Abstract
In Escherichia coli, the molecular chaperones of hsp60/hsp10 (GroEL/GroES) families are required not only for protein folding but also for the rapid degradation of certain abnormal proteins. The rate-limiting step in the degradation of the fusion protein CRAG by protease ClpP appears to be the formation of a complex with GroEL. We have isolated these complexes and found that each GroEL 14mer contained a short-lived fragment of CRAG plus a 50 kDa polypeptide, which we identified by sequencing and immunological methods as Trigger Factor (TF). Upon ATP addition, GroEL and TF dissociated together from CRAG but remained tightly associated with each other even upon gel filtration. TF was originally proposed to function in protein translocation across membranes but altering cellular content of TF did not affect this process in vivo. By contrast, low levels of TF expression markedly reduced CRAG degradation, while an overproduction of TF greatly stimulated this process. Furthermore, in extracts of cells expressing high levels of TF, the capacity of GroEL to bind to CRAG is greatly increased. Overproduction of TF also stimulated GroEL's ability to bind to other unfolded proteins (fetuin and histone). Thus, TF is a rate-limiting factor for CRAG degradation; it appears to regulate GroEL function and to promote the formation of TF-GroEL-CRAG complexes which are critical for proteolysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crooke E., Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem. 1992 Jan 15;203(1-2):9–23. doi: 10.1111/j.1432-1033.1992.tb19822.x. [DOI] [PubMed] [Google Scholar]
- Guthrie B., Wickner W. Trigger factor depletion or overproduction causes defective cell division but does not block protein export. J Bacteriol. 1990 Oct;172(10):5555–5562. doi: 10.1128/jb.172.10.5555-5562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U. Heat shock proteins in protein folding and membrane translocation. Semin Immunol. 1991 Jan;3(1):5–16. [PubMed] [Google Scholar]
