Abstract
Until recently, idiopathic pulmonary fibrosis (IPF) has been a devastating and generally fatal disease with no effective therapeutic. New developments in understanding the biology of the disease include a growing consensus that the lesions are mainly composed of cells that originated from resident fibroblasts. New developments in therapeutics include recommendations against several treatment regimes that have been previously used. On a positive note, the orally available drug pirfenidone has been approved for use in IPF in China, Japan, India, and the European Union, but not yet in the United States. Other possibilities for managing IPF include managing gastrointestinal reflux, and limiting excessive salt intake. A variety of potential therapeutics for IPF are in clinical trials; for instance in a Phase 1b trial, intravenous injections of a recombinant version of the normal human serum protein Serum Amyloid P (SAP, also known as PTX2) improved lung function in IPF patients.
Keywords: Fibrosing, Lung, Fibrocyte, Macrophage, Clinical trials, Modulation, Idiopathic pulmonary fibrosis, Treatment, Therapeutics, Salt intake
Introduction
Idiopathic pulmonary fibrosis (IPF), also referred to as usual interstitial pneumonia (UIP), is a devastating disease that, until recently, was essentially untreatable. Like asthma, it generally presents as shortness of breath, but the ~130,000 IPF patients in the US tend to be older, have relatively few flare-ups, and show a steady decline in lung function with ~80% mortality 5 years after the initial diagnosis [1]. Chest X-rays show diffuse increased density in the lungs, and high-resolution CT scans show a honeycomb-like arrangement of dense tissue forming in the lungs, with increased density typically at the periphery of the base of the lung [2]. The diagnosis however can be difficult and requires a careful exclusion of other possible lung diseases [2, 3]. Lung biopsies, the original gold standard for diagnosis (but now performed less frequently given the evolving role of high resolution CT scans, as well as the potential for this invasive diagnostic procedure to exacerbate lung damage) show scar-tissue like lesions in the alveoli that demonstrate the usual interstitial pneumonia pattern of relatively preserved lung tissue, established fibrosis, and intervening areas of active fibrogenesis called fibroblastic foci. There is a relatively small genetic component, and the lung function decline rate varies from patient to patient. Although some IPF is probably due to exposure to particulate irritants such as coal dust, the cause in many patients is unknown. Oxygen therapy and lung transplants remain a recommended treatment [2].
New developments in the biology of IPF
IPF is one of many different fibrosing diseases
Fibrosis involves the inappropriate formation of scar tissue-like lesions in tissues. Examples of fibrosing diseases include end-stage kidney disease, cardiac fibrosis, hypertrophic scarring, cirrhosis of the liver, and of course IPF. Fibrosis also occurs in asthma, causing a thickening of the airway walls [4], whereas in IPF the fibrosis is less in the airway walls and more around the alveoli. A growing awareness in the IPF field is that IPF has strong similarities to other fibrosing diseases. A 2009 Keystone meeting on fibrosis was, for some workers in the IPF field, their first chance to meet people working on related diseases such as cardiac and renal fibrosis. As with asthma, the consensus in the field is that fibrosis is due to an overreaction by a tissue defense/repair mechanism to a relatively minor insult.
Specialized profibrotic macrophages potentiate fibrosis
In animal models, cells of the innate immune system also participate in fibrosis. Monocytes leave the circulation, enter the lesion, and can differentiate into macrophages. The current consensus is that in rodents and humans there are at least three basic types of macrophages [5]. M1 macrophages participate in the defense against bacteria and participate in inflammation; alternatively activated (M2a) macrophages participate in fibrosis [6, 7]; while Mreg macrophages are regulatory and help to suppress inflammation and fibrosis [8]. A key problem in the macrophage field is that until very recently, most workers lumped M2a and Mreg macrophages together into a category called M2, and much of the earlier literature does not distinguish between the very different M2a and Mreg macrophages.
