Abstract
Osteocytes, the most abundant cells in bone, have been long postulated to detect and respond to mechanical and hormonal stimuli and to coordinate the function of osteoblasts and osteoclasts. The discovery that the inhibitor of bone formation sclerostin is primarily expressed in osteocytes in bone and it is downregulated by anabolic stimuli provided a mechanism by which osteocytes influence the activity of osteoblasts. Advances of the last few years provided experimental evidence demonstrating that osteocytes also participate in the recruitment of osteoclasts and the initiation of bone remodeling. Apoptotic osteocytes trigger yet to be identified signals that attract osteoclast precursors to specific areas of bone, which in turn differentiate to mature, bone resorbing osteoclasts. Osteocytes are also the source of molecules that regulate generation and activity of osteoclasts, such as OPG and RANKL; and genetic manipulations of the mouse genome leading to loss or gain of function, or to altered expression of either molecule in osteocytes, markedly affect bone resorption. This review highlights these investigations and discusses how the novel concept of osteocyte-driven bone resorption and formation impacts our understanding of the mechanisms by which current therapies control bone remodeling.
Keywords: osteocyte, osteoclast, osteoblast, bone remodeling, RANKL, OPG, Sost
1. Osteocytes and their functions in bone homeostasis
Osteocytes are former osteoblasts that become entombed during the process of bone deposition and remain regularly distributed throughout the mineralized bone matrix. Osteocytes comprise more than 90% of bone cells within the matrix or on the bone surfaces. It has been long hypothesized that osteocytes are the primary cells responsible for the adaptation of bone to mechanical force. Evidence accumulated in the last few years supports this notion and demonstrates that osteocytes are also involved in the response of bone to hormones. Few genes expressed in osteocytes have been identified as molecular mediators of the osteocyte-driven changes in bone remodeling. However, the molecular mechanisms by which osteocytes regulate the bone homeostasis are far from being understood.
Osteocytogenesis and the relationship between osteocyte shape and function
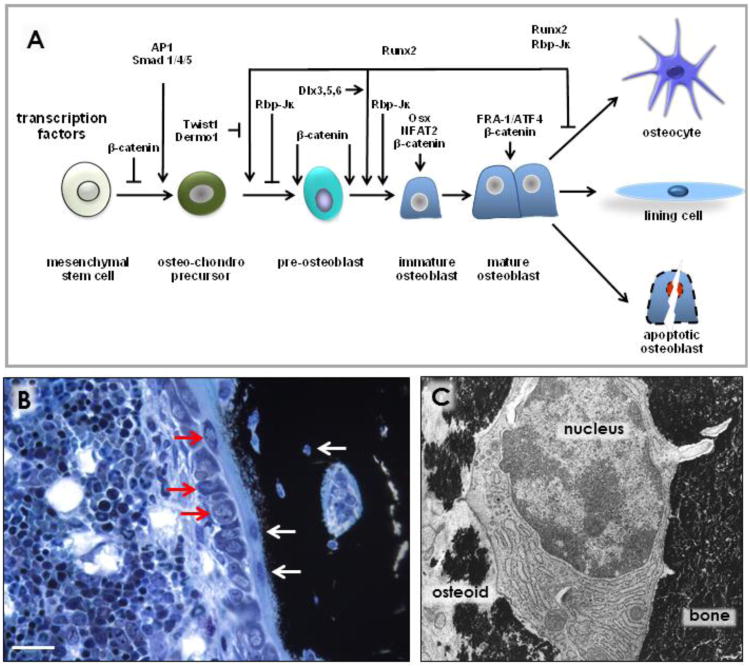
Between 5 to 20% of mature osteoblasts become entombed in the matrix that they generate and that subsequently mineralizes. The process of osteocyte formation was long thought to be stochastic. However, some osteoblasts might be prompted to extend cytoplasmic projections and to contact with already embedded cells, resulting in their differentiation into osteocytes. The mechanisms that regulate osteocytogenesis and osteocyte maturation have begun to be revealed. Expression of the membrane-associated proteins E11 and metalloproteinase MMP14 (also known as MT1-MMP) is required for the formation of osteocyte dendritic processes and canaliculi (1-3), suggesting that osteocytogenesis is an active process driven by changes in gene expression. Osteocyte formation is one of the three possible fates of mature osteoblasts, the other two being becoming lining cells or undergoing apoptosis (Figure 1). It is then expected that stimuli that alter one of the fates of osteoblasts would impact osteocyte formation. Consistent with this notion, inhibition of osteoblast apoptosis by intermittent administration of parathyroid hormone (PTH) leads to increased osteocyte density (4;5). However, it is still unknown whether this effect of the hormone is accompanied by changes in the expression of genes required for the osteoblast-osteocyte transition.
Figure 1. Osteocytogenesis and osteocyte maturation.
