Abstract
Blocking, desensitizing, or knocking out transient receptor potential vanilloid type 1 (TRPV1) receptors decreases immobility in the forced swim test, a measure of depressive behavior. We questioned whether enhancing TRPV1 activity promotes immobility in a fashion that is prevented by antidepressants. To test this we activated heat-sensitive TRPV1 receptors in mice by water that is warmer than body temperature (41°C) or a low dose of resiniferatoxin (RTX). Water at 41°C elicited less immobility than cooler water (26°C), indicating that thermoregulatory sites do not contribute to immobility. Although a desensitizing regimen of RTX (3–5 injections of 0.1 mg/kg s.c.) decreased immobility during swims at 26°C, it did not during swims at 41°C. In contrast, low dose of RTX (0.02 mg/kg s.c.) enhanced immobility, but only during swims at 41°C. Thus, activation of TRPV1 receptors, endogenously or exogenously, enhances immobility and these sites are activated by cold rather than warmth. Two distinct types of antidepressants, amitriptyline (10 mg/kg i.p.) and ketamine (50 mg/kg i.p.), each inhibited the increase in immobility induced by the low dose of RTX, verifying its mediation by TRPV1 sites. When desensitization was limited to central populations using intrathecal injections of RTX (0.25 µg/kg i.t.), immobility was attenuated at both temperatures and the increase in immobility produced by the low dose of RTX was inhibited. This demonstrates a role for central TRPV1 receptors in depressive behavior, activated by conditions (cold stress) distinct from those that activate TRPV1 receptors along thermosensory afferents (heat).
Keywords: TRPV1, depression, desensitization, RTX, forced swim test
1. Introduction
Transient receptor potential vanilloid type 1 (TRPV1) receptors are non-selective calcium-permeable cation channels. They are ubiquitous in the brain, spinal cord and periphery [1–4] where they participate in thermoregulation and pain [5–9], specifically models of thermal [10–15], inflammatory [16–19], neuropathic [20–23] and cancer nociception [24–28]. Several lines of evidence suggest that TRPV1 receptors also contribute to depressive behavior as removing TRPV1 receptor activity produces an antidepressant like effect in rodents [29–32]. For instance, TRPV1 knock out (KO) mice exhibit less immobility than their wild type littermates when tested in the forced swim test [29]. TRPV1 receptor knock out mice also have decreased latency times in the novelty-suppressed feeding paradigm compared to wild type mice demonstrating a decreased depressive response [29]. Furthermore, intracerebrovascular injection of capsazepine, a TRPV1 antagonist, decreases immobility time in mice during the forced swim test in a dose-dependent manner [32]. Capsazepine also enhances the antidepressant activity of a sub-threshold dose of fluoxetine in the forced swim test in mice [32]. Activating the TRPV1 receptor at doses that cause it to desensitize also produces an antidepressant effect. For example, olvanil, a TRPV1 receptor agonist, reduces immobility times of rats in the forced swim test in a dose-dependent manner when injected i.p. [30]. Similarly, an intracerebrovascular injection of a desensitizing dose of capsaicin decreases immobility of mice in the forced swim test [32]. Finally, capsaicin and olvanil each prevent the increase in immobility produced by nicotine in mice when tested in either the forced swim or tail suspension tests [30,33].
TRPV1 receptors are involved in thermoregulation where their activation leads to hypothermia and their desensitization leads to hyperthermia [8,9,34,35]. Using this, we monitored the degree of receptor activity verses desensitization produced by various doses of RTX by their effect on body temperature. Using these doses, we then queried whether activation or desensitization of TRPV1 sites alters depressive behavior in the forced swim test and whether increases in immobility are sensitive to antidepressants.
It is of interest that body temperature has been identified as a major factor influencing behavioral responses in the forced swim test. Rodents tend to float more in cold than in warmer water [36–39] and the cold swim causes a precipitous drop in body temperature [36,38,39]. Since TRPV1 receptors are involved in thermoregulation in the defense against hyperthermia [6,8,9,35], we also explored whether the activation of TRPV1 receptors along thermosensitive primary afferent fibers by a temperature that is warmer than the normal body temperature of mice (41°C) contributes to the difference in floating times produced during forced swims at two different water temperatures (26°C and 41°C).
2. Methods
2.1. Animals
Adult male Swiss Webster mice weighing 20–25 g (Harlan Sprague Dawley, INC; Indianapolis, IN) were housed four per cage and allowed to acclimate for at least one week prior to use. Mice were allowed free access to food and water, and housed in a room with a constant temperature of 23°C on a 12-h light–dark cycle. All procedures were performed according to the guidelines of the International Association for the Study of Pain (IASP), the University of Minnesota Animal Care and Use Committee, and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication NIH 78-23, revised 1995). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
2.2. Drugs and chemicals
Resiniferatoxin (RTX), a TRPV1 ligand and desensitizer, was obtained from LC Laboratories, Inc. (Woburn, MA), dissolved in 5% ethanol, 5% Tween and 90% saline solution (pH 5.5–6.0), and delivered intrathecally (i.t.) at a dose of 0.250 µg in a 5 µl volume or diluted in sesame oil (pH 4.5–5.0) and given subcutaneously (s.c.) at doses of 0.1 mg/kg or 0.02 mg/kg. Intrathecal injections were delivered at the L5–L6 intravertebral space using a 30-gauge, 0.5 inch disposable needle on a 50 µL Luer tip Hamilton syringe in lightly restrained, unanaesthetized mice [40].
