Abstract
Antibody technology has transformed drug development, providing robust approaches to producing highly targeted and active therapeutics that can routinely be advanced through clinical evaluation and registration. In parallel, there is an emerging need to access similarly targeted agents for diagnostic purposes, including non-invasive imaging in preclinical models and patients. Antibody engineering enables modification of key properties (immunogenicity, valency, biological inertness, pharmacokinetics, clearance route, site-specific conjugation) in order to produce targeting agents optimized for molecular imaging. Expanded availability of positron-emitting radionuclides has led to a resurgence of interest and applications of immunoPET (immuno-positron emission tomography). Molecular imaging using engineered antibodies and fragments provides a general approach for assessing cell surface phenotype in vivo and stands to play an increasingly important role in cancer diagnosis, treatment selection, and monitoring of molecularly targeted therapeutics.
Keywords: engineered antibody fragments, radiolabeling, positron emission tomography, immunoPET, site-specific conjugation
1. Introduction
The identification of cancer-specific monoclonal antibodies was swiftly followed by the recognition of their potential for detection and imaging of tumors in patients. Indeed, in the 1990s several radiolabeled antibodies or fragments, including satumomab pendetide (Oncoscint™), capromab pendetide (Prostascint™), arcitumomab (CEA-Scan™), and norfetumomab merpentan (Verluma™) were approved for cancer imaging by the FDA in the US [1]. However, the success of these first-generation imaging agents was limited by a number of shortcomings, including the murine origin of the antibodies, inconvenience of delayed imaging due to the serum persistence of intact antibodies, sub-optimal choice of targets, and limited detection and sensitivity of the agents. Despite initial interest, these first-generation antibody-based imaging agents were not highly successful in the clinic or commercially, with only one (ProstaScint) remaining on the market in the US, and interest in radioimmunoimaging flagged.
Fortunately, research and development in antibody-directed imaging has experienced a resurgence in recent years, due to scientific and technical advances across cancer biology, biotechnology, instrumentation and radionuclide availability, and clinical medicine [2, 3]. A fundamental shift is occuring in our understanding of the molecular origins and drivers of cancer, and a critical need has emerged to profile normal and disease biology in individual patients. Advances in laboratory-based diagnostic tests have brought the power of complex and comprehensive analyses such as genomic or proteomic profiling, to conventional serum and blood samples, biopsies, and circulating tumor cells. Such analyses have opened new insights into the complexities of cancers – not only highlighting key molecular targets that drive malignancy, but also compensatory pathways and resistance mechanisms. In addition, tumor profiling has led to a greater appreciation of genomic instability and its outcomes including tumor heterogeneity. At the same time, the ability of tumor biology to evolve over time and within and across lesions fundamentally limits the diagnositic and predictive value in vitro laboratory diagnostics, which are restricted by the specimens that can be obtained for analysis. Not all tumor tissues are biopsied; and it is not feasible to analyze all biopsy material to the same degree. Tissue samples are infrequently obtained when disease is extended or metastatic. Another obvious, yet often ignored subject, is that once tissue biopsies have been cut off from their blood supply and removed from the body, dramatic changes occur to the functional, metabolic, and signalling state of cells; in essence we are studying dying or dead tissue instead of viable cells. Delicate biomarkers are subject to further loss if tissue processing includes harsh chemical fixation and/or high temperatures. Blood and serum samples provide a source of complementary information, allowing sensitive and sophisticated monitoring of patient health or disease progression over time. However, no accompanying spatial information is provided. As a result, a large gap remains in our overall ability to profile the biology of active disease in a living patient.
Molecular imaging provides a means for non-invasive detection and measurement of molecular targets, pathways, and events in living organisms. Typically, molecular imaging employs highly specific tracer molecules labeled (inlcuding antibodies) with radionuclides to enable external detection of signals localized within the body, using cameras or scanners. Positron emission tomography (PET) has emerged as a mainstay in molecular imaging due to the sensitivity, resolution, and quantitation provided by this modality [4]. Other modalities including optical imaging (direct, fluorescent, or bioluminescent), MRI, ultrasound, have also been developed for specific molecular imaging applications [3].
Ultimately, the strength of molecular imaging is dependent on the availability of appropriate small molecule, peptide/aptamer, or protein probes that bind with high specificity and high affinity to the biological target of interest. In particular, the natural diversity of antibody binding specificities and their availability as high affinity reagents make antibodies a natural starting point for generating molecular imaging agents for the non-invasive detection and profiling of cancer. Furthermore, many of the lessons learned from engineering therapeutic antibodies for clinical use can be applied to development of antibody-based imaging agents.
This review will introduce the factors to consider when embarking on an antibody-based molecular imaging program. Here we focus on radioactive imaging modalities, in particular immunoPET, due to the inherent translatability of the approach. Strategies for developing an optimized imaging agent, suitable for clinical translation, will be discussed, including engineering the antibody itself and pairing with an appropriate radionuclide. Finally, present and future applications for antibody-based molecular imaging in oncology will be discussed.
2. Selection of targets for imaging
Many characteristics of a “good” target for imaging overlap with features that define good therapeutic targets. There are several “rules of thumb” that can guide the initiation of a molecular imaging project, but it is also important to keep an open mind because cancer biology seems to provide exceptions to every rule.
Development of antibody-based targeting agents has focused on cell-surface or extracellular targets; externally administered intact proteins such as antibodies do not have broad access to potential targets in the cytoplasm, nucleus or other subcellular components. In vivo imaging of cell surface biomarkers has nonetheless been fruitful, due to the broad classes of informative cell surface targets such as oncofetal antigens, growth factor receptors, adhesion molecules, lineage and differentiation markers, etc. Targets are not limited to malignant cells themselves, but can also be associated with any component of tumor tissue, including the extracellular matrix, stromal cells, vasculature, and infiltrating immune cells. Examples include fibroblast activation protein-α (FAP) [5], markers of angiogenesis and lymphangiogenesis (αvβ3 integrin, VEGF, fibronectin ED-B domain) [6]. This limitation is not absolute; exceptions to this apparent rule are not uncommon, due to the accumulation of many disruptions to normal biology in malignant cells [7]. Furthermore, several groups have harnessed cell-penetrating peptides (CPP) to transport antibodies into the cytoplasm or even to the nucleus of the cell (using a nuclear localizing signal, NLS) for imaging and therapy purposes [8, 9].
A perceived limitation of antibodies as delivery vectors is their inability to cross the blood-brain barrier. While generally true, this challenge has been addressed by taking advantage of active transcytosis mechanisms (TfR, IGFR) [10, 11]. Evidence suggests that the development primary and metastatic tumors in the brain is accompanied by abnormal development of vasculature, leaving open opportunities for imaging and therapeutic targeting using large biomolcules. In addition, a variety of approaches are under investigation to selectively disrupt the BBB, enhancing delivery of pharmaceuticals, including antibodies [12].