- Hellebust H., Uhlén M., Enfors S. O. Interaction between heat shock protein DnaK and recombinant staphylococcal protein A. J Bacteriol. 1990 Sep;172(9):5030–5034. doi: 10.1128/jb.172.9.5030-5034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
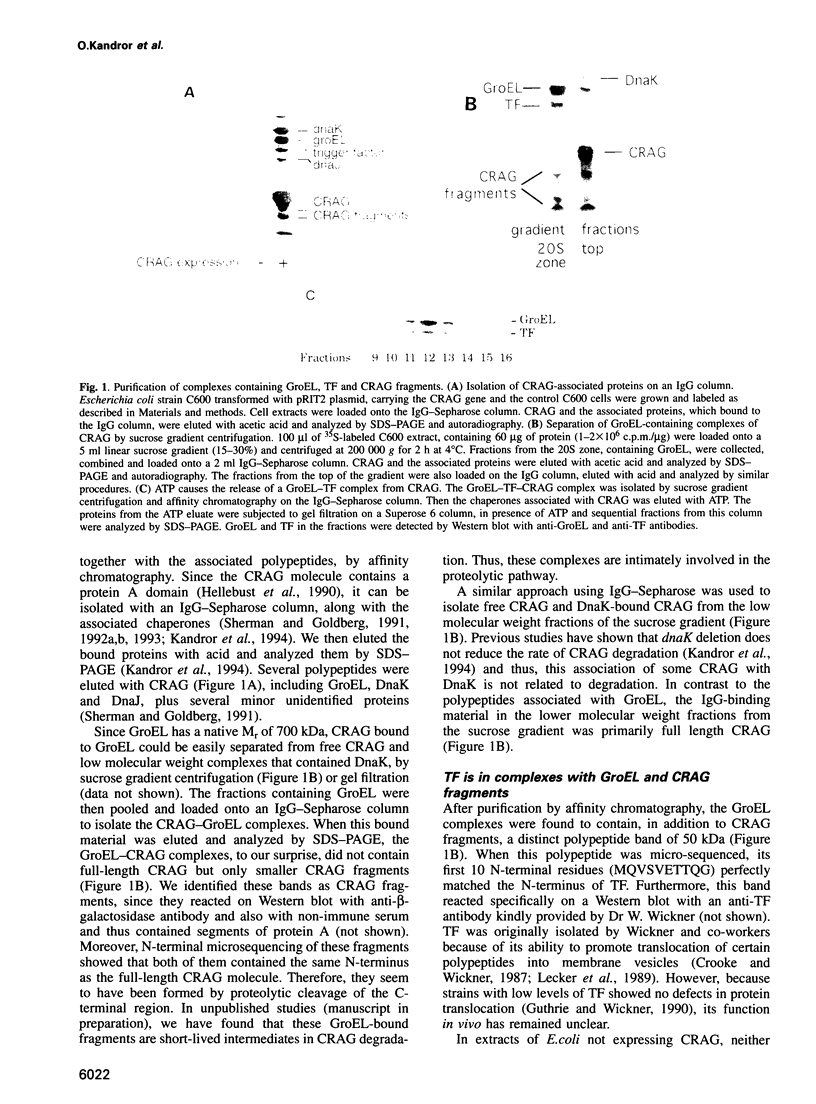
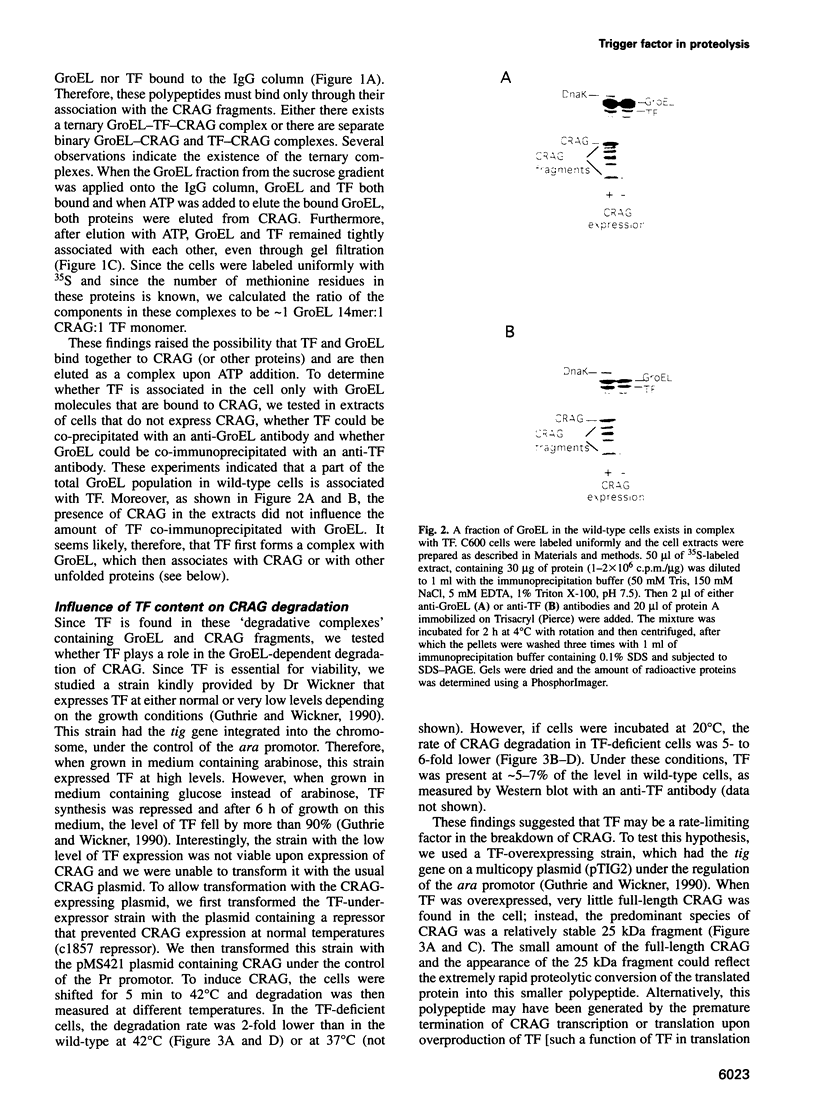
- Kandror O., Busconi L., Sherman M., Goldberg A. L. Rapid degradation of an abnormal protein in Escherichia coli involves the chaperones GroEL and GroES. J Biol Chem. 1994 Sep 23;269(38):23575–23582. [PubMed] [Google Scholar]
- Keller J. A., Simon L. D. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):31–41. doi: 10.1111/j.1365-2958.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Lill R., Crooke E., Guthrie B., Wickner W. The "trigger factor cycle" includes ribosomes, presecretory proteins, and the plasma membrane. Cell. 1988 Sep 23;54(7):1013–1018. doi: 10.1016/0092-8674(88)90116-x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Heat shock in Escherichia coli alters the protein-binding properties of the chaperonin groEL by inducing its phosphorylation. Nature. 1992 May 14;357(6374):167–169. doi: 10.1038/357167a0. [DOI] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. EMBO J. 1992 Jan;11(1):71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. Y., Goldberg A. L. Heat shock of Escherichia coli increases binding of dnaK (the hsp70 homolog) to polypeptides by promoting its phosphorylation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8648–8652. doi: 10.1073/pnas.90.18.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M., Goldberg A. L. Heat shock-induced phosphorylation of GroEL alters its binding and dissociation from unfolded proteins. J Biol Chem. 1994 Dec 16;269(50):31479–31483. [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Tilly K., Spence J., Georgopoulos C. Modulation of stability of the Escherichia coli heat shock regulatory factor sigma. J Bacteriol. 1989 Mar;171(3):1585–1589. doi: 10.1128/jb.171.3.1585-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol. 1991 Dec;3(6):1033–1038. doi: 10.1016/0955-0674(91)90125-i. [DOI] [PubMed] [Google Scholar]