Abstract
Th17 cytokines can play both protective and pathologic roles in the airway. An emerging theme in Th17 cytokine biology is that these responses can mediate tissue pathology when downstream effector cells are dysfunctional such as neutrophils lacking functional NADPH oxidase in the case of chronic granulomatous disease or epithelial cells lacking appropriate ion transport, as in the case of cystic fibrosis. In this mini-review we highlight recent advances in the protective and pathologic roles of Th17 cytokines in the context of pulmonary infection.
Introduction
With the recent discovery of a distinct subset of T helper cell called Th17 cells, in addition to the previously well characterized Th1 and Th2 cell subsets, came many new breakthroughs in the realm of innate and adaptive immunity. Th17 cells have been shown to differentiate from naïve CD4+ cells in the presence of IL-6 and TGFβ in mice, or IL-6 and IL-1 in humans, when stimulated with appropriate antigen via activation of transcription factor STAT3 [1]. As CD4+ cells commit to effector Th17 phenotype, the hallmark cytokines IL-17A, IL-17F, IL-21, and IL-22 are expressed via STAT-3-dependent activation of the critical transcription factor retinoid-related orphan receptor γt (RORγt). IL-23 is required at late stages of Th17 development to promote full differentiation and sustain IL-17 production [1, 2]. Signaling of these cytokines induces granulopoietic factors and chemokines including G-CSF, GM-CSF, and several CXC chemokines [3]. These elements then mediate immune response against extracellular bacteria and fungi via neutrophil recruitment to sites of infection. The recruitment of neutrophils to sites of infection is a key event in host defense against infection, as neutrophils are responsible for engulfing and killing foreign pathogens through production of reactive oxygen species, proteolytic enzymes, and other antimicrobial peptides [4]. This neutrophil recruitment and activation is ideally self-limited and leads to timely neutrophil apoptosis as part of normal resolution of inflammation. However, persistent responses can lead to release of these reactive oxygen species and proteolytic enzymes, which perpetuates local inflammation and is often very damaging to the surrounding tissues [4].
With regard to pulmonary infections, Th17 cells have been found to play a vital role in host defense against numerous pathogens, including Klebsiella pneumoniae [5]. However, while Th17 cells are paramount in the adaptive phases of host defense, several other cell types are able to generate these cytokines in the earlier phases of immune response and bridge the gap between innate and adaptive immunity in the lung. These cell types include γδ T cells, natural killer (NK) cells, NKT cells, and certain innate lymphoid cells (ILCs) [6].
The role of IL-17A and its complementary cytokines in lung immunity can be pathogenic or protective [7, 8]. Given the crucial task of IL-17 to recruit neutrophils to the site of infection and begin the cascade of phagocytosis and pathogen clearance, IL-17 mediated tissue inflammation is often beneficial for the host. However, there are circumstances in which IL-17 recruits neutrophils to sites of infection but does not bring about improved pathogen clearance, leading to dysregulated inflammation without benefit to the host and an ultimately in these circumstances IL-17 can play a pathogenic role.
Evidence for Protective Th17 Cytokine Responses in Pulmonary Infection
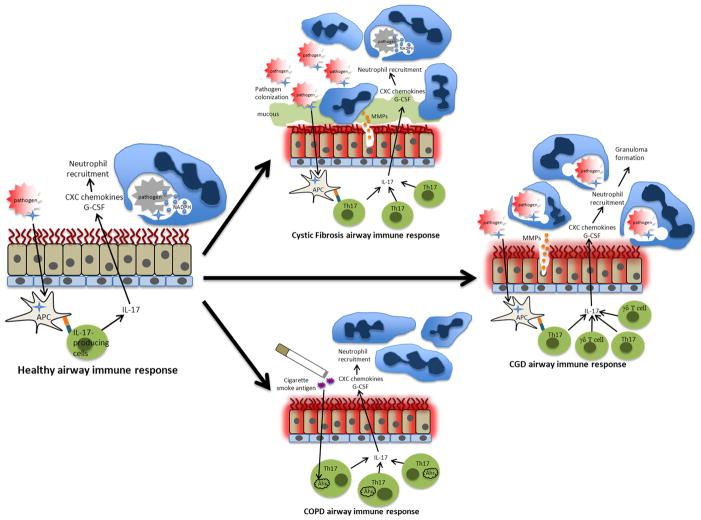
Th17 cytokines are essential for effective host defense against pulmonary pathogens by participating in the recruitment of pathogen-engulfing neutrophils and pathogen-killing molecules to the site of infection (see figure 1). Perhaps the most unambiguous clinical representation emphasizing the importance of Th17 cytokines in host defense against pulmonary infection is the autosomal dominant hyper IgE syndrome known as Job’s Syndrome. This disease is characterized by recurrent staphylococcal pneumonia and soft tissue infection, mucocutaneous candidiasis, atopic dermatitis, and elevated serum IgE levels. It has recently been found that hyper IgE syndrome is due to a dominant negative mutation in the transcription factor STAT3, and this results in a severe deficiency in production of Th17 cells [9, 10]. While STAT3 is also a critical transcription factor in several other cytokine and cellular function pathways, its involvement in Th17 cell differentiation appears to play a critical role in the pathogenesis and increased susceptibility to infections at mucosal surfaces in patients with Job Syndrome, particularly in mucocutaneous candidiasis [11, 12]. However it is important to note that the specific contributions of STAT3 signaling in various cells types and how these cells contribute to the phenotype of hyper IgE syndrome remains to be precisely determined.
