Abstract
Objectives
To assess the pattern of the adoption of internal mammary artery (IMA) grafting in the United States, test its association with clinical outcomes, and assess whether its effectiveness differs in key clinical subgroups.
Background
The effect of IMA grafting on major clinical outcomes has never been tested in a large randomized trial, yet it is now a quality standard for coronary artery bypass graft (CABG) surgery.
Methods
We identified Medicare beneficiaries aged ≥66 years who underwent isolated multivessel CABG between 1988 and 2008, and documented patterns of IMA use over time. We used a multivariable propensity score to match patients with and without an IMA, and compared rates of death, myocardial infarction (MI), and repeat revascularization. We tested for variations in IMA effectiveness using treatment by covariate interaction tests.
Results
IMA use in CABG rose slowly from 31% in 1988 to 91% in 2008, with persistent wide geographic variations. Among 60,896 propensity score matched patients over a median 6.8 year follow-up, IMA use was associated with lower all-cause mortality (adjusted hazard ratio 0.77, p<0.001), lower death or MI (adjusted hazard ratio 0.77, p<0.001), and fewer repeat revascularization over five years (8% vs. 9%, p<0.001). The association between IMA use and lower mortality was significantly weaker (p≤0.008) for older patients, women, and for patients with diabetes or peripheral arterial disease.
Conclusions
IMA grafting was adopted slowly and still shows substantial geographic variation. IMA use is associated with lower rates of death, MI and repeat coronary revascularization.
Keywords: Internal Mammary-Coronary Artery Anastomosis, Outcomes Research, Comparative Effectiveness Research
Coronary artery bypass graft (CABG) surgery was generally performed using saphenous veins as conduits until the pivotal study of Loop and colleagues reported improved long-term outcomes from using the internal mammary artery (IMA) as a conduit (1). Their single center, non-randomized comparison of 5,931 patients reported a 38% reduction in the risk of death over ten years of follow-up from use of the IMA. Similar results were subsequently reported by others using observational data (2–5). The only randomized trial of IMA grafting enrolled just 80 patients and lacked statistical power to assess its effect on hard cardiac outcomes (6). Perhaps because there has never been a large, definitive randomized trial, adoption of IMA grafting has been slow and uneven in the United States (7–11). The National Quality Forum in 2004 adopted use of an IMA graft as a measure of the quality of CABG (12), which is now reported publicly by several states. In this study, we sought to document the patterns of adoption of IMA use in coronary revascularization procedures performed in Medicare beneficiaries, and assess the association of IMA grafting with long-term outcomes in a “real world” population of patients undergoing CABG.
Methods
The overall study population consisted of Medicare beneficiaries who underwent CABG between 1988 and 2008 who were included in the 20% sample of Part A data. We identified patients using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM), procedure codes for multivessel CABG (36.12, 36.13, 36.14, 36.16 or 36.11 plus 36.15), and identified IMA grafts by procedure codes 36.15 or 36.16. We excluded patients who had single-vessel CABG, concomitant cardiac procedures (such as valve replacement) at the time of CABG, evidence of a prior coronary revascularization (a Medicare hospitalization for CABG or PCI since 1988, or a prior condition code of V.15.1, V45.81 or V45.82 in the index admission), were of unknown race, or who had end-stage renal disease receiving chronic dialysis.
We used the subset of this overall population who received CABG between 1992 and 2008 to assess the association of IMA use with clinical outcomes, since data on some key characteristics needed for risk adjustment were not available prior to 1992. To permit a one year look-back period and document the presence of co-morbid conditions, we restricted this portion of the study population to individuals 66 years of age or older who had both Part A and Part B Medicare coverage, and who were not enrolled in a Medicare Advantage Managed Care Program (in which diagnosis and procedure codes are not available) in the year prior to the procedure. Part B data from 1992 through 1997 were available from only a 5% sample of beneficiaries, rather than the 20% sample available from 1998 through 2008. We defined comorbid conditions using outpatient and inpatient encounters in the year prior to the index procedure. We considered a comorbidity to be present if it was recorded as a primary or secondary diagnosis code on an inpatient admission or outpatient encounter.