Desensitization of TRPV1 receptors to the higher s.c. dose of RTX (0.1 mg/kg) was validated by its inability to decrease body temperature after 4 or 5 injections when delivered at a dose that initially produced a profound decrease in body temperature. The intrathecal dose of RTX achieves an even greater degree of desensitization as it resulted in a persistent hyperthermia; a common side effect associated with the potential use of many TRPV1 antagonists as analgesics [8,9,35].
2.3. Forced swim test
The forced swim test was used, as this is one of the most commonly used assays of depressant-like activity in rodents, originally described by Porsolt [41– 43]. Each mouse was put individually into a large, 2.5-liter beaker (diameter: 15 cm, height: 20 cm) containing water maintained at 26°C or 41°C. Mice were forced to swim for 15 min on one or two consecutive days. The water level was deep enough (18 cm) so the tail of the mouse never touched the bottom. The first 5 min of the 15-min swim was recorded using a Flip (Cisco) or Handycam (Sony) video camera. After the swim, mice were removed from the water, their body temperature measured, and then mice were toweled dry and returned to their home cage. The amount of time spent immobile, including times during which small paw movements helped the mouse stay afloat rather than propel it forward, was evaluated by observers unaware of the treatment of each mouse.
2.4. Body temperature measurement
Body temperature measurement took place in a room with an ambient temperature of 25°C. The animal was removed from its cage, cupped on the counter under an open hand, and its colonic temperature measured using a rectal thermometer [44] (ETI Microtherma 2K Thermometer connected to a RET-3 rectal probe).
2.5. Behavioral data analysis
Mean values (± S.E.M.) are presented throughout the figures. Statistical analysis of the results was performed using a Student's paired t test between two groups or one-way ANOVA followed by post hoc Tukey’s test for comparison between multiple groups tested at the same time, as indicated. A difference was considered significant if the probability that it occurred because of chance alone was less than 5% (P<0.05).
3. Results
3.1. Validation of the effect of RTX by changes in body temperature
To determine the contribution of TRPV1 sites to depressive behavior, we injected RTX at a low dose that activated receptors but did not lead to desensitization, as indicated by its effect on body temperature. We then compared that to a higher dose that was delivered several times to produce a widespread desensitization of TRPV1 sites. A low dose of RTX (0.02 mg/kg s.c.) was deemed to produce a persistent activation of TRPV1 sites as it dramatically decreased body temperatures compared to their own pre-injection control values. The effect was maximal 1 h after injection but still present 24 h later (Fig. 1A). The high dose of RTX (0.1 mg/kg s.c.) was selected as it also produced hypothermia at 1 h after its injection, but hypothermia was no longer present 24 h later (Fig. 1A). A high degree of desensitization was confirmed by the smaller responses to subsequent injections delivered at 3–4 day intervals indicating that mice were completely tolerant to the hypothermic effect of the high dose of RTX after the third injection (Fig. 1B). When injected centrally via an intrathecal (i.t.) injection, RTX (0.25 µg) produced an initial decrease in body temperature 1 h after injection, after which the body temperature of mice was elevated compared to vehicle-injected controls when measured the next day (Fig. 1A). The persistent hyperthermia is consistent with complete desensitization of these pathways [8,9,34,35]. Subsequent injections of RTX (0.1 mg/kg s.c.) given 24 h after the central injection produced no decrease in body temperature, indicating that that the single intrathecal injection of RTX desensitized mice to the low dose of parenterally administered RTX (Fig. 1C).
Fig. 1.
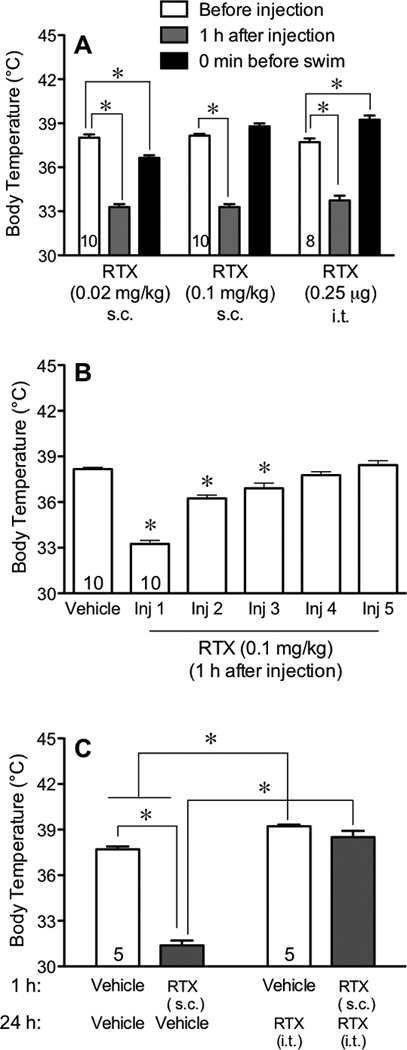
Effect of resiniferatoxin (RTX) on body temperature. Mice were injected subcutaneously (s.c.) with a single injection of a low dose RTX (0.02 mg/kg), a high dose of RTX (0.1 mg/kg) or an intrathecal (i.t.) injection of RTX (0.25 µ g). In panel A, body temperatures were measured before and 1 h after each injection as well as immediately prior to the forced swim (24 h after the last injection of RTX). In panel B, body temperatures were measured 1 h after receiving each of 5 injections of RTX (0.1 mg/kg s.c.) given at a 3–4 day intervals, to reveal the rate of desensitization to the hypothermic effect of RTX. In panel C, body temperatures were measured 24 h after an i.t. injection of RTX and 1 h after injection of 0.1 mg/kg of RTX s.c. The effect of RTX on body temperature was compared using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis, where an asterisk (*) indicates a difference from pre-injection temperatures or from vehicle. Throughout the figures, differences were statistically significant if the probability that it occurred because of chance alone was less than 5% (P<0.05).