(A) Stages of osteocytogenesis and main transcription factors involved in differentiation of osteoblast precursors towards mature osteocytes. (B) Row of osteoblasts (bottom red arrows); an osteocyte recently embedded (top red arrow); two osteocytes completely embedded in osteoid (bottom white arrows); an osteocyte fully embedded in mineralized bone matrix (top white arrow). Picture was contributed by Keith Condon, Indiana University School of Medicine, IN, USA. (C) Morphology of an early osteocyte being embedded in bone; with part of the cell surface partially embedded (left) and the other part totally embedded (right) in mineralized matrix, 10,000 ×, rat bone. Picture was contributed by Stephen B. Doty, Hospital for Special Surgery, New York, NY, USA. (Reprinted with kind permission of Elsevier, Basic and Applied Bone Biology, Chapter 2 Bone Cells, Bellido, Plotkin and Bruzzaniti).
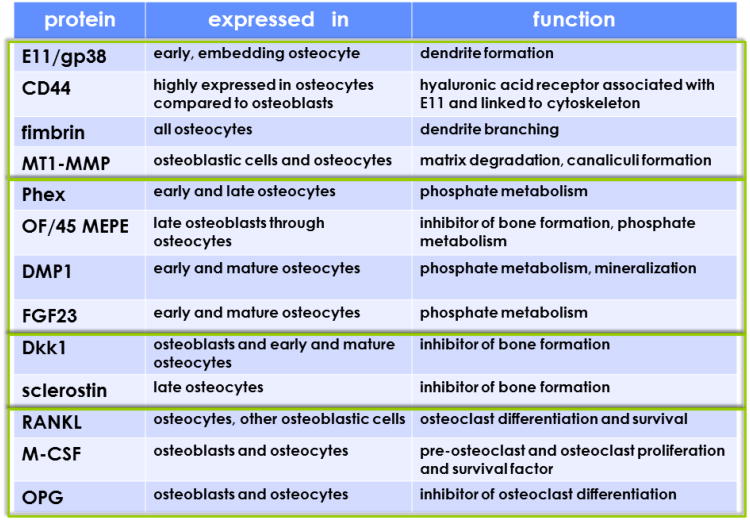
Osteocytes express most of the genes expressed by osteoblasts, including osteoblast-specific transcription factors and proteins, although the levels of expression may differ (Figure 2) (6). Thus, alkaline phosphatase and type I collagen expression is lower whereas osteocalcin expression is higher in osteocytes. Keratocan, an extracellular matrix protein that belongs to the small leucine rich proteoglycan family, has emerged as an osteoblast marker because its expression is greatly reduced in osteocytes compared to osteoblasts (6;7). Another gene that appears to be expressed preferentially in osteoblasts is integrin binding sialoprotein (8;9). Osteocytes, on the other hand, are richer than osteoblasts in genes related to mineralization and phosphate metabolism, including phosphate-regulating neutral endopeptidase (Phex), dentin matrix protein 1 (DMP1), matrix extracellular phosphoglycoprotein (MEPE) and fibroblast growth factor 23 (FGF23) (6;10). Osteocytes also express molecules that affect bone formation, including Dkk1, which also can be found in osteoblasts, and Sost, which is primarily expressed in osteocytes, but not in osteoblasts (10;11). The product of the sost gene sclerostin potently antagonizes several members of the bone morphogenetic protein (BMP) family of proteins. In addition, both sclerostin and Dkk1 bind to LRP5 and LPR6 preventing activation of Wnt signaling. BMPs and Wnts are critical for osteoblast generation and function as they induce commitment of multipotential mesenchymal progenitors towards the osteoblast lineage, stimulate osteoblast differentiation, and regulate osteoblast activity (Figure 1A). Thus, through the expression of Wnt and BMP antagonists, osteocytes have the potential to regulate the formation and activity of osteoblasts.
Figure 2. Gene expression at different stages of osteocyte development and maturation.
The osteocyte phenotype is characterized by the expression of groups of genes closely related to their morphology and function. Expression of some of these genes changes at different stages of osteocyte development and maturation. Boxes group four main categories: 1) Genes related to dendritic morphology and canaliculi formation; 2) genes related to phosphate metabolism and matrix mineralization; 3) genes that regulate bone formation; and 4) genes that regulate bone resorption. Please, note that several of these genes are also expressed in other cell types, besides cells of the osteoblastic lineage. (Reprinted with kind permission of Elsevier, Basic and Applied Bone Biology, Chapter 2 Bone Cells, Bellido, Plotkin and Bruzzaniti).
Osteocyte bodies are individually encased in lacunae and exhibit cytoplasmic dendritic processes that run along narrow canaliculi within the mineralized matrix (Figure 1) (10). Osteocyte morphology is dictated by the expression of genes involved in dendrite formation and branching, such as E11/gp38, CD44, and fimbrin (Figure 2), which are also expressed in neurons and give osteocytes their characteristic morphology in vivo as well as in culture. Numerous cytoplasmic projections (fifty in average in human bone) emerge from each osteocyte body. Projections from neighboring osteocytes touch each other within the canaliculi and establish intercellular communications through gap junctions. Osteocytic projections running inside canaliculi also reach the periosteal and endocortical surfaces of cortical bone as well as the surfaces adjacent to the bone marrow in cancellous bone. Thus, there is potential for direct cell-to-cell interactions between osteocytes and other bone cells (lining cells, osteoblasts and osteoclasts), and the marrow stroma. In turn, cells in the marrow establish contact with endothelial cells of the blood vessels. It appears that osteocytes also establish direct contact with blood vessels within the marrow and with capillaries derived from the Haversian canals in cortical bone (10). Nevertheless, it remains uncertain how proteins expressed by osteocytes reach their cellular targets. The fluid running in the lacunar-canalicular system could transport proteins secreted by osteocytes to their sites of action, provided that the osteocyte products are proteins up to 70kDa and 7 nm in diameter, as estimated by tracer experiments (9;12). However, it is less clear how and whether high molecular size proteins move through the osteocytic lacunar-canalicular system. Furthermore, in the case of membrane proteins which exert their effects through cell-to-cell interactions, direct contact between the osteocytic dendritic processes and the plasma membranes of the cellular targets must occur. The composition of the pericellular matrix surrounding osteocytes adds another layer of control over diffusion of molecules within the osteocyte network, as it will be discussed in section 2 latter in this review.
