those two groups (Table 4, compare lower vs. upper panels).
Table 4.
Cardiopulmonary chemoreflex responses to PBG (1–8 µg/kg) evoxed under control conditions (control), compared to the responses evoked after microinjection into the NTS of A2a receptor agonist (CGS 21680, 20 pmol/50 nl) and similar comparison performed after blockade of NTS A2a receptors with equimolar microinjections of the selective antagonist, ZM-241385 (40 pmol/100 nl).
| Control |
CGS 21680 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of animals |
PBG µg/kg |
MAP Δ% |
HR Δbpm |
RSNA Δ% |
pre- ASNA Δ% |
LSN A Δ% |
MAP Δ% |
HR Δbpm |
RSN A Δ% |
pre- ASNA Δ% |
LSNA Δ% |
| 9 | 1 | −11 ± 2 | −11 ± 3 | −36.7 ± 7.2 | −27.8 ± 7.2 | −23.4 ± 4.3 | −5 ± 1* | −11.3 ± 4.8 |
−12.6 ± 3.9* |
−11.9 ± 3.9* |
−8.5 ± 1.8* |
| 2 | −21 ± 2 | −25 ± 5 | −73.6 ± 4.1 | −51.1 ± 7.6 | −49.2 ± 4.6 | −5 ± 1* | −9.3 ± 1.7* | −24.7± 7.3* | −11.8 ± 1.8* |
−10.8 ± 2.0* | |
| 4 | −25 ± 2 | −48 ± 9 | −89.8 ± 3.0 | −65.2 ± 5.5 | −58.3 ± 3.8 | −9 ± 2* | −13.4 ± 2.0* | −35.9 ± 5.4* | −24.8 ± 3.2* | −21.4 ± 3.6* | |
| 8 | −33 ± 2 | −85 ± 19 | −95.5 ± 1.4 | −74.4 ± 6.4 | −60.2 ± 4.0 | −17 ± 2* | −28.4 ± 4.5* | −57.8 ± 4.7* |
−41.7 ± 7.9* | −38.7 ± 5.3* | |
| Control |
ZM 241385 + CGS 21680 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBG µg/kg |
MAP Δ% |
HR Δbpm |
RSNA Δ% |
pre- ASNA Δ% |
LSN A Δ% |
MAP Δ% |
HR Δbpm |
RSN A Δ% |
pre- ASNA Δ% |
LSNA Δ% |
|
| 6 | 1 | −16 ± 3 | −34 ± 20 | −54.3 ± 9.2 | −43.9 ± 16.9 | −23.6 ± 3.7 | −13 ± 3# | −26 ± 9 | −36.8 ± 6.8# | −40.3 ±13.0# | −20.0 ± 2.1# |
| 2 | −25 ± 2 | −34 ± 13 | −81.3. ± 4.3 | −64.5 ± 9.8 | −41.8 ± 3.7 | −19 ± 5# | −32 ± 6# | −69.7 ± 9.6# | −60.0 ± 14.3# | −29.6 ± 3.9# | |
| 4 | −30 ± 3 | −88 ± 35 | −87.9 ± 3.0 | −75.0 ± 7.7 | −43.8 ± 4.6 | −27 ± 5# | −74 ± 20# | −87.3 ± 3.2# | −59.8 ± 11.0# | −38.7 ± 0.1 | |
| 8 | −39 ± 3 | −146 ± 45 | −90.8 ± 2.4 | −84.5 ± 5.0 | −44.1 ± 4.3 | −35 ± 6# | −128 ± 41 # | −91.0 ± 2.3# | −74.1 ± 8.2# | −38.9 ± 3.3 | |
Values are mea ns ± SE. PBG, phenylbiguanide,
, P<0.05 vs. control,
, P<0.05 differences between two groups of animals. Note that blockade of NTS A2a adenosine receptors (lower panel) abolished the inhibition of the reflex responses caused by selective activation of these receptors with the highest dose of the agonist (CGS 21680, 20 pmpl/50 nl) (upper panel).