Practical challenges include identification of markers that are uniquely expressed (tumor-specific variants such as EGFRvIII) or over-expressed in malignancies, concomitant with little or no expression in normal tissues and organs. Requirements for differential expression can be more stringent for imaging studies compared to therapeutic protocols. For example, low-level expression of a target (e.g. a growth factor receptor such as EGFR) in normal organs can be tolerated in a therapeutic regimen as long as engagement of the target does not trigger a strong biological response in the normal tissues, resulting in unwanted side effects and toxicity. However, the same pattern of expression might preclude imaging with a radiolabeled antibody, since specific binding to target anywhere, including on normal tissues, would generate a background imaging signal. Presence of a large “antigen sink” in normal tissue can also impact pharmacokinetics and dosing, requiring higher administered protein doses in order to saturate non-target tissue binding.
Identification and validation of molecular imaging targets that are informative is a necessary first step. A candidate imaging target may itself play a key functional role, or have the potential to provide critical diagnostic information (i.e. prognostic, predictive). Non-invasive assessment of target expression in living subjects has become an important component of the development and implementation of molecularly targeted therapeutics, such as antibody-drug conjugates. Furthermore, a rapidly growing application area is the use of molecular imaging for specific assessment of mechanism and response to targeted drug therapy.
One question that is frequently raised is the minimal number of copies of target antigen per cell needed for detection by sensitive imaging modalities such as optical, SPECT, or PET imaging. The answer is highly dependent on many factors, including the biology and function of the target antigen, properties of the antibody probe including affinity and epitope recognized, amounts and routes of administration, physiology including vascularity of the target tissue (which can be notoriously variable), and numerous other properties. We have explored the threshhold of detection for tumor xenografts in systems including stably transfected Jurkat lymphoma cell lines bearing a transmembrane anchored form of CEA [13]. Quantitation of expression by flow cytometry and western blotting suggested that a minimum of 25,000 copies/cell were required for detection; tumor cell lines with lower levels of expression were not visualized by microPET using an 124I-labeled anti-CEA antibody fragment. Similar studies imaging prostate or pancreatic cancer xenografts expressing prostate stem cell antigen (PSCA) using an anti-PSCA fragment, or native CEA expression in pancreatic cancer xenografts also suggested a threshhold of 30–40,000 copies/cell (V. Kenanova, J. Tomlinson, A. Wu unpublished). It should be noted, however, that these microPET imaging studies were conducted in an idealized model, using human tumor cell line xenografts in immunocompromised mice, so the normal tissue background was very low. A more realistic minimum expression range for an imaging target might be 50–100,000 copies/cell. As additional quantitative studies are completed, a better definition of the threshhold for detection by PET should emerge, and applicability to target detection levels in human studies needs to be assessed carefully as well.
Secretion or shedding of target antigens or epitopes can also pose challenges to antibody-based targeting. Conventional wisdom holds that circulating antigen precludes effective targeting of tumor tissues, but as noted above, results in individual systems may vary and there are frequent exceptions to every rule. For example, CEA, which can be present at 10’s to 100’s of nanograms/ml in patients’ serum (or even higher in cases of medullary thyroid carcinoma), does not prevent targeting and imaging using radiolabeled anti-CEA fragments [14, 15]. Mucins, frequently bearing distinctive tumor-associated variations in glycosylation, can be present at even higher concentrations in the circulation of ovarian and breast cancer patients, yet are still viable targets for antibody-directed therapies and imaging. For example, intact antibodies and fragments recognizing the classic serum marker for pancreatic cancer, CA 19-9, have shown promise as PET imaging agents in xenograft models in mice [16, 17].
An additional consideration in the development of antibody imaging in a biological system is whether the target antigen internalizes – either constitutively, or upon engagement and/or cross-linking by an antibody. The kinetics of internalization and/or recycling will impact rate and extent of accumulation of an antibody at its target. Importantly, selection of a radionuclide and chemical conjugation approach will be strongly influenced by the binding, internalization, and metabolism of the protein-radiolabel conjugate. Nuclear imaging is based strictly on detection of the emissions from the radioactive isotope, regardless of its chemical or biological form. Thus it is important to understand the fate of a radiolabeled antibody once it is subject to degradation, whether due to instability in the circulation, or following localization either in target tissues or in organs primarily responsible for clearance (i.e. liver or kidneys). Following cellular internalization, catabolism of radiolabeled proteins will result in the generation of radioactive metabolites, which depending on their chemical and biological properties, can become trapped within the cell or released back into the circulation and subsequently cleared from the body [18]. PET or SPECT imaging will capture the totality of the radiolabeled protein and its metabolites. Thus, the choice of the linkage between radiolabel and protein, is of critical importance, and will be discussed in detail in a separate section below.
The availability of multiple epitopes on a target, and corresponding antibodies can add an additional degree of flexibility in designing a targeting strategy. For example, it has been possible to identify antibodies that recognize only cell-bound, and not shed versions of proteins that are established serum markers [19]. Engagement of different epitopes can lead to differing internalization outcomes, providing flexibility with regard to radiolabeling and delivery strategies [20]. In designing a companion imaging agent to a therapeutic antibody, matching the epitope can provide highly specific information about the availability of the exact therapeutic target in vivo, and enable assessment of delivery (coverage) of the therapeutic agent. This provides a means for individualized assessment of target expression to guide patient selection, and can also provide a rational, and personalized approach to establishing optimal dosing of therapeutic antibodies. Alternatively, there are clearly situations where use of an imaging agent recognizing a non-overlapping epitope can provide complementary information not otherwise accessible, such as a measure of the persistence of cells carrying the target even in the presence of saturating doses of the corresponding therapeutic antibody.
