SUMMARY
Objectives
During the 2009 influenza A (H1N1) pandemic, unusual influenza activity outside the typical winter season provided a unique opportunity to evaluate the association between influenza and pneumonia incidence. We sought to quantify the impact of the 2009 pandemic on the incidence of emergency department (ED) visits for pneumonia in the United States (US).
Methods
Using the Nationwide Emergency Department Survey, we estimated monthly counts and rates of excess all-cause pneumonia ED visits in the US attributable to the pandemic by comparing observed pneumonia ED visits during the pandemic (April 2009–March 2010) to expected values modeled from the three prior years.
Results
The pandemic was associated with an excess of 180,560 pneumonia ED visits or 0.59 excess pneumonia visits per 1,000 US population (95% confidence interval: 0.55, 0.62). These excess visits accounted for 7.0% of all pneumonia ED visits during the pandemic year. The greatest excess occurred during months with highest influenza activity (September - November 2009). Persons aged <65 years accounted for 94% of the excess pneumonia visits.
Conclusions
ED visits for pneumonia increased substantially during the 2009 pandemic, especially during peak influenza activity, suggesting a strong association between influenza activity and pneumonia incidence during the pandemic period.
Keywords: influenza, pneumonia, epidemiology
INTRODUCTION
Influenza infection is considered an important contributor to pneumonia pathogenesis and burden.1 Influenza can cause primary viral pneumonia and also predispose to secondary bacterial pneumonia with pathogens such as Streptococcus pneumoniae and Staphylococcus aureus.2–5 However, quantifying the burden of pneumonia attributable to influenza on a population level is challenging.6 Both influenza and pneumonia incidences peak in winter months,7–10 but several other factors may contribute to this temporal association between influenza and pneumonia, including activity of other respiratory viruses during the winter,11,12 environmental factors such as cold temperatures and decreased daylight in the winter,13 and increased person-to-person contact during the winter holidays.14
In 2009, the influenza A (H1N1) pandemic resulted in unusually high influenza activity in the United States (US) during the autumn, and to a lesser extent, during the preceding spring and summer.15,16 Influenza A (H1N1)pdm09 disproportionally affected children and young adults compared to the typical seasonal influenza pattern in which serious complications of influenza were more concentrated among older adults.15,16 These distinctive features of influenza activity during the 2009 pandemic provided a unique opportunity to evaluate the impact of influenza on pneumonia burden in the absence of winter-specific factors and in children and younger adult populations who do not typically contribute a high burden of influenza-associated pneumonia.
Weinberger et al16 demonstrated the 2009 influenza A (H1N1) pandemic was associated with increases in hospitalizations coded as pneumococcal pneumonia in the age groups most severely affected by the pandemic. Similar increases were recently reported for invasive pneumococcal pneumonia.17 Nevertheless, emergency department (ED) visits and hospitalizations are rarely coded specifically as pneumococcal pneumonia because most pneumonia episodes never have an etiology identified, partly due to the limitations of routine diagnostic tests.18 Furthermore, influenza infections can facilitate the development of non-pneumococcal pneumonia,4 but only pneumococcal disease was considered in previous studies. A comprehensive evaluation and quantification of the impact of the 2009 influenza pandemic on all-cause pneumonia burden is currently lacking.
With over 136 million US ED visits annually, the ED is an important venue for healthcare utilization, both as an entry point for hospitalizations and for outpatient treatment of serious illness.19 To quantify the impact of the 2009 influenza A (H1N1) pandemic on pneumonia burden in US EDs, we estimated excess all-cause pneumonia ED visits attributable to the pandemic by comparing rates of pneumonia ED visits observed during the pandemic year with expected rates estimated from modeling data from the three pre-pandemic years.
METHODS AND METHODS
Data source
We obtained ED visit data from the Nationwide Emergency Department Sample (NEDS), a component of the Healthcare Cost and Utilization Project (HCUP) maintained by the Agency for Healthcare Research and Quality (AHRQ).20 NEDS is the largest source of US ED data, and contains information from 25–30 million ED visits annually beginning in 2006. The sample, which includes data from 29 participating states and represents approximately 20% of all US ED visits, is stratified by hospital geographic region, trauma center designation, urban-rural status, teaching hospital status, and ownership. EDs represent the primary sampling units and all visits from sampled EDs are included. The NEDS sampling framework is updated annually and includes statistical weights and clustering elements to allow calculation of national estimates.20
For each ED visit, NEDS contains up to 15 diagnoses coded using the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM). Additionally, NEDS contains data on month and year of visit, disposition from the ED, and patient demographics.
Pneumonia case definition
A pneumonia ED visit was defined as an ED visit with a primary (first-listed) pneumonia diagnosis, or with a secondary pneumonia diagnosis (listed in diagnosis fields 2–15) with an accompanying primary diagnosis of respiratory failure, shock, septicemia, a sign or symptom consistent with pneumonia, another acute respiratory infection, or an acute exacerbation of a chronic pulmonary disease (Table 1).21 This case definition for all-cause pneumonia was not restricted to a specific pathogen.
Table 1.
