Abstract
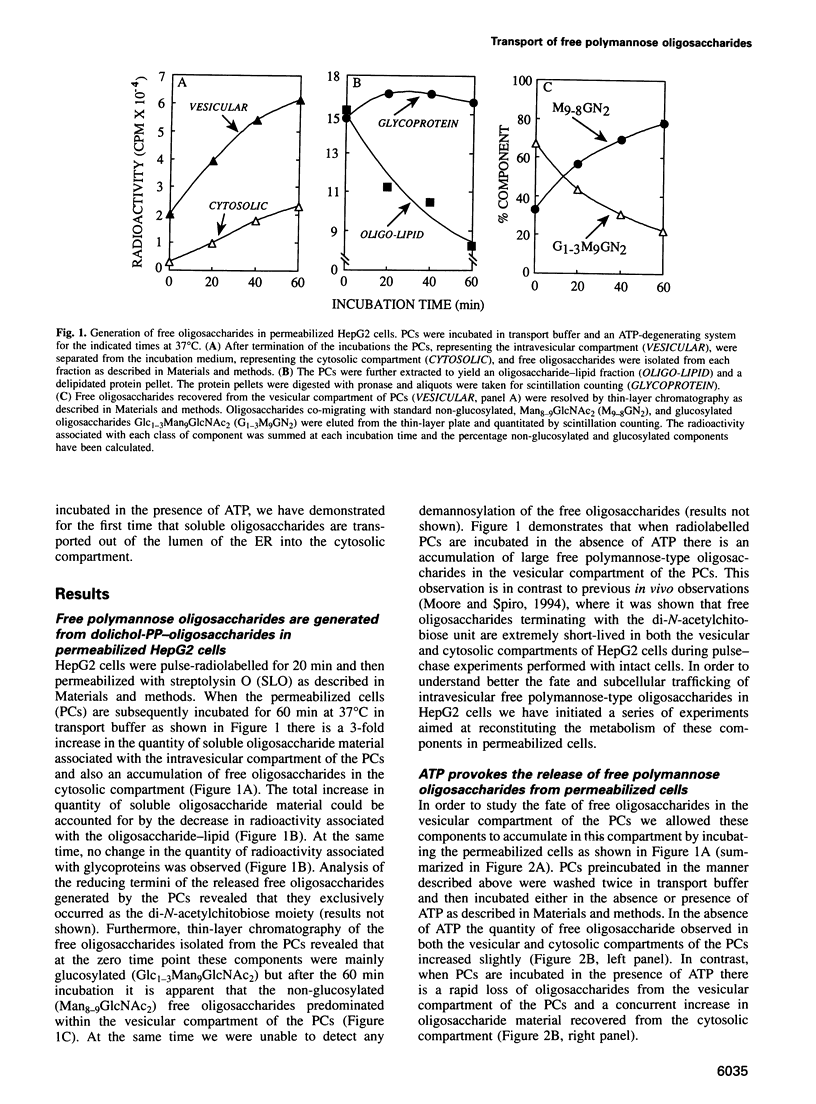
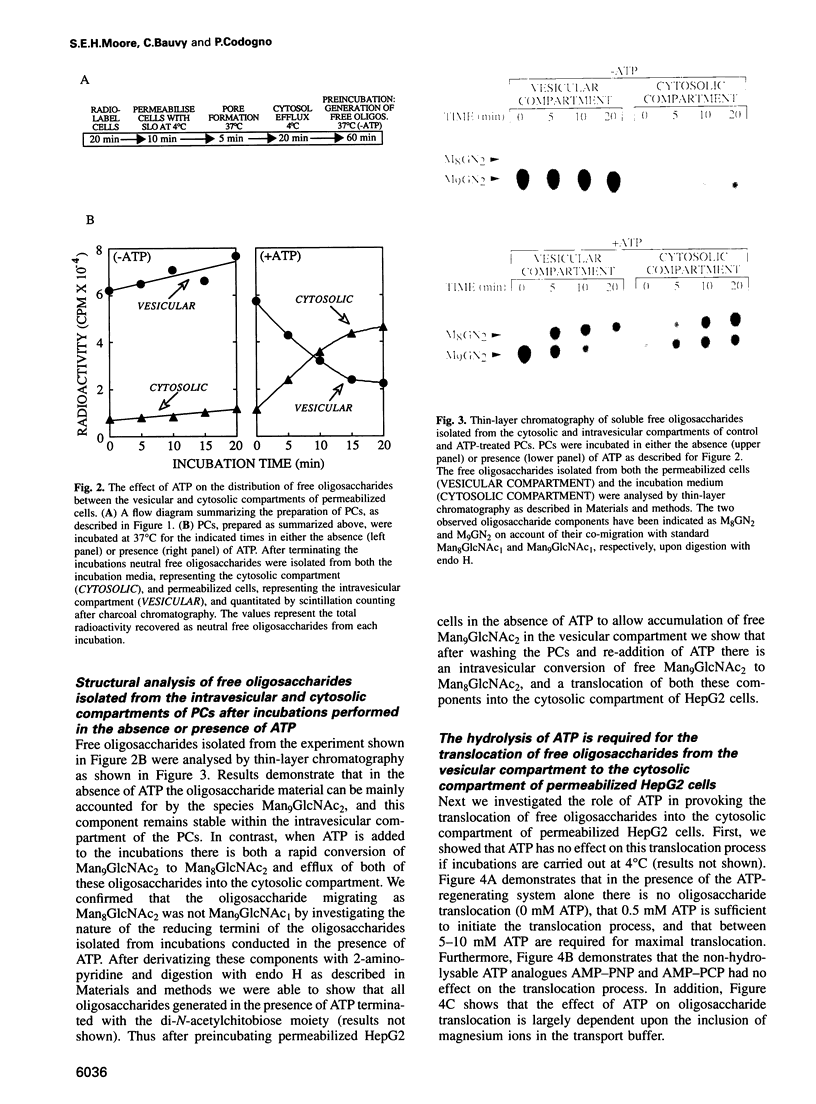
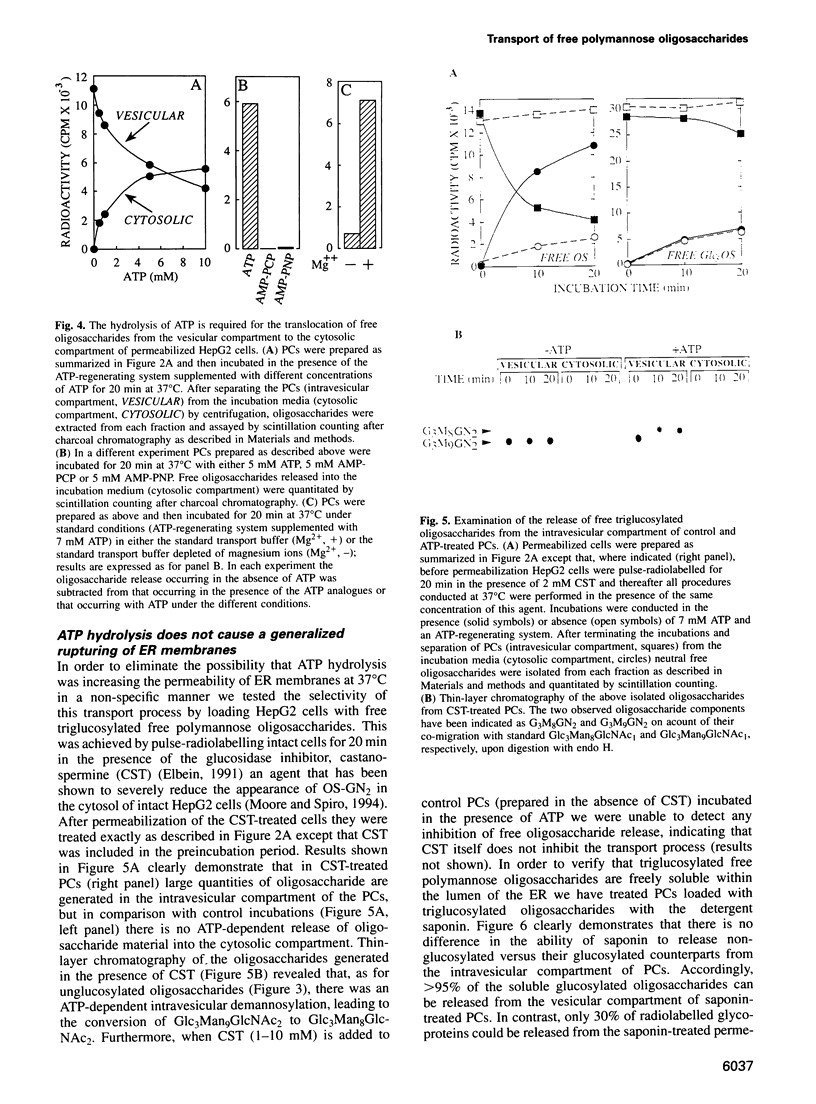
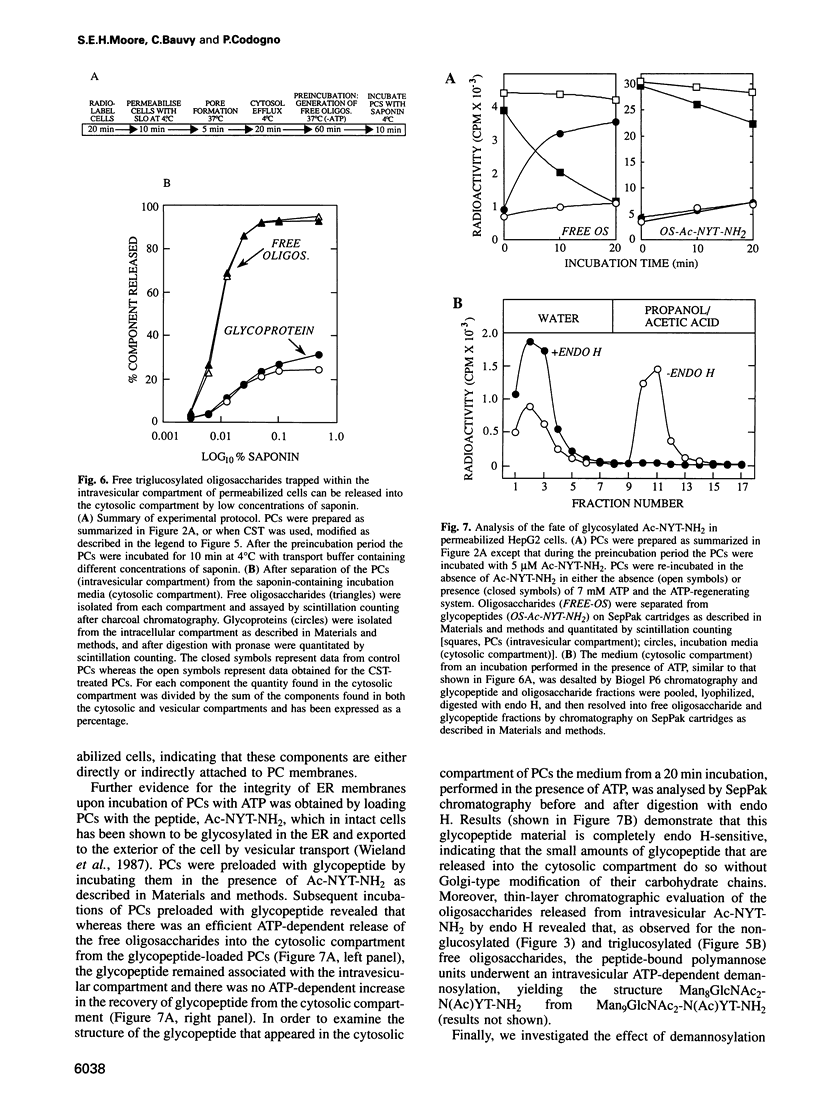
Free polymannose oligosaccharides have recently been localized to both the vesicular and cytosolic compartments of HepG2 cells. Here we investigated the possibility that free oligosaccharides originating in the lumen of the endoplasmic reticulum (ER) are transported directly into the cystosol. Incubation of permeabilized cells in the absence of ATP at 37 degrees C led to the intravesicular accumulation of free Man9GlcNAc2 which was generated from dolichol-linked oligosaccharide in the ER. This oligosaccharide remained stable within the permeabilized cells unless ATP was added to the incubations at which time the Man9GlcNac2 was partially converted to Man8GlcNAc2, and both these components were released from an intravesicular compartment into the cytosolic compartment of permeabilized cells. In contrast, when permeabilized cells, primed with either free triglucosyl-oligosaccharide or a glycotripeptide, were incubated with ATP both these structures remained associated with the intravesicular compartment. As the conditions in which free oligosaccharides were transported out of the intravesicular compartment into the cytosolic compartment did not permit vesicular transport of glycoproteins from the ER to the Golgi apparatus our data demonstrate the presence of a transport process for the delivery of free polymannose oligosaccharides from the ER to the cytosol.
Full text
PDF




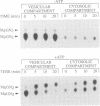