Figure 1. A model of protective versus pathologic Type 17 Immune responses in the lung.
Healthy bronchial epithelium can be exposed to pathogens, and intra-epithelial antigen presenting cells can present pathogenic antigens to T cells in both the bronchial submucosa and the lymph nodes, thereby activating IL-17 producing T cells. Cytokines produced by antigen presenting cells can also activate innate cells such as γδ T cells and other cells to produce Th17 cytokines. IL-17 and other cytokines such as TNF-α and IL-22 stimulate production of chemokines to recruit neutrophils to the site of infection. Neutrophils then participate in pathogen killing through multiple processes which include engulfment into phagolysosomes and production of reactive oxygen species in part by the enzyme NADPH. In cystic fibrosis, there is reduced chloride and bicarbonate secretion as well as excessive mucous production resulting in bronchial epithelial surfaces which are coated with inspissated mucous and dysfunctional cilia, creating an ideal environment for pathogen colonization. Chronic pathogen exposure can lead to chronic Th17 cell activation and augmented neutrophil infiltration which generate matrix metalloproteinases (MMPs) and and airway damage. In chronic granulomatous disease, there is a defect in NADPH function which results in phagocytosis of pathogens without effective pathogen killing, causing chronic inflammation by accumulation of dysfunctional neutrophils and ultimately granuloma formation. When the bronchial epithelium is exposed to cigarette smoke antigen, which contains the ligands for the aryl hydrocarbon receptor (Ahr), the activation of Ahr in Th17 cells enhances Th17 cytokine production, leading to chronic inflammation and ultimately may contribute to pathogenesis in chronic obstructive pulmonary disease.
One of the most common pulmonary bacterial pathogens in Job’s Syndrome patients is Staphylococcus aureus. After pulmonary S. aureus infection, IL-17RA knockout mice demonstrate decreased neutrophil recruitment to the lung and decreased production of downstream IL-17 effector chemokines and cytokines compared to wild type mice. Additionally, bacterial burden in the lung is markedly increased in IL-17RA, IL-17A, IL-17F, and IL-22 knockout mice compared to wild type [13]. This same study also established the requirement for IL-17 cytokines in host defense against S. aureus by demonstrating that when mice are infected with influenza A virus prior to S. aureus inoculation, S. aureus pneumonia is significantly worsened as demonstrated by increased bacterial burden, worsened pulmonary inflammation, and increased mortality compared to influenza infection alone or S. aureus infection alone. This study went on to show that one mechanism for influenza A infection causing worsened S. aureus pneumonia was through inhibition of the IL-17/IL-23 axis [13]. As an investigation into the role of γδ T cells in S. aureus pneumonia, a different study examined the effects of pulmonary S. aureus infection in TCR-δ knockout mice, and determined that bacterial burden in the lung as well as dissemination to the spleen was significantly increased in the TCR-δ knockout mice, and neutrophil counts in the lungs were significantly reduced, indicating that γδ T cells play a particularly important role in host defense against S. aureus pneumonia [14]. γδ T typically take on a Th1 phenotype or Th17 phenotype, and are thus potent producers of either IL-17 or IFNγ [15]. Flow cytometry analysis shows that while the percentage of IFNγ-producing γδ T cells decreases after S. aureus infection, the percentage of IL-17-producing γδ T cells markedly increases after infection, and in this study approximately 60% of the IL-17-producing cells in the lung after infection were TCR-δ+. Furthermore, TCR-δ knockout mice expressed significantly less IL-17 mRNA after infection compared to wild type infected mice and also expressed significantly less mRNA of downstream IL-17 effector molecules including CXCL1, CXCL2, GM-CSF, TNF-α, and IL-6 [14]. The impaired expression of these molecules is likely responsible for the decreased neutrophil counts in the lungs, resulting in impaired bacterial clearance.