International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) codes included in the case definitions for pneumonia ED visit and fracture ED visit.
| Category | ICD-9-CM Codes |
|---|---|
| Pneumonia | 480.xx–486.xx; 487.0 |
| Respiratory failure | 518.81; 518.83; 518.84 |
| Shock | 785.50; 785.51; 785.52; 785.59 |
| Septicemia | 003.1; 022.3; 036.2; 038.x; 054.5; 790.7; 995.91; 995.92 |
| Signs and symptoms consistent with pneumonia | 511.xx; 519.11; 780.3x; 780.6x; 780.97; 786.05; 786.06; 786.07; 786.2; 786.3x; 786.4; 786.5x; 786.7; 799.02; 799.1 |
| Another acute respiratory Infection | 381.0x; 382.0x; 382.9; 383.0x; 460.x–466.x; 487.x; 510.0; 510.9; 513.0 |
| Acute exacerbation of a chronic pulmonary disease | 491.21; 491.22; 493.01; 493.02; 493.11; 493.12; 493.21; 493.22; 493.91; 493.92; 494.1 |
| Fractures | 800.xx–829.xx |
Statistical analyses
NEDS data were used to calculate monthly counts of pneumonia ED visits nationwide from April 2006 through March 2010. Calculations were stratified by eight age groups: <2 years; 2–4 years; 5–17 years; 18–39 years; 40–64 years; 65–74 years; 75–84 years; and ≥85 years. For each age group we used Poisson regression models for survey data to calculate annualized incidence rates for each study month using monthly counts of pneumonia ED visits as the outcome and the respective July US population estimate22 as the model offset term. Indicators for calendar months and year were included as covariates. To account for the variability in duration of calendar months, the monthly denominators were adjusted by multiplying the annual US population estimate by the fraction of days in each month within a year.
The study period was divided into two time segments: the 36-month pre-pandemic period (April 2006–March 2009) and the 12-month pandemic period (April 2009–March 2010). We first used pre-pandemic data and our Poisson regression model to estimate the monthly pneumonia ED visit rates for each age group expected during the each month of the pandemic period in the absence of any perturbation (e.g. influenza A (H1N1) pandemic). We then estimated rates of excess pneumonia ED visits attributable to the pandemic in each age group by subtracting the expected monthly rates calculated with the pre-pandemic Poisson model from the observed monthly rates during the pandemic period. Thus, our analytic approach assumed that the number of pneumonia ED visits observed during the pandemic months above the historical monthly expectation was the disease burden or “excess” associated with the circulation of the pandemic virus. The overall (all-age) excess ED visit rate was calculated by summing the following product for each age group: (age specific excess rate) * (proportion of US population within respective age group). In order to report excess rates by season, we grouped the following months: April–June (Spring, pre-peak pandemic); July–August (Summer, pre-peak pandemic); September–November (Autumn, peak pandemic); and December–March (Winter, post-peak pandemic). Ninety five percent confidence intervals for excess rates were computed using a normal approximation for each age group individually and for the population overall. Variances were calculated by following general property of variance and variance under linear transformation rules.23
Subgroup analyses
To complement previous studies that focused only on hospitalizations, we performed a subgroup analysis of treat-and-release outpatient ED visits to quantify the proportion of excess pneumonia ED visits during the pandemic that resulted in ED discharge without hospitalization. Similar to the methods described above for total pneumonia ED visits, we fit Poisson regression models using pre-pandemic monthly pneumonia ED visit rates to predict pandemic period rates and then calculated excess monthly rates by subtracting predicted from observed rates during the pandemic period
Evaluation of secular trends
To assess for potential changes in ED utilization not associated with pandemic influenza activity during the study period, we also studied ED visits for fractures, a condition unlikely to increase with elevated influenza activity. ED visits for fractures were defined by ICD-9-CM coded diagnoses for a fracture in any diagnosis position (Table 1). Similar to our analysis of pneumonia ED visits, we calculated monthly rates of ED visits for fracture for each age group and compared observed rates in the pandemic period with expected rates from models using pre-pandemic period data.
This study was conducted with de-identified data and approved by the local institutional review board as nonhuman research. Statistical analyses accounted for the NEDS complex sampling design and were conducted with SAS 9.2 (SAS Institute, Cary, NC) and Stata 12 (Stata Corp, College Station, TX).
RESULTS
Characteristics of pneumonia ED visits
During the 48-month study period, there were approximately 9.4 million nationwide pneumonia ED visits, 39.5% of which resulted in ED discharge (treat-and-release outpatient pneumonia ED visits) (Table 2). Among adults, increasing age was associated with an increasing proportion of pneumonia ED visits resulting in hospitalization. Medicare and Medicaid were the primary payers for 43.5% and 21.2% of the pneumonia ED visits, respectively.
Table 2.