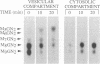
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anumula K. R., Spiro R. G. Release of glucose-containing polymannose oligosaccharides during glycoprotein biosynthesis. Studies with thyroid microsomal enzymes and slices. J Biol Chem. 1983 Dec 25;258(24):15274–15282. [PubMed] [Google Scholar]
- Arar C., Carpentier V., Le Caer J. P., Monsigny M., Legrand A., Roche A. C. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995 Feb 24;270(8):3551–3553. doi: 10.1074/jbc.270.8.3551. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Aegerter E., Mandel T., Hauri H. P. Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein). Carbohydr Res. 1986 Jun 1;149(1):23–33. doi: 10.1016/s0008-6215(00)90366-5. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Grimm K., Bächi T., Bosshart H., Kleene R., Watzele M. Double immunofluorescent staining of alpha 2,6 sialyltransferase and beta 1,4 galactosyltransferase in monensin-treated cells: evidence for different Golgi compartments? J Cell Biochem. 1993 Jul;52(3):275–288. doi: 10.1002/jcb.240520304. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Brenner M. B., Thomas D. Y., Williams D. B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994 Mar;19(3):124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Liscum L., Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986 Apr 5;261(10):4766–4774. [PubMed] [Google Scholar]
- Cacan R., Cecchelli R., Verbert A. Catabolic pathway of oligosaccharide-diphospho-dolichol. Study of the fate of the oligosaccharidic moiety in mouse splenocytes. Eur J Biochem. 1987 Jul 15;166(2):469–474. doi: 10.1111/j.1432-1033.1987.tb13539.x. [DOI] [PubMed] [Google Scholar]