Role of Th17 Cytokines in Host Protection against Bacterial Pneumonia
At a time when the scope of knowledge regarding IL-17 was still in its infancy, IL-17 was found to play a critical role in host defense against pulmonary K. pneumoniae infection. IL-17R knockout mice exhibit significant delays in neutrophil recruitment to the lung after K. pneumoniae infection, and bacteria are more highly disseminated to the spleen. This effect is mediated mainly through decreased production of downstream IL-17 effector molecules including G-CSF and macrophage inflammatory protein (MIP)-2 or CXCL2 [5]. The role of IL-23, which is a heterodimeric cytokine composed of two subunits, also shed light on the protective role of the IL-17/23 axis in K. pneumoniae infection. IL-23p19 is a subunit unique to IL-23 while IL-12p40 is a subunit which it shares with IL-12, another heterodimeric cytokine whose unique subunit is IL-12p35 [2]. Survival after pulmonary K. pneumoniae infection is severely reduced in IL23p19 knockout mice compared to wild type [16]. However, when IL-23p19 knockout mice receive intratracheal recombinant IL-17 rescue 12 hours after infection, bacterial burden in the lungs and dissemination to the spleen is significantly reduced compared to mice that receive vehicle control. This enhanced host defense is associated with increased downstream IL-17 effector molecules including G-CSF and CXCL1 [16].
In Mycobacterium tuberculosis infection, the involvement of IL-23 in host protection was suggested when IL-12p40 knockout mice were found to have higher bacterial burden after pulmonary infection and inferior ability to generate protective inflammatory response compared to IL-12p35 knockout mice [17, 18]. While IL-23p19 knockout mice do not demonstrate higher bacterial burden than wild type mice, IL-17 producing CD4+ cells and IL-17 mRNA expression in the lungs after infection are significantly attenuated [19]. The role of IL-17 is better defined in demonstrating that when IL-17 knockout mice are infected with M. bovis bacille Calmette-Guérin (BCG), granuloma formation in the lung is smaller, less frequent, and less densely packed with mononuclear cells compared to wild type [20]. Though granuloma formation is essential for host protection from disseminated infection, bacterial burden is similar in wild type mice versus IL-17 knockouts [20]. In vaccinated animals, IL-17 also aids in recruitment of IFNγ-producing Th1 cells to the lung after M. tuberculosis infection, which seems to ultimately limit bacterial growth [21]. While it remains unclear, there is evidence that the cells responsible for the majority of IL-17-induced granuloma formation are γδ T cells rather than Th17 cells during primary infection [22, 23]. Overall, the function of the IL-17 axis in mycobacterial infection appears to be mainly its downstream induction of neutrophil-recruiting cytokines and chemokines, contributing to granuloma formation and Th1 cell recruitment but it is relatively dispensable in primary host defense.