Patient characteristics for pneumonia ED visits in the United States, by age group, April 2006 through March 2010.
| < 2 years | 2–4 years | 5–17 years | 18–39 years | 40–64 years | 65–74 years | 75–84 years | ≥ 85 years | All Ages | |
|---|---|---|---|---|---|---|---|---|---|
| Pneumonia ED visits (n) | 953,169 | 673,917 | 658,129 | 977,197 | 2,351,573 | 1,247,866 | 1,492,850 | 1,041,016 | 9,395,717 |
| Rate of pneumonia ED visits per 1,000 population/year | 28.0 | 13.3 | 3.1 | 2.7 | 5.9 | 15.3 | 28.2 | 46.9 | 7.7 |
| Female (%) | 42.3% | 45.5% | 45.6% | 51.0% | 51.3% | 49.5% | 51.2% | 59.8% | 50.2% |
|
| |||||||||
| Disposition from ED (%) | |||||||||
| Discharged | 72.4 | 76.7 | 74.7 | 65.6 | 35.6 | 18.0 | 13.2 | 10.7 | 39.5 |
| Admitted | 22.6 | 19.4 | 21.3 | 32.3 | 61.7 | 78.6 | 83.4 | 85.7 | 57.2 |
| Other a | 5.1 | 3.9 | 4.0 | 2.1 | 2.7 | 3.2 | 3.5 | 3.7 | 3.3 |
|
| |||||||||
| Primary Payer (%) b | |||||||||
| Medicare | 0.2 | 0.2 | 0.3 | 7.1 | 25.4 | 85.1 | 91.9 | 93.7 | 43.5 |
| Medicaid | 61.2 | 51.5 | 43.7 | 25.2 | 19.8 | 2.6 | 1.4 | 1.2 | 21.2 |
| Private | 28.1 | 36.8 | 44.1 | 37.6 | 38.1 | 10.3 | 5.3 | 4.0 | 24.7 |
| Self Pay | 6.8 | 7.6 | 7.7 | 24.6 | 11.8 | 0.9 | 0.5 | 0.4 | 7.5 |
| Other | 3.7 | 3.9 | 4.2 | 5.4 | 4.9 | 1.1 | 0.9 | 0.8 | 3.1 |
|
| |||||||||
| Median household income by patient zip code (%) c | |||||||||
| 1st quartile | 34.3 | 31.8 | 30.2 | 32.3 | 34.0 | 31.0 | 27.3 | 25.7 | 31.0 |
| 2nd quartile | 31.1 | 28.5 | 27.6 | 29.0 | 28.4 | 29.0 | 28.2 | 27.1 | 28.5 |
| 3rd quartile | 22.3 | 23.2 | 23.3 | 23.0 | 21.9 | 22.6 | 23.8 | 23.6 | 22.8 |
| 4th quartile | 13.2 | 16.5 | 18.9 | 15.6 | 15.8 | 17.4 | 20.7 | 23.6 | 17.6 |
Other disposition included: transfer to a different hospital, died in the ED, and unknown disposition.
0.2% of visits had missing data for primary payer.
2.4% of visits had missing data for median household income by patient zip code.
Excess pneumonia ED visits during pandemic
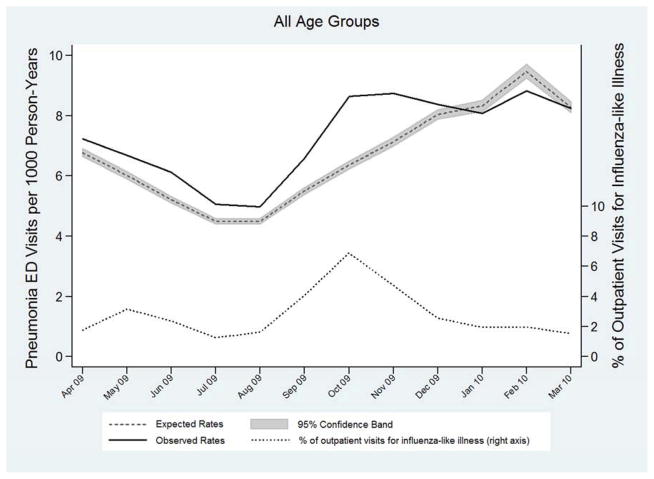
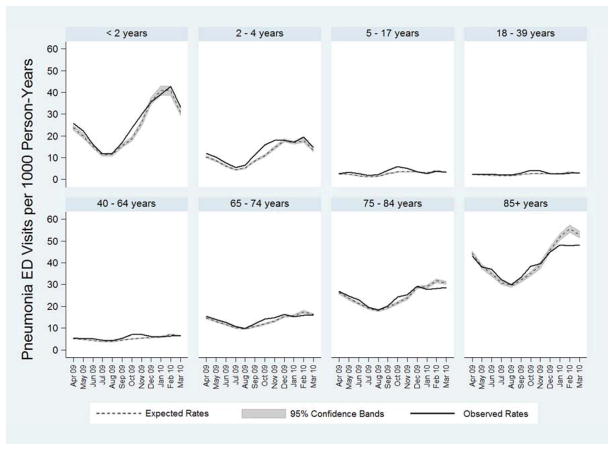
The pattern of excess pneumonia ED visits during the pandemic period showed the greatest excess corresponding to peak influenza activity from September through November, 2009 (Table 3, Figure 1A). Nationwide influenza activity during the pandemic period illustrated in Figure 1A was estimated based on proportion of outpatient visits for influenza-like illness reported by the Centers for Disease Control and Prevention (CDC).24 Similar monthly patterns of pneumonia ED visits were observed for each age group with higher than expected pneumonia ED visits in the autumn of 2009 (Figure 1B). Influenza activity was low during December 2009 through March 2010, and during these months there were fewer pneumonia ED visits than expected. Of note, the deficit of observed pneumonia ED visits compared to expected values during December through March was largely driven by patients ≥75 years old (Table 3, Figure 1B).
