Abstract
Problem
The goal of this study was to investigate the phenotype and functional responsiveness of CD4+ and CD8+ T-cells in the upper reproductive tract of healthy premenopausal women. The lower reproductive tract is frequently studied as a site of sexually transmitted infections; however, the upper tract may also be a portal of entry and dissemination for pathogens, including HIV-1.
Method of Study
Endometrial biopsy, endocervical curettage, cytobrush and blood were collected during mid-luteal phase from 23 healthy women. T-cells were isolated and analyzed by flow cytometry.
Results
As compared to their counterparts in blood, endometrial and endocervical T-cells had enhanced CCR5 expression, and were enriched for activated, effector memory cells. Endometrial T-cells were more responsive to polyclonal stimuli, producing a broad range of cytokines and chemokines.
Conclusions
These findings underscore the responsiveness of endometrial T-cells to stimulation, and reveal their activated phenotype. These findings also suggest susceptibility of the upper reproductive tract to HIV-1 infection.
Keywords: T-cell, CTL, HIV, STD, Endometrium, Endocervix
INTRODUCTION
The female reproductive tract (FRT) is unique in that it must be immunologically tolerant of a semi-allogeneic conceptus, but must also retain the ability to respond to potential pathogens. The lower FRT includes the vagina and ectocervix; the upper FRT includes the endocervix, uterine endometrium and fallopian tubes. The mucosal lining in the lower FRT is composed of multi-layered stratified squamous epithelium1, whereas the upper FRT is lined by single-layered columnar epithelium with tight junctions 2, 3. The mucosal immune system in the FRT consists of epithelial cells, stromal cells and immune cells that migrate into the uterus, cervix and vagina 4 and provide a robust physical and immunological barrier against sexually transmitted infections 5. Epithelial cells of the FRT express toll-like receptors 6 as well as intracellular pattern recognition receptors 7 and secrete a variety of cytokines, growth factors, chemokines and antimicrobial peptides that participate in host defense through interactions with immune cells that reside in the epithelium and the stromal layer 8, 9.
Lymphocytes and antigen presenting cells are present throughout the FRT. Leukocytes comprise 6–20% of total cells and are dispersed throughout the FRT tissues with the highest proportion seen in the uterine endometrium and fallopian tubes 10. The major population of leukocytes consists of T-lymphocytes, including CD3+ lymphocytes. Granulocytes are also present, particularly in the fallopian tubes. B-lymphocytes and monocytes are dispersed throughout the FRT, but are not as prominent as T-lymphocytes and granulocytes 11. In the upper FRT, reproductive hormone levels modulate immune functions including cytotoxic T lymphocyte (CTL) activity, secretion of immunoglobulins, cytokines, chemokines, growth factors and antimicrobial peptides over the course of the menstrual cycle 11–15. In contrast to the upper FRT, CTL activity and the frequency and distribution of immune cells in the lower FRT (i.e., ectocervix and vagina) remain constant throughout the menstrual cycle 15–17.
Previous studies suggest that the upper FRT might also be a portal for the entry of HIV-1 following sexual intercourse 5, 18–20. Susceptibility of the upper tract to infection likely varies throughout the menstrual cycle, with the highest susceptibility predicted at mid-luteal phase 5, 9, 21–24. Here, we have studied the phenotype and functionality of fresh CD4+ and CD8+ T cells isolated from the endometrium, endocervix, and peripheral blood (PB), collected from healthy premenopausal women during the mid-luteal (secretory) phase of the menstrual cycle. T-cells of both endocervix and endometrium had a predominant activated, effector memory phenotype with increased expression of CCR5 as compared to blood T-cells. Notably, mid-luteal endometrial CD4+ T-cells were significantly enriched for a CCR5+, activated, effector memory phenotype as compared to their counterparts in endocervix, suggesting high susceptibility to HIV-1 infection. In addition, endometrial T-cells (both CD4+ and CD8+) were more responsive to polyclonal stimuli, producing pro-inflammatory cytokines and chemokines, compared to blood T-cells. Taken together, these results reveal that endometrial T-cells have a phenotype that may render them highly susceptible to infection by HIV-1 and other sexually transmitted pathogens.
MATERIALS AND METHODS
Study participants and specimen collection
Participants were enrolled in two clinical research studies approved by University of California, San Francisco (UCSF) Committee on Human Research (CHR). The exclusion criteria for both studies were pregnancy, a current genital herpes outbreak or ≥4 or more herpes outbreaks in the last year, irregular menses, an abnormal Pap test within the past 12 months, recent use of intravaginal or intrauterine contraceptives, use of exogenous sex hormones, abnormal vaginal discharge or bleeding, immunocompromised status or use of immunosuppressive medications, or daily use of non-steroidal anti-inflammatory drugs. Within 7–11 days post luteinizing hormone surge during the mid-luteal phase approximately 20 mL of PB were collected in vacutainer tubes coated with EDTA (BD Pharmingen, SA Jose, CA) and endometrial biopsies were obtained using 3 mm Miltex brand Softflex endometrial biopsy cannula (1–2 passes). The cytobrush (Cytobrush Plus® cell collector, CooperSurgical, Trumbull, CT) and curettage specimens (using Kevorkian curette with basket square tip, CooperSurgical, Trumbull, CT) were obtained from the endocervix. FRT specimens were placed into R-15 medium (RPMI 1640 containing 15% fetal bovine serum, 100 IU/mL penicillin, 100 IU/mL streptomycin, 2 mM and glutamine). All specimens were transported on ice from UCSF within three hours of collection and processed immediately upon receipt.
Isolation of Mononuclear Cells
PBMC were isolated by Ficoll-Hypaque (Pfizer, New York, NY) density gradient centrifugation, and washed in phosphate-buffered saline (PBS). Cells contained in endocervical cytobrush samples were first dislodged from the brushes by rubbing the brushes together, then pipetting a stream of media on the brushes several times. After achieving a suspension, cells were passed through a 70-μM nylon cell strainer (Becton Dickinson Discovery Labware, Bedford, MA) followed by washing in R-15 medium. Cells from endocervical curettage samples were suspended by repeated pipetting, followed by straining and washing, as described above for cytobrush samples. Endometrial biopsies, being solid pieces of tissue, were subjected to two to three rounds of collagenase type II digestion (0.5 mg/mL; Sigma-Aldrich, St. Louis, MO). We previously tested collagenase for digestion of common T-cell surface markers and found it did not significantly affect detection of most markers used for routine T-cell phenotyping, with the exception of CD62L, a marker not included in the present study (C. Cox and B. Shacklett, unpublished data) 25, 26. Collagenase digestion was followed by mechanical disruption using an 18-gauge blunt-end needle and passage through a 70-μM nylon cell strainer. The pooled cells were washed in R-15 medium. Red blood cell lysis was performed when required on PBMC, endometrium and endocervical (cytobrush and curettage) cells using ammonium chloride-potassium carbonate-EDTA (ACK).