Many of the lesion cells appear to originate from resident fibroblasts
The source of the cells that form fibrotic lesions has been an active area of research [9]. Many of the lesion cells have characteristics of mesenchymal cells, express unusual markers including α-smooth muscle actin, and secrete copious amounts of collagen and other extracellular matrix proteins [10]. Although epithelial cells can transform into mesenchymal cells during embryogenesis, recent studies indicate that this, or the transformation of endothelial cells into mesenchyme, is not the source of the majority of the cells in the fibrotic lesion [11, 9]. The role of epithelial cells in fibrosis seems to be to secrete signals that then activate resident fibroblasts. In addition to becoming macrophages, some monocytes can differentiate into fibroblast-like cells called fibrocytes [12-14]. Fibrocytes produce a small amount of collagen, are rarely observed in normal tissue, but are prevalent in hypertrophic dermal scars and fibrotic lesions [15, 14]. A key function of fibrocytes is to produce signals that activate resident fibroblasts [16] The current consensus model is that the lesions are mainly composed of resident fibroblasts that proliferate and secrete collagen in response to damage, where the damage is sensed by the fibroblasts themselves, epithelial cells, and immune system cells.
A variety of signals mediate fibrosis
In response to perceived damage, epithelial cells and/or macrophages secrete signals such as tumor necrosis factor-α (TNF-α), interleukin-1β, interleukin-13, and transforming growth factor-β (TGF-β) [17]. These and other signals then appear to activate resident fibroblasts to form the fibrotic lesions. A simplistic overview is that epithelial cells, resident fibroblasts, and immune system cells signal to each other and to themselves in a complex mechanism that evolved to initiate and promote wound healing, and that in fibrosis this mechanism is inappropriately activated (Figure 1). An obvious, and unanswered, question is why the signaling persists in fibrosis and does not shut off as in normal wound healing. Compared to the variety of signals that promote fibrosis, relatively few signals have been found that counteract fibrosis. One such signal is interleukin-10, which is secreted by Mreg regulatory macrophages and other cells [18, 19]. As described below, a therapeutic that blocks fibrocyte and M2a macrophage differentiation, and potentiates Mreg macrophage differentiation, shows promise in clinical trials.
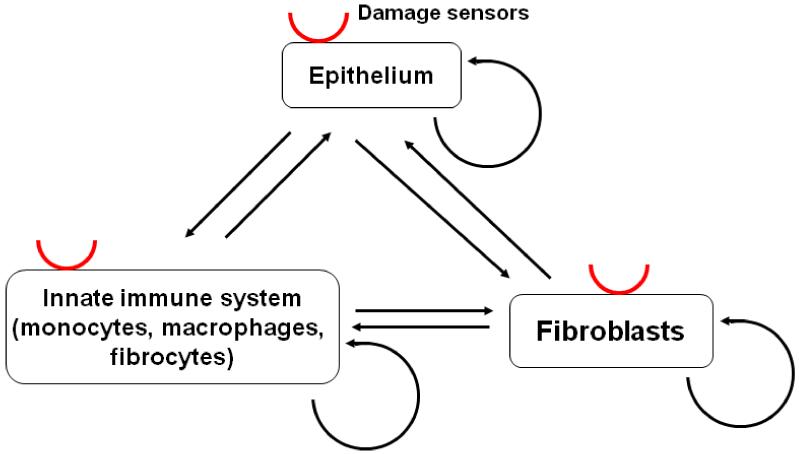
Figure 1.
Diagram of the known and potential interactions among the key cell types that participate in fibrosis. Red semicircles indicate damage sensors. Arrows indicate known or potential signal interactions, which could be either stimulatory or repressive. Fibroblasts have the potential to proliferate, and these interactions evolved to have the capacity to do two quite different things. First, negative feedback loops must exist to maintain homeostasis and prevent fibroblasts from proliferating, or at least limit fibroblast proliferation to just replacing dead fibroblasts, in a healthy adult tissue such as the lung. Second, activating pathways evolved to allow damage or wounding (sensed by some combination of epithelial cells, the innate immune system, and fibroblasts) to trigger fibrocyte differentiation, fibroblast activation, and fibroblast proliferation, all of which participate in wound contraction and the formation of scar tissue. The system has thus had to evolve a tricky balancing actremain quiescent in the absence of wounding, but respond quickly and vigorously when there is damage or wounding, and then stop the response when the damage has been repaired. Fibrosis appears to be a situation where this balancing act gets stuck in the repair mode. A probable scenario for fibrosis involves some sort of initial damage, a response from the innate immune system and resident fibroblasts, and then a positive feedback loop forming where, for instance, innate immune system cells activate fibroblasts, causing some sort of stress which is sensed by epithelial cells as damage, and these cells then activating the innate immune system. There are thus many possible targets to slow or stop this vicious cycle, and thus slow or stop fibrosis.