Table 3.
Rates per 1000 person-years of pneumonia ED visits in the United States by age group and season. The baseline period included April 2006 through March 2009. The pandemic period included April 2009 through March 2010. Excess rates during the pandemic period were calculated as the rate difference between the pandemic period and baseline period.
| Age Group (years) | April–June (Spring)
|
July–August (Summer)
|
September–November (Autumn)
|
December–March (Winter)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Period | Pandemic Period | Excess in pandemic period (95% CI) | Baseline Period | Pandemic Period | Excess in pandemic period (95% CI) | Baseline Period | Pandemic Period | Excess in pandemic period (95% CI) | Baseline Period | Pandemic Period | Excess in pandemic period (95% CI) | |
| < 2 | 19.63 | 21.38 | 1.75 (0.80, 2.71) | 12.75 | 13.55 | 0.80 (0.07, 1.54) | 26.85 | 29.71 | 2.86 (1.43, 4.29) | 37.52 | 38.37 | 0.86 (−0.72, 2.43) |
|
| ||||||||||||
| 2–4 | 8.38 | 9.98 | 1.60 (1.15, 2.04) | 6.06 | 7.73 | 1.67 (1.31, 2.02) | 14.46 | 17.23 | 2.77 (2.06, 3.48) | 16.02 | 17.13 | 1.11 (0.53, 1.70) |
|
| ||||||||||||
| 5–17 | 2.13 | 2.84 | 0.71 (0.56, 0.86) | 1.77 | 2.68 | 0.91 (0.78, 1.04) | 3.47 | 4.82 | 1.36 (1.17, 1.55) | 3.40 | 3.28 | −0.12 (−0.24, 0) |
|
| ||||||||||||
| 18–39 | 1.98 | 2.35 | 0.38 (0.30, 0.45) | 1.81 | 2.31 | 0.50 (0.42, 0.57) | 2.60 | 3.59 | 0.99 (0.89, 1.08) | 2.93 | 2.74 | −0.19 (−0.26, −0.12) |
|
| ||||||||||||
| 40–64 | 4.73 | 5.25 | 0.51 (0.39, 0.63) | 4.00 | 4.61 | 0.61 (0.50, 0.72) | 5.36 | 6.73 | 1.37 (1.23, 1.51) | 6.49 | 6.22 | −0.27 (−0.39, −0.15) |
|
| ||||||||||||
| 65–74 | 13.13 | 14.05 | 0.92 (0.56, 1.28) | 10.2 | 10.89 | 0.69 (0.37, 1.00) | 13.42 | 15.03 | 1.61 (1.23, 1.99) | 16.48 | 15.76 | −0.71 (−1.06, −0.36) |
|
| ||||||||||||
| 75–84 | 23.76 | 24.87 | 1.11 (0.50, 1.72) | 18.90 | 19.37 | 0.47 (−0.05, 0.99) | 24.71 | 26.29 | 1.57 (0.92, 2.23) | 30.52 | 28.19 | −2.33 (−2.91, −1.75) |
|
| ||||||||||||
| ≥ 85 | 39.16 | 39.47 | 0.31 (−0.84, 1.47) | 30.77 | 31.87 | 1.09 (0.14, 2.04) | 39.98 | 40.94 | 0.96 (−0.28, 2.20) | 53.52 | 48.10 | −5.42 (−6.60, −4.25) |
|
| ||||||||||||
| All Ages | 6.02 | 6.66 | 0.63 (0.56, 0.71) | 4.83 | 5.51 | 0.68 (0.62, 0.75) | 7.18 | 8.55 | 1.37 (1.28, 1.46) | 8.70 | 8.36 | −0.34 (−0.42, −0.26) |
Figure 1.
Observed (solid line) and expected (dashed line with shaded 95% confidence interval) incidence rates of pneumonia ED visits in the United States for all age groups combined (A) and stratified by age group (B), during the 2009 Influenza A (H1N1) pandemic, April 2009 through March 2010. Influenza activity, measured by the percentage of outpatient visits for influenza-like illness (ILI) reported by the Centers for Disease Control and Prevention (CDC),24 is represented by the dotted line in (A). Area under the observed rates line and above the expected rates line represents excess pneumonia ED visits.