Monoclonal Antibodies
Fluorochrome-labeled monoclonal antibodies used for the phenotypic and ICS assay include CD3 (clone UCHT1), CD4 (clone RPA-T4), CD8 (clone SK1), CCR7 (clone 3D12), CXCR4 (clone12G5), CCR5 (clone 2D7), CD8 (clone RPA-T8), IFNγ (clone B27), TNF-α (clone MAb11), MIP-1β (clone D21-1351) from Becton-Dickinson Pharmingen (San Diego, CA), CD45RA (2H4), CD4 (clone T4D11) from Beckman Coulter (Fullerton, CA), CD38 (clone HB7), CD107a (clone H4A3), and IL-10 (clone JES3-19F1) from BD Biosciences (San Jose, CA), HLADR (clone TU36), Aqua amino reactive dye from Invitrogen (Carlsbad, CA), CD66b (G10F5) Biolegend (San Diego, CA) and IL-17 (clone eBio64CAP17), IL-2 (clone MQ1-17H12), from eBioscience (San Diego, CA).
Phenotypic and Intracellular Cytokine Staining (ICS) and Flow Cytometry
PB, endometrial, and endocervical cells were stained the same day of isolation for cell surface phenotype. Fluorescence minus one (FMO) controls were included as needed 27. PBMC and endometrial cells required for the ICS assay were rested overnight in R-15 medium. Briefly, 2–3×106 cells in 200 μL complete medium were treated with anti-CD107a, monensin (1 μM GolgiStopTM, BD Biosciences), brefeldin A (5 μg/mL, Sigma), and staphylococcus enterotoxin B (SEB, 0.5 μg/mL) or phorbol myristate acetate (PMA, 50 ng/mL) and ionomycin (ION, 500 ng/mL). R-15 medium containing anti-CD107a, monensin (1 mM GolgiStopTM, BD Biosciences), and brefeldin A (5 mg/mL, Sigma) served as negative control. Following a 5 hour incubation, cells were incubated for 5 minutes in PBS/2% FCS/0.5 mM/EDTA, stained for surface markers and viable cells using aqua amino reactive dye in PBS/2% FCS for 20 min at 4°C, fixed in 4% formaldehyde, then permeabilized using FACS Perm 2 (BD Biosciences). Cells were then washed in PBS/2% FCS, stained for intracellular cytokines and CD3, washed again, then stored at 4°C in PBS/1% formaldehyde until analysis within 24 hours. The expression of CD107a, IL-2, IL-10, IL-17, TNF-α, IFN-γ and MIP-1β were measured as described previously 28. Flow cytometry data were acquired on an LSRII (BD Immunocytometry Systems, San Jose, CA) equipped with 405, 488, and 643 nm lasers and utilizing FACSDIVA software (BDIS).
Analysis of flow cytometry data was performed using FlowJo software (TreeStar, Ashland, OR). For phenotypic analysis of mucosal T-cells, a broad scatter gate was used to capture lymphocytes, followed by doublet discrimination, then selection of viable CD3+ cells. After excluding granulocytes using CD66b, CD4+ and CD8+ T-cells were identified and further analyzed. For analysis of cytokine production, CD4+ and CD8+ T-cells were gated using a similar strategy and then analyzed for individual responses. The cytokine response values obtained from cells stimulated with SEB or with PMA/ION were then background-corrected using the values obtained from cells treated with R-15 medium alone 29.
Statistical Analysis
CD4+ and CD8+ T-cell phenotypic markers between the tissues were compared using two-tailed Mann-Whitney test with Holms’ sequential Bonferroni correction.30 CD4+ and CD8+ T-cells cytokine production between endometrium and PBMC was compared using Wilcoxon matched pairs signed rank test. All analysis was done using GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS
Characteristics of the study subjects
Twenty-three participants were enrolled in the study, of whom 43% were White/Caucasian, 35% were Black/African-American, 9% were Asian or Pacific Islander and mixed, 4% were Hispanic/Latino. The median age was 36 years (range 28–40).
Flow cytometric analysis of mucosal T-cells
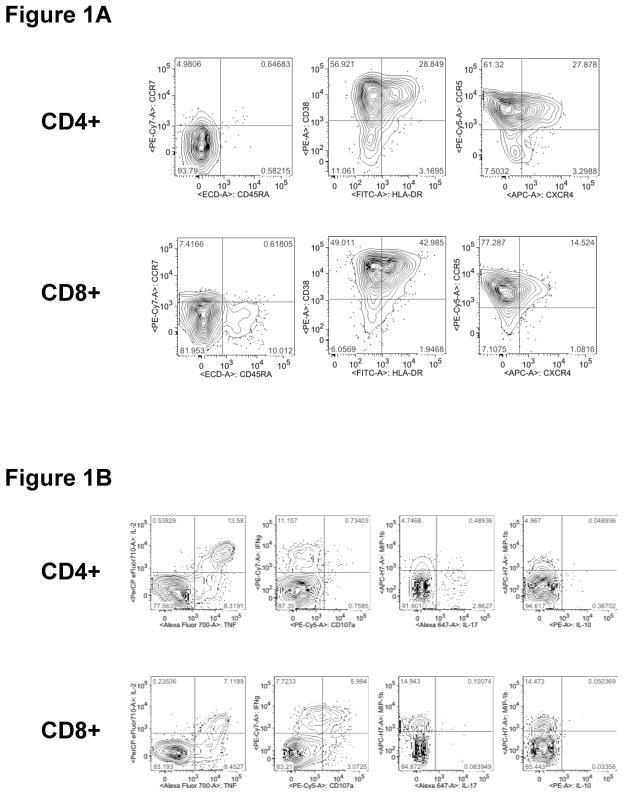
CD4+ and CD8+ T-cells were analyzed by cell surface staining for expression of CD45RA, CCR7, CD38, HLA-DR, CCR5 and CXCR4 as shown in Figure 1A. These phenotypic markers were further analyzed through Boolean combinations of markers for activation (CD38, HLADR), maturation (CCR7, CD45RA) and trafficking (CXCR4, CCR5). In separate experiments, CD4+ and CD8+ T-cells were analyzed for responsiveness to stimulation with SEB or PMA/ION. Seven functional responses were measured: CD107a, IL-2, IL-10, IL-17, TNF-α, IFN-γ and MIP-1β, as shown in Figure 1B and described in Materials and Methods.