3. Engineering antibodies for radionuclide delivery
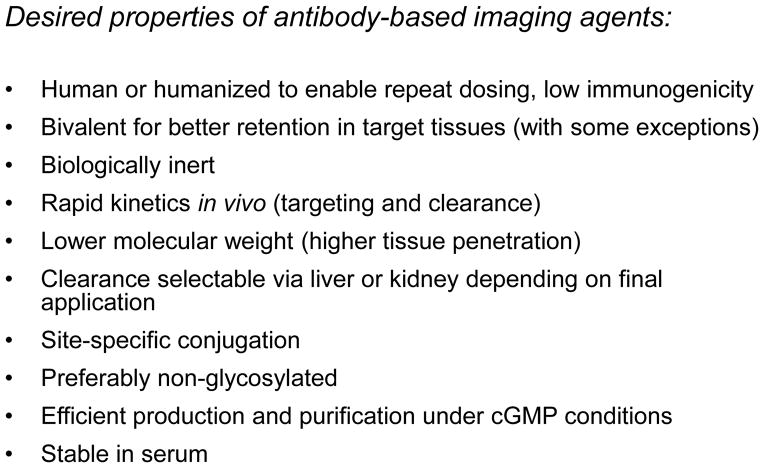
Advances in protein engineering have laid the groundwork for ongoing successful development of therapeutic antibodies and also provide the basis for developing antibody-based imaging agents. While some of the characteristics of an antibody-based imaging agent overlap with features that have been optimized and refined for therapeutic antibodies, there are also aspects which diverge. Figure 1 summarizes many of the desired properties of antibodies for imaging applications. A common requirements for clinical use is the availability of agents with minimal immunogenicity. This goal readily met through routine humanization or deimmunization of murine monoclonal antibodies, or through de novo production of human antibodies via display methodology (phage, yeast, in vitro) or transgenic mice. Where possible, generation of antibodies that also cross-react with the analogous rodent or non-human primate antigens can be useful for targeting and toxicity studies, since their normal tissue binding properties will more closely resemble the human setting.
Figure 1.
Key characteristics to consider when designing an antibody-based agent for imaging purposes.
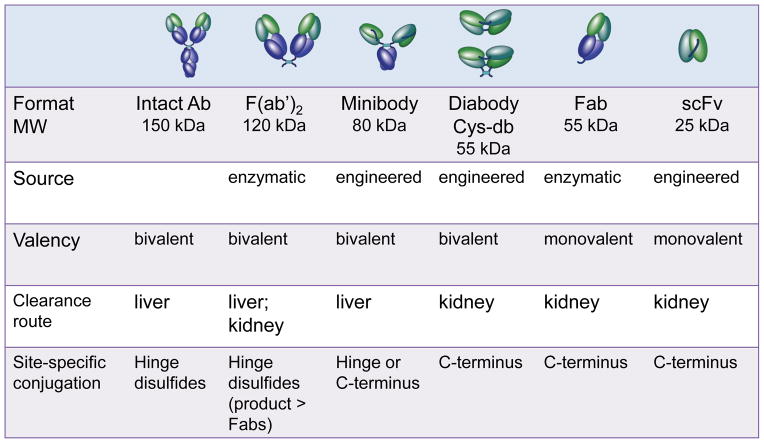
Beyond that, requirements for imaging vs. therapeutic antibodies diverge. An important criterion for an imaging agent is that it be biologically inert. It is necessary and sufficient for an antibody imaging agent to recognize its cognate target and to generate a signal that can be detected outside the body. Otherwise, any biological interaction resulting in functional perturbations (such as cell activition, induction of apoptosis, recruiting and engagement of immune effector cells) opens the door to side effects and safety concerns. An understanding of the many ways that therapeutic antibodies induce responses provides guidelines for abrogating biological activity in antibody imaging. Fc interactions with complement and Fc receptors on immune effector cells have been eliminated by removal of the Fc region (either through generation of conventional enzymatically-derived Fab or F(ab′)2 fragments, or by producing recombinant fragments lacking immunoglobulin Fc or CH2 domains; see Figure 2). Many therapeutic antibodies can bind bivalently to the cell surface, cross-linking their targets and inducing activation, signalling, and internalization. Either a different epitope should be targeted, or the possibility of developing high-affinity, monovalent binding agents should be explored. Other therapeutic antibodies have been specifically selected to display agonistic or antagonistic effects upon binding to their cognate receptors, and again, these may not be ideal for imaging purposes; it may be necessary to resort to an antibody recognizing a different epitope on the same antigen. It should be noted that the significantly lower protein doses used for antibody imaging could also lessen the likelihood of a biological or immunological response. Potential biological interactions are reduced even further by the use of antibody fragments, which due to the absence of Fc regions, clear quickly from the circulation, reducing overall exposure of tissues and organs to the agent.
Figure 2.
Overview of salient features of intact antibodies, enzymatic fragments such as F(ab′)2 and Fab, and engineered fragments including minibody, diabody/cys-diabody (cys-db), and scFv.
Pharmacokinetics is a second key areas where therapeutic vs. imaging applications for antibodies require differing approaches. Native antibodies make excellent drugs; their innate long circulation times (typically 1–3 weeks) ensure steady levels of drug exposure without the need for frequent dosing. The presence of the agent at significant concentrations in the blood does not impact, and indeed enhances, overall therapeutic outcomes. However, when a payload such as a therapeutic radionuclide (e.g. an alpha- or strong beta-emitting radioisotope) is attached to the antibody, circulating activity becomes a liability, resulting in dose deposition in key organs and tissues including bone marrow, kidney, and/or liver. Instead, antibody fragments and engineered antibodies with shorter circulating half-lives have been employed for radionuclide delivery [21]. Fragments typically retain the specificity and affinity of the corresponding parental antibody. Yet they exhibit much more rapid kinetics than intact antibodies, with circulating half-lives measured in hours instead of days. A large component of the accelerated clearance of antibody fragments can be attributed to lack of an Fc region. The Fc regions of intact antibodies interact in a pH-dependent fashion with the FcRn recycling receptor on the vascular endothelium, in liver, and in many other tissues. Following endocytosis, FcRn interactions divert intact antibodies back to the cell surface for release, prolonging their persistence in the circulation. In contrast, fragments lacking the Fc are not protected from transport to the lysosomal compartment and subsequent degradation.
Proteins including antibody fragments, with molecular weights below about 60 kDa, are subject to rapid renal clearance and elimination, further accelerating the kinetics of small fragments. Larger antibody-based fragments, above the threshhold for renal clearance, are removed by the liver, with the rate controlled by the presence or absence of the Fc domain (Figure 2). Clearance rates can be further modulated by introducing mutations into the Fc region at sites of interaction with the FcRn. Mutations which enhance binding to the FcRn have been identified, and their incorporation into intact antibodies can be used for half-life extension of therapeutic antibodies [22]. Alternatively, mutations can be introduced replacing key amino acid residues required for FcRn binding, reducing interactions and accelerating clearance and metabolism. Kenanova et al. used this approach to develop scFv-Fc variants exhibiting short to intermediate circulating half lives (with terminal half-lives ranging from 8 to 80 hours in mouse models) [23].
Interactions of intact immunoglobulins with class-specific Fc receptors on immune cells can also play a significant role in persistance of exogenously administered antibodies, with both circulating cells as well as well as pools of resident immune cells in lymphoid tissues such as the spleen and lymph nodes. This reinforces the importance of considering the pharmokinetic as well as biologic properties of an antibody when designing an agent for targeted delivery.