We developed a multivariable logistic regression in the analysis subset to identify baseline clinical factors that predicted receipt of an IMA graft, and assessed the discrimination of the model with the C-statistic (13). We used the results of this analysis to assign each patient a propensity score, indicating the probability that the individual would receive an IMA graft. We then applied a greedy pair matching algorithm (14) to match each patient who did not receive an IMA graft with another patient who did. The algorithm first matched patients at seven digits of the propensity score, then matched the remaining patients at six digits, and so forth, down to a two digit match (i.e., to agreement of 0.01 or better). We additionally required that patients match on the calendar year they underwent CABG (to control further for any secular trends in outcome), and to be the same age within one year.
Patients were followed until death, or December 31, 2008. We identified all-cause mortality from the Medicare Denominator file, admissions for acute myocardial infarction in Part A data by an ICD9-CM primary hospital discharge diagnosis code of 410.x, repeat CABG in Part A data by ICD9-CM procedure codes 36.1x, and percutaneous coronary interventions by ICD9-CM procedure codes 36.01, 36.02, 36.05, 36.06, 36.07 and, after October 2005, by procedure code 00.66. For the endpoints of MI and repeat procedures, patients were censored if they entered a Medicare Advantage Plan or lost Part A coverage, as data on hospitalization would no longer be available.
We described event-free survival data using the Kaplan-Meier method (15), and we compared outcomes in the matched population by IMA status using the Cox proportional hazards model. We initially performed the comparison among the propensity score matched cohort without additional adjustment for baseline clinical characteristics, and subsequently adjusted for the baseline characteristics shown in Table 1, since they may provide additional prognostic information. We tested for interactions between each of four pre-specified clinical characteristics (age, sex, diabetes, and peripheral arterial disease), treatment with an IMA graft, and mortality using a model that contained treatment, the selected covariate, and their interaction. We repeated the interaction tests in models that contained other baseline characteristics.
Table 1.
Baseline Clinical Characteristics of Adults Undergoing Isolated CABG, by Use of IMA Graft (%)
| Characteristic | All Patients*
|
Propensity Score Matched Patients†
|
||
|---|---|---|---|---|
| IMA
|
No IMA
|
IMA
|
No IMA
|
|
| N=151,909 | N=34,542 | N=30,448 | N=30,448 | |
|
|
|
|||
| Age | ||||
| 66–70 years | 32.1 | 24.0 | 24.5 | 24.7 |
| 71–75 years | 32.5 | 28.5 | 29.3 | 29.4 |
| 76–80 years | 23.7 | 27.5 | 27.6 | 27.6 |
| 81–85 years | 9.9 | 15.8 | 15.3 | 15.1 |
| ≥86 years | 1.8 | 4.1 | 3.3 | 3.3 |
| Female | 32.2 | 42.5 | 41.5 | 41.9 |
| Race | ||||
| White | 92.8 | 91.7 | 92.6 | 91.9 |
| Black | 4.2 | 4.9 | 4.5 | 5.0 |
| Other | 3.0 | 3.3 | 2.9 | 3.2 |
| Diabetes | 36.4 | 33.8 | 34.0 | 34.3 |
| Hypertension | 78.4 | 74.0 | 74.4 | 75.0 |
| Hyperlipidemia | 30.2 | 23.5 | 23.8 | 24.4 |
| Tobacco Abuse | 18.9 | 14.0 | 14.7 | 15.0 |
| Chronic Kidney Disease | 5.1 | 5.4 | 5.4 | 5.4 |
| Peripheral Arterial Disease | 21.4 | 22.9 | 23.0 | 23.2 |
| Cerebrovascular Disease | 21.9 | 22.4 | 22.1 | 22.8 |
| Prior MI | 12.7 | 16.7 | 16.0 | 16.3 |
| Heart Failure | 12.1 | 18.3 | 17.3 | 17.5 |
| Unstable Angina | 38.4 | 39.5 | 38.6 | 38.7 |
| Atrial Fibrillation | 9.3 | 10.8 | 10.5 | 10.6 |
| Primary diagnosis of MI | 20.8 | 26.0 | 25.7 | 25.8 |
| Metropolitan Area | 72.7 | 71.1 | 71.2 | 71.2 |
| US Census Division | ||||
| New England | 4.8 | 2.9 | 2.9 | 2.9 |
| Middle Atlantic | 13.9 | 12.0 | 12.1 | 12.5 |
| South Atlantic | 21.4 | 22.7 | 22.5 | 22.7 |
| East South Central | 8.6 | 9.9 | 10.1 | 10.1 |
| West South Central | 11.0 | 17.4 | 15.9 | 15.9 |
| East North Central | 20.3 | 17.9 | 19.0 | 18.4 |
| West North Central | 8.6 | 7.5 | 7.7 | 7.7 |
| Mountain | 3.9 | 2.9 | 2.7 | 2.9 |
| Pacific | 7.4 | 6.8 | 7.0 | 7.0 |
| Year of Procedure | ||||
| 1992–1995 | 7.9 | 21.8 | 16.4 | 16.4 |
| 1996–1999 | 17.2 | 23.6 | 24.6 | 24.6 |
| 2000–2002 | 29.0 | 28.3 | 30.6 | 30.6 |
| 2003–2005 | 26.5 | 17.8 | 19.2 | 19.2 |
| 2006–2008 | 19.4 | 8.5 | 9.2 | 9.2 |
CABG = Coronary artery bypass graft surgery
IMA = Internal mammary artery
MI = Myocardial infarction
N = Number US = United States
All p-values <0.0001, except chronic kidney disease (P=0.005) and cerebrovascular disease (p=0.02).