Figure 1. Flow cytometry gating used in the analysis of endometrial T-cell phenotype and function.
Panel (A): For phenotypic analysis, after initial gating of endometrial lymphocytes and doublet discrimination, viable cells were gated for CD3+CD66b− (not shown) and then for CD4+ (top row) and CD8+ (bottom row) T-cells. The resulting populations were gated for CD45RA, CCR7, CD38, HLA-DR, CCR5 and CXCR4. Panel (B): For analysis of endometrial T-cell responses to polyclonal stimulation, seven functional responses (CD107a, IL-2, IL-10, IL-17, TNF-α, IFN-γ and MIP-1β) were measured. Initial gating was performed to identify lymphocytes and doublet discrimination (not shown), followed by gating of viable cells on CD4+ (top row) and CD8+ (bottom row) T-cells, and finally for individual responses.
Enhanced expression of CCR5 on endometrial CD4+ and CD8+ T-cells
The percentages of T-cells from endometrium, endocervix and PB expressing chemokine receptors, CCR5 and CXCR4 were measured by flow cytometry (Fig. 2). As compared to PB, CD4+ T-cells from endometrium and endocervix displayed significantly higher percentages of CCR5-positive cells (Fig. 2A). In contrast, the percentage of endometrial and endocervical CD4+ and CD8+ T-cells expressing CXCR4 was significantly lower compared to PB (Fig. 2C & 2D). Interestingly, expression of CCR5 was significantly greater and CXCR4 expression was significantly lower on endometrial CD4+ (Fig. 2A & 2C) and CD8+ T-cells (Fig. 2B & 2D) compared to cytobrush T-cells. Furthermore, the CCR5 median fluorescence intensity (MFI) of endometrial CD4+ T-cells was significantly greater than for PB, (P = 0.0082, Fig. 3) suggesting greater CCR5 receptor density. Elevated expression of CCR5 by CD4+ T-cells in the gastrointestinal tract has also been reported, and is believed to partly explain the high susceptibility of these cells to HIV-1 infection31.
Figure 2. CCR5-expressing T-cells were enriched in endometrium compared to endocervix and peripheral blood (PB).
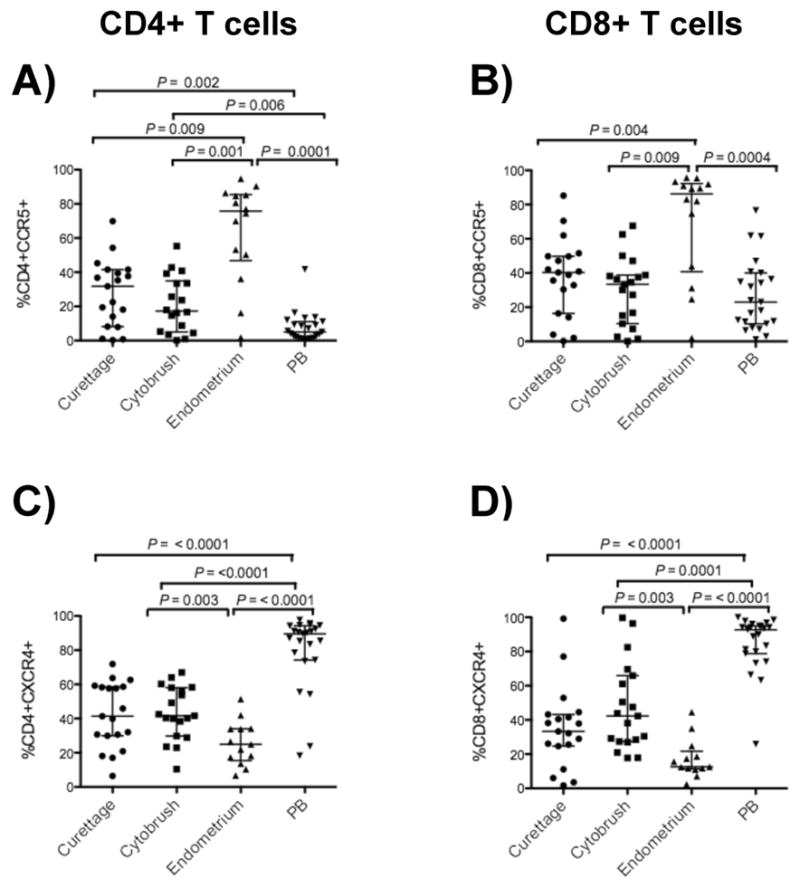
The figures show the percentages of CD4+ (left panels A, C) and CD8+ T-cells (right panels B, D) from curettage, cytobrush, endometrium and peripheral blood (PB) expressing CCR5 and CXCR4. Horizontal lines correspond to the median values and the vertical lines and whiskers represent 25th and 75th percentiles. Two tailed Mann-Whitney tests and Holms’ sequential Bonferroni corrections were performed.
Figure 3. Median fluorescence intensity (MFI) of CCR5 on CD4+ T-cells.
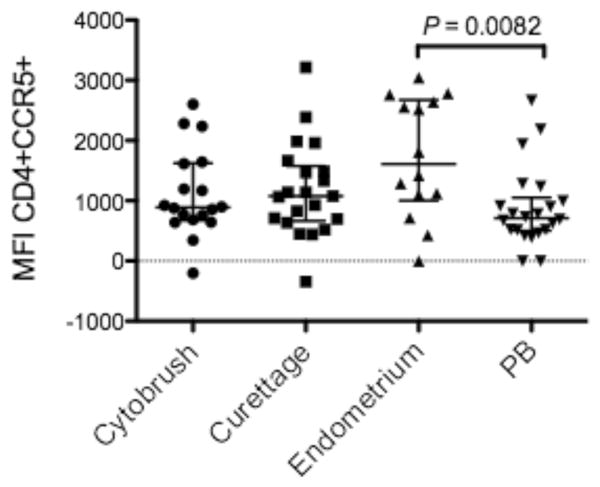
CCR5 MFI was significantly higher on CD4+ T-cells in endometrium compared to PB. Similar trends were observed when comparing endocervical curettage CD4+ T-cells vs PB (P=0.09) and endometrial vs endocervical cytobrush CD4+ T-cells (P = 0.05). MFI was calculated using FlowJo software (Tree Star, Inc., Ashland, OR). Horizontal lines correspond to the median values; vertical lines and whiskers represent 25th and 75th percentiles. Two tailed Mann-Whitney tests and Holms’ sequential Bonferroni corrections were performed.