3.2. Effects of RTX on immobility in the forced swim test
Because TRPV1 sites are activated by exposure to heat, we tested mice in water that is warmer (41°C) than the normal resting body temperature of mice (37–38°C) as well as cooler water (26°C) to determine whether TRPV1 populations that are activated by cutaneous heat sensors contribute to immobility. We found that saline-injected control mice were immobile longer in water maintained at 26°C (Fig. 2A, 168.7 +/− 15.07 s, n=13), than identical mice in 41°C water (Fig. 2C, 82.39 +/− 7.37 s, n=34, P=6.75e−5 analyzed using a Student’s unpaired t-test).
Fig. 2.
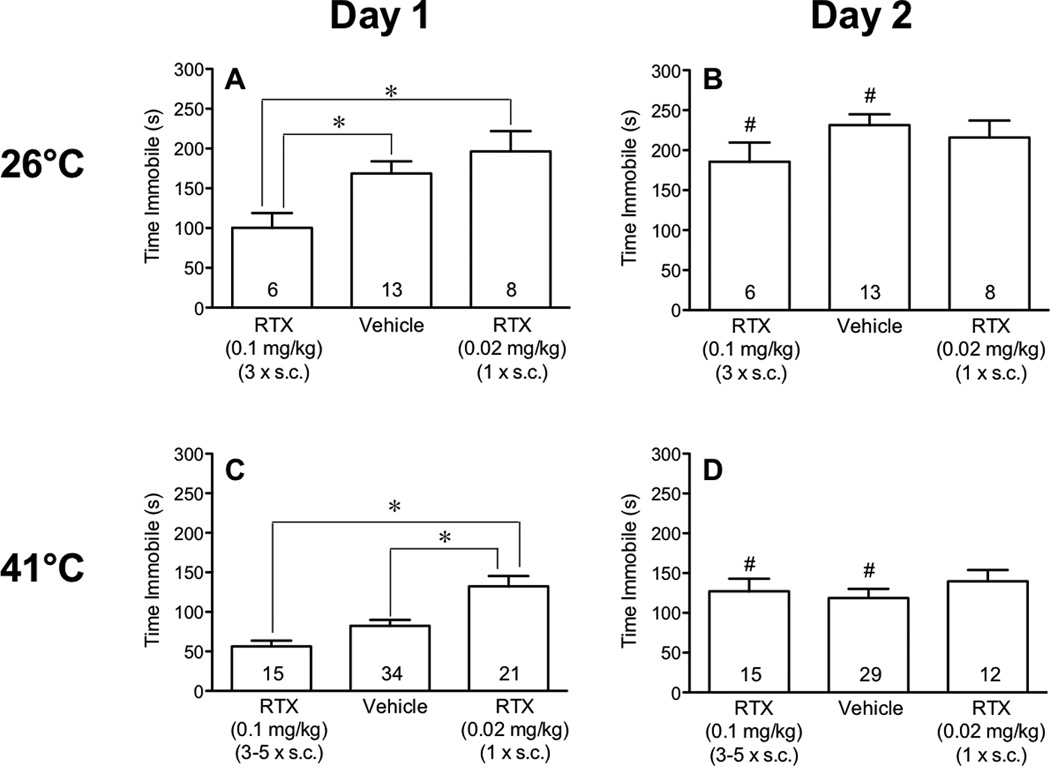
Effect of RTX delivered s.c. on immobility in the forced swim test. Mice were injected with vehicle or either a low dose of RTX (0.02 mg/kg) or 3–5 injections of a high dose of RTX (0.1 mg/kg) and then subjected to 2 daily 15-min swims in baths maintained at either 26°C (panels A and B) or 41°C (panels C and D). Immobility in the two vehicle-injected control groups did not differ from each other, so they were pooled. The first forced swim occurred 24 h after the final injection of RTX. Comparisons between the effects of the two doses of RTX and vehicle (on the same day and at the same swim temperature) were performed using one-way ANOVA followed by Tukey’s post-hoc analysis and marked with an asterisk (*) when significant (panels A and C). Comparisons between immobility during swims on day 1 and day 2 (the same treatment and water temperature) were performed using Student’s t-test and marked with a pound sign (#) when significant (panels B and D).
Compared to saline-injected controls, pretreatment with the desensitizing regimen of RTX (3–5 injections of 0.1 mg/kg s.c. at 3–5 day intervals) decreased the time that mice spent floating on day 1 of the 26°C swim (Fig. 2A) but had no effect on the time spent immobile in the 41°C bath (Fig. 2C). In contrast, activation of the TRPV1 receptors by pretreatment (24 h) with the low dose of RTX (0.02 mg/kg) increased immobility of mice in 41°C water (Fig. 2C) but had no effect on immobility in 26°C water when compared to vehicle-injected controls (Fig. 2A). We then determined whether the potentiative effect of the low dose of RTX was greater at 3 h, when the effect on body temperature peaked and the degree of desensitization minimal, than at 24 h. The same magnitude of a potentiative effect was observed when mice were tested in 41°C water at 3 h as at 24 h after the injection of a low dose of RTX (data not shown). These data document that it is the activation of TRPV1 sites rather than their desensitization that enhances immobility. At both water temperatures, mice were more immobile after the low dose of RTX than after the higher desensitizing dose of RTX (Fig. 2A,C).