- Carpentier V., Vassard C., Plessis C., Motta G., Monsigny M., Roche A. C. Characterization and cellular localization by monoclonal antibodies of the 60 kDa mannose specific lectin of human promyelocytic cells, HL60. Glycoconj J. 1994 Aug;11(4):333–338. doi: 10.1007/BF00731206. [DOI] [PubMed] [Google Scholar]
- Colombo M. I., Lenhard J. M., Mayorga L. S., Stahl P. D. Reconstitution of endosome fusion: identification of factors necessary for fusion competency. Methods Enzymol. 1992;219:32–44. doi: 10.1016/0076-6879(92)19007-s. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991 Dec;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Parton R. G., Kellner R., Etzold T., Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J. 1994 Apr 1;13(7):1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Simons K. A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins. Cell. 1994 Jun 3;77(5):625–626. doi: 10.1016/0092-8674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995 May 5;81(3):309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Godelaine D., Spiro M. J., Spiro R. G. Processing of the carbohydrate units of thyroglobulin. J Biol Chem. 1981 Oct 10;256(19):10161–10168. [PubMed] [Google Scholar]
- Grard T., Saint-Pol A., Haeuw J. F., Alonso C., Wieruszeski J. M., Strecker G., Michalski J. C. Soluble forms of alpha-D-mannosidases from rat liver. Separation and characterization of two enzymic forms with different substrate specificities. Eur J Biochem. 1994 Jul 1;223(1):99–106. doi: 10.1111/j.1432-1033.1994.tb18970.x. [DOI] [PubMed] [Google Scholar]