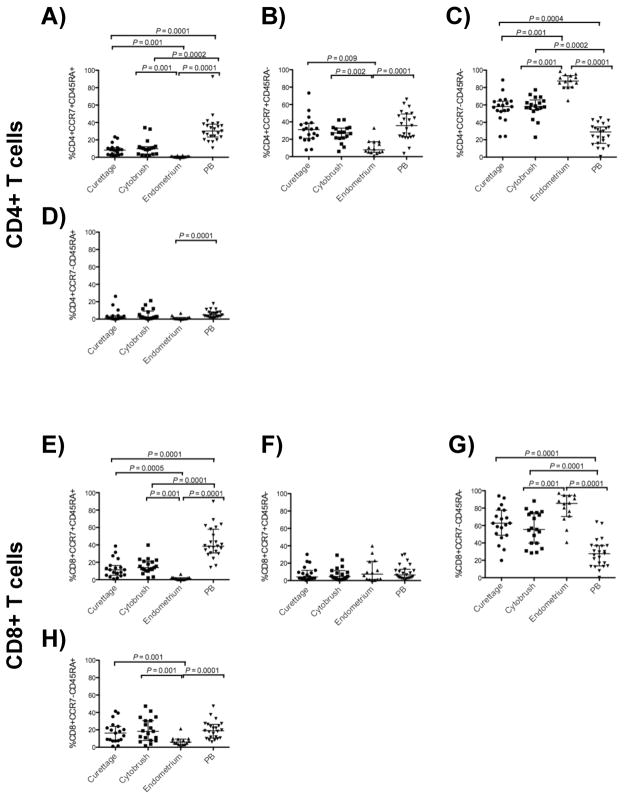
CD4+ and CD8+ T-cells from the endometrium display a memory phenotype
Resting and effector memory CD4+ T-cells have the greatest susceptibility to infection with CCR5-utilizing HIV-132, 33, and different memory/effector T-cell subsets display different effector functions in vitro 34. We measured expression of CCR7 and CD45RA on endometrium, endocervix and PB T-cells to phenotypically characterize memory/effector T-cells from the upper FRT. Populations were defined as naïve (CCR7+CD45RA+), central memory (TCM, CCR7+CD45RA−), effector memory (TEM, CCR7−CD45RA−) and terminally differentiated effector memory (TEMRA, CCR7−CD45RA+) CD4+ and CD8+ T-cells (Fig. 4) 35, 36.
Figure 4. Endometrial CD4+ and CD8+ T-cells display a memory phenotype.
The figure shows percentages of CD4+ (upper panels, A–D) and CD8+ (lower panels, E–H) T-cells with the phenotypes CCR7+CD45RA+ (naïve), CCR7+CD45RA− (central memory), CCR7−CD45RA− (effector memory), and CCR7−CD45RA+ (terminally differentiated memory) from curettage, cytobrush, endometrium and peripheral blood (PB). Horizontal lines correspond to median values; vertical lines and whiskers represent the 25th and 75th percentiles. For statistical analysis, two-tailed Mann-Whitney tests were performed with Holms’ sequential Bonferroni corrections.
Significantly lower percentages of naïve CD4+ and CD8+ T-cells (Fig. 4A, E) were detected in the endocervix and endometrium as compared to PB. Furthermore, the percentage of naïve CD4+ and CD8+ T-cells was significantly lower in endometrium than in endocervical curettage or cytobrush (Fig. 4A, E). Central memory CD4+ T-cells were least abundant in endometrium, but present at similar frequencies in other sites (Fig. 4B), and central memory CD8+ T-cell frequencies were similar at all sites (Fig. 4F). Consistent with a prior report 37, the proportions of TEM CD4+ and CD8+ T-cells were greater in the endocervix compared to PB; we also found this to be true for endometrial cells (Fig. 4C, G). The frequencies of TEMRA CD4+ and CD8+ T-cells were significantly lower in endometrium compared to PB (Fig. 4D, H). The percentages of TEMRA CD4+ T-cells in endocervical samples did not differ significantly from endometrium or blood, but the frequency of TEMRA CD8+ T-cells in endocervix was similar to blood, and significantly greater than in endometrium (Fig. 4H).
Endometrial CD4+ and CD8+ T-cells are highly activated
Coexpression of CD38 and HLA-DR on T-cells is commonly used to assess T-cell activation; expression of these markers is a strong correlate of disease progression in HIV-1 infected individuals 38, 39. In healthy individuals, coexpression of these markers is significantly elevated in T-cells of the gastrointestinal tract, compared to PB31. We measured expression of these markers both individually and in combination. Activated CD4+ and CD8+ T-cells co-expressing CD38/HLA-DR were present at significantly higher frequency in the endometrium and endocervix compared to PB. In addition, CD38/HLA-DR coexpression was significantly higher on endometrial CD4+ and CD8+ T-cells as compared to endocervical T-cells (Fig. 5).
Figure 5. Endometrial CD4+ and CD8+ T cells are highly activated.
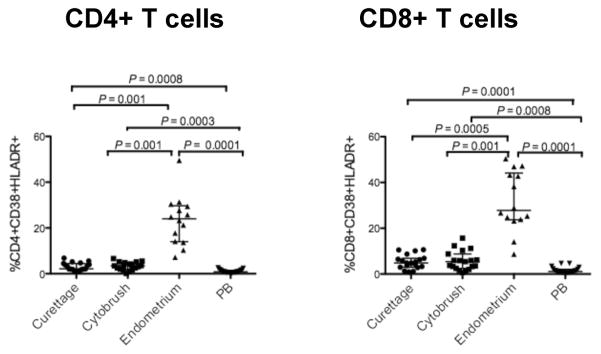
The figure shows the percentage of CD4+ (left, A) and CD8+ (right, B) T-cells expressing CD38 and HLA-DR from curettage, cytobrush, endometrium and PB. Horizontal lines correspond to median values; vertical lines and whiskers represent the 25th and 75th percentiles. For statistical analysis, two-tailed Mann-Whitney tests and Holms’ sequential Bonferroni corrections were performed.