Comparison of observed pneumonia ED visit rates during the pandemic period (April 2009–March 2010) with expected rates from the pre-pandemic period demonstrated an excess rate of 0.59 pneumonia ED visits per 1,000 US population (95% CI: 0.55, 0.62) and a total excess of 180,560 pneumonia ED visits during the pandemic year (Table 4). These excess visits represented 7.0% of all 2,572,544 pneumonia ED visits during the pandemic year. Excess pneumonia ED visit rates were greatest in children < 5 years old and lowest in older adults. In fact, 94% of the excess visits were in people < 65 years old. Adults ≥ 85 years old had fewer pneumonia ED visits during the pandemic year than expected based on projections from the pre-pandemic period. This deficit was largely due to lower than expected numbers of pneumonia ED visits during December 2009 through March 2010, which were months with relatively low influenza activity following peak pandemic influenza activity in the autumn of 2009 (Figure 1B).
Table 4.
Counts and incidence rates per 1,000 person-years of excess total pneumonia ED visits, excess outpatient pneumonia ED visits, and excess pneumonia ED visits resulting in hospitalization during the influenza A (H1N1) pandemic year in the United States by age group, April 2009–March 2010.a
| Age Group (years) | Excess Total Pneumonia ED Visits | Excess Outpatient Pneumonia ED Visits | Excess Hospitalized Pneumonia ED Visits | |||
|---|---|---|---|---|---|---|
| Counts | Rates per 1000 (95% CI) | Counts | Rates per 1000 (95% CI) | Counts | Rates per 1000 (95% CI) | |
| < 2 | 13,158 | 1.57 (0.96, 2.18) | 10,365 | 1.24 (0.69, 1.78) | 2,982 | 0.36 (0.07, 0.64) |
|
| ||||||
| 2–4 | 22,999 | 1.79 (1.52, 2.06) | 18,760 | 1.46 (1.22, 1.69) | 4,374 | 0.34 (0.22, 0.46) |
|
| ||||||
| 5–17 | 38,072 | 0.71 (0.64, 0.79) | 30,917 | 0.58 (0.52, 0.64) | 6,500 | 0.12 (0.09, 0.16) |
|
| ||||||
| 18–39 | 38,606 | 0.42 (0.38, 0.46) | 25,205 | 0.27 (0.24, 0.30) | 14,183 | 0.15 (0.13, 0.17) |
|
| ||||||
| 40–64 | 56,151 | 0.56 (0.49, 0.62) | 18,534 | 0.18 (0.15, 0.22) | 37,662 | 0.37 (0.33, 0.42) |
|
| ||||||
| 65–74 | 13,301 | 0.62 (0.45, 0.80) | 1,006 | 0.05 (−0.02, 0.12) | 11,503 | 0.54 (0.38, 0.70) |
|
| ||||||
| 75–84 | 2,718 | 0.21 (−0.09, 0.50) | −652 | −0.05 (−0.16, 0.06) | 2,716 | 0.21 (−0.06, 0.47) |
|
| ||||||
| ≥ 85 | −4,446 | −0.77 (−1.33, −0.20) | −901 | −0.16 (−0.33, 0.02) | −3,846 | −0.66 (−1.16, −0.17) |
|
| ||||||
| All Ages | 180,560 | 0.59 (0.55, 0.62) | 103,232 | 0.33 (0.31, 0.36) | 76,073 | 0.25 0.22, 0.27) |
Separate models were developed for each age group and each outcome (total ED visits, outpatient ED visits, and hospitalized ED visits). Separate estimations for other ED dispositions (e.g. hospital transfers and ED deaths) were not performed. There, the excess total ED visits may not precisely equal the sum of outpatient visits and hospitalized visits.
Outpatient (treat-and-release) ED visits
During the pandemic year, there were 1,037,021 treat-and-release outpatient pneumonia ED visits; 103,232 (10.0%) of these outpatient ED pneumonia visits were excess visits attributable to the influenza pandemic. Outpatient visits accounted for 57.2% of all the excess pneumonia ED visits during the pandemic period. Nearly all of these excess outpatient pneumonia ED visits were in patients <65 years old (Table 4). In comparison, 76,073 of the 1,451,754 (5.2%) pneumonia ED visits resulting in hospitalization during the pandemic period were excess visits attributable to the pandemic.
Evaluation of secular trends
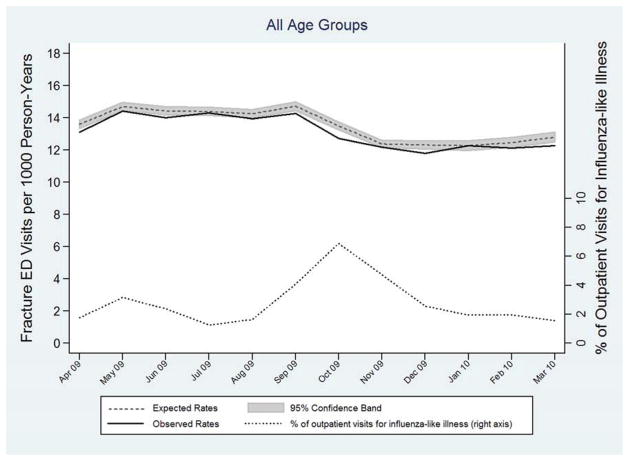
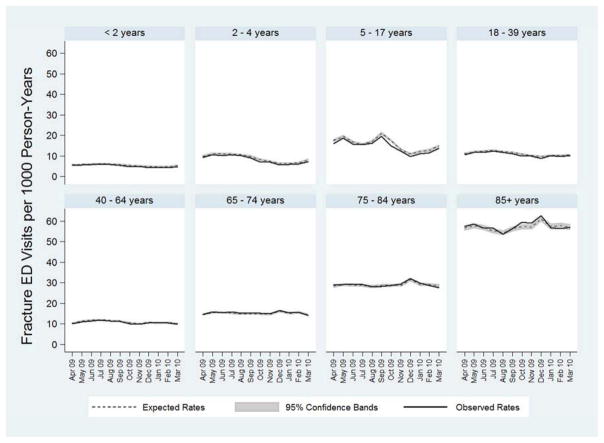
Evaluation of ED visits for fractures did not demonstrate excess ED visits during the pandemic period or a monthly pattern of ED utilization that matched influenza activity, suggesting there were not concurrent changes in general ED utilization that could explain the excess pneumonia ED visits observed during the pandemic period (Figures 2A & 2B).