All p-values >0.05, except race (p=0.005) and cerebrovascular disease (p=0.05).
Results
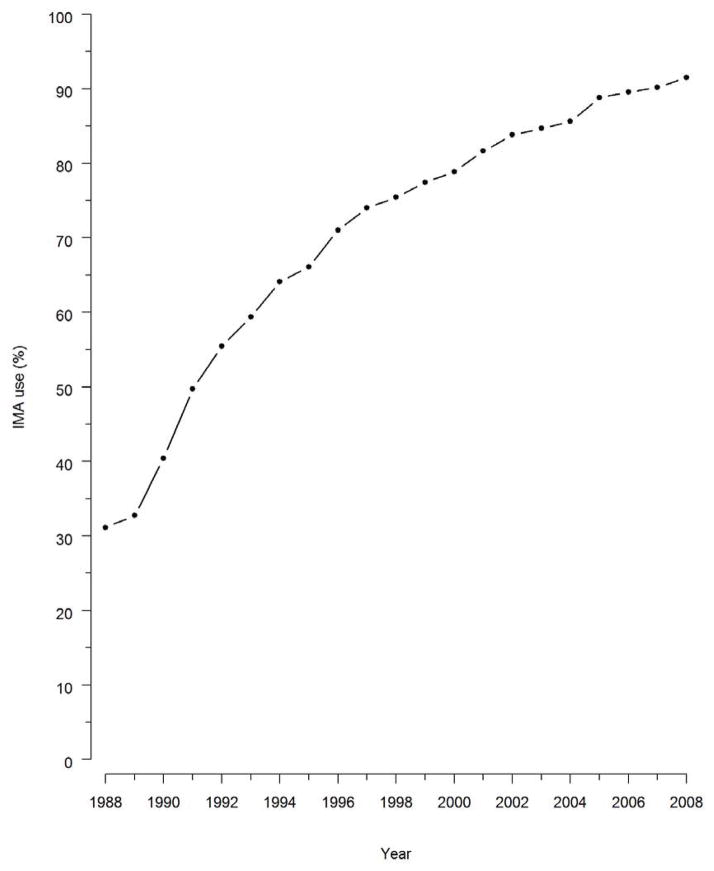
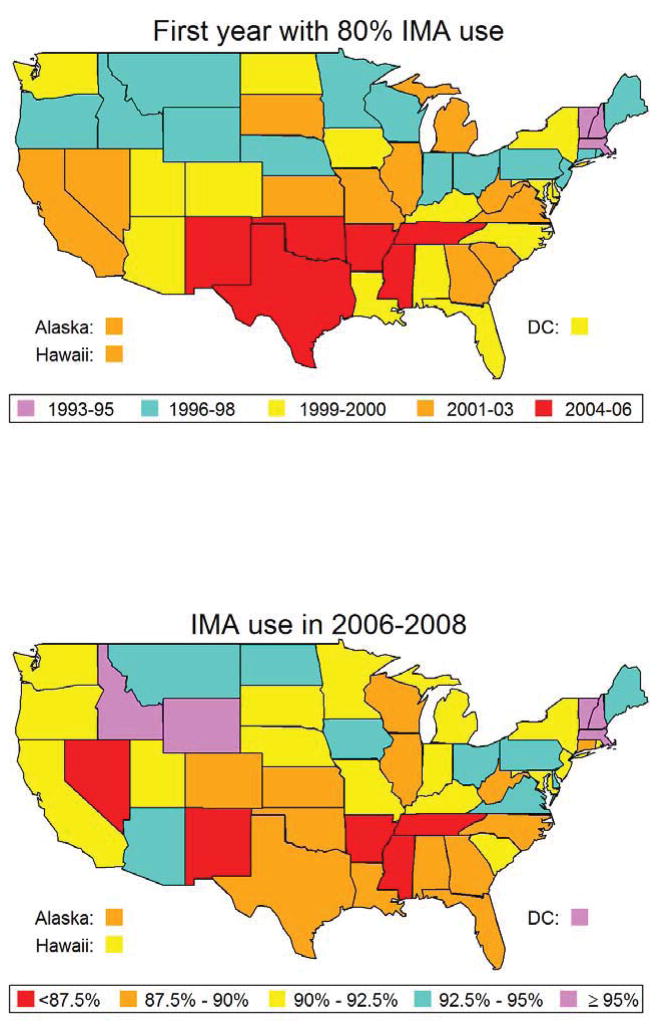
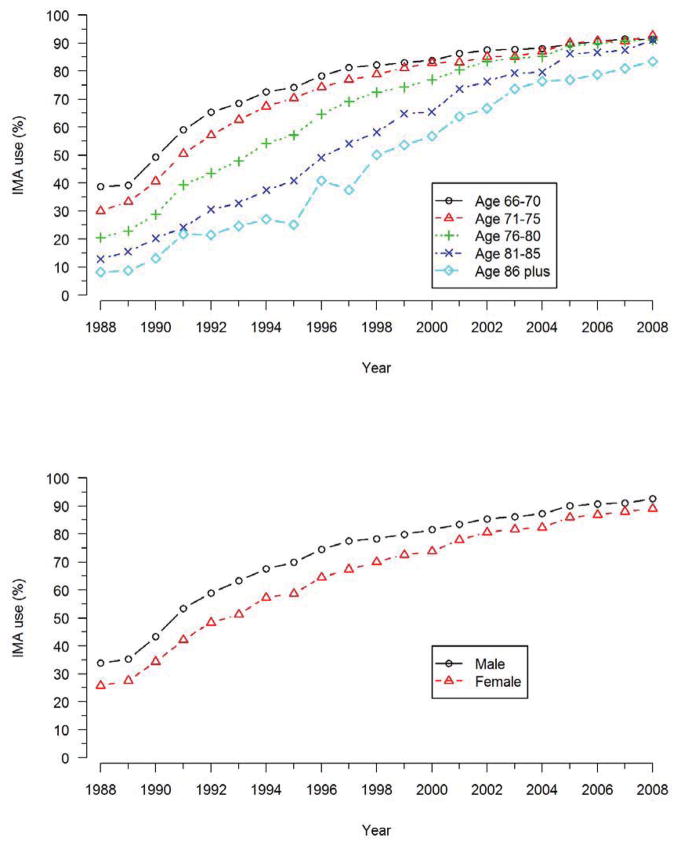
A total of 374,918 patients in the study population underwent isolated multivessel CABG between 1988 and 2008, and 260,119 (69%) patients received at least one IMA graft. Overall, use of the IMA graft during CABG increased steadily over time (Figure 1), from 31% in 1988 to 91% in 2008. There was an additional increase in use of the IMA after its adoption in late 2004 as a quality measure for CABG (Figure 1). The use of IMA grafting differed substantially among different regions of the United States (Figure 2), and while these differences narrowed over time they were still evident in 2008. Use of IMA grafts was highest among patients aged 66 to 70 years, and lowest among patients 86 years and older (Figure 3), and the difference in IMA use by age narrowed progresusively over time. By contrast, rates of IMA grafting among women was slightly lower than among men throughout the study period (Figure 3).
Figure 1. IMA adoption, 1988–2008.
Use of internal mammary artery (IMA) grafts in multivessel CABG over time.
Figure 2. Geographic variation in IMA use.
The percentage of patients with IMA grafts used in CABG in each state in 2006–2006 (top panel) is indicated by different colors. The first year that 80% of patients in each state had an IMA graft (bottom panel) is indicated by different colors.
Figure 3. IMA adoption by age and sex.
Use of IMA grafts in multivessel CABG over time by age (top panel) and sex (bottom panel). The growth of IMA grafting use over time differed greatly according to age (p<0.0001), but only slightly according to sex (p=0.04).