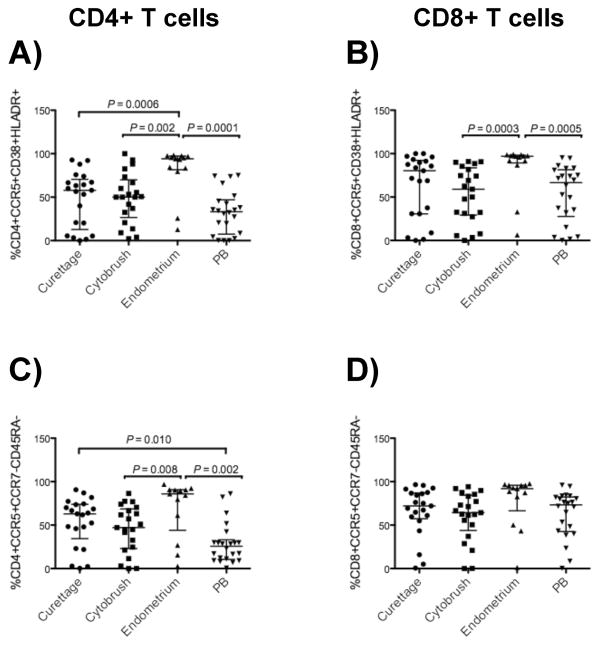
CCR5-expressing TEM and activated T-cells are enhanced in endometrium
CCR5 expression by CD4+ T-cells is associated with an activated, memory phenotype as well as susceptibility to HIV-1 infection. Indeed, activated, memory CD4+ T-cells have been associated with susceptibility to infection with R5-tropic virus and produce the majority of R5-tropic HIV-133, 40–43. We measured the frequencies of CCR5+CD38+HLADR+ and CCR5+CCR7−CD45RA−(TEM) CD4+ and CD8+ T-cells in endometrium, endocervix and PB (Fig. 6). The CCR5− expressing activated and effector memory (TEM) CD4+ T-cell population was significantly more abundant in endometrium compared to endocervix and PB (Fig. 6A, C), suggesting that cells in endometrial tissue may be particularly susceptible to HIV-1 infection. CCR5-expressing TEM CD4+ T-cells were also more abundant in the endocervix (curettage, P = 0.010) compared to PB (Fig. 6C). Most endometrial CD8+ T-cells were also CCR5-positive and expressed activation markers CD38 and HLA-DR, although no difference between tissues was seen in the percentage of CCR5-expressing TEM CD8+ T-cells (Fig. 6B, D).
Figure 6. Enhanced CCR5-expressing activated and effector memory CD4+ and CD8+ T-cells in endometrium.
Shown here are the frequencies of CD4+ (left panels, A and C) and CD8+ (right panels, B and D) T-cells with the phenotypes CCR5+CD38+HLADR+ and CCR5+CCR7−CD45RA−. Horizontal lines correspond to median values; vertical lines and whiskers represent the 25th and 75th percentiles. Two-tailed Mann-Whitney tests and Holms’ sequential Bonferroni corrections were performed for statistical analysis.
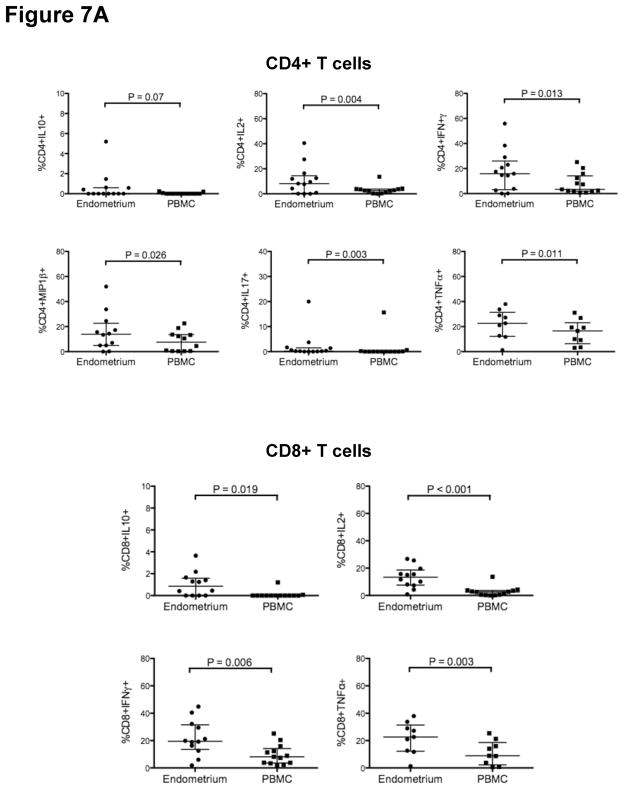
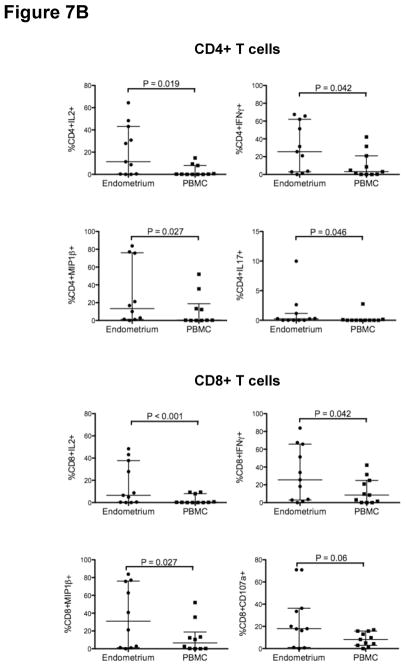
Endometrial CD4+ and CD8+ T-cell responses
A pro-inflammatory mucosal environment has been associated with an increased risk of HIV-1 acquisition 44–46. We measured CD4+ and CD8+ T-cell responses by stimulating cells freshly isolated from PB and endometrial biopsy with SEB and PMA/ION and staining with fluorescently labeled monoclonal antibodies to measure the production of cytokines, chemokines and a marker of degranulation (CD107a). Functional analysis was not performed on endocervical tissue, as the numbers of cells obtained from cytobrush and curettage were insufficient for these assays. As compared to PBMC, endometrial CD4+ T-cells produced significantly higher levels of IL-2, IL-17, IFN-γ and MIP1-β (Fig. 7A, B) following stimulation with either PMA/ION or SEB, and higher levels of TNF-α after SEB stimulation (Fig. 7A). Endometrial CD8+ T-cells were significantly more responsive than PBMC to SEB in the production of IL-10, IFN-γ, IL-2 and TNF-α (Fig. 7A) and to PMA/ION in the production of IFN-γ, IL-2 and MIP1-β (Fig. 7B). Endometrial CD4+ T-cells also produced increased IL-10 relative to PBMC following SEB stimulation, and CD8+ T-cells produced increased CD107a relative to PBMC after PMA/ION stimulation; however, these trends did not reach significance.