New developments in the treatment of IPF
Several possible therapeutics should not be used to treat IPF patients
Although there is some association between IPF and thromboembolism [20], treatment of IPF patients with warfarin increases mortality [21]. Until recently, many physicians prescribed a combination of prednisone, azathioprine, and N-acetylcysteine [22], but a clinical trail comparing the combination to placebo showed that the prednisone/ azathioprine/ N-acetylcysteine combination significantly increases mortality [23]. Recent guidelines also recommend against treating IPF with corticosteroids, colchicine, cyclosporine A, combination corticosteroid and immunomodulator therapy, interferon-γ, bosentan, or etanercept [2]. Clinical trials with imatinib showed no improvement of IPF symptoms [24], and trials of everolimus and ambrisentan showed both of these drug worsening IPF symptoms [25, 26].
Managing gastrointestinal reflux
Approximately 90% of IPF patients also have gastrointestinal reflux [27], and an intriguing possibility is that microaspiration of the refluxed material may cause a chronic lung injury that exacerbates and/or potentiates IPF [28]. A study of four IPF patients suggested that treatment of the gastrointestinal reflux stabilized or reversed the decline in lung function [29], and a retrospective study indicated that patients who were being actively treated for reflux had a slower decline in lung function compared to patients who were not being treated for reflux [30]. Although a prospective study on managing acid reflux in IPF is clearly needed, managing acid reflux in IPF should be considered; an important caveat is that microaspiration of refluxed stomach contents may still occur, and act as an irritant, even if the acidity is controlled with proton pump inhibitors or H2 histamine receptor antagonists [28].
Managing salt intake
Excessive NaCl (salt) intake is associated with cardiac and renal fibrosis [31]. Although salt is generally thought of as being deleterious due to its effect of increasing blood pressure, in some cases salt appears to promote fibrosis in the absence of an effect on blood pressure [32-35]. We found that in culture, NaCl promotes the differentiation of human monocytes into fibrocytes, and also inhibits the ability of the serum protein serum amyloid P (described in more detail below) to inhibit fibrocyte differentiation [36]. Based on the combined results, as with most people, healthy or not, helping an IPF patient to limit excessive salt intake should be useful.
Sildenafil is useful in IPF patients with right ventricular hypertrophy or right ventricular systolic dysfunction
One effect of sildenafil (Viagra) is to improve blood flow into the lungs, and sildenafil is used to treat pulmonary artery hypertension [37]. In a clinical trial with IPF patients, sildenafil did not significantly improve exercise capacity, but it did improve dyspnea and quality of life [38]. However, in the subset of IPF patients in this trial who had right ventricular hypertrophy or right ventricular systolic dysfunction, sildenafil did significantly reduce the IPF-associated decline in exercise capacity [39].
Pirfenidone has been approved as a therapeutic for IPF in Europe, Japan, India, and China
Pirfenidone is a small molecule drug (the backbone resembles two connected benzene rings). It was originally discovered as having analgesic and anti-inflammatory activity, and was then found to be efficacious in animal studies as an orally available anti-fibrotic [40]. Pirfenidone appears to inhibit fibrosis by regulating a remarkably broad array of pathways, including inhibiting cell proliferation, T-cell activation, collagen production, TGF-β1 signaling, and the production of a variety of cytokines including TNF-α, interferon-γ, and basic fibroblast growth factor, while increasing production of the anti-inflammatory cytokine interleukin-10 [41]. There have been three Phase III clinical trials of pirfenidone, one in Japan and two in the US. The trials resulted in approval of pirfenidone for use in IPF in Japan, China, India, and Europe, but because of mixed results in the two US trials [42, 43], the US FDA declined to approve pirfenidone. Some patients taking pirfenidone experience side effects including liver enzyme abnormalities, nausea, diarrhea, dizziness, photosensitivity, and rashes [41, 44].