4. Discussion
The present study showed that activation of facilitatory A2a adenosine receptors in the NTS inhibited CCR hemodynamic and regional sympathetic responses. Although stimulation of NTS A2a receptors evokes contrasting effects on regional sympathetic outputs (it decreases RSNA, increases pre-ASNA and does not markedly alter LSNA) the inhibition of the reflex responses of these sympathetic outputs was similar. This study indicated that NTS A2a receptors are indeed present on NTS neurons/nerve terminals participating in CCR neurotransmission and inhibit this neurotransmission similarly as the inhibitory A1 adenosine receptors did in our previous study (Ichinose et al., 2012). To our knowledge, this is the first report showing that antagonistic A1 and A2a adenosine receptors may act synergistically in modulation of cardiovascular reflexes although both receptor subtypes operate via different mechanisms. NTS A2a receptors do not exert a significant, tonic inhibition of the reflex under normal conditions; however, these receptors may be activated upon release of adenosine into the NTS, for example during severe hypotension leading to ischemia as we observed it previously during hypotensive stage of severe hemorrhage (Scislo and O'Leary, 2006;Minic et al., 2013).
4.1. Differential modulation of sympathoinhibitory reflexes by adenosine receptor subtypes
Selective stimulation of NTS A2a adenosine receptors evokes contrasting changes in the baseline levels of efferent sympathetic nerve activity: decreases in RSNA and post-ASNA, increases in pre-ASNA, and no changes in LSNA were observed (Scislo and O'Leary, 1998b;Scislo and O'Leary, 1998a). Therefore, we initially expected that activation of these receptors may differentially inhibit CCR responses of these sympathetic outputs (pre-ASNA>LSNA>RSNA). However, the CCR responses of all these sympathetic outputs were inhibited similarly. In addition there were no differences in A2a receptor mediated inhibition of different components of the adrenal nerve (pre-ASNA, int-ASNA and post-ASNA). This indicates that the contrasting shifts in baseline levels of regional sympathetic nerve activity and the inhibition of CCR-evoked responses in these sympathetic outputs are mediated by two different, most likely independent mechanisms.
Previous studies showed that NTS A1 adenosine receptors inhibit glutamatergic transmission in the arterial baroreflex arc and in the CCR, which is consistent with direct inhibition of glutamate release from nerve terminals or central neurons by presynaptic and postsynaptic A1 receptors, respectively (Scislo et al., 2008;Ichinose et al., 2012). In contrast, activation of NTS A2a receptors did not alter arterial baroreflex responses (Ichinose et al, 2009); however, it did inhibit the CCR responses to a similar extent as A1 adenosine receptors did, although via a different, likely indirect mechanism.
Taken together, the present and previous data show that there are contrasting functional differences between A1 vs. A2a-adenosine receptors in neuromodulation of the CCR and arterial baroreflex mechanisms located in the NTS: 1) direct inhibition of neurotransmission in both reflex pathways is mediated by A1 receptors, 2) selective, indirect inhibition of CCR, but not arterial baroreflex, pathway is provided by A2a receptors, and 3) contrasting shifts in baseline levels of sympathetic activity mediated by A2a adenosine receptors are independent of the reflex mechanisms. These functional differences most likely reflect differential localization of adenosine receptor subtypes on NTS neurons/nerve terminals mediating both sympathoinhibitory reflexes and targeting different regional sympathetic outputs.
It should be stressed that in the NTS A1 adenosine receptors play smaller role than the functionally dominating A2a receptors (Barraco et al., 1991;Barraco and Phillis, 1991;Abdel-Rahman and Tao, 1996;Mosqueda-Garcia et al., 1989). The present study showed that activation of these dominating, facilitatory A2a receptors inhibits CCR control of regional sympathetic outputs although it does not inhibit baroreflex control of these outputs (Ichinose et al., 2009). This indicates that both, usually antagonistic, A1 and A2a adenosine receptor subtypes may act in concert by powerfully inhibiting the CCR but not arterial baroreflex mechanisms. The adenosinergic inhibition of the CCR at the level of the NTS is much more potent than the inhibition of baroreflex pathway which is mediated only by less functionally relevant A1 adenosine receptors. This difference may have a physiologic basis because cardiopulmonary chemoreflex (or Bezold-Jarisch reflex) may cause more severe bradycardia and hypotension than that normally evoked by activation of the arterial baroreflex which makes smaller beat to beat corrections of arterial pressure. Powerful activation of the depressor Bezold-Jarisch reflex may lead to severe hemodynamic imbalance, ischemia and consequently to the release of adenosine into the NTS. Then, adenosine may act as especially powerful negative feedback regulator for this reflex, via both less potent A1 adenosine receptors and dominating A2a receptors. The strong adenosinergic inhibition of the CCR may prevent potentially dangerous consequences of activation of this reflex, for example, fainting or life threatening bradyarrhythmias, arising from simultaneous activation of vagal and sympathetic control of the heart, which may lead even sudden cardiac death of young athletes (Salo et al., 2007;Benton and Maginot, 2007;Mark, 1983;Greenberg, 1984).