While the mechanisms for protection against Bordatella pertussis are not entirely clear, several types of T helper cell as well as B cell responses appear to be involved, even after vaccine-induced antibody response fades [24]. More recent evidence in vaccine models indicate a robust in vitro IL-17 response to B. pertussis antigen by splenocytes from mice that had been immunized with whole cell pertussis vaccine, an effect that was further augmented by the presence of IL-23 in culture medium [25]. In vivo, when immunized mice are treated with anti-IL-17 antibody before and after an aerosolized B. pertussis challenge, bacterial burden is significantly higher compared to infected immunized mice that did not receive anti-IL-17 antibody, further indicating a protective role for IL-17-producing cells in B. pertussis host defense [25]. This study specifically demonstrates the vital role of toll-like receptor 4 in activating signaling pathways from dendritic cells which promote Th1 and Th17 cell differentiation to fully protect the host against B. pertussis after whole cell vaccination. In a model of natural infection, when IL-17 knockout mice are infected with B. pertussis, lung bacterial burden is nearly 100-fold higher compared to wild type mice [26]. A baboon model of B. pertussis infection demonstrates that intratracheal administration of bacteria induces a marked rise in IL-17, detected by nasopharyngeal washes, as early as day 5 after infection. This rise in IL-17 is preceded by increases in IL-6, IL-23, and IL-1β which may indicate a priming of Th17 cell differentiation. Downstream effector molecules IL-8, G-CSF, MCP-1, and MIP-1α are also significantly increased after infection [27]. Furthermore, a large population of IL-17 producing cells was detected by ELISPOT in peripheral blood mononuclear cells harvested from convalescent baboons after ex-vivo stimulation with heat-killed B. pertussis compared to naïve baboons, indicating an important role for memory Th17 cells in B. pertussis immunity [27]. Taken together, these studies indicate a protective role for IL-17 producing cells in host defense against B. pertussis infection, but further studies investigating which cell types are producing this IL-17 are necessary.
Evidence also exists for an important role for IL-17 in host defense against Mycoplasma pneumoniae. Mice that received intranasal inoculation with M. pneumoniae were found to have significantly increased leukocyte counts in bronchoalveolar lavage (BAL) fluid, which correlated with markedly increased IL-17 protein levels and IL-23p19 mRNA expression that was at least 100 times greater in lung tissue compared to uninfected mice [28]. Since this increase in cytokine expression does not alone indicate a direct causal relationship between IL-17 and host defense against Mycoplasma pneumoniae, a study using IL-17A or IL-17 RA knockout mice would be useful. However, a study using a different species of mycoplasma showed that when IL-17-receptor deficient mice are infected intranasally with Mycoplasma pulmonis, bacterial burden in the lung is approximately 10 times greater compared to wild type infected mice [29]. While this evidence is convincing for IL-17 playing a role in host defense against pulmonary mycoplasma infections, more investigations are required to better delineate the mechanism of protection.
IL-17 has been shown to be relevant in host defense against Streptococcus pneumoniae, one of the most common and clinically relevant pathogens of all. After S. pneumoniae whole cell vaccination, protection against S. pneumoniae colonization is significantly impaired in IL-17A receptor knockout mice compared to IFNγ or IL-4 knockout mice, who are well protected against colonization [30]. In another study, intranasal Th17 specific S. pneumoniae antigen immunizations were shown to be markedly protective against airway colonization. Additionally, after intraperitoneal administration of anti-IL-17A antibody, the protection against this colonization was effectively abrogated [31].
Role of Th17 Cytokines in Protective Host Responses Against Fungal Pneumonia
Pneumocystis jirovecii and P. murina (the mouse Pneumocystis pathogen) is a fungal pathogen that is typically opportunistic, classically affecting patients with HIV, and its effects can be devastating and frequently fatal. Prior to identification of Th17 cells, it was noted that mice deficient in either Th1 or Th2 cellular responses were still protected against P. murina infection [32]. Pneumocystis pneumonia was later linked to the IL-17/23 axis when it was shown that intratracheally-infected IL-23p19 knockout mice or wild type mice treated with anti-IL-23 antibody have higher fungal burdens, decreased expression of several associated lymphocyte chemokines, and reduced effector T cells in the lung compared with control mice [33]. When wild type mice receive IL-17 neutralizing antibody after infection, there is a 10,000-fold increase in P. murina rRNA expression in the lung compared to control [33], suggesting that the mechanism for IL-23 protection against P. murina pneumonia may be through promotion of full effector Th17 cell function
Evidence for the protective roles of Th17 cytokines in endemic mycoses has recently been presented. In mice infected intranasally with Histoplasma capsulatum, fungal burden in the lungs is increased when anti-IL-17A monoclonal antibody is administered at the time of infection. This effect is not evident until day 7 of infection but continues until day 30, highlighting the role of IL-17 in adaptive immune response to this organism [34]. Additionally, mice deficient in both IL-12 and IL-23 (IL-12p40 knockout) exhibit significantly decreased survival compared to mice deficient in IL-12 alone (IL-12p35 knockout), but when IL-12p35 knockout mice are treated with anti-IL17A antibody during infection, their survival is reduced to the level of the IL-12p40 knockout mice. This would suggest that IL-23 is protective in H. capsulatum infection, and that effect appears to be dependent upon IL-17 [34]. In a mouse model of Cryptococcus neoformans, when mice are given anti-IL-17A antibody during pulmonary infection, pulmonary fungal burden is increased at day 7 compared to mice that did not receive anti-IL-17A antibody, but this difference is abolished at day 14. Additionally, IL-17RA knockout mice do not demonstrate decreased survival compared to wild type mice when infected with Cryptococcus [35]. Deletion of Th2-driving cytokines IL-4 and IL-13 results in a robust Th1 and Th17 cytokine response in mice infected with Cryptococcus that results in decreased fungal burden in the lung and reduced pulmonary inflammation. However, deletion of Th2 immune responses did not improve survival or Cryptococcal dissemination to the brain resulting in severe meningoencephalitis [36]. These studies appear to indicate that IL-17 immune responses are important for optimum host defense against Cryptococcus neoformans, especially in the lung, but that they are not sufficient to protect against mortality and severe dissemination.