Serum Amyloid P (PTX2) improved lung function in a small clinical trial
While looking for factors secreted by human white blood cells, we noticed that in serum-free culture conditions, some monocytes differentiate into fibrocytes. The differentiation is inhibited by serum, and we isolated the inhibitor from human serum and identified it as Serum Amyloid P (SAP, also known as PTX2) [45]. Since fibrocytes contribute to fibrosis, we tested SAP injections in mouse and rat models of pulmonary fibrosis, and a mouse model of cardiac fibrosis [46, 47]. In all three cases, SAP injections prevented fibrosis, and in the rat pulmonary fibrosis model, SAP injections improved lung function in animals with an established fibrosis. Subsequent tests in animal models of renal fibrosis, asthma, radiation-induced fibrosis, and experimental autoimmune encephalomyelitis showed that SAP can prevent fibrosis [48-52], and in a mouse model of pulmonary fibrosis, SAP also improved lung function in an established disease [53]. Since fibrocytes participate in wound healing, SAP injections, as expected, slowed dermal wound healing in mice [54].
SAP is a constitutive component of serum, with levels ranging from 10 to 60 μg/ ml [55]. Patients with keloid scars (a dermal fibrosis) have normal serum levels of SAP, but their monocytes tend to be abnormally insensitive to the ability of SAP to inhibit fibrocyte differentiation [56]. Patients with IPF or kidney disease tend to have low serum SAP levels [53, 48]. Together, these results suggest that for some fibrosing diseases, low SAP levels, or a decreased ability of SAP to inhibit fibrocyte differentiation, may contribute to the disease.
In addition to inhibiting the differentiation of monocytes into fibrocytes, SAP has several other properties which could be useful for its use as an antifibrotic. First, SAP binds to a variety of debris molecules such as chromatin, DNA, and apoptotic cells, and helps phagocytes to clear the debris [57-59]. Since debris could act to induce local inflammation, clearance of debris should counteract this source of a localized insult. Second, SAP promotes the differentiation of Mreg macrophages [48, 50, 8]. Finally, SAP inhibits neutrophil adhesion to basement membrane components, and in a mouse model, SAP injections inhibited neutrophil influx into the lungs in a mouse model of early-stage IPF [60]. A Phase I trial of single doses of intravenous recombinant human SAP showed no significant adverse events [61], and a preliminary report of a Phase 1b trial of intravenous injections of recombinant SAP (14 patients treated with SAP, 6 treated with placebo) on days 1, 3, 5, 8, and 15 indicated that SAP injections caused improvements of forced vital capacity, FEV1, and distance in a 6-minute walk test at day 57 [62].
A variety of potential therapeutics for IPF are in clinical trails
Several clinical trials are currently underway using, as a potential therapeutic, antibodies against cytokines that promote fibrosis, such as TGF-β1, connective tissue growth factor, IL-4, and IL-13 [63, 64]. Other trials target a variety of other pathways, such as angiogenesis, collagen synthesis, and growth hormone. A Phase II trial of the orally-available kinase inhibitor nintedanib (BIBF1120) showed that this drug significantly slowed the decline in lung function in IPF patients [65], and Phase III trials are underway. One of the more interesting trials is the use of brief exposure to inhaled carbon monoxide. At the time of this writing (mid-June 2013), a search of clinicaltrials.gov showed 34 clinical trials actively recruiting for IPF, and of these, 17 are testing a possible therapeutic.
Conclusions
Significant advances are being made in understanding the mystery of how scar tissue starts forming in the lungs of IPF patients. New therapies include pirfenidone, and for a subset of IPF patients, sildenafil may be useful. Managing reflux and salt intake are also simple possibilities that a physician might consider. Finally, a variety of clinical trials hold promise for additional therapeutics for IPF.
Acknowledgments
The author wishes to thank Rachel Sterling, Erica Herzog, and Mark Lupher for helpful comments and corrections to the manuscript.