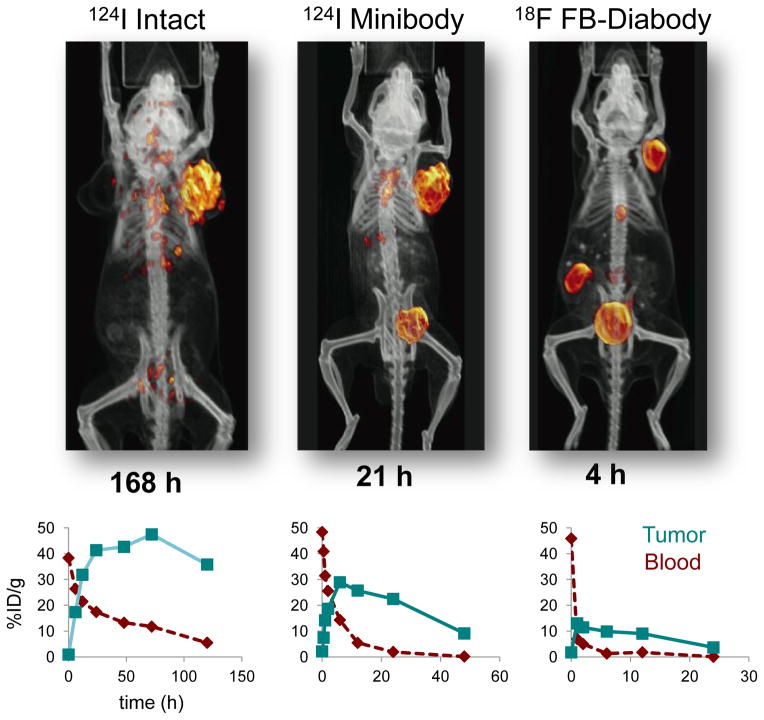
Engineered fragments based on antibody variable regions provide a versatile approach for retaining the specificity and affinity of the parental antibody while accelerating clearance from the circulation, which should lead to high contrast between antigen-expressing target tissues and background activity. The use of antibody fragments for imaging can replace current protocols requiring a 5–7 day delay between administration of intact antibodies and image acquisition, with next-day or even same-day imaging. Building upon single-chain antibody variable fragments (scFv) has facilitated the production of a spectrum of larger, bivalent single-chain formats, including scFv-Fc fusion proteins (105–110 kDa), minibodies (~80 kDa), and diabodies (~55 kDa). In our work, we have focused on minibody and diabody (including a cysteine-modified diabody; see below) due to their advantageous combinations of rapid targeting and faster clearance than intact antibodies (terminal half-lives in mice of about 5–10 h for minibodies and 3–4 h for diabodies, respectively) [24, 25]. This is illustrated in Figure 3, which shows microPET images acquired using 124I- or 18F-radiolabeled antibodies and fragments recognizing prostate stem cell antigen (PSCA) in athymic mice carrying LAPC-9 human prostate cancer xenografts [26, 27]. Antigen-driven targeting and imaging is evident for all three formats, but requires 168 h for intact antibody, whereas use of radiolabeled minibody or diabody results in high contrast images within 21 or 4 h respectively. Imaging using antigen-negative control tumors (e.g. PC-3), or a control (anti-CD20) fragment in the prostate cancer model showed minimal localization, confirming specificity. The potential for same-day imaging using and F-18 radiolabeled antibody fragment provides a path for establishing routine immunoPET in the clinic [28, 29].
Figure 3.
Accelerated targeting, clearance, and imaging afforded by minibody and diabody formats compared to intact antibodies. Top row shows representative microPET images using 124I- radiolabeled intact antibody and minibody, and 18F-radiolabeled diabody recognizing prostate stem cell antigen (PSCA) in athymic mice bearing LAPC-9 human prostate cancer tumor xenografts [2, 26, 27]. Imaging times post injection are indicated below each image. Bottom row shows typical tumor and blood biodistribution curves for radiolabeled intact, minibody, and diabody formats in s.c. xenograft models in mice (anti-CEA in LS174T human colon cancer tumors) [21].
Control over the location and extent of conjugation of antibodies destined for the clinic is increasingly recognized as central, not only in order to provide homogenous, well-characterized products, but also assure to uniform and predictable activity. This has emerged as a key issue in the development of antibody-drug conjugates [30] but is also highly pertinent in the advancement of antibody imaging. This is frequently accomplished by site-specific chemical conjugation, which requires the identification or introduction of selectively reactive sites in an antibody, remote from the target binding site or other functional regions of the protein. Antibodies have been modified via endogenous sites including the hinge or inter-chain disulfide bridges following mild reduction, or by chemical modification of the carbohydrate groups characteristicaly found on CH2 domains. Alternatively, cysteine residues can be engineered into proteins to provide novel sites for chemical conjugation [31]. Unnatural amino acids providing uniquely reactive chemical groups can be introduced using in vitro transcription/translation systems or by engineering the production cells themselves [32, 33].
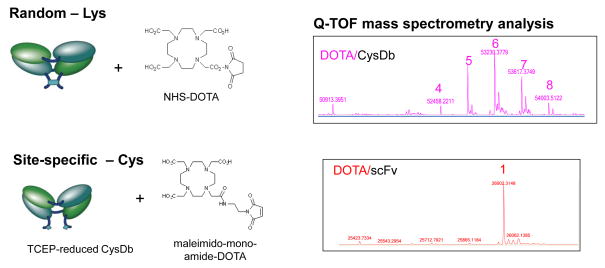
An example of an approach to site-specific conjugation is shown in Figure 4. Cysteine residues were introduced at the C-termini of the polypeptides that comprise an anti-ALCAM diabody [34]. Upon dimerization, oxidation results in formation of a disulfide bridge. Chemical conjugation of a metal chelating moiety, DOTA, was accomplished at random surface lysine residues of the anti-ALCAM cys-diabody using NHS-DOTA. Alternatively, following mild reduction of the cys-diabody with TCEP, the C-terminal cysteines were reacted with maleimido-mono-amide-DOTA. The extent of modification of the proteins was assessed by mass spectrometry. As can be seen in Figure 4, randomly conjugated cys-diabodies carried from 4 to 8 DOTA moieties per 50 kDa dimer, whereas site specific conjugation produced a well-defined product with 1 DOTA per 25 kDa monomer (2 per dimer), as anticipated (K. McCabe, R. Moore, A. Wu, unpublished). Importantly, the modifications are located on the opposite face of the protein from the antigen-binding site; thus, this approach facilitates high retention of immunoreactivity of the conjugated and radiolabeled protein.
Figure 4.