Evidence for Pathologic Th17 Cytokine Responses in Pulmonary Infection
Since Th17 cytokines are responsible for recruiting microbe-clearing cells and microbicidal peptides to the sites of infection, they tend to have an inherently protective function. However, when there is any breakdown or defect in the immune system physiology, typically resulting from a disease process, these same functions of Th17 cytokines can become pathogenic. For example, cystic fibrosis (CF) is a disease involving impaired host defense against pulmonary infection in which Th17 cytokines have been shown to play an important role in pathogenesis. The airways of patients with CF are coated with a dense film of inflammatory cells, most of which are neutrophils that produce lung-damaging protease enzymes such as elastases and matrix metalloproteinases (MMPs) (see figure 1) [37]. As IL-17 is known to initiate a signal transduction pathway that ultimately recruits neutrophils to sites of infection, it is hypothesized that production of IL-17 by leukocytes from human airway submucosa is responsible for recruitment of these neutrophils in response to Pseudomonas aeruginosa colonization in CF [38]. Indeed, several lymphocyte populations have been shown to produce IL-17 and its downstream chemokines in the human CF airway in all different stages of disease, and levels of IL-17 in bronchoalveolar lavage fluid correlate with neutrophil counts [38]. Pseudomonas aeruginosa is a mainly opportunistic bacterium that infects immunocompromised hosts, and is thus a common pulmonary infection in cystic fibrosis (CF). Patients colonized with the Pseudomonas have increased levels of IL-17 in sputum samples, which decreases after treatment with appropriate anti-pseudomonas antibiotics [39]. Studies on CF patients having pulmonary exacerbations have shown increased levels of IL-17A and IL-17F as well as upregulated downstream IL-17 effector molecules such as IL-8 and G-CSF [39]. Lung draining lymph nodes from CF patients at the time of lung transplant also contain T cells with a strong Th17 phenotype. These patients demonstrate an increase in IL-17 producing T cells and IL-22 producing T cells in response P. aeruginosa antigens [40]. In mouse models of chronic P. aeruginosa infection using intratracheally administered pseudomonas-coated beads meant to mimic the effects of decreased pathogen clearance in CF, IL-23 gene expression is elevated early after infection, followed by significant elevations of IL-17 compared to uninfected mice [41]. In IL-23p19 knockout mice, downstream IL-17 effector molecules CXCL1, MIP-1α, CXCL10, and MMP9 are significantly inhibited compared to wild type mice after P. aeruginosa infection, and neutrophil recruitment to the lung is significantly decreased, further emphasizing the role of IL-17 induced chemokines in neutrophil recruitment and lung inflammation [41]. However, there is no difference in bacterial burden suggesting that the IL-17/23 pathway in chronic P. aeruginosa infection is central to generation of inflammatory response and thus bronchiectasis, but does not participate in primary host defense and may therefore be detrimental to the host. However, the role of IL-17 in acute pulmonary infection with P. aeruginosa may in fact be protective. Intraperitoneal administration of anti-IL-17 monoclonal antibody 24 hours prior to acute infection results in decreased levels of IL-17 as well as downstream effector molecules G-CSF and CXCL1 compared to those that received control antibody resulting in reduced neutrophil recruitment to the lung. However, contrary to the chronic infection model, anti-IL-17 resulted in higher bacterial burden, suggesting that IL-17 may play a protective role in acute P. aeruginosa infection [42]. While this cumulative evidence may suggest that IL-17 plays a pathogenic role in CF in response to pathogen colonization via excess neutrophil recruitment to the airway epithelium, direct causality between IL-17 production and the development of bronchiectasis has yet to be established.