- Hebert D. N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995 May 5;81(3):425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. E., Spiro R. G. Demonstration that Golgi endo-alpha-D-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990 Aug 5;265(22):13104–13112. [PubMed] [Google Scholar]
- Moore S. E., Spiro R. G. Intracellular compartmentalization and degradation of free polymannose oligosaccharides released during glycoprotein biosynthesis. J Biol Chem. 1994 Apr 29;269(17):12715–12721. [PubMed] [Google Scholar]
- Nauseef W. M., McCormick S. J., Clark R. A. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. J Biol Chem. 1995 Mar 3;270(9):4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F., Hämmerling G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993 Aug 6;261(5122):769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Pierce R. J., Spik G., Montreuil J. Cytosolic location of an endo-N-acetyl-beta-D-glucosaminidase activity in rat liver and kidney. Biochem J. 1979 Jun 15;180(3):673–676. doi: 10.1042/bj1800673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpaneau V., Midoux P., Monsigny M., Roche A. C. Characterization and isolation of an intracellular D-mannose-specific receptor from human promyelocytic HL60 cells. Carbohydr Res. 1991 Jun 25;213:95–108. doi: 10.1016/s0008-6215(00)90601-3. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Transport of proteins across the endoplasmic reticulum membrane. Science. 1992 Nov 6;258(5084):931–936. doi: 10.1126/science.1332192. [DOI] [PubMed] [Google Scholar]