In the subset of 186,451 patients who underwent isolated multivessel CABG between 1992 and 2008, for whom baseline clinical data on comorbid conditions were available, there were several significant differences between patients who received an IMA graft and those who did not (Table 1). In addition to differences in age, geographic region, and year of procedure, patients who received an IMA graft were more often male and white, but less likely to have diabetes. In a multivariable logistic regression model, the strongest independent predictors of receiving an IMA graft were calendar year, United States census division, age and sex, as well as comorbidity measures indicating heart failure, chronic obstructive lung disease, recent myocardial infarction, and a history of systemic cancer. The overall c-statistic of the propensity score model was 0.71.
Outcomes
In order to examine the association of IMA grafting with outcomes, we matched 30,448 patients who did not receive an IMA graft (88% of all patients without an IMA graft) with a patient who did receive an IMA graft and who underwent CABG in the same year, was within one year of age, and within 0.01 units of propensity score. As expected from the matching process, the clinical characteristics of the two groups were quite similar after matching (Table 1).
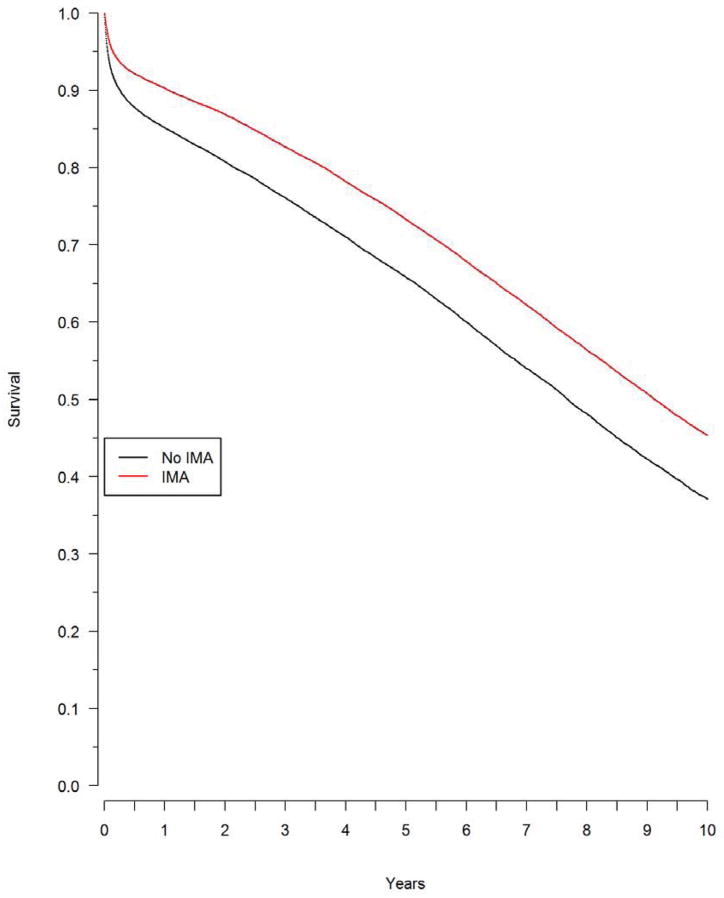
Over a median follow-up of 6.8 years, there were 13,930 (46%) deaths among patients who received an IMA and 16,208 (53%) deaths among patients who did not receive an IMA. The Kaplan-Meier survival rates at five years were 73% among patients who received an IMA, and 66% in those who did not, and at ten years survival was 45% among patients who received an IMA and 37% among patients who did not (Figure 4). The unadjusted hazard ratio for death from any cause in the propensity score matched population was 0.77 (95% confidence limits (CL) 0.75 to 0.79, p<0.001), and was essentially unchanged by further adjustment for baseline characteristics (adjusted hazard ratio 0.77, CL 0.75 to 0.78). The hazard ratio for the composite outcomes of death or myocardial infarction was 0.78 (CL 0.76 to 0.80), which was also virtually unchanged by further adjustment for baseline factors (HR=0.77, CL 0.75 to 0.79). The rate of repeat revascularization at five years was significantly lower among surviving patients who had received an IMA compared with those who had not received an IMA (8% vs. 9%, P<0.001).
Figure 4. Survival by IMA use.
Survival after multivessel CABG, by IMA use, in the propensity matched population. The difference in survival was significant (p<0.0001).