4.2. Potential mechanisms
In contrast to A1 adenosine receptors which exert uniform inhibition of glutamatergic transmission in both CCR and arterial baroreflex pathways via decrease in Ca++ influx into the synaptic terminals or postsynaptic hyperpolarization of NTS neurons (Ralevic and Burnstock, 1998), the facilitatory A2a receptors may exert the inhibition of the CCR via more complex mechanisms. The inhibition of the CCR by A2a adenosine receptors may be mediated, for example, via facilitation of GABA release from NTS GABA-ergic neurons and/or terminals via postsynaptic and/or presynaptic A2a receptors, respectively. A2a receptors may also disinhibit GABA release by removing dopaminergic inhibition of GABA release via direct A2a-D2 receptor interactions as it was suggested for basal ganglia (Fuxe et al., 2007;Dayne et al., 1996). Since dopamine itself has been reported to inhibit afferent activation of NTS neurons (Kline et al., 2002;Kline, Hendricks et al., 2009) it is also possible that A2a receptors may facilitate this inhibitory mechanism. A2a receptors may also facilitate a vasoactive intestinal peptide dependent release of GABA as it has been shown in hippocampal nerve terminals (Cunha-Reis et al., 2008). Since NTS contains glycinergic terminals/receptors, given that glycine inhibits arterial chemoreflex at the level of the NTS in the rat, and considering that A2a adenosine receptors may activate glycine release from central neurons, it is possible that A2a adenosine receptors may inhibit the CCR via facilitation of glycine release which then would inhibit transmission in this reflex pathway (Edwards and Robertson, 1999;Baptista and Varanda, 2005;Pimentel et al., 2003). It is also possible that adenosine A2a receptors facilitate both GABA and glycine release as co-localization of glycinergic and GABA-ergic mechanisms in the NTS has been reported (Batten et al., 2010); however, only GABA but not glycine mediate CCR responses at the level of the rostral ventrolateral medulla (Verberne and Guyenet, 1992) and activation of NTS GABAA receptors inhibits the CCR reactivity (Sartor and Verberne, 2007). Which of those potential mechanisms is indeed responsible for A2a receptor mediated inhibition of the CCR pathway remains to be elucidated in future studies.
The CCR is mediated via excitatory amino acids at the level of the NTS and both non-NMDA and NMDA receptors are involved (Sartor and Verberne, 2007;Verberne and Guyenet, 1992). It is possible that CGS 21680 may inhibit these mechanisms directly via some unknown mechanism(s) rather than through stimulation of A2a receptors. However, this seems unlikely as the selective blockade of NTS A2a receptors applied in the present study virtually abolished the inhibitory effects of CGS 21680 on the CCR responses.