2. Modulation of bone formation and resorption by osteocyte-derived molecules
Regulation of bone formation by osteocytes: role of sclerostin
Mature osteocytes embedded in the matrix selectively secrete sclerostin, the product of the Sost gene, which antagonizes several members of the BMP family of proteins and also binds to LRP5/LRP6 preventing canonical Wnt signaling (11;13;14). Sclerostin is a potent inhibitor of bone formation (15). Genetic and pharmacologic evidence supports this mechanism. Loss of SOST expression in humans causes the high bone mass disorders Van Buchem's disease (16) and sclerosteosis (17). Mice with targeted deletion of the Sost gene display progressive high bone mass and increased bone strength (18;19); whereas, conversely, transgenic mice overexpressing human SOST exhibit low bone mass (15;20;21). These findings demonstrate conservation throughout the species of the inhibitory effect of sclerostin on bone formation. Pharmacologic inhibition of sclerostin with neutralizing antibodies leads to marked anabolic effects in several preclinical osteopenic animal models and it is currently in clinical trials for the treatment of postmenopausal osteoporosis, validating the high potential of targeting osteocytes for increasing bone mass and strength (22-25).
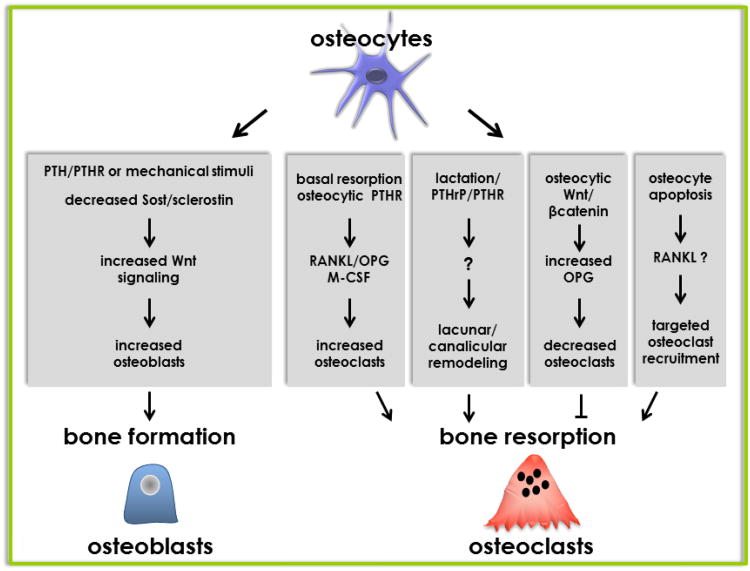
Sclerostin is also regulated by stimuli with anabolic effects on the skeleton. In particular, elevation of parathyroid hormone (PTH), either in an intermittent or a continuous mode, downregulates sclerostin expression in osteocytes in mice and decreases the circulating levels of the protein in humans (11;26-29) (Figure 3). Furthermore, changes in sclerostin expression are responsible for the adaptive responses of the skeleton to mechanical stimulation. Thus, cortical bone areas exposed to high mechanical strain exhibit a reduction in sclerostin-positive osteocytes that is associated with higher bone formation on adjacent periosteal surfaces (30). Conversely, sost/sclerostin expression is high in unloaded bones (30). Taken together, these findings suggest that osteocytes coordinate the osteogenic response to mechanical force by downregulating sclerostin, thereby locally unleashing Wnt signaling. In fact, mice overexpressing a human SOST transgene in osteocytes, which cannot be downregulated by loading, failed to exhibit activation of the Wnt pathway and the anabolic response to mechanical stimulation (31). Therefore, Sost downregulation is an obligatory step for mechanotransduction.
Figure 3. Regulation of osteoblast and osteoclast production and function by osteocytes.
Osteocytes regulate bone formation through sost/sclerostin. Thus, bone formation induced by systemic elevation of PTH or local mechanical loading is associated with decreased expression of sclerostin. Osteocytes regulate bone resorption through pro- and anti-osteoclastogenic cytokines. Resorption under basal conditions, induced by PTH elevation or by PTHrP increased during lactation is regulated by RANKL through the PTH receptor (PTHR) expressed in osteocytes. Activation of Wnt signaling in osteocytes increases OPG expression leading to inhibition of resorption. Osteocyte apoptosis induced by immobilization, fatigue loading, sex steroid deficiency, or genetically induced by activating diphtheria toxin receptor signaling, is sufficient to recruit osteoclasts to specific bone areas and increase resorption; likely through a mechanism that increases RANKL expression in still-living osteocytes surrounding dead osteocytes. (Reprinted with kind permission of Elsevier, Basic and Applied Bone Biology, Chapter 2 Bone Cells, Bellido, Plotkin and Bruzzaniti).