Thirty-day mortality was slightly lower among patients who received an IMA graft, 3.8% vs. 6.4% (p<0.001). We next performed a secondary analysis to assess the association of IMA grafting with long-term outcomes among patients who survived at least 30 days after CABG. In this analysis of 28,451 patient pairs, the five year survival was 76% among patients who received an IMA and 70% among patients who did not. The unadjusted hazard ratio for death comparing IMA use versus non-use among 30-day survivors was 0.79 (CL 0.77 to 0.81, p<0.001), and was essentially unchanged by additional adjustment for baseline characteristics (0.79, CL 0.77 to 0.81).
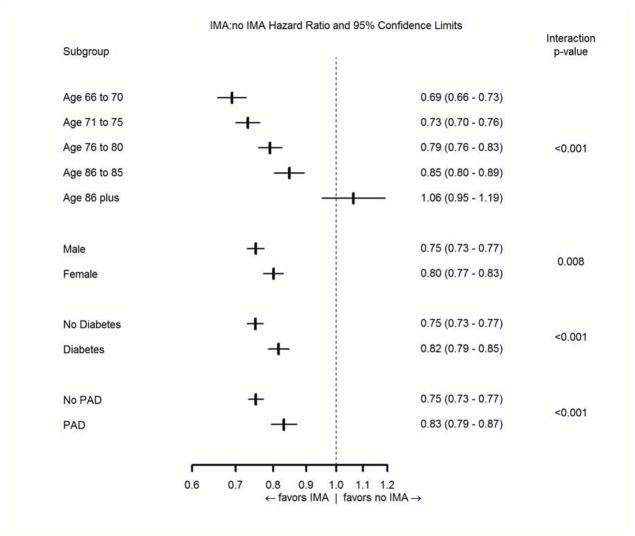
The use of an IMA graft was associated with a smaller survival advantage among patients with diabetes (p-value for the treatment-by-covariate interaction test (Pint = 0.001), patients with peripheral arterial disease (pint <0.001), among older patients (Pint <0.001), and women (pint = 0.008). After additional adjustments for the baseline factors in Table 1, the interactions remained significant for diabetes (pint = 0.011), peripheral arterial disease (pint = 0.027), and age (pint <0.001), but not for women (pint = 0.083). Use of an IMA graft was associated with improved survival in all pre-specified subgroups, however, except for patients aged 86 years and older (Figure 5).
Figure 5. IMA effectiveness in subgroups.
Hazard ratios for mortality (95% confidence limits) associated with use of an IMA graft among pre-specified clinical subgroups.
Discussion
This study documents the slow adoption of IMA grafting among Medicare beneficiaries undergoing isolated multivessel CABG. In 1988, two years after publication of the pivotal paper by Loop and associates (1), IMA grafts were used in only about 30% of CABG procedures, and five years later had increased only to about 50% of patients. Patient-related factors were relatively weak predictors of IMA use, apart from age. The considerable geographic variation in IMA use, and the weak predictive power of patient characteristics in determining its use, suggests that surgical practice style was the key factor determining adoption of IMA grafting during CABG. The recognition of IMA grafting as a quality measure for CABG (12, 16) is likely to further increase its use and attenuate any residual geographic variation.
The slow adoption of IMA grafting contrasts with the rapid uptake of drug eluting stents during percutaneous coronary intervention, which were used in over 50% of procedures within six months of approval by the Food and Drug Administration, and in 90% of procedures within two years (17). There are several notable differences between these two innovations that might have affected their adoption. Use of a drug eluting stent instead of a bare metal stent does not extend the time or difficulty of the procedure, whereas dissection of the IMA and performing the anastomosis do extend the duration and difficulty of CABG. Also, there were several large randomized trials showing the use of drug eluting stents reduced the rate of repeat revascularization (18), but there have been no large randomized trials of IMA grafting, so the evidence for its efficacy was weaker. Finally, drug eluting stents were vigorously promoted by the manufacturers to cardiologists, whereas there was no concerted marketing of IMA grafts to surgeons.
Our study confirms the association of IMA grafting with improved outcomes after CABG. The adjusted hazard ratio for mortality of 0.77 we observed in this study was somewhat less favorable than the hazard ratio of 0.61 reported by the original study of Loop and associates (1), but was almost identical to the more contemporary estimate of 0.77 derived from 3,087 patients enrolled in eight trials of CABG and percutaneous coronary intervention (4). The present study is much larger than any prior study of the association of IMA grafting with outcomes, and is based on a representative population of older patients and treating surgeons in the United States. The present study is also based on more contemporary patients than earlier reports. In addition to the association of IMA graft use with lower mortality, we also found that IMA use was associated with a lower rate of death or myocardial infarction (adjusted hazard ratio of 0.78), and with a slightly lower use of repeat coronary revascularization procedures. These data are consistent with observations that the IMA is more durable than a saphenous vein graft, and less likely to develop stenosis or occlusion in long-term follow-up (19, 20).