Figure 7. Endometrial T-cells are highly responsive to polyclonal stimulation.
Figure 7A shows the percentage of endometrial and PB CD4+ (upper panels) and CD8+ T cells (lower panels) responding to staphylococcus enterotoxin B (SEB) stimulation. Figure 7B shows the percentage of endometrial and PB CD4+ (left) and CD8+ T-cells (right) responding to phorbol myristate acetate (PMA) and ionomycin (ION) stimulation. Horizontal lines correspond to median values; vertical lines and whiskers denote the 25th and 75th percentiles. Statistical analyses were performed using Wilcoxon-matched pairs signed rank test and P values ≤0.05 were considered significant.
DISCUSSION
The tissues of the upper FRT are rich in immune effector cells, including CD4+ and CD8+ T-cells; however, little is known of the phenotype or functionality of these cells due to the difficulties inherent in obtaining fresh tissue samples. The uterine endometrium and endocervix are lined by a single layer of columnar epithelium and may be readily exposed to agents deposited in the lower FRT; accordingly, the upper FRT may serve as a portal of entry for HIV-1 and other pathogens 18, 19. Understanding the immunological milieu of upper FRT may therefore be important for the design of effective strategies to prevent sexually transmitted infections and for assessing the safety of future microbicide candidates. In the present study, we demonstrate that during the mid-luteal phase of menstrual cycle, T-cells from the endometrium and endocervix have enhanced expression of CCR5 and are predominantly of an activated, effector memory phenotype, compared to PB T-cells. Furthermore, in comparison to endocervix, T-cell expression of memory and activation markers, as well as the HIV-1 coreceptor CCR5, are enhanced in the endometrium. Endometrial T-cells are also more responsive to polyclonal stimulation than cells from PB, producing a wide range of pro-inflammatory cytokines and chemokines. These findings suggest that the upper FRT is rich in potential HIV-1 target cells and immune responsive effector cells. Further studies are warranted to determine the extent to which the endometrium is a site of HIV-1 replication during natural infection. Importantly, this study also demonstrates the feasibility of utilizing endometrial biopsy material to explore the phenotype and functionality of T-cell populations localized to the upper FRT.
Expression of CCR5 co-receptors is reportedly enhanced on endocervical T-cells during the secretory phase of the menstrual cycle 47–49. The mid-luteal (secretory) phase of the cycle has also been proposed to represent a ‘window of vulnerability’ to sexually transmitted infections, notably HIV-1 5. This speculation is based on the hormone-dependent alterations including suppression of innate and adaptive immune responses, reduced epithelial barrier integrity and reduced viscosity of mucus, all of which contribute to optimizing conditions for embryo implantation 5, 9, 21–23. In support of this hypothesis, susceptibility of reproductive tract to HIV-1 and SIV infection was reported to be high during the luteal phase of the menstrual cycle 50, 51 Previous studies have also shown that the use of progestin-based contraceptives increases susceptibility to HIV-1 acquisition in women 52, although this topic remains controversial 53, 54.
In the present analysis, during the mid-luteal phase of the menstrual cycle, both endometrium and endocervix had higher percentages of CCR5 expressing CD4+ T-cells compared to PB. The CCR5 receptor density was also higher in endometrial CD4+ T-cells compared to that of PB. While there are reports demonstrating increased CCR5 expression on CD4+ T-cells in endocervical cells compared to PB 49, 55, 56, to our knowledge the comparisons within the upper FRT presented herein have not been reported previously. CCR5 expression was elevated on endometrial compared to endocervical T-cells, and CXCR4 expression was enhanced in endocervical T-cells compared to endometrium. A recent study reported preferential binding of HIV-1 gp120 to cervical T-cells expressing CCR555. Our finding that CD4+ T-cells from the endometrium and endocervix express high levels of CCR5 suggests that the upper FRT may be a site of transmission for R5 tropic HIV-1. The upper FRT could theoretically also be susceptible to X4 tropic HIV-1 due to increased CXCR4 expression on endocervical CD4+ T-cells compared to endometrium as observed in our analysis; however, levels of CXCR4 have not been associated with initial transmission of HIV-1, and preferential transmission of R5 over X4 tropic viruses has been well documented, although the precise mechanism remains unresolved 57. Uterine epithelial cells also express CD4, CCR5 and CXCR4 depending on the stage of the menstrual cycle 58 and may play a role in HIV-1 transmission via transcytosis. However, given the reduction of epithelial barrier integrity that occurs during the mid-luteal phase 9, HIV-1 may also directly contact underlying CCR5-expressing target cells.
Increased TEM and activated T-cells in mucosal tissues have been associated with increased HIV-1 infection and replication 32, 40, 42, 59. Several studies of HIV-1 and SIV have shown that the initial burst of viral replication following transmission occurs in CCR5-expressing TEM and activated CD4+ T-cells 33, 40–43. In a recent study by Saba and colleagues,51 the frequency of CCR5 expression was found to be similar on cervical T-cells from ‘productive’ (i.e., productively infected) vs ‘non-productive’ cervical tissue explants. Both cell types were of effector memory phenotype. Interestingly, T-cells from non-productive explants produced higher levels of CCR5-binding chemokines than T-cells from productive explants, suggesting a role for these chemokines in preventing HIV-1 infection 51.
In the present analysis, TEM and activated CD4+ and CD8+ T-cells were elevated in the endometrium and endocervix of the upper FRT compared to PB. The observed increase in TEM cells in endometrium and endocervix was accompanied by a decrease in naïve, TCM and TEMRA cells. Thus, not only do endometrial CD4+ T-cells express high levels of CCR5, but they also have the highly activated, effector memory phenotype associated with HIV-1 susceptibility in some studies. Previous studies have also demonstrated higher percentages of TEM and activated CD4+ T-cells in the endocervix compared to PB 49, 60. The reasons for the observed differences between endometrium and endocervix in the frequency of CCR5− expressing T-cells, TEM and activated T-cells remain unclear, but are likely related to differential hormone-mediated regulation of immune cells in distinct regions of the FRT 15, 58, 61, 62.