Other diseases that result in chronic lung inflammation and damage in the form of bronchiectasis are chronic granulomatous disease (CGD) and chronic obstructive pulmonary disease (COPD). CGD is a rare immunodeficiency caused by defective nicotinamide dinucleotide phosphate (NADPH) oxidase, causing failure of phagocytes to create reactive oxygen species and kill their engulfed pathogen which results in chronic inflammation and granuloma formation [43] (see figure 1). Patients with CGD suffer from recurrent pulmonary infections and long-term lung damage. While the defect in this disease is downstream of IL-17 and its effector chemokines and cytokines, patients with CGD demonstrate increased circulating Th17 cells compared to healthy controls which may be due to the defects in pathogen clearance. However, this phenomenon can be corrected by hematopoietic stem cell transplant [44], indicating that the over activation of Th17 cells due to the insufficient pathogen killing is likely contributing to the significant inflammation which is a hallmark of this disease. A common pulmonary pathogen for patients with CGD is Aspergillus fumigatus. Though there is some conflicting data regarding the pathogenic function of IL-17 in pulmonary aspergillosis [45], experiments in mouse models of chronic granulomatous disease have corroborated a pathogenic role for IL-17. In these mouse models of CGD, IL-17 is mainly produced by aberrant γδ T cells in response to aspergillus infection, and there appears to be improved survival when treated with anti-IL-17 antibody [46].
COPD is a common destructive lung disease that typically results from long-term tobacco smoke exposure, characterized by increased mucous production, airway inflammation, and obstructive bronchitis (see figure 1). Patients with COPD express increased numbers of IL-22+ cells in bronchial epithelium and increased numbers of IL-17A+ cells in bronchial submucosa [47]. Perhaps stronger evidence for a pathogenic role of Th17 cells in COPD is that patients with COPD also demonstrate increased Th17 signatures in peripheral blood which significantly correlates with presence and severity of airflow limitation as measured by pulmonary function tests [48]. Furthermore, cigarettes smoke extract has been shown to be a strong Th17 adjuvant and IL-17 receptor deficient mice are protected from smoke induced emphysema [49]. While these findings indicate that IL-17-producing cells may be pathogenic in these diseases, more work is necessary to clarify this point, as there are profound therapeutic implications.
Role of Th17 Cytokines in Pathologic Host Responses against Fungal Pneumonia
As mentioned above, Aspergillus fumigatus is a fungal pathogen which often causes pneumonia in immunocompromised hosts. The IL-17/23 axis was found to be important in increasing pathogenesis of this organism by administering intraperitoneal anti-IL-23 and anti-IL-17 antibodies in mice that had been infected intranasally with the organism. Either treatment decreases fungal burden and decreases Th17 cells in the lung while increasing pulmonary Th1 cells, indicating that the IL-17/23 pathway increases pathogenesis by inhibiting protective Th1 responses [50]. Conversely, when IL-22 is neutralized after acute pulmonary aspergillus infection or IL-22 knockout mice are infected with aspergillus, fungal clearance is impaired [51]. However, in a model of chronic infection, mice that are chronically exposed to aspergillus demonstrate improved lung function when treated with IL-22 neutralizing antibody [52], indicating that IL-22 may play a protective or restorative role in acute pulmonary aspergillus infection but a pathogenic role in chronic pulmonary aspergillus infection. In mouse models of Th-2-mediated allergic inflammatory responses to pulmonary aspergillus infection, IL-17 deficient mice show improved A. fumigatus clearance while the presence of IL-17 seems to be associated with increased pulmonary eosinophilia [53]. This pattern of increased pulmonary inflammation without improved pathogen clearance is pathogenic in lung immunity and is a potential cause for lasting lung damage.