Figure 2.
Observed (solid line) and expected (dashed line with shaded 95% confidence interval) incidence rates of ED visits for fractures in the United States for all age groups combined (A), and stratified by age group (B), during the 2009 Influenza A (H1N1) pandemic period, April 2009 through March 2010. Influenza activity, measured by the percentage of outpatient visits for influenza-like illness (ILI) reported by the Centers for Disease Control and Prevention (CDC),24 is represented by the dotted line in (A). Area under the observed rates line and above the expected rates line represents excess ED visits for fractures.
DISCUSSION
Several features of the 2009 influenza A (H1N1) pandemic distinguished it from typical seasonal influenza epidemics and allowed for a unique evaluation of the association between influenza and pneumonia. Peak influenza activity during the pandemic occurred in the autumn, months earlier than typical seasonal influenza activity in the winter, allowing for evaluation of the association between influenza and pneumonia in the absence of other winter-related factors.15,16 Additionally, the attack rate for Influenza A (H1N1)pdm09 was unusually high among children, young adults, and middle-aged adults, with relative sparing of older adults.15,16
We demonstrated that ED visits for pneumonia spiked during periods of peak pandemic influenza activity among age groups most significantly affected by the pandemic—children and young and middle-aged adults. These autumn spikes in pneumonia ED visits were unique to 2009 and not present in the three pre-pandemic years used to create the baseline period in our regression models. These findings suggest that high influenza activity in the autumn of 2009 was associated with unusually high rates of ED visits for pneumonia and indicate that influenza is an important contributor to overall pneumonia burden. During the 12-month period following onset of the pandemic in April 2009, there were 180,560 excess pneumonia ED visits attributable to the influenza pandemic, accounting for 7.0% of all pneumonia ED visits during that time. Approximately 94% of the excess pneumonia ED visits occurred in patients less than 65 years old; 57% of these excess pneumonia visits were treat-and-release outpatient ED visits. This substantial burden of outpatient visits for pneumonia among patients less than 65 years old is often underappreciated and not assessed by studies that focus only on hospitalizations.
Our findings complement those of Weinberger et al16 and Fleming-Dutra et al.17 Weinberger et al16 reported rates of excess pneumococcal pneumonia hospitalizations attributed to the 2009 influenza A (H1N1) pandemic in the autumn of 2009 using data from selected states. They found rates of 0.47, 0.52, and 1.25 excess pneumococcal pneumonia hospitalizations per 100,000 population in age groups 5–19 years old, 20–39 years old, and 40–64, respectively. No excess pneumococcal pneumonia hospitalizations were found in children < 5 years old or adults ≥ 65 years old. Similarly, Fleming-Dutra et al17 analyzed data from sites participating in the US Active Bacterial Core Surveillance system and found increases in the rates of hospitalizations for invasive pneumococcal pneumonia (pneumonia with S. pneumoniae isolated from a normally sterile site) during months with peak pandemic influenza activity for all age groups other than children < 5 years old. They reported excess rates of invasive pneumococcal pneumonia hospitalizations from 0.21 cases per 100,000 population in the 25–49 year old group to 0.44 cases per 100,000 population in the 50–64 year old age group. Our study of nationwide data reveals that the pandemic’s impact on pneumonia was not restricted to pneumonia identified as pneumococcal disease or hospitalizations, but broadly increased the burden for all-cause pneumonia in both the inpatient and outpatient settings. Rates of excess all-cause pneumonia ED visits attributed to the 2009 influenza pandemic reported here are approximately 100-times larger than excess pneumococcal pneumonia hospitalizations reported by Weinberger et al16 and Fleming-Dutra et al.17 Moreover, unlike these previous reports conducted in selected populations, our national study, which used the largest available dataset for US ED visits, accrued enough statistical power to show that the pandemic was also associated with increased pneumonia incidence among young children < 5 years old.