When these groups of mice were tested again 24 h later at their respective temperatures, but with no additional injections of RTX, floating times increased in vehicle-injected control mice, consistent with the potentiative effect of a conditioning swim on immobility [45]. Although desensitization of TRPV1 sites is reported to persist for long periods of time in most studies, mice injected several times with the high dose RTX prior to day 1 also floated more on day 2 than on day 1 (Fig. 2B,D), in spite of the antidepressant effect of RTX on day 1. The immobility of mice injected with a low dose RTX (0.02 mg/kg) did not increase further on day 2 than on day 1 (Fig. 2B,D), a time when responses were already near maximal. Immobility times on day 2 did not differ between treatment groups when compared at either temperature.
3.3. Desensitization of central TRPV1 receptors
To determine whether the potentiative effect of the low dose of RTX (0.02 mg/kg s.c.) on depressive behavior was brought about by an action in the central nervous system (CNS), we compared the effect of the low dose in mice injected with vehicle to those whose TRPV1 sites in the spinal cord and lower brainstem were desensitized by pretreatment with RTX (0.25 µg i.t.). Twenty-four h later, mice were subjected to forced swims at either 26 or 41°C.
Compared to vehicle-injected mice, the central injection of RTX (0.25 µg i.t.) decreased immobility of mice when measured using baths at either water temperature (Fig. 3 A,C). Pretreatment with RTX injected i.t. also attenuated the increased immobility caused by pretreatment (24 h) with the low dose RTX (0.02 mg/kg s.c.) in 41°C water (Fig. 3C), confirming that centrally located TRPV1 receptors are responsible for the enhanced depressive behavior produced by the low dose of RTX.
Fig. 3.
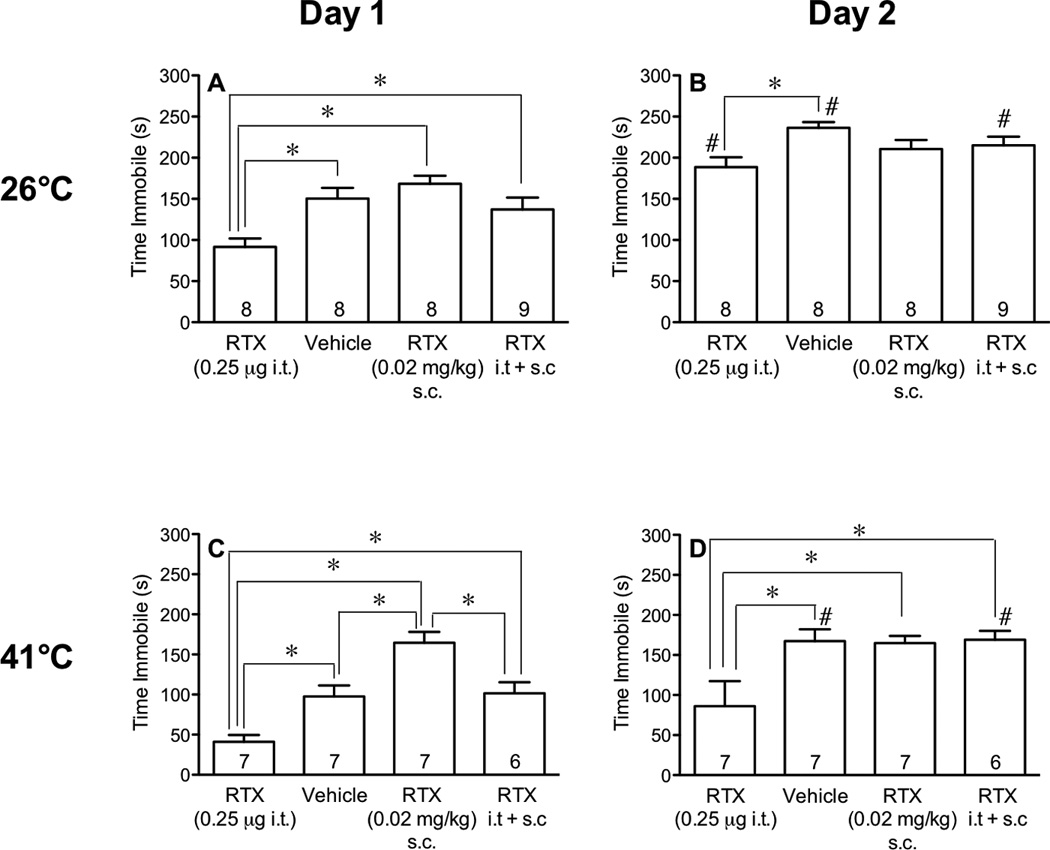
Effect of desensitization of central TRPV1 receptors on forced swims in 26°C and 41°C water. Mice were pretreated i.t. with vehicle or RTX (0.25 µg). The next day, mice were injected with vehicle or a low dose of RTX (0.02 mg/kg s.c.) and tested 24 and 48 h later in a 15-min forced swims in water maintained at either 26°C or 41°C. The time spent immobile during the first 5 min of each swim was measured. Comparisons between different treatments were performed using one-way ANOVA followed by Tukey’s post-hoc analysis and marked with an asterisk (*) when different from each other. Comparisons between same treatments on different days were performed using a paired Students t-test and when significantly different indicated with a pound sign (#) on day 2 (panels B and D).