Role of Th17 Cytokines in Pathologic Host Responses against Viral Pneumonia
Respiratory Syncytial Virus (RSV) is a very common respiratory pathogen, and has been linked to Th17 cytokines through various mechanisms. IL-17 production in murine models of RSV infection appears to be regulated through STAT-1 signaling as well as TLR7 activity, and is associated with worsened airway hyperresponsiveness and increased mucous production [54, 55]. IL-17 and several of its related cytokines and transcription factors are increased after RSV infection in mice via upregulation of the anaphylatoxin C3a [56]. Neutralization of IL-17 after RSV infection results in down-regulation of mucus-associated genes Muc5ac and Gob5 and a correlating decrease in mucus and goblet cell staining by lung histology. IL-17 neutralization after RSV infection also results in decreased lung neutrophilia and lower viral load in the lungs. Further, development of protective RSV antigen-specific cytotoxic CD8 T cells appears to be impaired by IL-17 [57]. Taken together, there is significant data to indicate that IL-17 is regulated at multiple levels because it has a significantly pathogenic role in RSV infection by impairing viral clearance while worsening inflammation and hindering antiviral response capacity by the host.
The involvement of Th17 cytokines in influenza infection was shown to be clinically relevant in the 2009 H1N1 flu pandemic that affected much of the world when serum samples from H1N1 patients demonstrated significant elevations in IL-17 as well as several Th-17 mediators including IL-6, IL-8, G-CSF, and GM-CSF [58, 59]. The mechanisms and extent of the involvement of IL-17 were clarified when IL-17RA knockout mice that were infected with PR/8 H1N1 influenza virus exhibit less weight loss than wild type infected mice. IL-17RA knockout mice also exhibit attenuated pulmonary inflammation by histology, less lung damage, decreased IL-17 neutrophil-recruiting chemokines KC and G-CSF, and therefore decreased neutrophil counts in BAL samples [60]. This combination of factors leads to a conclusion that much like RSV, influenza virus appears to induce IL-17 in a pathogenic capacity with regard to pulmonary host defense. By contrast, IL-22 has been found to aid in epithelial repair after influenza infection, as IL-22 knockout mice have worsened lung damage after influenza infection. Additionally, while IL-22Ra1 expression is typically only found in airway epithelium, its expression appears to be induced in the parenchyma after influenza infection. IL-22 knockout mice also demonstrate increased collagen deposition in the lung parenchyma and correlating increased lung stiffness [61]. IL-22 knockout mice also demonstrate increased susceptibility to secondary bacterial infection after influenza exposure [62]. While the cell types responsible for IL-22 production have not been fully elucidated, IL-22 does appear to play a reparative role in the lung after influenza infection.
Conclusions
Though much work has yet to be done towards better defining the role of Th17 cytokines in pulmonary host defense, animal models as well as human disease processes have provided us with much insight into this question. These cytokines appear to act through neutrophil recruitment to protect the host against such pathogens as Klebsiella pneumoniae, Mycobacterium tuberculosis, Bordatella pertussis, Mycoplasma pneumoniae, Staphylococcus aureus, Streptococcus pneumoniae, and fungal pathogens including Candida albicans and possibly Pneumocystis carinii, Histoplasmosis capsulatum, and Cryptococcus neoformans. However, by acting through the same process of chemokine and cytokine production resulting in neutrophil recruitment, Th17 cytokines can contribute to pathogenesis in Pseudomonas aeruginosa, Aspergillus fumigatus, Respiratory syncytial virus, and influenza virus. Recently, a significant amount of information has become available to define some of the phenotypic distinctions between pathogenic and classical Th17 cells [63–66]. While these distinctions in models of lung infection have not yet been elucidated, it is possible that such differences are involved in the discrepant roles of Th17 cells in lung infection. Future scientific endeavors examining mechanisms behind the differential outcomes of pathogenesis versus protection in these infections would be particularly useful, as there may be significant therapeutic strategies and prognostic implications that could be extracted from this information.
Acknowledgments
This work was supported by NIH grants R37-HL079142 (KC and JKK) and T32- 5T32AR052282-10.
Footnotes
The authors have no conflicts of interests to disclose.