Regulation of bone resorption by osteocytes: RANKL and OPG
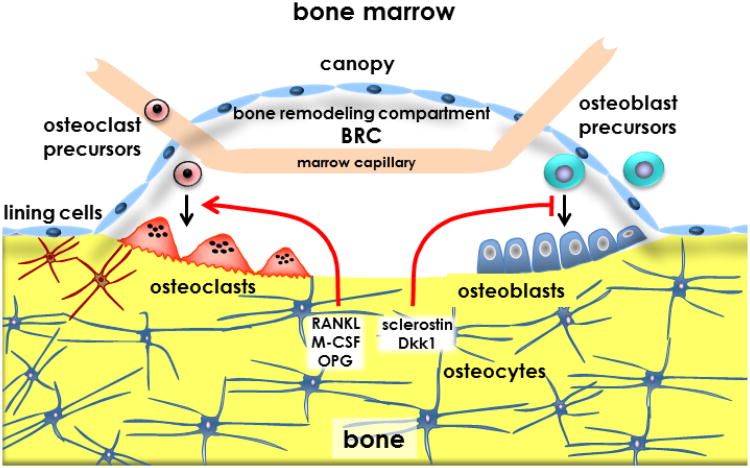
The cues that signal bone resorption are not completely understood. One important event in the regulation of remodeling appears to be the apoptosis of osteocytes, which might prompt lining cells to form the bone remodeling compartment (BRC) (Figure 3 and Figure 4) and could also signal to neighboring cells (osteocytes or other osteoblast lineage cells) to change the expression of pro- and anti-osteoclastogenic genes. Apoptotic osteocytes could regulate the recruitment of osteoclast precursors and their differentiation in two ways. Osteocyte apoptosis may indirectly stimulate osteoclastogenesis by inducing stromal/osteoblastic cells to secrete RANKL. In addition, osteocytes can directly produce and potentially secrete RANKL. Indeed, in vitro, purified osteocytes express higher levels of RANKL than osteoblasts and bone marrow stromal cells (32). The osteopetrotic phenotype observed in mice lacking RANKL in osteocytes supports the notion that osteocytes are a major source of RANKL in remodeling bone (32;33). As it will be discussed below, these conditional knockout mice are resistant to bone loss induced by tail suspension demonstrating that osteocytic RANKL contributes to disuse osteopenia (33). It remains unknown however whether osteocytic membrane-bound or soluble RANKL is involved in osteocyte-driven bone resorption. The decreased bone resorption exhibited by mice lacking RANKL in osteocytes was accompanied by lower expression of RANKL in bone but no decreased soluble RANKL in the circulation (33).
Figure 4. Osteocytes and the bone remodeling compartment (BRC).
Apoptotic osteocytes might initiate bone remodeling by sending signals to lining cells, which retract from the bone surface to form a structure named the bone remodeling compartment (BRC). Osteoclast precursors are transported to the BRC by marrow capillaries, differentiate to mature osteoclasts under the influence of pro- and anti-osteoclastogenic cytokines (RANKL, M-CSF and OPG) derived from osteocytes, and initiate bone remodeling. Osteoblast precursors from the bone marrow or the circulation differentiate into mature, bone synthesizing cells in response to factors released from the bone matrix by resorption. Differentiation and function of osteoblasts is controlled by molecules derived from osteocytes, including sclerostin and Dkk1. (Reprinted with kind permission of Elsevier, Basic and Applied Bone Biology, Chapter 2 Bone Cells, Bellido, Plotkin and Bruzzaniti).
Moreover, no changes in circulating soluble RANKL were found in tail suspended mice [(34) and Bellido et al, unpublished]. Furthermore, the contribution of soluble RANKL to osteoclastogenesis in a novel, 3D-coculture system between osteocytes and osteoclast precursors was found minimal. Instead, direct contact between membrane-bound RANKL expressed in the osteocytic dendrites and RANK expressed in osteoclast precursors appears to be required to initiate osteoclast development (35). Resorption induced by exclusive activation of the PTH receptor in osteocytes in transgenic mice is also associated to increased RANKL (36-38); and the bone loss induced during lactation, which is accompanied by increased PTHrP, is dependent on the expression of osteocytic PTH receptor (36;39) (Figure 3). In the latter case, it was shown that osteocytes can remove bone matrix by remodeling their perilacunar/canalicular matrix. Osteocytes from lactating animals exhibit elevated expression of genes known to be utilized by osteoclasts to remove bone, including tartrate-resistant acid phosphatase (TRAP) and cathepsin K, suggesting that osteocytes remove mineralized matrix through molecular mechanisms similar to those utilized by osteoclasts (39). Considering that the matrix surrounding osteocytes is a likely sieve regulating the diffusion of cellular products, control by osteocytes of their own pericellular matrix might have implications for the molecular transport within the lacunar-canalicular system. Thus, changes in matrix composition or mineral deposition surrounding osteocytes due to altered osteocyte function or decreased osteocyte viability might add an additional level of regulation to the movement of osteocytic products within the lacunae-canalicular system.