3.4. Effect of antidepressants on RTX-induced increase in immobility
We questioned whether the enhanced immobility of mice after injection with a low dose of RTX (0.02 mg/kg s.c.) was sensitive to traditional antidepressants. We examined the effect of pretreatment with amitriptyline (2 × 10 mg/kg i.p.), a tricyclic antidepressant, on the increased immobility produced by the low dose of RTX in the 41°C swim. Mice were injected twice with amitriptyline or vehicle 1 h before the injection of RTX (0.02 mg/kg) and 1 h before the swim. We found that amitriptyline inhibited immobility of mice and prevented the increased immobility produced by the low dose of RTX (Fig. 4A).
Fig. 4.
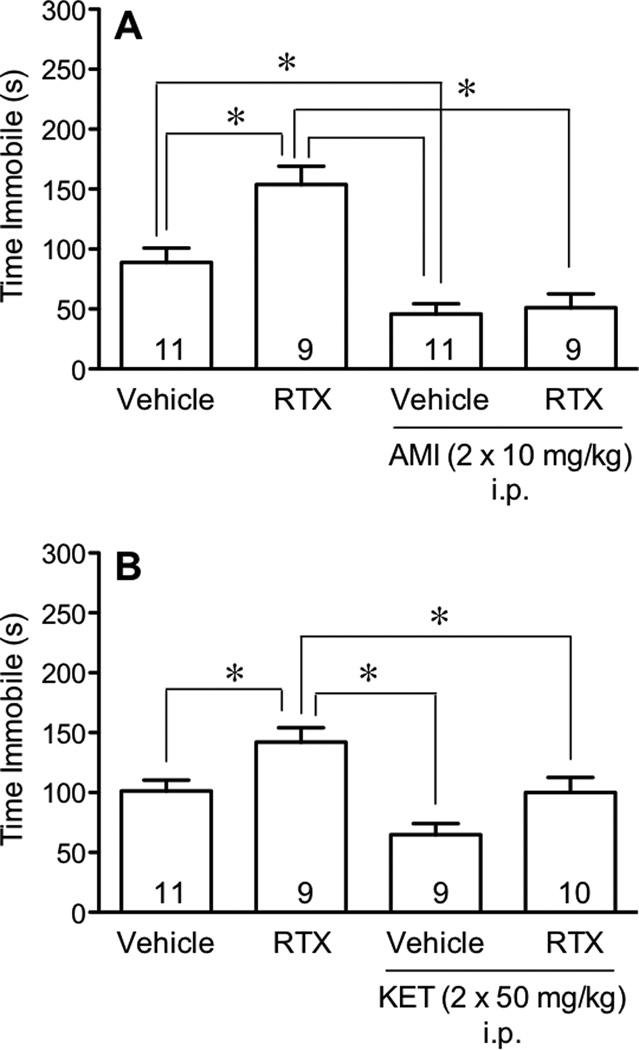
Effect of antidepressants on RTX-induced increases in immobility. Mice were injected with RTX (0.02 mg/kg s.c.) and tested 24 h later in the forced swim test at 41°C. In panel A, in addition to RTX, mice were injected with either saline or amitriptyline (AMI, 10 mg/kg i.p.) 1 h before the RTX and 1 h before the swim. In panel B, mice were injected with saline or ketamine (KET, 50 mg/kg i.p.) 45 min before the RTX and 45 min before the swim. Comparisons between different treatments were performed using one-way ANOVA followed by Tukey’s post-hoc analysis and marked with an asterisk (*) when they differed.
To determine whether the enhanced immobility of mice after injection with a low dose of RTX was sensitive to the action of newer antidepressants [46], we examined the effect of pretreatment with ketamine (2 × 50 mg/kg i.p.), an N-methyl-D-aspartic acid (NMDA) antagonist, on the increased immobility time produced by RTX in the 41°C swim. Ketamine was given twice, 45 min before the RTX injection and 45 min before the swim. When injected alone, ketamine had no effect on immobility when compared to vehicle-injected mice (Fig. 4B), however, ketamine attenuated the increased immobility produced by the low dose of RTX (Fig. 4B).
4. Discussion
This study investigates the role of TRPV1 receptors on immobility in the forced swim test, one of many screens for antidepressant activity [47]. We confirmed the antidepressant-like effect produced by inhibition of TRPV1 receptors [29–32] by desensitizing these sites using RTX injected s.c. at a high dose. RTX was even more effective when given i.t., indicating that depressive behavior is enhanced by TRPV1 receptor activity located centrally. In addition, by showing that a low, non-desensitizing dose of RTX enhances immobility, we provide a new additional line of evidence to support the role of TRPV1 in depressive behavior. Because the increased immobility produced by RTX is inhibited by only two injections of amitriptyline (acute treatment), whereas chronic treatment with traditional antidepressants is required to treat depression or models of depression, the potentiative effect of RTX does not reflect depression per se but merely a behavioral response that is sensitive to the acute effects of antidepressant compounds. We further document that the effects produced by RTX differ during forced swims in water baths that are colder or warmer than body temperature, indicating the involvement of a population of TRPV1 receptors that are unique from those along thermoregulatory pathways.