Inhibition of the CCR via nonselective activation of A1 receptors by A2a receptor agonist CGS 21680 seems also unlikely. Barraco and Phillis showed in their classical studies (Barraco et al., 1991;Barraco and Phillis, 1991) that even higher concentrations of CGS 21680 (up to 1 nmol/50 nl) than those used in the present study (20 pmol/50 nl) evokes decreases in MAP whereas selective activation of NTS A1 receptors with N6cyclopentyl adenosine (0.03–1.5 nmol/50 nl) evokes the opposite, pressor effects. We have also shown the contrasting A1 vs. A2a responses in RSNA, post-ASNA and LSNA using the same concentration of CGS 21680 microinjected into the NTS as in the present study (20 pmol/50 nl) (Scislo and O'Leary, 2002;Scislo and O'Leary, 1998b;Scislo and O'Leary, 1998a). Decreases in arterial pressure evoked by microinjections of this concentration of the agonist (20 pmol/50 nl) into the NTS were virtually abolished by pretreatment with the A2a receptor antagonist, CGS 15943A, but did not affect the responses evoked by selective stimulation of A1 receptors (Barraco et al., 1991). Finally, selective blockade of NTS A2a receptors with a newer antagonist, ZM-241385, abolished the inhibitory effects of CGS 21680 in the present study indicating that the inhibition of CCR hemodynamic and regional neural responses was mediated mainly by A2a receptors and some unknown inhibitory mechanisms downstream of these receptors as suggested above.
Although stimulation of NTS A2a receptors evoked powerful inhibition of the hemodynamic and regional sympathetic CCR responses, the blockade of these receptors exerted only a very small, if any, facilitation of the reflex responses indicating that A2a receptors do not exert a significant tonic inhibition on this reflex at the level of the NTS. Lack of marked, tonic adenosine modulation of mechanisms of cardiovascular control at the level of the NTS and in other medullary cardiovascular centers was usually observed in our laboratory and by others (Ichinose et al., 2009;Ichinose et al., 2012;Scislo and O'Leary, 2006;Thomas et al., 2000). This is not surprising, as adenosine release into the NTS during normal, physiological conditions is negligible. However, adenosine is released into the central nervous system, including the NTS, during stress/hypothalamic defense response, severe hemorrhage, ischemia and hypoxia (Dale et al., 2002;Phillis et al., 1987;Scislo and O'Leary, 2006;St Lambert et al., 1997;St Lambert et al., 1994;Thomas et al., 2000;Van Wylen et al., 1988;Winn et al., 1979;Yan et al., 1995). During the severe hemodynamic imbalances the rising adenosine levels modulate neural control of cardiovascular system (Phillis et al., 1987;Scislo and O'Leary, 2006;Van Wylen et al, 1988;Winn et al., 1979;Yan et al., 1995). In the present study we used relatively small, short lasting activation of only one subset of receptors participating in the reflex to construct the most specific, dose dependent hemodynamic and regional neural stimulus-response function curves. Therefore the CCR depressor responses observed in the present study were too small and too short lasting by design to expect release of adenosine as a consequence of the hypotension induced by the CCR stimulations. However, in a preliminary report, we showed that during severe hemorrhage naturally released adenosine does inhibit neural and hemodynamic CCR responses and this inhibition is removed by microinjections into the NTS of nonselective A1/A2a adenosine receptor antagonist, 8-(p-sulfophenyl)theophylline (Minic et al., 2013).
4.3. Methodological considerations
Major limitations of the method were described in detail in our previous study concerning NTS A1 adenosine receptor mediated inhibition of the CCR (Ichinose et al., 2012). Briefly, we believe that this method of evoking the CCR with increasing doses of PBG, which activate only a subset of CCR afferents, is a good, well controlled experimental tool widely accepted in the field (Cao and Morrison, 2000;Dias et al., 2005;Ditting et al., 2006;Veelken et al., 1993;Higuchi et al., 1988;Kashihara et al., 2003;Salo et al., 2007;Scislo and DiCarlo, 1994;Scislo et al., 1993). Selective activation of serotonin 5HT3 receptors on polymodal vagal afferents from cardiopulmonary area dissects the activation of CCR from other reflexes and humoral mechanisms which may be triggered by cardiac ischemia which is known to naturally activate the CCR (Lupinski et al., 2011;Rocha et al., 2003;Schultz, 2001;Hainsworth, 1991;Burnstock et al., 1988;Oei et al., 1983). Although we could not distinguish between adrenal nerve fibers supplying adrenergic vs. noradrenergic chromafin cells in the adrenal medulla it has been shown previously that both these types of adrenal nerve fibers are similarly inhibited by activation of the CCR (Cao and Morrison, 2000).