Osteocytes also secrete OPG, which competes with RANKL for its receptor RANK on osteoclast precursors. In osteocytes, as in osteoblasts, OPG secretion is regulated by the Wnt/β-catenin pathway and mice lacking β-catenin in osteocytes are osteoporotic due to increased osteoclast numbers and bone resorption (9). In addition, emerging evidence also points to osteocytes as an additional source of secreted M-CSF in bone (40). Together, these novel findings suggest that osteocytes have the potential to control bone resorption through direct and indirect regulation of osteoclast differentiation and function under physiological and pathological conditions.
3. Osteocyte apoptosis: Regulation and consequences
That osteocytes perceive changes in the level of both physical stimuli as well as circulating factors is evidenced by studies on the regulation of their life span (41-43). Osteocytes are long-lived cells. However, like osteoblasts and osteoclasts, they die by apoptosis. The early work of Noble and colleagues showed an association between osteocyte apoptosis and estrogen withdrawal (44). Subsequent studies by this and other research groups demonstrated the role of estrogen and SERMS preserving osteocyte viability (45-49). It is now recognized that decreased osteocyte viability accompanies not only the bone fragility syndrome that characterizes estrogen withdrawal, but also glucocorticoid excess, mechanical disuse, and aging (34;50;51). Conversely, preservation of osteocyte viability might result from physiological levels of mechanical stimulation (34;52) and is associated with the anti-fracture effects of treatment with sex steroids (48;49) or bisphosphonates (53).
Inhibition of osteocyte apoptosis by mechanical stimulation and activation of Wnt signaling
Mechanical stimulation of osteocytic cells or authentic osteocytes protects them from the pro-apoptotic action of death inducers including glucocorticoids (54;55). Mechanotransduction is accomplished by a signalsome assembled at caveolin-rich domains of the plasma membrane and composed of integrins, cytoskeletal proteins, the focal adhesion kinase FAK, and the Src kinase. Downstream activation of the ERK pathway results in preservation of osteocyte viability (54). Activation of Wnt signaling is an early response of osteocytes (and osteoblasts) to mechanical loading (5;56-58) that also promotes osteocyte survival by activating ERKs (59). Moreover, there is interaction between the caveolin-1/ERK and Wnt/β-catenin signaling pathways in osteocytes (60). ERK nuclear translocation and anti-apoptosis induced by mechanical stretching or fluid flow is abolished by the Wnt antagonist DKK1 and the stimulator of β-catenin degradation Axin2. Conversely, glycogen synthase kinase 3β (GSK3β) phosphorylation and β-catenin accumulation induced by mechanical stimulation are abolished either by pharmacologic inhibition of ERKs or by silencing caveolin-1. The simultaneous requirement of β-catenin for ERK activation and of ERK activation for β-catenin accumulation suggests a bidirectional crosstalk between the caveolin-1/ERKs and the Wnt/β-catenin pathways in mechanotransduction leading to osteocyte survival (60). Remarkably, the inhibitor of transcription induced by canonical Wnt signaling dominant negative T cell factor (TCF) does not alter ERK nuclear translocation or survival induced by mechanical stimulation. Thus, β-catenin accumulation is an essential component of the mechanotransduction machinery in osteocytes, albeit β-catenin/TCF-mediated transcription is not required.
Dying osteocytes: primary culprits for the bone loss induced by physical inactivity
Mechanical forces also regulate osteocyte life span in vivo. Apoptotic osteocytes are found in unloaded bones (34) or in bones exposed to high levels of mechanical strain (41;61;62). In both cases, increased osteocyte apoptosis precedes osteoclastic resorption; and apoptotic osteocytes accumulate in areas subsequently removed by osteoclasts (34). These findings suggest that dying osteocytes become the beacons for osteoclast recruitment to the vicinity and the resulting increase in bone resorption (63) (Figure 4). Indeed, targeted ablation of osteocytes by genetic means is sufficient to induce osteoclast recruitment and resorption leading to bone loss (64). It is possible that osteocytes produce molecules that restrain osteoclast recruitment and/or formation; thereby when osteocytes die, there is a spontaneous increase in osteoclast genesis. A potential candidate mediating this phenomenon is OPG, the decoy receptor for RANKL, which is expressed in osteocytes at least at similar levels than in osteoblasts (9). Alternatively, in the process of undergoing apoptosis, osteocytes might produce molecular signals that attract osteoclast precursors and/or factors that stimulate osteoclast development. A potential molecular mediator in this case is the osteoclast chemotactic factor high mobility group box 1 (HMGB1) protein (65), which is released by osteocytes undergoing apoptosis and that upregulates the expression of RANKL, TNF and, IL6, and also decreases OPG expression. Apoptotic bodies released from dying osteocytes are another potential signal leading to changes in gene expression in surrounding cells (66). Apoptotic bodies produced by osteocytic cells, but not by osteoblastic cells, have been shown to stimulate osteoclast differentiation and to initiate localized bone resorption, although RANKL was apparently not involved (67). Furthermore, in overloaded rat bones, dead osteocytes are surrounded by still-living osteocytes in which the expression of VEGF and RANKL is elevated (68), suggesting that signals emanated from apoptotic cells alter the expression of molecules that influence angiogenesis and potentially osteoclast precursor recruitment, as well as osteoclast differentiation.