Random vs. site-specific conjugation of a chelating agent to an ALCAM-specific cys-diabody. Conjugation to random lysine residues was accomplished using NHS-DOTA; site-specific conjugation was conducted by mild reduction using TCEP followed by reaction with maleimido-DOTA. On the right, mass spectrometry analysis reveals a range of modification (4–8) by the random method, and uniform addition of a single DOTA moiety to each monomer chain of the cys-diabody following site-specific conjugation (K. McCabe, R. Moore., T. Lee, unpublished).
4. Selection of radionuclide and conjugation strategy
A growing spectrum of radionuclides has become available for attachment to molecularly targeted agents for radioactive imaging, emitting either single-photons (for single photon emission computed tomography; SPECT) or positrons (for PET). The first wave of nuclear medicine instrumentation and probes utilized radionuclides that emit single photons (gamma rays), and clinical detection and imaging is conducted using planar gamma cameras, or 3-dimensional SPECT. The most widely used radioisotope has been 99mTc, due to the convenience of generator production, ensuring a steady and reliable source on site in hospital or clinic radiopharmacies. Its 6 h half-life is also convenient and practical for routine use. Arcitumomab combined a fast-clearing anti-CEA Fab fragment with 99mTc to provide a clinical imaging agent for colon cancer. Longer-lived gamma emitters such as 111In (2.8 d), 131I (8.0 d), and 177Lu (6.7 d) are better matched to the circulation times of intact antibodies, and 111In was the radionuclide selected for labeling the FDA-approved imaging products satumomab pendetide (anti-TAG-72) and capromab pendetide (anti-PSMA). 131I and 177Lu also emit beta particles and represent combined imaging/therapeutic radionuclides.
More recently, the development of new radiotracers labeled with positron-emitting radionuclides has dramatically increased. Positron-emission tomography offers inherent advantages over single-photon imaging, including higher sensitivity, improved resolution, and quantitation. Decay of certain proton-rich nuclides results in the ejection of a positron (β+), which will lose energy through scattering in surounding tissue until it encounters an electron and the particles annihilate. The result is emission of two 511 keV photons in coincidence at approximately 180°, which can be detected by a ring of detectors. Thus, decay events that release a positron have a higher information content due to production of two photons with a defined geometrical relationship, and image reconstruction can more accurately localize and quantify the source of the emission.
Spurred by the broad availability and utility of 18F-fluoro-deoxyglucose as a molecular imaging probe for elevated metabolism, there is now a comprehensive infrastructure in place for production of PET tracers (cyclotrons, cGMP radiopharmacies) and use in hospitals and clinics worldwide. PET probe development has been weighted towards small molecules labeled with F-18, due to alignment between the typical pharmacokinetics of drugs and analogs, with the physical half-life of the isotope (1.9 h). Availability of additional positron-emitting radionuclides with a range of physical properties (Figure 5) has significantly expanded in recent years.
Figure 5.
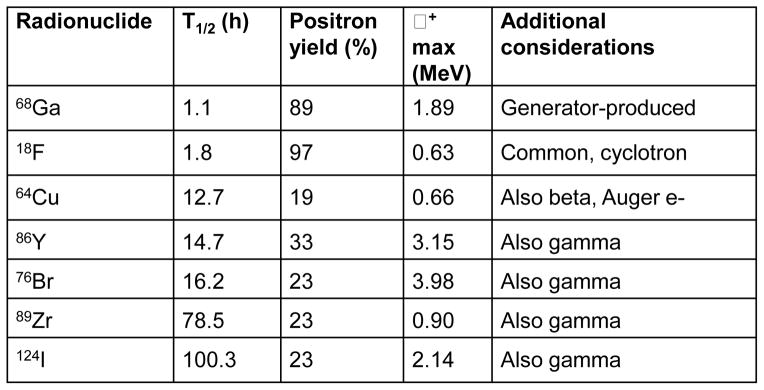
Properties of positron-emitting radionuclides commonly used for labeling proteins, including antibodies and fragments. See also [35].
Selection of an appropriate radionuclide tag for an antibody imaging application will depend on many factors. Several sources are available which provide an overview of different classes of radionuclides that have been used in conjunction with antibodies for imaging and/or therapy for PET imaging in particular [35]. With regard to PET isotopes, the physical properties of a specific radionuclide, including its half-life, positron yield, positron range, and overall decay scheme (including additional emissions) will determine suitability for an antibody imaging application (Figure 5). In general, matching the physical half-life of the radionuclide with the biological half-life of the targeting agent is desirable, such that sufficient counts are available for detection at the end of the uptake and localization period. Radionuclides with high positron yield, such as F-18, reduce the overall activity that must be administered in a study; conversely, radionuclides that decay with lower positron yields also release other emissions (beta, gamma) which can complicate quantitation and contribute to absorbed dose.
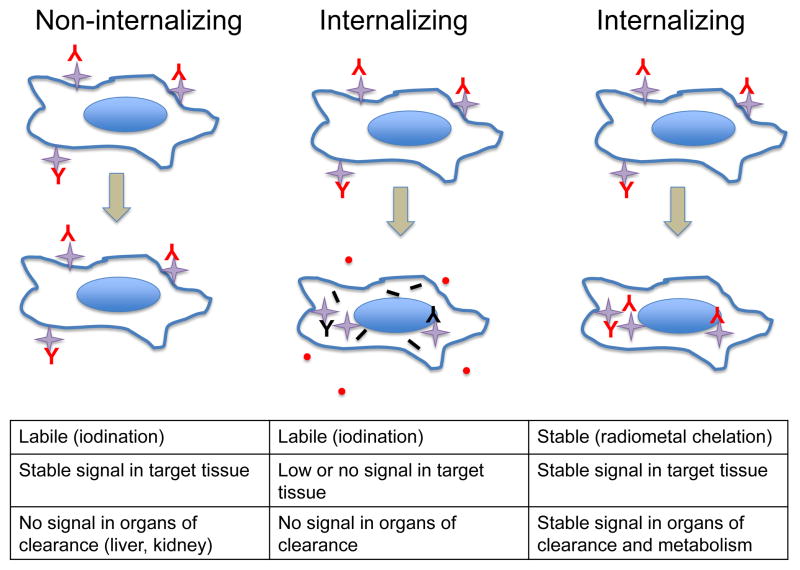
Chemical properties will drive the selection of a conjugation approach, whether the isotope of interest is a halogen or radiometal. Biological properties are also important, particularly with regard to serum stability, metabolism and disposition of radioactive catabolites, since the detectors in imaging instruments will strictly record the physical decay of the radionuclide, regardless of what it might be attached to. As a result, it is important to consider the fate of the radiolabeled tracer (e.g. antibody) upon interaction with its target on cells and tissues, in particular with regard to internalization and catabolism, as this will influence the selection of radionuclide and conjugation approach. For example, standard iodination methods, which result in attachment of iodine to tyrosine residues in proteins under oxidizing conditions (e.g. using Chloramine-T or Iodogen), result in a labile linkage between radionuclide and protein. Upon internalization and catabolism, degradation releases radioactive iodotyrosine that is readily released from the cell [18]. If the cell surface target is non-internalizing (including GPI-linked proteins such as CEA and PSCA, or some transmembrane proteins such as CD20), this can be advantageous since activity will be retained at the target tissue, and any non-bound radiotracer will be cleared via liver or kidney where subsequent metabolism will result in the disappearance of normal tissue signal (Figure 6, left). However, if the target internalizes, labile iodination would not be favorable, since signal would also be lost at the target tissue (Figure 6, middle). Instead, it is necessary to use a residualizing approach for radiolabeling the antibody, either employing a radiometal-chelate complex or stable iodination chemistry. In this case, following cellular internalization and catabolism of the labeled antibody, the resulting radioactive metabolites remain trapped in the lysosomal compartment of the cell, and signal is retained in the target tissue (Figure 6, right). A downside of using a residualizing label is that radioactivity will also remain trapped in the primary organ of clearance (typically liver for larger proteins, or kidneys for lower molecular weight agents).