Changes in body temperature were used to validate that the low s.c. dose of RTX activates while the higher dose and the intrathecal dose desensitize TRPV1 receptors. A decrease in body temperature 1 h after injection of RTX reflects its agonistic effect [9,35,48,49] that persisted for 24 h after injection of the low s.c. dose of RTX [48,49]. In contrast, the diminished response to repeated injections of the higher s.c. dose of RTX indicates desensitization of thermosensitive receptors [49,50]. The central injection of RTX appears to have produced an even greater degree of desensitization of the critical receptor field than that produced by the high s.c. dose of RTX as evidenced by the hyperthermia that developed after the i.t. but not the s.c. injection. This suggests that the protection against hyperthermia that TRPV1 sites tonically subserve [8,9,34,35] was not fully desensitized when RTX was injected peripherally, but it was when injected intrathecally. The antidepressant effect of intrathecally injected RTX inhibited immobility at both temperatures on day 2 when compared to vehicle-injected controls. In contrast, desensitization using s.c. injected RTX (0.1 mg/kg) was not antidepressant on day 2. Together these data reflect the greater degree of desensitization produced by the i.t. than by the s.c. injections used. They also indicate a greater contribution of TRPV1 receptors to immobility on day 1 than on day 2 as desensitization would be expected to persist for at least 48 h after either route of injection.
Desensitizing [30,33], pharmacologically antagonizing [32], or making mice deficient in the expression of the TRPV1 receptors [29] is antidepressant in the more traditional swims in cooler water (22–25°C). Consistent with this, we confirmed that desensitization of TRPV1 receptors using multiple injections of a high s.c. dose of RTX decreased immobility in the forced swim test at 26°C. Together these data provide additional evidence to support the conclusion that depressive behavior is modulated by the activation of TRPV1 receptors by endogenously released TRPV1 receptor ligands.
Endogenously occurring TRPV1 receptor ligands, called endovanilloids, are lipids in nature and derived from the metabolism of arachidonic acid and unsaturated N-acyldopamines (NADA) [51]. Endovanilloids are produced when needed through a Ca2+-dependent mechanism and hydrolyzed by fatty acid amide hydrolase (FAAH) that co-localizes with TRPV1 receptors [52–54]. Endovanilloids activate TRPV1 receptors in various regions of the brain that are responsible for regulating multiple brain functions, including depression. For instance, TRPV1 receptors modulate input to the locus coeruleus [55], a site that is implicated in major depression [56], stress [57,58], attention [59], and memory [57,58]. Immobility in rats in a forced swim is decreased by electrical stimulation of the locus coeruleus but increased by 6-hydroxydopamine-induced lesions of that area [60]. TRPV1 receptor activation in the locus coeruleus leads to neuronal activation and enhancement of the release of glutamate and epinephrine/norepinephrine in that region [61].
It is possible that TRPV1 receptor populations involved in thermoregulation might be involved in depressive behavior, as it is known that rodents tend to float more in 26°C water than in more tepid water [36–39] but to our knowledge, baths that are above the normal body temperature of mice (41°C), and thus likely to activate TRPV1 receptors, have not been tested. To determine whether activation of TRPV1 sites along thermoregulatory primary afferent fibers influences immobility, we subjected mice to forced swims in 41°C water, that would activate this population of TRPV1 receptors, compared to 26°C water that should not. Mice subjected to 41°C baths did not float as much as mice subjected to a swim at 26°C. This greater immobility in 26°C water may result from either the activation of TRPV1 sites by warmer water inhibiting immobility or by the recruitment of additional TRPV1 receptor activity by cooler water (26°C) that potentiates immobility. Our data support the latter conclusion as desensitization of TRPV1 receptors (using s.c. or i.t. injections of RTX) inhibited immobility in the cold swim but did not potentiate immobility in the 41°C swim. The more complete desensitization brought about by RTX injected intrathecally even inhibited immobility in both the 41°C and 26°C swims while the partial desensitization by s.c. RTX had no effect on 41°C swims. Together these data indicate that endogenous activation of TRPV1 receptors plays a greater role in immobility during the cooler than during the warmer swims. Furthermore, the antidepressant effects of desensitizing doses of RTX indicate that endogenous TRPV1 receptor activity evoked by cold stress promotes depressive behavior. It is somewhat paradoxical that the greater immobility in 26°C water results from activation of pathways mediated by TRPV1 activity as these receptors have been traditionally associated with heat perception. However, the inability of TRPV1 receptor activity induced by a bath temperature (41°C) above the normal body temperature (around 38°C) to potentiate immobility to the same degree as that during a colder stress (26°C) clearly differentiates TRPV1 receptor populations during cold stress from those involved in thermoregulation.
In contrast to the desensitizing effect of RTX at high doses, activation of TRPV1 sites using a low dose of RTX increased depressive behavior in the 41°C water, mimicking the effect of the colder forced swim stress. Prior desensitization of these sites using either s.c. or i.t. injections of RTX prevented this potentiative effect of RTX on immobility during the 41°C swim, confirming that enhancement of depressive behavior is due to activation TRPV1 sites. These data indicate that immobility during a 41°C swim remains subject to the same positive modulatory effect of TRPV1 receptor activity as that during a 26°C swim stress. The relevant receptor population involved is merely not activated by heat. In contrast to the potentiative effect of the low dose of RTX in a 41°C swim, when mice were tested at 26°C, there was no additional increase in depressive behavior at this temperature. This could be due to the release of sufficient endogenous ligands during cold stress activating TRPV1 receptors maximally during the forced swim, resulting in a ceiling effect.