In contrast to the increasing knowledge about the role of osteocyte apoptosis in remodeling bone, whether osteocyte apoptosis plays any role in bone modeling remains unknown. Future studies specifically designed to answer this question are still warranted.
Mechanical loading is critical for the maintenance of bone mass; and skeletal unloading as with reduced physical activity with old age, immobilization of bed rest, and total or partial motor paralyses, causes bone loss leading to disuse osteoporosis (69). Furthermore, the bone loss that ensues under microgravity conditions represents the most significant hindrance for long-term space flying (70). The rapid decrease in osteocyte viability with unloading had suggested that osteocytes are the first responders to the change in mechanical forces (34). Consistent with this notion, mice depleted from osteocytes are protected from the bone loss induced by tail suspension indicating that in the absence of osteocytes the skeleton is unable to elicit the normal osteoclastogenic response (64). Mice with conditional deletion of RANKL in osteocytes are also protected from unloading-induced elevation in osteoclasts and bone loss (33), suggesting that osteocytes provide the required RANKL for osteoclast formation during skeletal disuse. These findings confirm that osteocytes are the primary culprit of the negative bone balance that ensues with weightlessness.
Regulation of osteocyte survival by sex steroids and bisphosphonates
Loss of sex steroids leads to increased prevalence of osteocyte apoptosis; and conversely, estrogens and androgens inhibit apoptosis of osteocytes as well as osteoblasts (5;48). This anti-apoptotic effect is due to rapid activation of the Src/Shc/ERK and PI3K signaling pathways through non-genotropic actions of the classical receptors for sex steroids (48;71). Bisphosphonates also preserve viability of osteocytes (and osteoblasts) in vitro and in vivo, by a mechanism that in this case involves opening of connexin (Cx) 43 hemichannels and ERK activation (42;53;72;73). The fact that apoptotic osteocytes trigger bone resorption, taken together with the evidence that osteocyte apoptosis is inhibited by estrogens and bisphosphonates, raises the possibility that preservation of osteocyte viability contributes to the anti-remodeling properties of these agents.
Aging and osteocyte apoptosis
One of the functions of the osteocyte network is to detect microdamage and trigger its repair (74;75). During aging, there is accumulation of microdamage and a decline in osteocyte density accompanied by decreased prevalence of osteocyte-occupied lacunae, an index of premature osteocyte death (76). Reduced osteocyte density might be a direct consequence of increased osteoblast apoptosis; whereas increase in the prevalence of apoptotic osteocytes might result from the decline in physical activity with old age leading to reduced skeletal loading, accumulation of reactive oxygen species (ROS) in bone (77) and/or increased levels of endogenous glucocorticoids with age (78). Age-related loss of osteocytes could be then at least partially responsible for the disparity between bone quantity and quality that occurs with aging.
Connexin-43 and osteocyte survival
Osteocytic expression of the gap junction channel/hemichannel protein C×43 is required in a cell autonomous fashion to preserve the viability of osteocytes, as well as to control in osteocytes the levels of proteins that regulate the generation and activity of osteoclast and osteoblasts (79;80). C×43 deficiency causes an intrinsic reduction in OPG expression and loss of viable osteocytes, with the consequent decrease in local levels of the bone formation inhibitor sclerostin. Anatomical mapping of apoptotic osteocytes, osteocytic protein expression, and resorption and formation suggests that C×43 controls osteoclast and osteoblast activity by regulating OPG and sclerostin levels, respectively, in osteocytes located in specific areas of cortical bone. Whereas empty lacunae and living osteocytes lacking OPG are distributed throughout cortical bone of mice lacking osteocytic C×43, apoptotic osteocytes preferentially locate in areas containing osteoclasts, suggesting that osteoclast recruitment requires active signaling from dying osteocytes. Furthermore, cultured osteocytic cells lacking C×43 exhibit increased rate of apoptosis, decreased OPG and increased RANKL expression (79;81). Similar molecular changes are observed in bones of mice lacking C×43 in osteocytes. Moreover, these conditional knockout mice display increased endocortical resorption and exaggerated periosteal bone apposition resulting in altered cortical bone geometry. As a consequence, long bones from mice deficient in C×43 in osteocytes exhibit enlarged bone marrow cavities and increased cross sectional diameter (79;81;82). Accumulation of apoptotic osteocytes and empty lacunae, increased endocortical resorption and periosteal expansion of the long bones resemble bones from aging rodents and humans (51;83). C×43 is a Wnt target gene (84) and Wnt signaling as well as C×43 expression decrease with age in bone [(51) and Plotkin et al, unpublished]. Therefore, reduced C×43 expression might mediate at least some of the changes induced by aging in the skeleton.