We previously reported that incidence rates of ED visits attributable to influenza during the 2009 pandemic were approximately 10 per 1000 person-years.15 Similarly, our group and others have estimated that the rate of influenza-related hospitalizations during the pandemic to be approximately 1 per 1000 person-years.25,26 Our current study complements these previous estimations and suggests that sizable fractions of ED visits (approximately 3%) and hospitalizations (approximately 25%) attributable to pandemic influenza were due to influenza-associated pneumonia. Furthermore, the patterns of influenza activity and pneumonia ED visits illustrate an important shift in the typical distribution of pneumonia burden during the pandemic that matched influenza activity, with higher burden in the autumn and lower burden in the winter.
Limitations to our study include reliance on ICD-9-CM codes to identify pneumonia cases and the ecological study design. Our case definition captured ED visits with a primary ICD-9 code for pneumonia and visits with a secondary code for pneumonia with an accompanying primary code for selected signs, symptoms and other acute respiratory diseases. The goal of using this case definition was to capture ED visits in which pneumonia was the primary reason for ED evaluation. Nevertheless, this retrospective identification of pneumonia ED visits may be subject to misclassification. Secondly, in this ecological study design, patients who experienced a pneumonia ED visit during the pandemic period were not proven to have a preceding or concurrent influenza infection. However, the specific pattern of spikes in pneumonia ED visits during the months of peak influenza activity and in age groups most severely affected by the pandemic strengthens our confidence in a causal association between influenza infection and pneumonia ED visits. Furthermore, ED visits for fractures, which are unlikely to increase with influenza activity, did not increase during the pandemic period, suggesting our pneumonia findings were not the result of a generalized increase in ED utilization. The use of weekly instead of monthly data would allow for more precise characterization of influenza activity; however, weekly data are not available in NEDS. The high burden of influenza during the pandemic year may have been associated with a concomitant decrease in the activity of other respiratory viruses compared to previous years.27–28 If disease burden of non-influenza viruses during the pandemic were lower than the historical baseline, our calculations would have underestimated the true burden of influenza-associated disease. Such underestimation would be highest at the extremes of age, when rates of non-influenza respiratory viral disease are greatest.29–31 Finally, since our baseline data consisted of time periods with seasonal influenza epidemics, we were able to calculate the excess in pneumonia visits associated with the pandemic compared to years with seasonal influenza, but could not determine the total burden of influenza-associated pneumonia during the pandemic, which would require a baseline comparator period devoid of all influenza.
In conclusion, the influenza A (H1N1) pandemic in the US was associated with a large increase in ED visits for pneumonia compared to the previous three years. Excess pneumonia ED visits spiked during periods of greatest influenza activity in the autumn and in age groups most commonly infected with the pandemic virus, suggesting a strong association between influenza activity and pneumonia ED visits during the pandemic period.
Acknowledgments
We are grateful to Daniel Weinberger for helpful suggestions regarding regression modeling strategies and to Matthew Moore and Cynthia Whitney for their critical review. The authors had no writing assistance during preparation of this manuscript.
Funding: This work was supported in part by Intergovernmental Personnel Agreements from the Centers for Disease Control and Prevention (CDC) [grant numbers 11-IPA1110211 and 12-IPA1210402 to MRG, and 12-IPA1210402 to CGG] and the National Center for Advancing Translational Sciences [grant number KL2TR000446 to WHS]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. These funding sources had no role in the study design, data collection, data analysis, interpretation of the data, or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wesley H. Self, Department of Emergency Medicine, Vanderbilt University, Nashville, Tennessee, USA 37232.
Marie R. Griffin, Department of Preventive Medicine, Vanderbilt University, and the Mid-South Geriatric Research Education and Clinical Center, VA TN Valley Health Care System, Nashville, Tennessee, USA 37232.
Yuwei Zhu, Department of Biostatistics, Vanderbilt University, Nashville, Tennessee, USA 37232.
William D. Dupont, Department of Biostatistics, Vanderbilt University, Nashville, Tennessee, USA 37232.
Tyler W. Barrett, Department of Emergency Medicine, Vanderbilt University, Nashville, Tennessee, USA 37232.
Carlos G. Grijalva, Department of Preventive Medicine, Vanderbilt University, and the Mid-South Geriatric Research Education and Clinical Center, VA TN Valley Health Care System, Nashville, Tennessee, USA 37232.