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Compliance with Ethics Guidelines
Conflict of Interest
Richard H. Gomer is a co-founder of, receives royalties from, and holds stock options in Promedior, a company that is developing SAP as a therapeutic for idiopathic pulmonary fibrosis. He also is a member of the Science Advisory Board for Promedior.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277–84. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- 2••.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. latest official guidelines for diagnosis and treatment of IPF
- 3•.Wells AU. Managing diagnostic procedures in idiopathic pulmonary fibrosis. Eur Respir Rev. 2013;22(128):158–62. doi: 10.1183/09059180.00001213. points out difficulties with the diagnosis
- 4.Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 5•.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–37. doi: 10.1038/nri3073. shows the presence of peribronchiolar fibrosis in asthma
- 6.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173(7):781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 7•.Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11(1):29. doi: 10.1186/1478-811X-11-29. shows that signals from human M2a macrophages stimulate proliferation of human (dermal) fibroblasts
- 8.Murray LA, Rosada R, Moreira AP, Joshi A, Kramer MS, Hesson DP, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5(3):e9683. doi: 10.1371/journal.pone.0009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301(6):G950–5. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucala R, Spiegel L, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8(2):145–50. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 14.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38(7):548–56. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Scott P, Giuffre J, Shankowsky H, Ghahary A, Tredget E. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82(9):1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 16.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–21. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2(2):103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5(4):e1000371. doi: 10.1371/journal.ppat.1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedrich CM, Bream JH. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res. 2010;47(1-3):185–206. doi: 10.1007/s12026-009-8150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sode BF, Dahl M, Nielsen SF, Nordestgaard BG. Venous thromboembolism and risk of idiopathic interstitial pneumonia: a nationwide study. Am J Respir Crit Care Med. 2010;181(10):1085–92. doi: 10.1164/rccm.200912-1951OC. [DOI] [PubMed] [Google Scholar]
- 21.Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186(1):88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peikert T, Daniels CE, Beebe TJ, Meyer KC, Ryu JH. Assessment of current practice in the diagnosis and therapy of idiopathic pulmonary fibrosis. Respir Med. 2008;102(9):1342–8. doi: 10.1016/j.rmed.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Raghu G, Anstrom KJ, King TE, Jr., Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181(6):604–10. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 25.Malouf MA, Hopkins P, Snell G, Glanville AR. An investigator-driven study of everolimus in surgical lung biopsy confirmed idiopathic pulmonary fibrosis. Respirology. 2011;16(5):776–83. doi: 10.1111/j.1440-1843.2011.01955.x. [DOI] [PubMed] [Google Scholar]
- 26.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158(9):641–9. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 27.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27(1):136–42. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G. Idiopathic pulmonary fibrosis: increased survival with “gastroesophageal reflux therapy”: fact or fallacy? Am J Respir Crit Care Med. 2011;184(12):1330–2. doi: 10.1164/rccm.201110-1842ED. [DOI] [PubMed] [Google Scholar]
- 29.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129(3):794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390–4. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23(6):363–84. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 32.Yuan BX, Leenen FH. Dietary sodium intake and left ventricular hypertrophy in normotensive rats. Am J Physiol. 1991;261(5 Pt 2):H1397–401. doi: 10.1152/ajpheart.1991.261.5.H1397. [DOI] [PubMed] [Google Scholar]
- 33.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98(23):2621–8. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 34.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi: 10.1136/bmj.b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He FJ, Burnier M, Macgregor GA. Nutrition in cardiovascular disease: salt in hypertension and heart failure. Eur Heart J. 2011;32(24):3073–80. doi: 10.1093/eurheartj/ehr194. [DOI] [PubMed] [Google Scholar]