A pro-inflammatory mucosal environment has consistently been associated with an increased risk of HIV-1 acquisition 44–46 by modulating the expression of HIV-1 receptors of available target cells 63, 64. Elevated levels of pro-inflammatory cytokines and inflammatory immune cells have been demonstrated in the cervical fluid and biopsies compared to PB 44, 55, 65, 66. With limited reports on T-cell responses in the endometrium 67, we have shown here that the endometrial CD4+ and CD8+ T-cells are more highly responsive than PBMC to PMA/ION and SEB stimulation in the production of pro-inflammatory cytokines and chemokines. The secreted pro-inflammatory cytokines and chemokines may be beneficial to the host by blocking infectivity of microbial pathogens; however, they may also contribute to pathogen dissemination by recruiting and/or activating additional susceptible target cells. There is evidence that viral particles can ascend into the upper FRT through the endocervical canal 19, 68 and substances such as semen, sperm and microbicides reach the endometrium from the vagina within hours of deposition 69–72. The fact that these agents introduced into the lower FRT can gain access to the upper FRT and the present observation that this site contains high levels of pro-inflammatory cytokines and HIV-1 target cells implies its inherent vulnerability to HIV-1 infection.
In summary, during mid-luteal phase T-cells from the endometrium and endocervix were observed to have a phenotype that is distinct from that of PB T-cells, with a predominance of highly activated, effector memory T-cells expressing CCR5. In comparison to endocervix, T-cell expression of memory and activation markers, as well as the HIV-1 coreceptor CCR5, are enhanced in the endometrium. In addition, compared to PBMC, endometrial T-cells were highly responsive to polyclonal stimuli with the production of pro-inflammatory cytokines and chemokines. Taken together, these studies demonstrate the feasibility of using biopsy samples to evaluate CD4+ and CD8+ T-cell populations, including antigen-specific T-cells, in the uterine endometrium. They also reveal that CD4+ T-cells in the endometrium have a phenotype associated with high susceptibility to HIV-1 infection. Further studies to evaluate the potential role of the upper FRT in HIV-1 transmission, viral replication and pathogenesis are warranted.
Acknowledgments
This research was supported by NIH Grant NIH/NIAID P01 AI083050. We thank the study subjects for their willingness to participate in this work. We thank Warner C. Greene, Gladstone Institute of Virology and Immunology, UCSF, for thoughtful comments and suggestions and Tara Ilsley for helping to recruit the participants for this study.
References
- 1.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstetrics and gynecology. 2000;96:431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 2.Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. The Journal of reproductive medicine. 1984;29:269–271. [PubMed] [Google Scholar]
- 3.Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasu K, Narahara H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediators Inflamm. 2010;2010:976024. doi: 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh M, Shen Z, Fahey JV, Crist SG, Patel M, Smith JM, Wira CR. Pathogen recognition in the human female reproductive tract: expression of intracellular cytosolic sensors NOD1, NOD2, RIG-1, and MDA5 and response to HIV-1 and Neisseria gonorrhea. Am J Reprod Immunol. 2013;69:41–51. doi: 10.1111/aji.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt JS. Immunologically relevant cells in the uterus. Biol Reprod. 1994;50:461–466. doi: 10.1095/biolreprod50.3.461. [DOI] [PubMed] [Google Scholar]
- 9.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 11.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. American journal of obstetrics and gynecology. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 13.Keller MJ, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, Cole AL, Cole AM, Profy AT, Wira CR, Hogarty K, Herold BC. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 14.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–749. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 15.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 16.White HD, Yeaman GR, Givan AL, Wira CR. Mucosal immunity in the human female reproductive tract: cytotoxic T lymphocyte function in the cervix and vagina of premenopausal and postmenopausal women. Am J Reprod Immunol. 1997;37:30–38. doi: 10.1111/j.1600-0897.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, Lu FX, Torten M, Miller CJ. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol. 2001;100:240–249. doi: 10.1006/clim.2001.5058. [DOI] [PubMed] [Google Scholar]
- 18.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 19.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Skinner PJ, Ha SJ, Duan L, Mattila TL, Hage A, White C, Barber DL, O’Mara L, Southern PJ, Reilly CS, Carlis JV, Miller CJ, Ahmed R, Haase AT. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science. 2009;323:1726–1729. doi: 10.1126/science.1168676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 23.De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11:205–214. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- 24.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 25.Shacklett BL, Yang O, Hausner MA, Elliott J, Hultin L, Price C, Fuerst M, Matud J, Hultin P, Cox C, Ibarrondo J, Wong JT, Nixon DF, Anton PA, Jamieson BD. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 26.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77:5621–5631. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. Journal of virology. 2007;81:5460–5471. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Critchfield JW, Young DH, Hayes TL, Braun JV, Garcia JC, Pollard RB, Shacklett BL. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 31.Shacklett BL, Greenblatt RM. Immune responses to HIV in the female reproductive tract, immunologic parallels with the gastrointestinal tract, and research implications. Am J Reprod Immunol. 2011;65:230–241. doi: 10.1111/j.1600-0897.2010.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groot F, van Capel TM, Schuitemaker J, Berkhout B, de Jong EC. Differential susceptibility of naive, central memory and effector memory T cells to dendritic cell-mediated HIV-1 transmission. Retrovirology. 2006;3:52. doi: 10.1186/1742-4690-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 37.Gumbi PP, Jaumdally SZ, Salkinder AL, Burgers WA, Mkhize NN, Hanekom W, Coetzee D, Williamson A, Passmore JS. CD4 T cell depletion at the cervix during HIV infection is associated with accumulation of terminally differentiated T cells. Journal of virology. 2011;85:13333–13341. doi: 10.1128/JVI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo JJ, Bach BA. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clinical and experimental immunology. 1992;90:376–382. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorgi JV, Hausner MA, Hultin LE. Detailed immunophenotype of CD8+ memory cytotoxic T-lymphocytes (CTL) against HIV-1 with respect to expression of CD45RA/RO, CD62L and CD28 antigens. Immunology letters. 1999;66:105–110. doi: 10.1016/s0165-2478(98)00170-9. [DOI] [PubMed] [Google Scholar]