Our study was much larger than previous studies of outcomes after IMA grafting, so we were also able to assess outcomes in several key clinical subgroups (Figure 5). The association of IMA grafting with lower mortality was strikingly modified by patient age, with a progressively less favorable association at older ages. Indeed, our data show no association of IMA grafting with mortality among patients aged 86 years or more (Figure 5). The apparent effect of age on the hazard ratio for mortality may be the result of the inherently lower life expectancy of older patients, so there is less opportunity for IMA grafting to improve long-term survival. While residual selection bias cannot be dismissed, it seems more likely that the “healthiest” older patients would be selected for IMA grafting, not the “sickest.” Limited life expectancy might also explain the weaker association of IMA grafting upon survival among patients with diabetes and peripheral arterial disease, as they also have higher rates of mortality than patients without these conditions, but does not explain the weaker association among women, who generally have longer life expectancy than men.
The five year survival rate in this study of patients who received an IMA (73%) was considerably lower than the five year survival rate of patients assigned to CABG in randomized trials. The five year survival rate was 90% after CABG in the pooled data from ten randomized trials of CABG versus PCI (21) and 89% in the SYNTAX trial (22). The better survival after CABG in these trials than after CABG in the community is likely due to the stringent selection of patients for the randomized trials and, to a lesser extent, the limitation of CABG to centers of excellence in the trials. For instance, patients in the ten pooled trials had a median age of 61 years, versus a median age of 75 years in the present study. The representative nature of the patients and providers in the present study is strength, and the outcomes are likely to be more representative. The study population was, however, limited to patients aged 66 years and older.
Use of internal mammary arteries among Medicare beneficiaries was 91% in 2008, but still far from universal. The reasons why IMA grafts are not used in all contemporary CABG procedures are uncertain. Several large registries have shown that IMA use decreases with increasing procedural risk estimated by multivariable scores (7, 23), suggesting that surgeons may not wish to prolong the procedure by grafting the IMA in higher risk cases. Operative notes contained a specific comment in the reason for non-use of the IMA in 53% of cases in one registry (3), with the most common reasons being damage or injury to the IMA on harvesting, poor flow in the IMA, poor lung function, and unstable symptoms. It is likely that patients or technical factors will lead to a few patients not receiving an IMA during CABG even when universal use is the goal, and clinical registries could record the reasons for non-use of the IMA (16).
This study has several limitations. Our comparison of the outcomes of patients who did and did not receive an IMA graft was based on observational data, not a randomized trial. We did use several methods to improve comparability of the treatment groups, starting with our restriction of the population to patients with multivessel procedures, isolated CABG, and no prior cardiac surgery. We matched patients on year of procedure and on multivariable propensity score, which also controls for potential confounding factors. The consistency of our results in a secondary analysis of patients who survived the first 30 days after CABG, and across multiple endpoints, is also reassuring. Nevertheless, we cannot exclude the possibility that residual selection bias in the use of the IMA affected our estimates of its effectiveness. This study was based on Medicare claims, and lacks data on several important clinical variables, such as the location and extent of coronary lesions, and the level of left ventricular function. In particular, we do not have specific information on which patients had disease in the left anterior descending coronary artery, or where the IMA graft was placed. While we excluded patients who had either a Medicare claim for cardiac surgery since 1988 or who had a condition code for prior cardiac surgery, we cannot exclude the possibility that some patients may have had prior CABG.
In conclusion, our analysis confirms the association of IMA grafting with better outcomes among older patients receiving multivessel CABG, and reinforces the choice of IMA grafting as a quality measure. The reasons for the relatively slow and uneven adoption of IMA grafting in CABG should be further studied, in order to better understand the barriers to diffusion of treatment advances.