In the present paper we did not provide the microinjection volume control for the hemodynamic and regional neural responses evoked by the CCR. However, this volume control was completed in our previous study and did not show any effects on the hemodynamic and regional neural reflex responses evoked by activation of the CCR and the arterial baroreflex (Ichinose et al., 2009;Ichinose et al., 2012;Scislo et al., 2008). In addition, the lowest dose of A2a receptor agonist (CGS-21680, 0.2 pmol) did not alter the reflex reactivity in all recorded variables; therefore there is no doubt that gradual activation of NTS A2a receptors evoked dose dependent inhibition of the reflex.
4.4. Summary
Stimulation of facilitatory NTS A2a adenosine receptors evoked dose dependent inhibition of regional sympathetic responses evoked by graded stimulation of the CCR. The inhibition was uniform for all recorded sympathetic outputs (renal, adrenal and lumbar) despite the reciprocal effect of stimulation of NTS A2a adenosine receptors on baseline activity of these sympathetic outputs. This suggests that A2a receptor mediated modulation of CCR reactivity and changes in baseline levels of regional sympathetic activity are mediated via different mechanisms. The inhibition of CCR control of regional sympathetic outputs occurs most likely via A2a receptor mediated facilitation of release of an inhibitory neurotransmitter which in turn inhibits neurotransmission in the CCR network.
Acknowledgments
The authors gratefully acknowledge technical help of Miss Cailian Li and the generous gift of Arfonad by Hoffmann-La Roche Ltd., Basel, Switzerland. This study was supported by grant HL 67814 from National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors report no conflicts of interest.
REFERENCES
- Abdel-Rahman AA, Tao S. Differential alteration of neuronal and cardiovascular responses to adenosine microinjected into the nucleus tractus solitarius of spontaneously hypertensive rats. Hypertension. 1996;27:939–948. doi: 10.1161/01.hyp.27.4.939. [DOI] [PubMed] [Google Scholar]
- Baptista V, Varanda WA. Glycine binding site of the synaptic NMDA receptor in subpostremal NTS neurons. J. Neurophysiol. 2005;94:147–152. doi: 10.1152/jn.00927.2004. [DOI] [PubMed] [Google Scholar]
- Barraco R, el Ridi M, Ergene E, Parizon M, Bradley D. An atlas of the rat subpostremal nucleus tractus solitarius. Brain Res. Bull. 1992;29:703–765. doi: 10.1016/0361-9230(92)90143-l. [DOI] [PubMed] [Google Scholar]
- Barraco RA, el Ridi MR, Ergene E, Phillis JW. Adenosine receptor subtypes in the brainstem mediate distinct cardiovascular response patterns. Brain Res. Bull. 1991;26:59–84. doi: 10.1016/0361-9230(91)90192-m. [DOI] [PubMed] [Google Scholar]
- Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J. Auton. Nerv. Syst. 1996;59:113–124. doi: 10.1016/0165-1838(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Phillis JW. Subtypes of adenosine receptors in the brainstem mediate opposite blood pressure responses. Neuropharmacology. 1991;30:403–407. doi: 10.1016/0028-3908(91)90067-l. [DOI] [PubMed] [Google Scholar]
- Batten TF, Pow DV, Saha S. Co-localisation of markers for glycinergic and GABAergic neurones in rat nucleus of the solitary tract: implications for co-transmission. J. Chem. Neuroanat. 2010;40:160–176. doi: 10.1016/j.jchemneu.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Benton Ng, Maginot KR. Sudden Cardiac Death in Young Athletes:Trying to Find the Needle in the Haystack. Wisconsin Medical Journal. 2007;106:335–342. [PubMed] [Google Scholar]
- Bisserbe JC, Patel J, Marangos PJ. Autoradiographic localization of adenosine uptake sites in rat brain using [3H]nitrobenzylthioinosine. J. Neurosci. 1985;5:544–550. doi: 10.1523/JNEUROSCI.05-02-00544.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Lincoln J, Feher E, Hopwood AM, Kirkpatrick K, Milner P, Ralevic V. Serotonin is localized in endothelial cells of coronary arteries and released during hypoxia: a possible new mechanism for hypoxia-induced vasodilatation of the rat heart. Experientia. 1988;44:705–707. doi: 10.1007/BF01941035. [DOI] [PubMed] [Google Scholar]