Figure 6.
Radiolabeling strategies for non-internalizing vs. internalizing targets. Left column depicts targeting of a non-internalizing cell-surface target using a radioiodinated antibody or fragment; signal at the target cells and tissues is retained, and whole body-background is minimized due to loss of signal in clearance organs (liver, kidney) following internalization. Center column indicates that this strategy is not effective for cell-surface targets that internalize; signal will be lost from the target tissue as well. Right column demonstrates that use of a residualizing radiolabel (radiometal chelate complex or stable iodination) will ensure retention of the signal at the target tissue, at the cost of retention in organs/tissue of clearance and metabolism.
Nonetheless, there are at least two major advantages of developing molecular imaging agents based on radiolabeled antibodies as opposed to small molecules. First, despite the extensive diversity of their binding specificities, antibodies are structurally and biochemically very similar. As a result, the standardized methods for conjugation and radiolabeling that have been established and optimized over the years can be applied to essentially any antibody. Secondly, since proteins are quite large compared to the radioactive, optical or other tags that have been attached, their overall binding and biological properties can be maintained. In contrast, development of small molecule radiotracers can be significantly more challenging; new radiochemistry must be developed to incorporate radionuclides into each candidate small molecule. There is also a significant risk that alteration of a single atom, or appendage of a larger functional group, can dramatically alter the binding specificity, targeting, and metabolism/clearance of a candidate small molecule radiotracer. Evidence of the breadth of applicability of antibodies and their engineered fragments as molecular imaging agents for PET is shown in Figure 7. Shown are eight examples of recombinant antibody fragments (diabodies/cys-diabodies or minibodies) generated from parental antibodies recognizing cell surface markers that are highly relevant to different tumor types, with potential applications in breast, colon, pancreatic, ovarian, and prostate cancers as well as lymphoma [2, 17, 29, 34, 36–39]. In all cases, fragment engineering was straightforward and successful, and a variety of positron-emitting radionuclides (including 18F, 64Cu, 89Zr, and 124I) have all been employed as labels for immunoPET.
Figure 7.
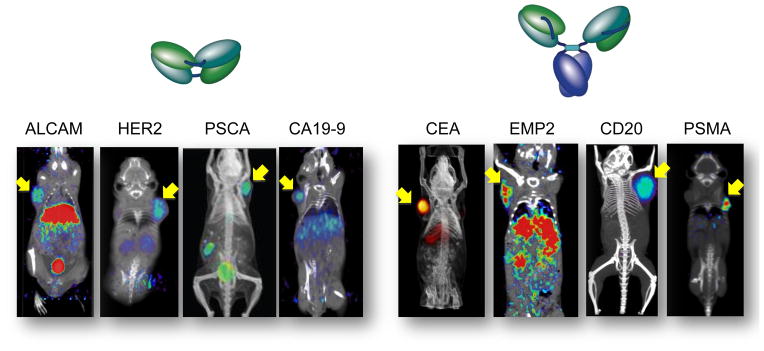
Application of microPET imaging using diabodies and minibodies in a panel of subcutaneous tumor xenograft models in immunocompromised mice. ImmunoPET probes used include: 64Cu-DOTA anti-ALCAM cys-diabody; 18F-anti-Her2 diabody; 18F-anti-PSCA diabody; 124I-anti-CA19-9 diabody; 124I-anti-CEA minibody; 64Cu-DOTA-anti-EMP2 minibody; 124I-anti-CD20 minibody; 124I-anti-PSMA minibody. Further details are in the literature references provided in the text.
Further development, including clinical translation and eventual commercialization of immunoPET agents, requires assessment of additional, practical considerations. The method of production and half-life of a radionuclide impose constraints on availability. Short-lived radionuclides (exemplified by 18F) are typically produced in biomedical cyclotrons, in hospitals or commercial radiopharmacies, for local use or regional delivery. Generators have also provided a local source of radionuclides, facilitating the establishment of 99mTc as the dominant single-photon emitter for general clinical use. The PET radionuclide 68Ga is also generator-produced, providing a path for broader adoption. Radionuclides with slightly longer half-lives (such as 64Cu) can pose challenges if they must be shipped; there is little room for error in the case of a missed connection. Longer-lived PET radionuclides such as 89Zr and 124I are gaining favor due to wider availability and greater flexibility in scheduling. They also are an excellent match for labeling intact antibodies, as evidenced by expanding examples of clinical immunoPET [40–43]. Although it may seem counterintuitive, the longer-lived radionuclides are also excellent candidates labeling antibody fragments (with accelerated kinetics) as clinical imaging tracers, primarily because this approach lends itself to centralized radiolabeling and shipping.
5. Applications
A major impetus behind recent interest in molecular imaging has been the development and maturation of molecularly-targeted therapeutics, spanning small molecules, biologicals, vaccines, and cell-based therapies. In order to tailor treatments specifically to a patient’s individual disease, equally sophisticated diagnostics are needed. As discussed above, antibody-based molecular imaging agents can be developed for highly sensitive, non-invasive detection and imaging of cell-surface biomarkers. The power of immunoPET is vested in the multitude of applications that can be addressed. For example, the availability of more sensitive and specific imaging agents will enhance the determination of whether tumors are present or have spread, providing essential information to inform decisions between local and systemic therapies. Moreover, phenotypic information supplied by immunoPET can be exploited to provide information on the tissue of origin and expression of a variety of biological markers including receptors, adhesion molecules, activation markers, etc. If there is a corresponding targeted therapeutic (which can be a biomolecule, small molecule, or other agent), target expression can play a central role in therapy selection.