- 36.Cox N, Pilling D, Gomer RH. NaCl potentiates human fibrocyte differentiation. PLoS One. 2012;7(9):e45674. doi: 10.1371/journal.pone.0045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh TP. Clinical use of sildenafil in pulmonary artery hypertension. Expert Rev Respir Med. 2010;4(1):13–9. doi: 10.1586/ers.09.71. [DOI] [PubMed] [Google Scholar]
- 38.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363(7):620–8. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, et al. Sildenafil Preserves Exercise Capacity in Patients With Idiopathic Pulmonary Fibrosis and Right-sided Ventricular Dysfunction. Chest. 2013;143(6):1699–708. doi: 10.1378/chest.12-1594. describes the subset of IPF patients for whom sildenafil may be useful
- 40.Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125(6):779–85. [PubMed] [Google Scholar]
- 41.Gan Y, Herzog EL, Gomer RH. Pirfenidone treatment of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. 2011;7:39–47. doi: 10.2147/TCRM.S12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 43.Raghu G, Thickett DR. Pirfenidone for IPF: pro/con debate; the ‘con’ viewpoint. Thorax. 2013 doi: 10.1136/thoraxjnl-2011-201269. [DOI] [PubMed] [Google Scholar]
- 44.Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012;7(10):e47024. doi: 10.1371/journal.pone.0047024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. Journal Of Immunology. 2003;17(10):5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179(6):4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103(48):18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castaño AP, Lin S-L, Surowy T, Nowlin BT, Turlapati SA, Patel T, et al. Serum Amyloid P Inhibits Fibrosis Through FcγR-Dependent Monocyte-Macrophage Regulation in Vivo. Science Translational Medicine. 2009;1(5):5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W, Wu J, Qiao B, Xu W, Xiong S. Amelioration of lupus nephritis by serum amyloid P component gene therapy with distinct mechanisms varied from different stage of the disease. PLoS One. 2011;6(7):e22659. doi: 10.1371/journal.pone.0022659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira AP, Cavassani KA, Hullinger R, Rosada RS, Fong DJ, Murray L, et al. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J Allergy Clin Immunol. 2010;4:712–21. doi: 10.1016/j.jaci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Murray LA, Kramer MS, Hesson DP, Watkins BA, Fey EG, Argentieri RL, et al. Serum amyloid P ameliorates radiation-induced oral mucositis and fibrosis. Fibrogenesis Tissue Repair. 2010;3:11. doi: 10.1186/1755-1536-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji Z, Ke ZJ, Geng JG. SAP suppresses the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray LA, Chen Q, Kramer MS, Hesson DP, Argentieri RL, Peng X, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154–62. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Naik-Mathuria B, Pilling D, Crawford JR, Gay AN, Smith CW, Gomer RH, et al. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16(2):266–73. doi: 10.1111/j.1524-475X.2008.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson S, Tennent G, Sethi D, Gower P, Ballardie F, Amatayakul-Chantler S, et al. Serum amyloid P component in chronic renal failure and dialysis. Clinica Chimica Acta. 1991;200(2-3):191–9. doi: 10.1016/0009-8981(91)90090-y. [DOI] [PubMed] [Google Scholar]
- 56.Naylor MC, Lazar DA, Zamora IJ, Mushin OP, Yu L, Brissett AE, et al. Increased in vitro differentiation of fibrocytes from keloid patients is inhibited by serum amyloid P. Wound Repair Regen. 2012;20(3):277–83. doi: 10.1111/j.1524-475X.2012.00782.x. [DOI] [PubMed] [Google Scholar]
- 57.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. JImmunol. 2001;166(11):6735–41. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 58.Bijl M, Horst G, Bijzet J, Bootsma H, Limburg PC, Kallenberg CG. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthritis Rheum. 2003;48(1):248–54. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- 59.Familian A, Zwart B, Huisman HG, Rensink I, Roem D, Hordijk PL, et al. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001;167(2):647–54. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- 60.Maharjan AS, Roife D, Brazill D, Gomer RH. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair. 2013;6(1):2. doi: 10.1186/1755-1536-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dillingh MR, van den Blink B, Moerland M, van Dongen MG, Levi M, Kleinjan A, et al. Recombinant human serum amyloid P in healthy volunteers and patients with pulmonary fibrosis. Pulm Pharmacol Ther. 2013 doi: 10.1016/j.pupt.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 62.Van Den Blink B, Burggraaf J, Morrison LD, Ginns LC, Wijsenbeek MS, Moerland M, et al. A Phase I Study Of PRM-151 In Patients With Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2013;187:A5705. [Google Scholar]
- 63.Adamali HI, Maher TM. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel Ther. 2012;6:261–72. doi: 10.2147/DDDT.S29928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5(1):48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079–87. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]