References
- 1.Bisno AL, Griffin JP, Van Epps KA, Niell HB, Rytel MW. Pneumonia and Hong Kong influenza: A prospective study of the 1968–1969 epidemic. Am J Med Sci. 1971;261:251–63. doi: 10.1097/00000441-197105000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Pop-Vicas A. Clinical review: Primary influenza viral pneumonia. Crit Care. 2009;13:235. doi: 10.1186/cc8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCullers JA. Insights into the interaction between influenza and pneumococcus. Clin Microbiol Rev. 2006;19:571–82. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G, et al. Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med. 2009;53:358–65. doi: 10.1016/j.annemergmed.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Walter ND, Taylor TH, Shay DK, Thompson WW, Brammer L, Dowell SF, et al. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50:175–83. doi: 10.1086/649208. [DOI] [PubMed] [Google Scholar]
- 6.Grijalva CG, Griffin MR. Unveiling the burden of influenza-associated pneumococcal pneumonia. J Infect Dis. 2012;205:355–7. doi: 10.1093/infdis/jir753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 8.Marrie TJ. Pneumococcal pneumonia epidemiology and clinical features. Semin Respir Infect. 1999;14:227–36. [PubMed] [Google Scholar]
- 9.Gray BM, Converse GM, Dillon HC. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 10.Nicoli EJ, Trotter CL, Turner KM, Colijn C, Waight P, Miller E. Influenza and RSV make a modest contribution to invasive pneumococcal disease incidence in the UK. J Infect. 2013;66:512–20. doi: 10.1016/j.jinf.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Edwards KE, et al. Seasonality of invasive pneumococcal disease: Temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med. 2005;118:285–91. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Techasaensiri B, Techasaensiri C, Mejias A, McCraken GH, Ramilo O. Viral coinfections in children with invasive pneumococcal disease. Pediatr Infect Dis J. 2010;29:519–23. doi: 10.1097/INF.0b013e3181cdafc2. [DOI] [PubMed] [Google Scholar]
- 13.Dowell SF, Whitney CG, Wright C, Rose CE, Jr, Schuchat A. Seasonal patterns of invasive pneumococcal disease. Emerg Infect Dis. 2003;9:573–9. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter ND, Taylor TH, Jr, Dowell SF, Mathis S, Moore MR Active Bacterial Core Surveillance Systems Team. Holiday spikes in pneumococcal disease among older adults. N Engl J Med. 2009;361:2584–5. doi: 10.1056/NEJMc0904844. [DOI] [PubMed] [Google Scholar]
- 15.Self WH, Grijalva CG, Zhu Y, Talbot HK, Jules A, Widmer KE, et al. Emergency department visits for influenza A (H1N1)pdm09, Davidson County, Tennessee, USA. Emerg Infect Dis. 2012;18:863–5. doi: 10.3201/eid1805.111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberger DM, Simonsen L, Jordan R, Steiner C, Miller M, Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205:458–65. doi: 10.1093/infdis/jir749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming-Dutra KE, Taylor T, Link-Gelles R, Garg S, Jhung MA, Finelli L, et al. Effect of the 2009 influenza A(H1N1) pandemic on invasive pneumococcal pneumonia. J Infect Dis. 2013;207:1135–43. doi: 10.1093/infdis/jit008. [DOI] [PubMed] [Google Scholar]
- 18.Marston BJ, Plouffe JF, File TM, Jr, Hackman BA, Salstrom SJ, Lipman HB, et al. Incidence of community-acquired pneumonia requiring hospitalization. Arch Intern Med. 1997;157:1709–18. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) [Accessed August 22, 2012];National Hospital Ambulatory Medical Care Survey: 2009 Emergency department summary. http://www.cdc.gov/nchs/fastats/ervisits.htm.
- 20.Agency for Healthcare Quality and Research (AHRQ) [Accessed 22 August 2012];Overview of the National Emergency Department Sample (NEDS) Available at: http://www.hcup-us.ahrq.gov/nedsoverview.jsp.
- 21.Self WH, Grijalva CG, Zhu Y, McNaughton CD, Barrett TW, Collins SP, et al. Rates of emergency department visits due to pneumonia in the United States, July 2006–June 2009. Acad Emerg Med. 2013 doi: 10.1111/acem.12203. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) [Accessed 3 September 2012];National Vital Statistics System: US census population with bridged race categories. Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm.
- 23.Roberts MJ, Riccardo R. A student’s guide to analysis of variance. Routledge: 1999. [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) [Accessed 21 February 2013];FluView: A Weekly Influenza Surveillance Report Prepared by the Influenza Division. Available at: www.cdc.gov/flu/weekly.
- 25.Jules A, Grijalva CG, Zhu Y, Talbot HK, Williams JV, Dupont WD, et al. Estimating age-specfiic influenza-related hospitalization rates during the pandemic (H1N1) 2009 in Davidson Co, TN. Influenza Other Respi Viruses. 2012;6:e63–71. doi: 10.1111/j.1750-2659.2012.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed C, Angulo FJ, Swerdlow DL. Estimates of the prevalence of pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis. 2009;15:2004–7. doi: 10.3201/eid1512.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casalegno JS, Ottmann M, Bouscambert-Duchamp M, Valette M, Morfin F, Lina B. Impact of the 2009 influenza A(H1N1) pandemic wave on pattern of hibernal respiratory virus epidemics, France, 2009. Euro Surveill. 2010;15 pii:19485. [PubMed] [Google Scholar]
- 28.Anestad G. Interference between outbreaks of respiratory syncytial virus and influenza virus infection. Lancet. 1982;1:502. doi: 10.1016/s0140-6736(82)91466-0. [DOI] [PubMed] [Google Scholar]
- 29.Kahn JS. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–57. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law BJ, Carbonell-Estrany X, Simoes EAF. An update on respiratory syncytial virus epidemiology: a developed country perspective. Respir Med. 2002;96 (Suppl B):S1–S7. [PubMed] [Google Scholar]
- 31.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]