During the development of therapeutic antibodies (including antibody-drug conjugates), radiolabeling and PET imaging of the agent itself can provide quantitative information on targeted delivery, pharmacokinetics, and normal tissue retention. It also opens the way for in vivo blocking studies, modeled on extensive use of PET imaging in neurosciences for assessing receptor occupancy. Importantly, quantitiative imaging may provide an important approach for antibody dose determination through traditional biodistribution and competitive-binding assays. This concept can be further extended by employing a rapidly targeted and penetrating antibody fragment in an “enhanced competition experiment” (ECE) format, as described by Beckman et al. [44].
Another practical application will be the ability to assess the rate and extent of internalization and catabolism of an antibody:antigen complex in vivo. Internalization and metabolism are key proccess that must be understood in order to develop effective therapeutics such as antibody-drug conjugates, and most studies are currently conducted using cell culture models. In an extension of this concept to the in vivo setting, comparisons of the uptake and retention of an antibody conjugated with residualizing vs. non-residualizing radiolabels can be used to establish the rate and extent of internalization non-invasively in living organisms or patients, providing critical information not readily available by other means [39].
Response to therapy can be assessed using antibody-targeted imaging, which can be employed whether the drug is a small molecule, antibody, or cell-based or immunological agent. Antibody-directed imaging can provide measure of decrease in tumor size, complementary to anatomic imaging such as CT or MRI. Loss of antigen-positive cells and/or target downregulation can also be imaged. If the therepeutic itself is an antibody, one can also employ antibody agents with non-overlapping epitope specificity to image in the presence of blocking or saturating doses of the therapeutic. The breadth of applicability of antibody-based imaging of response to therapy has been highlighted by studies focusing cell surface expression of targets that serve as readouts of intracellular events. For example, Smith-Jones assessed the activity of hsp90 inhibitors by imaging cell surface expression of its downstream client Her2, using 68Ga-labeled F(ab′)2 fragments of trastuzumab [45]. Similarly, upregulation of PSMA is a downstream effect of inhibition of the androgen-receptor axis, as shown using 89Zr-J591 antibodies in mouse models treated by castration or administration of the anti-androgen MDV3100 [46].
Clinical development of antibodies for imaging cancer is not without its limitations and challenges. The current resolution of commercial PET scanners in clinical use is typically around 4–5 mm; CT and MRI are capable of much higher resolution imaging. Development costs of a biological imaging agent are high, particularly if a recombinant format must be produced for clinical use. Clinical imaging studies are more cumbersome and much more expensive than standard laboratory diagnostic tests, and the value of the potential information provided must be high in order to justify the effort and expense. Nonetheless, the potential of antibody-based imaging to generate highly specific, quantitative biological and functional information about disease in individual patients seems worthy of pursuit, as a component of precision medicine in the future.
6. Conclusion and perspectives
In summary, non-invasive imaging of cell-surface phenotypes using antibodies or engineered antibody fragments can facilitate broad new inquiries into biological activity and function at the molecular level. The information provided by molecular imaging can inform and enhance disease management at many points in the cancer care continuum. One step where molecular imaging is not likely to play a major role is in general screening for cancer, due to the costs and the risks associated with administering low levels of radioactivity. However, once cancer is detected, molecular imaging can enhance detection of regional and distant metastases, information critical for appropriate staging and treatment selection. Non-invasive phenotypic assessment of disease can contribute to prognostic and predictive knowledge to guide therapy selection, and provide mechanistic and molecular monitoring response to therapy as described above. If disease recurs, the whole-body information provided by molecular imaging can assist in determining whether loco-regional or systemic treatment is required. Importantly, phenotypic (and thus biologic) heterogeneity can be assessed across different lesions, providing important early insights into treatment selection and the potential for failure.
What is the best combination of antibody or fragment with radionuclide for antibody-targeted imaging? This is a question with a multitude of answers, and in the end, design of an immunoPET (or SPECT) agent must be driven by the clinical problem that is being addressed. This is because the development of radiolabeled antibody tracers is a complex, expensive, and time-consuming process combining the manufacturing, regulatory and clinical challenges of a biological agent that is also a radiopharmaceutical. Therefore, a new immunoPET tracer, or indeed any new imaging probe, will only succeed in situations where it provides information that will impact treatment decisions and outcomes. Innovations in non-invasive molecular imaging will be in constant competition with developments in laboratory-based in vitro diagnostics, which currently bring unenviable power and sophistication to the analysis of markers in biopsies, blood, circulating tumor cells, or other specimens, through the use of high-throughput assays and profiling of DNA, RNA, and proteins, etc. Nonetheless, molecular imaging is able to provide unique insights into the function of living tissue, in the whole organism or patient.
Selection of an optimal antibody format for a imaging agent will depend on the specific question asked. Radiolabeling and detection of a therapeutic antibody or ADC would be the best means to assess targeting, biodistribution, metabolism and clearance of the drug itself. Alternatively, in vivo assay of target expression level and dose coverage lend themselves to alternative formats such as engineered antibody fragments, which can offer additional features including rapid kinetics, lack of biological activity, and site-specific conjugation. An expanding list of radionuclides with a spectrum of properties (single-photon vs. positron emitter, half-life, available radiolabeling chemistries) provides many choices and combinations that are straightforward to adapt to antibodies or fragments with the desired specificity. Fortunately, the options available are rapidly expanding and examples preclinical and clinical applications of immunoPET are becoming commonplace. Importantly, the ability to query the molecular characteristics of living tissue enables assessment of not only the tumor cells that signify malignancy, but also vital components including the stroma, vasculature and resident and infiltrating immune cells.
Acknowledgments
Many thanks are due to the members of my laboratory, past and present, as well as the collaborators whom I have had the pleasure of working with over the past years at the Beckman Research Institute of the City of Hope, David Geffen School of Medicine at UCLA, and many other institutions (too many to list individually). Funding has been provided by NIH grants CA092131, CA016042, and CA174294.