4. Therapeutic implications of osteocyte-driven bone remodeling and closing remarks
In closing, research from the last decade greatly increased our understanding of the biology of osteocytes and revealed previously unrecognized mechanisms by which bone acting stimuli regulate the skeleton through effects on these cells. We now know that some of the most profound effects that hormones, such as PTH or glucocorticoids, exert on bone are mediated by actions on osteocytes. The fact that osteocyte apoptosis underlies osteocyte-driven bone resorption raises the possibility that bisphosphonates and estrogens, which prevent osteocyte apoptosis, exert part of their anti-remodeling effects acting on osteocytes. Moreover, the discovery that osteocytes are the major source of RANKL in remodeling bone strongly suggest that the potent antiresorptive effects of the anti-RANKL antibody are exerted by inhibiting osteolytic RANKL. Our current knowledge of the molecular events by which osteocytes influence the function of osteoblasts and osteoclasts has opened new opportunities for developing therapeutic strategies to regulate bone remodeling targeting osteocytes. The neutralizing antibody against sclerostin is the best example of an approach that positively impacts the skeleton by controlling an osteocytic product. It is expected that future investigations will identify new genes expressed in osteocytes, thereby increasing the number of potential targets of pharmacological intervention towards an improved management of bone diseases.
Acknowledgments
This research was supported by the National Institutes of Health (R01-AR053643, KO2-AR02127, R03 TW006919, R01-DK076007, and P01-AG13918).
Footnotes
The author has stated that there is no conflict of interest.
Disclosures: None
References
- 1.Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 3.Holmbeck K, Bianco P, Pidoux I, Inoue S, Billinghurst RC, Wu W, Chrysovergis K, et al. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 4.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce BF, Xing L, Jilka RL, Bellido T, Weinstein RS, Parfitt AM, Manolagas SC. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Academic Press; San Diego, San Francisco, New York, London, Sydney, Tokyo: 2002. pp. 151–168. [Google Scholar]
- 6.Paic F, Igwe JC, Nori R, Kronenberg MS, Franceschetti T, Harrington P, Kuo L, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igwe JC, Gao Q, Kizivat T, Kao WW, Kalajzic I. Keratocan is Expressed by Osteoblasts and Can Modulate Osteogenic Differentiation. Connect Tissue Res. 2011;52:401–407. doi: 10.3109/03008207.2010.546536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson MD, Aubin JE, Xiao G, Thomas PE, Franceschi RT. Cloning of a 2.5 kb murine bone sialoprotein promoter fragment and functional analysis of putative Osf2 binding sites. J Bone Miner Res. 1999;14:396–405. doi: 10.1359/jbmr.1999.14.3.396. [DOI] [PubMed] [Google Scholar]
- 9.Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonewald LF. The Amazing Osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, et al. Chronic elevation of PTH in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Ciani C, Doty SB, Fritton SP. Delineating bone's interstitial fluid pathway in vivo. Bone. 2004;34:499–509. doi: 10.1016/j.bone.2003.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole KE, Van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 14.Van Bezooijen RL, Roelen BA, Visser A, Wee-Pals L, de Wilt E, Karperien M, Hamersma H, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 17.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 19.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 20.Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res. 2011;26:1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warmington K, Morony S, Sarosi I, Gong G, Stepphens P, Winkler DG, Sutherland MK, et al. Sclerostin antagonism in adult rodents, via monoclonal antibody mediated blockade, increases bone mineral density and implicates sclerostin as a key regulator of bone mass during adulthood. J Bone Min Res. 2004;19:S56. [Google Scholar]
- 23.Warmington K, Ominsky M, Bolon B, Cattley R, Stephens P, Lawson A, Lightwood D, et al. Sclerostin monoclonal antibody treatment of osteoporotic rats completely reverses one year of ovariectomy-induced systemic bone loss. J Bone Min Res. 2005;20:S22. [Google Scholar]
- 24.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 25.Jilka RL. Inhibiting the inhibitor: a new route to bone anabolism. J Bone Miner Res. 2009;24:575–577. doi: 10.1359/jbmr.090228. [DOI] [PubMed] [Google Scholar]
- 26.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.van Lierop AH, Witteveen J, Hamdy N, Papapoulos S. Patients with primary hyperparathyroidism have lower circulating sclerostin levels than euparathyroid controls. Eur J Endocrinol. 2010;163:833–837. doi: 10.1530/EJE-10-0699. [DOI] [PubMed] [Google Scholar]
- 28.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95:1991–1997. doi: 10.1210/jc.2009-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MJ, Alam I, Mantila SM, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 31.Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012;50:209–217. doi: 10.1016/j.bone.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 33.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Min Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 35.Honma M, Ikebuchi Y, Kariya Y, Hayashi M, Hayashi N, Aoki S, Suzuki H. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J Bone Miner Res. 2013 doi: 10.1002/jbmr.1941. [DOI] [PubMed] [Google Scholar]
- 36.Bellido T, Saini V, Divieti Pajevic P. 2012. Effects of PTH on osteocyte function. Bone. 2012 Sep 24; doi: 10.1016/j.bone.2012.09.016. pii: S8756-3282(12)01245-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee Y, Allen MR, Condon K, Plotkin LI, Lezcano V, Vyas K, O'Brien CA, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling: divergent role of Sost and the Wnt pathway. J Bone Min Res. 2009;24:S78. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien CA, Plotkin LI, Galli C, Goellner J, Gortazar AR, Allen MR, Robling AG, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris SE, MacDougall M, Horn D, Woodruff K, Zimmer SN, Rebel VI, Fajardo R, et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone. 2012;50:42–53. doi: 10.1016/j.bone.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble BS, Peet N, Stevens HY, Brabbs A, Mosley JR, Reilly GC, Reeve J, et al. Mechanical loading: biphasic osteocyte survival and the targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–C943. doi: 10.1152/ajpcell.00234.2002. [DOI] [PubMed] [Google Scholar]