- 40.Begaud E, Chartier L, Marechal V, Ipero J, Leal J, Versmisse P, Breton G, Fontanet A, Capoulade-Metay C, Fleury H, Barre-Sinoussi F, Scott-Algara D, Pancino G. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, Mawhinney S, Campbell TB, Lie Y, Coakley E, Levy DN, Connick E. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. Journal of virology. 2011;85:10189–10200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pancino G, Saez-Cirion A, Scott-Algara D, Paul P. Natural resistance to HIV infection: lessons learned from HIV-exposed uninfected individuals. The Journal of infectious diseases. 2010;202 (Suppl 3):S345–350. doi: 10.1086/655973. [DOI] [PubMed] [Google Scholar]
- 43.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nature medicine. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 44.Gumbi PP, Nkwanyana NN, Bere A, Burgers WA, Gray CM, Williamson AL, Hoffman M, Coetzee D, Denny L, Passmore JA. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. Journal of virology. 2008;82:8529–8536. doi: 10.1128/JVI.00183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, Broliden K, Hirbod T. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009;23:309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 46.Chege D, Chai Y, Huibner S, Kain T, Wachihi C, Kimani M, Barasa S, McKinnon LR, Muriuki FK, Kariri A, Jaoko W, Anzala O, Kimani J, Ball TB, Plummer FA, Kaul R. Blunted IL17/IL22 and pro-inflammatory cytokine responses in the genital tract and blood of HIV-exposed, seronegative female sex workers in Kenya. PloS one. 2012;7:e43670. doi: 10.1371/journal.pone.0043670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson BK, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski MA, Garcia P. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. The American journal of pathology. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheffield JS, Wendel GD, Jr, McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16:20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 49.Meditz AL, Moreau KL, MaWhinney S, Gozansky WS, Melander K, Kohrt WM, Wierman ME, Connick E. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–228. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 51.Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A, Lisco A, Grivel JC, Margolis L, Poli G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet infectious diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1–86. [PubMed] [Google Scholar]
- 54.U. S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of hormonal contraception among women at high risk for HIV infection or infected with HIV. MMWR Morb Mortal Wkly Rep. 2012;61:449–452. [PubMed] [Google Scholar]
- 55.McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S, Gelmon L, Block KE, Cicala C, Anzala AO, Arthos J, Kimani J, Kaul R. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 56.Prakash M, Kapembwa MS, Gotch F, Patterson S. Higher levels of activation markers and chemokine receptors on T lymphocytes in the cervix than peripheral blood of normal healthy women. Journal of reproductive immunology. 2001;52:101–111. doi: 10.1016/s0165-0378(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 57.Lawrence P, Portran D, Terrasse R, Palle S, Olivier T, Fantini J, Bourlet T, Pozzetto B, Delezay O. Selective transmigration of monocyte-associated HIV-1 across a human cervical monolayer and its modulation by seminal plasma. AIDS. 2012;26:785–796. doi: 10.1097/QAD.0b013e328351426e. [DOI] [PubMed] [Google Scholar]
- 58.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O’Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. The Journal of clinical investigation. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nkwanyana NN, Gumbi PP, Roberts L, Denny L, Hanekom W, Soares A, Allan B, Williamson AL, Coetzee D, Olivier AJ, Burgers WA, Passmore JA. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–757. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS research and human retroviruses. 1998;14 (Suppl 1):S57–62. [PubMed] [Google Scholar]
- 62.Mselle TF, Meadows SK, Eriksson M, Smith JM, Shen L, Wira CR, Sentman CL. Unique characteristics of NK cells throughout the human female reproductive tract. Clin Immunol. 2007;124:69–76. doi: 10.1016/j.clim.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Eslahpazir J, Jenabian MA, Bouhlal H, Hocini H, Carbonneil C, Gresenguet G, Mbopi Keou FX, LeGoff J, Saidi H, Requena M, Nasreddine N, de Dieu Longo J, Kaveri SV, Belec L. Infection of macrophages and dendritic cells with primary R5-tropic human immunodeficiency virus type 1 inhibited by natural polyreactive anti-CCR5 antibodies purified from cervicovaginal secretions. Clinical and vaccine immunology : CVI. 2008;15:872–884. doi: 10.1128/CVI.00463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saidi H, Magri G, Carbonneil C, Nasreddine N, Requena M, Belec L. IFN-gamma-activated monocytes weakly produce HIV-1 but induce the recruitment of HIV-sensitive T cells and enhance the viral production by these recruited T cells. Journal of leukocyte biology. 2007;81:642–653. doi: 10.1189/jlb.0406278. [DOI] [PubMed] [Google Scholar]
- 65.Hladik F, Lentz G, Delpit E, McElroy A, McElrath MJ. Coexpression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ Th1 phenotype. J Immunol. 1999;163:2306–2313. [PubMed] [Google Scholar]
- 66.Passmore JA, Milner M, Denny L, Sampson C, Marais DJ, Allan B, Gumbi PP, Hitzeroth, Rybicki EP, Williamson AL. Comparison of cervical and blood T-cell responses to human papillomavirus-16 in women with human papillomavirus-associated cervical intraepithelial neoplasia. Immunology. 2006;119:507–514. doi: 10.1111/j.1365-2567.2006.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shacklett BL. Cell-mediated immunity to HIV in the female reproductive tract. J Reprod Immunol. 2009;83:190–195. doi: 10.1016/j.jri.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joag SV, Adany I, Li Z, Foresman L, Pinson DM, Wang C, Stephens EB, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. Journal of virology. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnhart KT, Stolpen A, Pretorius ES, Malamud D. Distribution of a spermicide containing Nonoxynol-9 in the vaginal canal and the upper female reproductive tract. Hum Reprod. 2001;16:1151–1154. doi: 10.1093/humrep/16.6.1151. [DOI] [PubMed] [Google Scholar]
- 70.Barnhart KT, Pretorius ES, Shaunik A, Timbers K, Nasution M, Mauck C. Vaginal distribution of two volumes of the novel microbicide gel cellulose sulfate (2.5 and 3. 5 mL) Contraception. 2005;72:65–70. doi: 10.1016/j.contraception.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70:498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Kunz G, Beil D, Deiniger H, Einspanier A, Mall G, Leyendecker G. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Advances in experimental medicine and biology. 1997;424:267–277. [PubMed] [Google Scholar]