References
- 1.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordon J, Aliberti S, Fernandez-Botran R, Uriarte SM, Rane MJ, Duvvuri P, Peyrani P, Morlacchi LC, Blasi F, Ramirez JA. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013;17:e76–83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 7.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson ML, Freeman AF, Holland SM. Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr Opin Allergy Clin Immunol. 2008;8:527–533. doi: 10.1097/ACI.0b013e3283184210. [DOI] [PubMed] [Google Scholar]
- 10.Milner JD, Sandler NG, Douek DC. Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr Opin HIV AIDS. 2010;5:179–183. doi: 10.1097/COH.0b013e328335ed3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puel A, Cypowyj S, Marodi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12:616–622. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol. 2011;186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Yin ZN, Mao XH, Guo G, Shi Y, Zou QM. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 2012;13:38. doi: 10.1186/1471-2172-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 18.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167:6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 19.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 20.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 21.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto Yoshida Y, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 23.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 24.Mahon BP, Brady MT, Mills KH. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J Infect Dis. 2000;181:2087–2091. doi: 10.1086/315527. [DOI] [PubMed] [Google Scholar]
- 25.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 26.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, Iwakura Y, Tschopp J, Sebo P, Mills KH. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185:1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 27.Warfel JM, Merkel TJ. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.117. [DOI] [PubMed] [Google Scholar]
- 28.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieve AN, Meeks KD, Bodhankar S, Lee S, Kolls JK, Simecka JW, Berg RE. A novel IL-17-dependent mechanism of cross protection: respiratory infection with mycoplasma protects against a secondary listeria infection. Eur J Immunol. 2009;39:426–438. doi: 10.1002/eji.200838726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffitt KL, Gierahn TM, Lu YJ, Gouveia P, Alderson M, Flechtner JB, Higgins DE, Malley R. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9:158–165. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deepe GS, Jr, Gibbons RS. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J Infect Dis. 2009;200:142–151. doi: 10.1086/599333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS One. 2011;6:e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang F, Tompkins KC, McNamara A, Jain AV, Moore BB, Toews GB, Huffnagle GB, Olszewski MA. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175:2489–2500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 38.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J, Kolls JK. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, Pilewski JM, Kolls JK. Patients with cystic fibrosis have inducible IL-17(+)IL-22(+) memory cells in lung draining lymph nodes. J Allergy Clin Immunol. 2013;131:1117–1129. e1115. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L519–528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, Wan H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 43.Rieber N, Hector A, Kuijpers T, Roos D, Hartl D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin Dev Immunol. 2012;2012:252460. doi: 10.1155/2012/252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horvath R, Rozkova D, Lastovicka J, Polouckova A, Sedlacek P, Sediva A, Spisek R. Expansion of T helper type 17 lymphocytes in patients with chronic granulomatous disease. Clin Exp Immunol. 2011;166:26–33. doi: 10.1111/j.1365-2249.2011.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 47.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D’Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargas-Rojas MI, Ramirez-Venegas A, Limon-Camacho L, Ochoa L, Hernandez-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med. 2011;105:1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 49.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, Zheng M. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 51.Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun. 2012;80:410–417. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol. 2012;189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect Immun. 2012;80:1424–1436. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimoto K, Durbin JE, Zhou W, Collins RD, Ho SB, Kolls JK, Dubin PJ, Sheller JR, Goleniewska K, O’Neal JF, Olson SJ, Mitchell D, Graham BS, Peebles RS., Jr Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol. 2005;116:550–557. doi: 10.1016/j.jaci.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 55.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bera MM, Lu B, Martin TR, Cui S, Rhein LM, Gerard C, Gerard NP. Th17 cytokines are critical for respiratory syncytial virus-associated airway hyperreponsiveness through regulation by complement C3a and tachykinins. J Immunol. 2011;187:4245–4255. doi: 10.4049/jimmunol.1101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Yang P, Sun Y, Li T, Wang C, Wang Z, Zou Z, Yan Y, Wang W, Chen Z, Xing L, Tang C, Ju X, Guo F, Deng J, Zhao Y, Tang J, Wang H, Zhao Z, Yin Z, Cao B, Wang X, Jiang C. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramirez P, Martin-Loeches I, Varillas D, Gallegos MC, Seron C, Micheloud D, Gomez JM, Tenorio-Abreu A, Ramos MJ, Molina ML, Huidobro S, Sanchez E, Gordon M, Fernandez V, Del Castillo A, Marcos MA, Villanueva B, Lopez CJ, Rodriguez-Dominguez M, Galan JC, Canton R, Lietor A, Rojo S, Eiros JM, Hinojosa C, Gonzalez I, Torner N, Banner D, Leon A, Cuesta P, Rowe T, Kelvin DJ. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Macho Fernandez E, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol. 2013 doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]