- Römisch K., Schekman R. Distinct processes mediate glycoprotein and glycopeptide export from the endoplasmic reticulum in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7227–7231. doi: 10.1073/pnas.89.15.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Itin C., Zerial M., Lottspeich F., Hauri H. P. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur J Cell Biol. 1993 Jun;61(1):1–9. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Potential regulation of N-glycosylation precursor through oligosaccharide-lipid hydrolase action and glucosyltransferase-glucosidase shuttle. J Biol Chem. 1991 Mar 15;266(8):5311–5317. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Substrate specificities of rat kidney lysosomal and cytosolic alpha-D-mannosidases and effects of swainsonine suggest a role of the cytosolic enzyme in glycoprotein catabolism. J Biol Chem. 1987 May 15;262(14):6506–6514. [PubMed] [Google Scholar]
- Villers C., Cacan R., Mir A. M., Labiau O., Verbert A. Release of oligomannoside-type glycans as a marker of the degradation of newly synthesized glycoproteins. Biochem J. 1994 Feb 15;298(Pt 1):135–142. doi: 10.1042/bj2980135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware F. E., Vassilakos A., Peterson P. A., Jackson M. R., Lehrman M. A., Williams D. B. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995 Mar 3;270(9):4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- van Leyen K., Widemann M., Wieland F. Glycotripeptides are released by yeast but not by mammalian microsomes. FEBS Lett. 1994 Nov 28;355(2):147–150. doi: 10.1016/0014-5793(94)01167-2. [DOI] [PubMed] [Google Scholar]