One possible mechanism by which TRPV1 activity might be induced by cold stress is via activation of the TRPV1 receptors along primary afferent fibers projecting to brown adipose. Cold and stress initiate thermogenesis in brown adipose via sympathetic activity [62]. TRPV1 receptors along primary afferent C-fibers innervating brown adipose detect the heat generated from uncoupling protein-1 activity and feedback to inhibit sympathetic tone. One might speculate that this or similar regulatory input to the CNS also helps shape behavioral responses to cold stress.
Some drugs are capable of enhancing immobility in the forced swim test without inducing depression per se. For instance, psychogenic drugs like phencyclidine (PCP), also increase immobility in the forced swim test when given repeatedly [63], but in a manner that is insensitive to antidepressants [64]. To determine whether the increase in immobility produced by RTX during the 41°C swim was depression or merely depressive behavior, we used acute injections of the antidepressant amitriptyline [65]. Amytriptyline decreased immobility when compared to saline-injected control mice and attenuated the depressive behavior induced by the low dose of RTX, suggesting that the enhanced immobility induced by the low dose of RTX was a form of depressive behavior, but not depression per se. Newer antidepressant compounds, as typified by ketamine, CPP (3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid) and dizocilpine (MK-801), inhibit NMDA receptors to produce antidepressant activity in the forced swim test [46,66–68]. When the potentiative effect of a low dose of RTX was tested at 41°C, ketamine did not affect depressive behavior in saline-injected mice, but did in mice injected with the low dose of RTX. This suggests that the effect of RTX is mediated by NMDA receptor activity and is even more sensitive to the effects of these new antidepressants than is the basal immobility.
In conclusion, the greater degree of depressive behavior during 26°C than during 41°C swims appears to result from centrally located TRPV1 receptors that are activated by endogenous ligands during forced swims in water temperatures that are lower (26°C) than the normal body temperature (around 38°C) but less so during swims in water maintained at a temperature that is higher (41°C). Exogenous TRPV1 ligands also enhance depressive behavior in the forced swim test in a fashion that is sensitive to acutely administered antidepressant compounds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- 2.Menigoz A, Boudes M. The Expression Pattern of TRPV1 in Brain. J Neurosci. 2011;31:13025–13027. doi: 10.1523/JNEUROSCI.2589-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, et al. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–R76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishnoi M, Bosgraaf CA, Abooj M, Zhong L, Premkumar LS. Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: role of transient receptor potential vanilloid 1(TRPV1) and inflammatory mediators. Mol Pain. 2011;7:52. doi: 10.1186/1744-8069-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishnoi M, Bosgraaf CA, Premkumar LS. Preservation of acute pain and efferent functions following intrathecal resiniferatoxin-induced analgesia in rats. J Pain. 2011;12:991–1003. doi: 10.1016/j.jpain.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai Y, Hara T, Imai A, Sakakibara A. Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia. J Pharm Pharmacol. 2007;59:733–738. doi: 10.1211/jpp.59.5.0015. [DOI] [PubMed] [Google Scholar]
- 17.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neubert JK, Mannes AJ, Karai LJ, Jenkins AC, Zawatski L, Abu-Asab M, et al. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Mol Pain. 2008;4:3. doi: 10.1186/1744-8069-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, et al. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watabiki T, Kiso T, Tsukamoto M, Aoki T, Matsuoka N. Intrathecal administration of AS1928370, a transient receptor potential vanilloid 1 antagonist, attenuates mechanical allodynia in a mouse model of neuropathic pain. Biol Pharm Bull. 2011;34:1105–1108. doi: 10.1248/bpb.34.1105. [DOI] [PubMed] [Google Scholar]
- 21.Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Watabiki T, Kiso T, Kuramochi T, Yonezawa K, Tsuji N, Kohara A, et al. Amelioration of neuropathic pain by novel transient receptor potential vanilloid 1 antagonist AS1928370 in rats without hyperthermic effect. J Pharmacol Exp Ther. 2011;336:743–750. doi: 10.1124/jpet.110.175570. [DOI] [PubMed] [Google Scholar]
- 23.Zakir HM, Mostafeezur RM, Suzuki A, Hitomi S, Suzuki I, Maeda T, et al. Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One. 2012;7:e44023. doi: 10.1371/journal.pone.0044023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamata T, Niiyama Y, Yamamoto J, Furuse S. Reduction of bone cancer pain by CB1 activation and TRPV1 inhibition. J Anesth. 2010;24:328–332. doi: 10.1007/s00540-010-0919-0. [DOI] [PubMed] [Google Scholar]
- 27.Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102:251–258. doi: 10.1093/bja/aen347. [DOI] [PubMed] [Google Scholar]