- Cao WH, Morrison SF. Responses of adrenal sympathetic preganglionic neurons to stimulation of cardiopulmonary receptors. Brain Res. 2000;887:46–52. doi: 10.1016/s0006-8993(00)02964-4. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC, Dangel A, Kidd C, Luck JC, Sleight P. Impulses in slowly conducting vagal fibers from afferent endings in the veins, atria, and arteries of dogs and cats. Circ. Res. 1973;33:87–97. doi: 10.1161/01.res.33.1.87. [DOI] [PubMed] [Google Scholar]
- Cunha-Reis D, Ribeiro JA, Sebastiao AM. A1 and A2a receptor activation by endogenous adenosine is required for VIP enhancement of K+-evoked [3H]-GABA release from rat hippocampal nerve terminals. Neurosci. Lett. 2008;430:207–212. doi: 10.1016/j.neulet.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, Spyer KM. Rapid adenosine release in the nucleus tractus solitarii during defence response in rats: real-time measurement in vivo. J. Physiol. 2002;544:149–160. doi: 10.1113/jphysiol.2002.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayne MR, Larson G, Orona RA, Zahniser NR. Opposing actions of adenosine A2a and dopamine D2 receptor activation on GABA release in the basal ganglia: evidence for an A2a/D2 receptor interaction in globus pallidus. Synapse. 1996;22:132–138. doi: 10.1002/(SICI)1098-2396(199602)22:2<132::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Dias AC, Vitela M, Colombari E, Mifflin SW. Nitric oxide modulation of glutamatergic, baroreflex, and cardiopulmonary transmission in the nucleus of the solitary tract. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H256–H262. doi: 10.1152/ajpheart.01149.2003. [DOI] [PubMed] [Google Scholar]
- Ditting T, Hilgers KF, Scrogin KE, Linz P, Veelken R. Influence of short-term versus prolonged cardiopulmonary receptor stimulation on renal and preganglionic adrenal sympathetic nerve activity in rats. Basic Res.Cardiol. 2006;101:223–234. doi: 10.1007/s00395-005-0572-1. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ. The function of A2 adenosine receptors in the mammalian brain: evidence for inhibition vs. enhancement of voltage gated calcium channels and neurotransmitter release. Prog. Brain Res. 1999;120:265–273. doi: 10.1016/s0079-6123(08)63561-x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Greenberg HM. Bradycardia at onset of sudden death: potential mechanisms. Ann. N. Y. Acad. Sci. 1984;427:241–252. doi: 10.1111/j.1749-6632.1984.tb20788.x. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol. Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Morgan DA, Mark AL. Contrasting reflex effects of chemosensitive and mechanosensitive vagal afferents. Hypertension. 1988;11:674–679. doi: 10.1161/01.hyp.11.6.674. [DOI] [PubMed] [Google Scholar]
- Ichinose TK, Minic Z, Li C, O'Leary DS, Scislo TJ. Activation of NTS A1 adenosine receptors inhibits regional sympathetic responses evoked by activation of cardiopulmonary chemoreflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:539–R550. doi: 10.1152/ajpregu.00164.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose TK, O'Leary DS, Scislo TJ. Activation of NTS A2a adenosine receptors differentially resets baroreflex control of renal vs. adrenal sympathetic nerve activity. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1058–H1068. doi: 10.1152/ajpheart.00906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara K, Kawada T, Yanagiya Y, Uemura K, Inagaki M, Takaki H, Sugimachi M, Sunagawa K. Bezold-Jarisch reflex attenuates dynamic gain of baroreflex neural arc. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H833–H840. doi: 10.1152/ajpheart.01082.2002. [DOI] [PubMed] [Google Scholar]
- Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J. Neurophysiol. 2009;101:2270–2278. doi: 10.1152/jn.91304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J. Neurophysiol. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Lupinski SL, Schlicker E, Pedzinska-Betiuk A, Malinowska B. Acute myocardial ischemia enhances the vanilloid TRPV1 and serotonin 5-HT3 receptor-mediated Bezold-Jarisch reflex in rats. Pharmacol. Rep. 2011;63:1450–1459. doi: 10.1016/s1734-1140(11)70709-5. [DOI] [PubMed] [Google Scholar]
- Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J. Am. Coll. Cardiol. 1983;1:90–102. doi: 10.1016/s0735-1097(83)80014-x. [DOI] [PubMed] [Google Scholar]
- Minic Z, Li C, O'Leary DS, Scislo TJ. Severe hemorrhage attenuates cardiopulmonary chemoreflex (CCR) control of renal and adrenal sympathetic nerves via adenosine operating in the nucleus of the solitary tract (NTS) FASEB J. 2013;27 [Google Scholar]
- Mosqueda-Garcia R, Tseng CJ, Appalsamy M, Robertson D. Modulatory effects of adenosine on baroreflex activation in the brainstem of normotensive rats. Eur. J. Pharmacol. 1989;174:119–122. doi: 10.1016/0014-2999(89)90882-0. [DOI] [PubMed] [Google Scholar]
- Oei HH, Hughes WE, Schaffer SW, Longenecker GL, Glenn TM. Platelet serotonin uptake during myocardial ischemia. Am. Heart J. 1983;106:1077–1081. doi: 10.1016/0002-8703(83)90655-5. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Vagal sensory receptors and their reflex effects. Physiol. Rev. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Effects of drugs on chemoreceptors, pulmonary and cardiovascular receptors. Pharmacol. Ther. B. 1977;3:41–63. doi: 10.1016/0306-039x(77)90003-4. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Walter GA, O'Regan MH, Stair RE. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J. Cereb. Blood Flow Metab. 1987;7:679–686. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- Pimentel FF, Bonagamba LG, Machado BH. Pressor response to chemoreflex activation before and after microinjection of glycine into the NTS of awake rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1000–R1009. doi: 10.1152/ajpregu.00310.2002. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ribeiro JA, Sebastiao AM, de Mendonca A. Adenosine receptors in the nervous system: pathophysiological implications. Prog. Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Rocha I, Rosario LB, de Oliveira EI, Barros MA, Silva-Carvallho L. Enhancement of carotid chemoreceptor reflex and cardiac chemosensitive reflex in the acute phase of myocardial infarction of the anesthetized rabbit. Basic Res. Cardiol. 2003;98:175–180. doi: 10.1007/s00395-003-0407-x. [DOI] [PubMed] [Google Scholar]
- Salo LM, Woods RL, Anderson CR, McAllen RM. Nonuniformity in the von Bezold-Jarisch reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R714–R720. doi: 10.1152/ajpregu.00099.2007. [DOI] [PubMed] [Google Scholar]
- Sartor DM, Verberne AJM. The role of NMDA and non-NMDA receptors in the NTS in mediating three distinct sympathoinhibitory reflexes. N-S Arch. Pharmacol. 2007;376:241–252. doi: 10.1007/s00210-007-0203-5. [DOI] [PubMed] [Google Scholar]
- Schultz HD. Cardiac vagal chemosensory afferents. Function in pathophysiological states. Ann. N. Y. Acad. Sci. 2001;940:59–73. [PubMed] [Google Scholar]
- Scislo TJ, DiCarlo SE. Gender difference in cardiopulmonary reflex inhibition of sympathetic nerve activity. Am. J. Physiol. Heart Circ. Physiol. 1994;267:H1537–H1543. doi: 10.1152/ajpheart.1994.267.4.H1537. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, DiCarlo SE, Collins HL. Daily spontaneous running did not alter vagal afferent reactivity. Am. J. Physiol. Heart Circ. Physiol. 1993;265:H1564–H1570. doi: 10.1152/ajpheart.1993.265.5.H1564. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, Ichinose TK, O'Leary DS. Stimulation of NTS A1 adenosine receptors differentially resets baroreflex control of regional sympathetic outputs. Am. J. Physiol. Heart. Circ. Physiol. 2008;294:H172–H182. doi: 10.1152/ajpheart.01099.2007. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, Kitchen AM, Augustyniak RA, O'Leary DS. Differential patterns of sympathetic responses to selective stimulation of nucleus tractus solitarius purinergic receptor subtypes. Clin. Exp. Pharmacol. Physiol. 2001;28:120–124. doi: 10.1046/j.1440-1681.2001.03404.x. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Activation of A2a adenosine receptors in the nucleus tractus solitarius inhibits renal but not lumbar sympathetic nerve activity. J. Auton. Nerv. Syst. 1998a;68:145–152. doi: 10.1016/s0165-1838(97)00135-5. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A2a and P2x purinoceptors. Am. J. Physiol. Heart Circ. Physiol. 1998b;275:H2130–H2139. doi: 10.1152/ajpheart.1998.275.6.H2130. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P2x and A2a receptor stimulation. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H2057–H2068. doi: 10.1152/ajpheart.2000.278.6.H2057. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A1 adenosine receptors. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1588–H1599. doi: 10.1152/ajpheart.00897.2001. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol. Res. 2005;27:182–194. doi: 10.1179/016164105X21959. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O'Leary DS. Adenosine receptors located in the NTS contribute to renal sympathoinhibition during hypotensive phase of severe hemorrhage in anesthetized rats. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2453–H2461. doi: 10.1152/ajpheart.00158.2006. [DOI] [PubMed] [Google Scholar]
- St Lambert JH, Dashwood MR, Spyer KM. Role of brainstem adenosine A1 receptors in the cardiovascular response to hypothalamic defence area stimulation in the anaesthetized rat. Br. J. Pharmacol. 1996;117:277–282. doi: 10.1111/j.1476-5381.1996.tb15187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Lambert JH, Dawid-Milner MS, Silva-Carvalho L, Spyer KM. Action of adenosine receptor antagonists on the cardiovascular response to defence area stimulation in the rat. Br. J. Pharmacol. 1994;113:159–164. doi: 10.1111/j.1476-5381.1994.tb16188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Lambert JH, Thomas T, Burnstock G, Spyer KM. A source of adenosine involved in cardiovascular responses to defense area stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1997;272:R195–R200. doi: 10.1152/ajpregu.1997.272.1.R195. [DOI] [PubMed] [Google Scholar]
- Thomas T, St Lambert JH, Dashwood MR, Spyer KM. Localization and action of adenosine A2a receptors in regions of the brainstem important in cardiovascular control. Neuroscience. 2000;95:513–518. doi: 10.1016/s0306-4522(99)00473-x. [DOI] [PubMed] [Google Scholar]
- Thoren P, Noresson E, Ricksten SE. Cardiac receptors with non-medullated vagal afferents in the rat. Acta Physiol. Scand. 1979a;105:295–303. doi: 10.1111/j.1748-1716.1979.tb06344.x. [DOI] [PubMed] [Google Scholar]
- Thoren P, Noresson E, Ricksten SE. Resetting of cardiac C-fiber endings in the spontaneously hypertensive rat. Acta Physiol. Scand. 1979b;107:13–18. doi: 10.1111/j.1748-1716.1979.tb06437.x. [DOI] [PubMed] [Google Scholar]
- Thoren PN. Characteristics of left ventricular receptors with nonmedullated vagal afferents in cats. Circ. Res. 1977;40:415–421. doi: 10.1161/01.res.40.4.415. [DOI] [PubMed] [Google Scholar]
- Van Wylen DG, Park TS, Rubio R, Berne RM. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am. J. Physiol. Heart Circ. Physiol. 1988;255:H1211–H1218. doi: 10.1152/ajpheart.1988.255.5.H1211. [DOI] [PubMed] [Google Scholar]
- Veelken R, Hilgers KF, Leonard M, Scrogin K, Ruhe J, Mann JF, Luft FC. A highly selective cardiorenal serotonergic 5-HT3-mediated reflex in rats. Am. J. Physiol. Heart Circ. Physiol. 1993;264:H1871–H1877. doi: 10.1152/ajpheart.1993.264.6.H1871. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992;263:R1195–R1202. doi: 10.1152/ajpregu.1992.263.6.R1195. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 seconds of ischemia. Circ. Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Yan S, Laferriere A, Zhang C, Moss IR. Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets. J. Appl. Physiol. 1995;79:405–410. doi: 10.1152/jappl.1995.79.2.405. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ectonucleotidases in the nervous system. Prog. Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]