Footnotes
Financial Disclosure: Anna M. Wu is a founder and board member of ImaginAb, Inc. which has licensed some of the technologies described herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40(3):167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles SM, Wu AM. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012;30(31):3884–92. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur S, et al. Recent trends in antibody-based oncologic imaging. Cancer Lett. 2012;315(2):97–111. doi: 10.1016/j.canlet.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy S, Robinson MK. Immuno-positron emission tomography in cancer models. Semin Nucl Med. 2010;40(3):182–9. doi: 10.1053/j.semnuclmed.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly T, et al. Fibroblast activation protein-alpha: a key modulator of the microenvironment in multiple pathologies. Int Rev Cell Mol Biol. 2012;297:83–116. doi: 10.1016/B978-0-12-394308-8.00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Schliemann C, Neri D. Antibody-based targeting of the tumor vasculature. Biochim Biophys Acta. 2007;1776(2):175–92. doi: 10.1016/j.bbcan.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Guo K, et al. Monoclonal antibodies target intracellular PRL phosphatases to inhibit cancer metastases in mice. Cancer Biol Ther. 2008;7(5):750–7. doi: 10.4161/cbt.7.5.5764. [DOI] [PubMed] [Google Scholar]
- 8.Costantini DL, et al. (111)In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48(8):1357–68. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen B, et al. Imaging DNA damage in vivo using gammaH2AX-targeted immunoconjugates. Cancer Res. 2011;71(13):4539–49. doi: 10.1158/0008-5472.CAN-10-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–92. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- 12.Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39(3):261–9. doi: 10.1016/j.ctrv.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenanova V, et al. Recombinant carcinoembryonic antigen as a reporter gene for molecular imaging. Eur J Nucl Med Mol Imaging. 2009;36(1):104–14. doi: 10.1007/s00259-008-0921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldenberg DM. Carcinoembryonic antigen as a targent cancer antigen for radiolabeled antibodies: Prospects for cancer imaging and therapy. Tum Biol. 1995;16:62–73. doi: 10.1159/000217930. [DOI] [PubMed] [Google Scholar]
- 15.Beatty JD, et al. Preoperative imaging of colorectal carcinoma with 111-In-labeled anticarcinoembryonic antigen monoclonal antibody. Canc Res. 1986;46:6494–6502. [PubMed] [Google Scholar]
- 16.Girgis MD, et al. CA19-9 as a Potential Target for Radiolabeled Antibody-Based Positron Emission Tomography of Pancreas Cancer. Int J Mol Imaging. 2011;2011:834515. doi: 10.1155/2011/834515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girgis MD, et al. Anti-CA19-9 diabody as a PET imaging probe for pancreas cancer. J Surg Res. 2011;170(2):169–78. doi: 10.1016/j.jss.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 18.Shih LB, et al. The processing and fate of antibodies and their radiolabels bound to the surface of tumor cells in vitro: a comparison of nine radiolabels. J Nucl Med. 1994;35(5):899–908. [PubMed] [Google Scholar]
- 19.Pichinuk E, et al. Antibody targeting of cell-bound MUC1 SEA domain kills tumor cells. Cancer Res. 2012;72(13):3324–36. doi: 10.1158/0008-5472.CAN-12-0067. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt MM, Thurber GM, Wittrup KD. Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability. Cancer Immunol Immunother. 2008;57(12):1879–90. doi: 10.1007/s00262-008-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–46. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 22.Dall’Acqua WF, et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169(9):5171–80. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 23.Kenanova V, et al. Tailoring the pharmacokinetics and positron emission tomography imaging properties of anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments. Cancer Res. 2005;65(2):622–31. [PMC free article] [PubMed] [Google Scholar]
- 24.Hu S, et al. Minibody: A novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 1996;56(13):3055–61. [PubMed] [Google Scholar]
- 25.Wu AM, et al. Anti-carcinoembryonic antigen (CEA) diabody for rapid tumor targeting and imaging. Tumor Targeting. 1999;4:47–58. [Google Scholar]
- 26.Leyton JV, et al. Humanized radioiodinated minibody for imaging of prostate stem cell antigen-expressing tumors. Clin Cancer Res. 2008;14(22):7488–96. doi: 10.1158/1078-0432.CCR-07-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepin EJ, et al. An affinity matured minibody for PET imaging of prostate stem cell antigen (PSCA)-expressing tumors. Eur J Nucl Med Mol Imaging. 37(8):1529–38. doi: 10.1007/s00259-010-1433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu K, et al. Microfluidic-based 18F-labeling of biomolecules for immuno-positron emission tomography. Mol Imaging. 2011;10(3):168–76. 1–7. [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe KE, Wu AM. Positive progress in immunoPET--not just a coincidence. Cancer Biother Radiopharm. 2010;25(3):253–61. doi: 10.1089/cbr.2010.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strop P, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20(2):161–7. doi: 10.1016/j.chembiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Olafsen T, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17(1):21–7. doi: 10.1093/protein/gzh009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swartz JR. Universal cell-free protein synthesis. Nat Biotechnol. 2009;27(8):731–2. doi: 10.1038/nbt0809-731. [DOI] [PubMed] [Google Scholar]
- 33.Young TS, Schultz PG. Beyond the canonical 20 amino acids: expanding the genetic lexicon. J Biol Chem. 2010;285(15):11039–44. doi: 10.1074/jbc.R109.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe KE, et al. An engineered cysteine-modified diabody for imaging activated leukocyte cell adhesion molecule (ALCAM)-positive tumors. Mol Imaging Biol. 2012;14(3):336–47. doi: 10.1007/s11307-011-0500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nayak TK, Brechbiel MW. Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges. Bioconjug Chem. 2009;20(5):825–41. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundaresan G, et al. 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med. 2003;44(12):1962–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Fu M, et al. Positron emission tomography imaging of endometrial cancer using engineered anti-EMP2 antibody fragments. Mol Imaging Biol. 2013;15(1):68–78. doi: 10.1007/s11307-012-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olafsen T, et al. Recombinant anti-CD20 antibody fragments for small-animal PET imaging of B-cell lymphomas. J Nucl Med. 2009;50(9):1500–8. doi: 10.2967/jnumed.108.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olafsen T, et al. Assessment of in vivo internalization by microPET imaging of prostate specific membrane antigen (PSMA)-expressing xenografts using 64Cu vs 124I radiolabeled minibodies. Journal of Nuclear Medicine. 2011;52s:156P. [Google Scholar]
- 40.van Dongen GA, et al. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12(12):1379–89. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 41.Vugts DJ, Visser GW, van Dongen GA. 89Zr-PET radiochemistry in the development and application of therapeutic monoclonal antibodies and other biologicals. Curr Top Med Chem. 2013;13(4):446–57. doi: 10.2174/1568026611313040005. [DOI] [PubMed] [Google Scholar]
- 42.Divgi CR, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol. 2013;31(2):187–94. doi: 10.1200/JCO.2011.41.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deri MA, et al. PET imaging with (8)(9)Zr: from radiochemistry to the clinic. Nucl Med Biol. 2013;40(1):3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckman RA, von Roemeling R, Scott AM. Monoclonal antibody dose determination and biodistribution into solid tumors. Ther Deliv. 2011;2(3):333–44. doi: 10.4155/tde.10.91. [DOI] [PubMed] [Google Scholar]
- 45.Smith-Jones PM, et al. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol. 2004;22(6):701–6. doi: 10.1038/nbt968. [DOI] [PubMed] [Google Scholar]
- 46.Evans MJ, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108(23):9578–82. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]