- 42.Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011;49:50–55. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, Manolagas SC. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Academic Press; San Diego, San Francisco, New York, London, Sydney, Tokyo: 2008. pp. 237–261. [Google Scholar]
- 44.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 45.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Min Res. 1998;13:1243–1250. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 46.Huber C, Collishaw S, Mosley JR, Reeve J, Noble BS. Selective estrogen receptor modulator inhibits osteocyte apoptosis during abrupt estrogen withdrawal: implications for bone quality maintenance. Calcif Tissue Int. 2007;81:139–144. doi: 10.1007/s00223-007-9049-6. [DOI] [PubMed] [Google Scholar]
- 47.Mann V, Huber C, Kogianni G, Collins F, Noble B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone. 2007;40:674–684. doi: 10.1016/j.bone.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 49.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin LI, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellido T. Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J Cell Biochem. 2010;111:1–6. doi: 10.1002/jcb.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–C643. doi: 10.1152/ajpcell.00278.2004. [DOI] [PubMed] [Google Scholar]
- 55.Bakker A, Klein-Nulend J, Burger E. Shear stress inhibits while disuse promotes osteocyte apoptosis. Biochem Biophys Res Commun. 2004;320:1163–1168. doi: 10.1016/j.bbrc.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 56.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armstrong VJ, Muzylak M, Sunters A, Zaman G, Saxon LK, Price JS, Lanyon LE. Wnt/β-catenin signaling Is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor α. J Biol Chem. 2007;282:20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- 58.Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS. Mechano-transduction in osteoblastic cells involves strain-regulated, Estrogen Receptor {alpha&rcub -mediated, control of IGF-IR sensitivity to ambient IGF, leading to PI3-K/ AKT dependent, Wnt/LRP5 receptor-independent activation of { beta} -catenin signaling. J Biol Chem. 2010;285:8743–8758. doi: 10.1074/jbc.M109.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- 60.Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/ERKs and ß-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem. 2013 doi: 10.1074/jbc.M112.437921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verborgt O, Gibson G, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Min Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 62.Verborgt O, Tatton NA, Majeska RJ, Schaffler MB. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–914. doi: 10.1359/jbmr.2002.17.5.907. [DOI] [PubMed] [Google Scholar]
- 63.Bellido T. Osteocyte apoptosis induces bone resorption and impairs the skeletal response to weightlessness. BoneKEy-osteovision. 2007;4:252–256. [Google Scholar]
- 64.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Shah R, Robling AG, Templeton E, Yang H, Tracey KJ, Bidwell JP. HMGB1 is a bone-active cytokine. J Cell Physiol. 2008;214:730–739. doi: 10.1002/jcp.21268. [DOI] [PubMed] [Google Scholar]
- 66.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2012 doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localised bone destruction. J Bone Miner Res. 2008;23:915–927. doi: 10.1359/jbmr.080207. [DOI] [PubMed] [Google Scholar]
- 68.Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50:1115–1122. doi: 10.1016/j.bone.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcus R. Mechanisms of exercise effects on bone. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Academic Press; San Diego: 2002. pp. 1477–1488. [Google Scholar]
- 70.Bikle DD, Halloran BP, Morey-Holton E. Spaceflight and the skeleton: lessons for the earthbound. Gravit Space Biol Bull. 1997;10:119–135. [PubMed] [Google Scholar]
- 71.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–1664. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 73.Plotkin LI, Bellido T. Bisphosphonate-induced, hemichannel-mediated, anti-apoptosis through the Src/ERK pathway: a gap junction-independent action of connexin43. Cell Adhes Commun. 2001;8:377–382. doi: 10.3109/15419060109080757. [DOI] [PubMed] [Google Scholar]
- 74.Parfitt AM. Life history of osteocytes: relationship to bone age, bone remodeling, and bone fragility. J Musculoskelet Neuronal Interact. 2002;2:499–500. [PubMed] [Google Scholar]
- 75.Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 76.Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manolagas SC. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in 21-month-old mice. Aging Cell. 2009;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun L, Rhee Y, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Min Res. 2012;27:374–389. doi: 10.1002/jbmr.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–166. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y, Paul EM, Sathyendra V, Davidson A, Bronson S, Srinivasan S, Gross TS, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE. 2011;6:e23516. doi: 10.1371/journal.pone.0023516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grimston SK, Brodt MD, Silva MJ, Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the Connexin43 gene ( Gja1. J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu S, Rao DS, Palnitkar S, Parfitt AM. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone. 2002;31:313–318. doi: 10.1016/s8756-3282(02)00819-0. [DOI] [PubMed] [Google Scholar]
- 84.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, et al. WNT/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]