- 28.Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, et al. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You IJ, Jung YH, Kim MJ, Kwon SH, Hong SI, Lee SY, et al. Alterations in the emotional and memory behavioral phenotypes of transient receptor potential vanilloid type 1-deficient mice are mediated by changes in expression of 5-HT(1)A, GABA(A), and NMDA receptors. Neuropharmacology. 2012;62:1034–1043. doi: 10.1016/j.neuropharm.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Kasckow JW, Mulchahey JJ, Geracioti TD., Jr Effects of the vanilloid agonist olvanil and antagonist capsazepine on rat behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:291–295. doi: 10.1016/j.pnpbp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Kulisch C, Albrecht D. Effects of single swim stress on changes in TRPV1-mediated plasticity in the amygdala. Behav Brain Res. 2013;236:344–349. doi: 10.1016/j.bbr.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Manna SS, Umathe SN. A possible participation of transient receptor potential vanilloid type 1 channels in the antidepressant effect of fluoxetine. Eur J Pharmacol. 2012;685:81–90. doi: 10.1016/j.ejphar.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Hayase T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011;11:6. doi: 10.1186/1471-2210-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 35.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, et al. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linthorst AC, Flachskamm C, Reul JM. Water temperature determines neurochemical and behavioural responses to forced swim stress: an in vivo microdialysis and biotelemetry study in rats. Stress. 2008;11:88–100. doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- 37.Peeters BW, Smets RJ, Broekkamp CL. The involvement of glucocorticoids in the acquired immobility response is dependent on the water temperature. Physiol Behav. 1992;51:127–129. doi: 10.1016/0031-9384(92)90213-l. [DOI] [PubMed] [Google Scholar]
- 38.Arai I, Tsuyuki Y, Shiomoto H, Satoh M, Otomo S. Decreased body temperature dependent appearance of behavioral despair in the forced swimming test in mice. Pharmacol Res. 2000;42:171–176. doi: 10.1006/phrs.2000.0672. [DOI] [PubMed] [Google Scholar]
- 39.Taltavull JF, Chefer VI, Shippenberg TS, Kiyatkin EA. Severe brain hypothermia as a factor underlying behavioral immobility during cold-water forced swim. Brain Res. 2003;975:244–247. doi: 10.1016/s0006-8993(03)02695-7. [DOI] [PubMed] [Google Scholar]
- 40.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 41.Porsolt RD. Animal model of depression. Biomedicine. 1979;30:139–140. [PubMed] [Google Scholar]
- 42.Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. Eur J Pharmacol. 1979;57:201–210. doi: 10.1016/0014-2999(79)90366-2. [DOI] [PubMed] [Google Scholar]
- 43.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 44.Abdelhamid RE, Kovacs KJ, Pasley JD, Nunez MG, Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology. 2013;72:29–37. doi: 10.1016/j.neuropharm.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 46.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries DJ, Blumberg PM. Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci. 1989;44:711–715. doi: 10.1016/0024-3205(89)90382-2. [DOI] [PubMed] [Google Scholar]
- 49.Woods AJ, Stock MJ, Gupta AN, Wong TT, Andrews PL. Thermoregulatory effects of resiniferatoxin in the rat. Eur J Pharmacol. 1994;264:125–133. doi: 10.1016/0014-2999(94)00445-5. [DOI] [PubMed] [Google Scholar]
- 50.Szallasi A, Joo F, Blumberg PM. Duration of desensitization and ultrastructural changes in dorsal root ganglia in rats treated with resiniferatoxin, an ultrapotent capsaicin analog. Brain Res. 1989;503:68–72. doi: 10.1016/0006-8993(89)91705-8. [DOI] [PubMed] [Google Scholar]
- 51.Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 52.Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- 53.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 54.Cristino L, Starowicz K, De Petrocellis L, Morishita J, Ueda N, Guglielmotti V, et al. Immunohistochemical localization of anabolic and catabolic enzymes for anandamide and other putative endovanilloids in the hippocampus and cerebellar cortex of the mouse brain. Neuroscience. 2008;151:955–968. doi: 10.1016/j.neuroscience.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 55.Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64:286–292. doi: 10.1016/j.biopsych.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 56.Benarroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–1704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- 57.Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karolewicz B, Szebeni K, Stockmeier CA, Konick L, Overholser JC, Jurjus G, et al. Low nNOS protein in the locus coeruleus in major depression. J Neurochem. 2004;91:1057–1066. doi: 10.1111/j.1471-4159.2004.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hajos M, Jancso G, Engberg G. Capsaicin-induced excitation of locus coeruleus neurons. Acta Physiol Scand. 1987;129:415–420. doi: 10.1111/j.1748-1716.1987.tb08086.x. [DOI] [PubMed] [Google Scholar]
- 60.Plaznik A, Danysz W, Kostowski W. Mesolimbic noradrenaline but not dopamine is responsible for organization of rat behavior in the forced swim test and an anti-immobilizing effect of desipramine. Pol J Pharmacol Pharm. 1985;37:347–357. [PubMed] [Google Scholar]
- 61.Mello-Carpes PB, Izquierdo I. The Nucleus of the Solitary Tract --> Nucleus Paragigantocellularis --> Locus Coeruleus --> CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem. 2013;100:56–63. doi: 10.1016/j.nlm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 63.Noda Y, Yamada K, Furukawa H, Nabeshima T. Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol. 1995;116:2531–2537. doi: 10.1111/j.1476-5381.1995.tb15106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noda Y, Mamiya T, Furukawa H, Nabeshima T. Effects of antidepressants on phencyclidine-induced enhancement of immobility in a forced swimming test in mice. Eur J Pharmacol. 1997;324:135–140. doi: 10.1016/s0014-2999(97)00067-8. [DOI] [PubMed] [Google Scholar]
- 65.Guaiana G, Barbui C, Hotopf M. Amitriptyline for depression. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004186.pub2. CD004186. [DOI] [PubMed] [Google Scholar]
- 66.Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Jr, Cohen BM, et al. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology (Berl) 2011;215:689–695. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- 67.Popik P, Kos T, Sowa-Kucma M, Nowak G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl) 2008;198:421–430. doi: 10.1007/s00213-008-1158-z. [DOI] [PubMed] [Google Scholar]
- 68.Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (sigma) receptors in the antidepressant-like effects of ketamine in vitro and in vivo. Eur Neuropsychopharmacol. 2012;22:308–317. doi: 10.1016/j.euroneuro.2011.08.002. [DOI] [PubMed] [Google Scholar]