Acknowledgments
Funding Sources. This study was supported by Grant HL099872 from the National Heart, Lung and Blood Institute, Bethesda, MD, with additional support from Grant 0875162N from the American Heart Association, Dallas, TX
Abbreviations
- CABG
Coronary artery bypass graft surgery
- CL
95% confidence limit
- ICD9-CM
International Classification of Diseases 9th Edition, Clinical Modification
- IMA
Internal mammary artery
- MI
Myocardial infarction
- US
United States
- PAD
Peripheral arterial disease
- pint
p-value for the treatment-by-covariate interaction test
- PCI
Percutaneous coronary intervention
- SYNTAX
SYNergy Between PCI with TAXus and Cardiac Surgery
Footnotes
Disclosures. None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 2.Cameron A, Davis KB, Green G, Schaff HV. Coronary bypass surgery with internal thoracic-artery grafts–effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 3.Karthik S, Srinivasan AK, Grayson AD, Jackson M, Mediratta NK. Left internal mammary artery to the left anterior descending artery: Effect on morbidity and mortality and reasons for nonusage. Ann Thorac Surg. 2004;78:142–148. doi: 10.1016/j.athoracsur.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Hlatky MA, Shilane D, Boothroyd DB, et al. The effect of internal thoracic artery grafts on long-term clinical outcomes after coronary bypass surgery. J Thorac Cardiovasc Surg. 2011;142:829–835. doi: 10.1016/j.jtcvs.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AAC, Green GE, Brogno DA, Thornton J. Internal thoracic artery grafts: 20-year clinical follow-up. J Am Coll Cardiol. 1995;25:188–192. doi: 10.1016/0735-1097(94)00332-k. [DOI] [PubMed] [Google Scholar]
- 6.Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: Prospective randomized study with 10-year follow-up. Ann Thorac Surg. 1988;45:533–536. doi: 10.1016/s0003-4975(10)64526-2. [DOI] [PubMed] [Google Scholar]
- 7.Edwards FH, Clark RE, Schwartz M. Impact of internal mammary artery conduits on operative mortality in coronary revascularization. Ann Thorac Surg. 1994;57:27–32. doi: 10.1016/0003-4975(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson TB, Coombs LP, Peterson ED. Internal thoracic artery grafting in the elderly patient undergoing coronary artery bypass grafting: Room for process improvement? J Thorac Cardiovasc Surg. 2002;123:869–880. doi: 10.1067/mtc.2002.121679. [DOI] [PubMed] [Google Scholar]
- 9.Holman WL, Allman RM, Sansom M, et al. Alabama coronary artery bypass grafting project: Results of a statewide quality improvement initiative. JAMA. 2001;285:3003–3010. doi: 10.1001/jama.285.23.3003. [DOI] [PubMed] [Google Scholar]
- 10.Tabata M, Grab JD, Khalpey Z, et al. Prevalence and variability of internal mammary artery graft use in contemporary multivessel coronary artery bypass graft surgery. Analysis of the Society of Thoracic Surgeons National Cardiac Database. Circulation. 2009;120:935–940. doi: 10.1161/CIRCULATIONAHA.108.832444. [DOI] [PubMed] [Google Scholar]
- 11.Wennberg DE, Birkmeyer JD, Birkmeyer NJO, et al. The Dartmouth Atlas of Cardiovascular Care. Chicago, IL: AHA Press; 1999. [Google Scholar]
- 12.National Quality Forum. A consensus report. 2004. National voluntary consensus standards for cardiac surgery. [Google Scholar]
- 13.Harrell FE. Regression modeling strategies, with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer; 2001. [Google Scholar]
- 14.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Preesented at 26th Annual SAS Users Group International Conference; Long Beach, California. April 22–25 2001. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Karthik S, Fabri BM. Left internal mammary artery usage in coronary artery bypass grafting: A measure of quality control. Ann R Coll Surg Engl. 2006;88:367–369. doi: 10.1308/003588406X98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krone RJ, Rao SV, Dai D, et al. Acceptance, panic, and partial recovery. The pattern of usage of drug-eluting stents after introduction in the U.S (A report from the American College of Cardiology/National Cardiovascular Data Registry) J Am Coll Cardiol Intv. 2010;3:902–910. doi: 10.1016/j.jcin.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Babapulle MN, Joseph L, Bélisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004;364:583–591. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 19.Sabik JF, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–551. doi: 10.1016/j.athoracsur.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 20.Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery. Results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 21.Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: A collaborative analysis of individual patient data from ten randomized trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 22.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 23.Leavitt BJ, O’Connor GT, Olmstead EM, et al. Use of the internal mammary artery graft and in-hospital mortality and other adverse outcomes associated with coronary artery bypass surgery. Circulation. 2001;103:507–512. doi: 10.1161/01.cir.103.4.507. [DOI] [PubMed] [Google Scholar]