Abstract
We previously reported that a 4-6 week low-fat fish oil (LFFO) diet did not affect serum IGF-1 levels (primary outcome) but resulted in lower omega-6 to omega-3 fatty acid ratios in prostate tissue and lower prostate cancer proliferation (Ki67) as compared to a Western diet (WD). In this post-hoc analysis, the effect of the LFFO intervention on serum pro-inflammatory eicosanoids, LTB4 and 15(S)-HETE, and the cell cycle progression (CCP) score were investigated. Serum fatty acids and eicosanoids were measured by gas chromatography and ELISA. CCP score was determined by RT-PCR. Associations between serum eicosanoids, Ki67, and CCP score were evaluated using partial correlation analyses. BLT1 (LTB4 receptor) expression was determined in prostate cancer cell lines and prostatectomy specimens. Serum omega-6 fatty acids and 15(S)-HETE levels were significantly reduced, and serum omega-3 levels were increased in the LFFO group relative to the WD group, whereas there was no change in LTB4 levels. The CCP score was significantly lower in the LFFO compared to the WD group. The 15(S)-HETE change correlated with tissue Ki67 (R=0.48; p<0.01) but not with CCP score. The LTB4 change correlated with the CCP score (r=0.4; p=0.02) but not with Ki67. The LTB4 receptor BLT1 was detected in prostate cancer cell lines and human prostate cancer specimens. In conclusion, a LFFO diet resulted in decreased 15(S)-HETE levels and lower CCP score relative to a WD. Further studies are warranted to determine whether the LFFO diet anti-proliferative effects are mediated through the LTB4/BLT1 and 15(S)-HETE pathways.
INTRODUCTION
Prostate cancer is a leading cause of cancer death among men in the United States (1). It is estimated that 238,590 men will be diagnosed with prostate cancer and 29,720 men will die from the disease in 2013 (2). There is an ever-growing need to find new strategies to prevent the development of prostate cancer or to slow disease progression. Pre-clinical studies utilizing xenografts and genetically engineered mouse models demonstrated that reducing dietary fat from corn oil (omega-6 fatty acids) and increasing fish oil intake (omega-3 fatty acids) delays the development and progression of prostate cancer (3-6). Epidemiologic studies also found that a high-fat diet and low intake of omega-3 fatty acids were associated with increased risk of developing prostate cancer and increased risk of advanced disease (7-10). However, this association is not supported by other reports (11, 12). Intake of fish and marine-derived omega-3 fatty acids has been shown to be associated with decreased prostate cancer mortality (13, 14). Epidemiologic studies have yielded conflicting results with regard to the association of circulating omega-3 fatty acid levels and prostate cancer risk (12, 15-20). In a prospective randomized trial involving men diagnosed with prostate cancer, serum from men consuming a low-fat diet reduced the proliferation of LNCaP cells in an ex-vivo bioassay compared to men on a high-fat diet. In the same study, serum omega-6 fatty acid levels were positively associated with proliferation, whereas serum omega-3 fatty acid levels were inversely associated (21).
The proportion of omega-6 to omega-3 fatty acids in adipose tissue and blood lipids reflect the dietary intake of fatty acids (22). Through a series of steps, cyclooxygenases and lipoxygenases convert the fatty acids to metabolically active eicosanoids including prostaglandins, thromboxanes, hydroxyeicosatetraenoic acids (HETEs), and leukotrienes. Eicosanoids derived from dietary omega-6 polyunsaturated fatty acids, including 15(S)-HETE and leukotriene B4 (LTB4), have pro-inflammatory effects whereas those formed from omega-3 polyunsaturated fatty acids are less inflammatory and/or anti-inflammatory in nature (23). Inflammation is under active investigation as an important component of cancer development and progression (22, 24, 25) including prostate cancer (26). Eicosanoids regulate inflammatory responses by selective interaction with the BLT receptors. The BLT2 receptor is expressed ubiquitously and is known to bind both LTB4 and 15(S)-HETE (27). Ligand binding to the receptor induces signaling pathways involved in cell proliferation (28). It was recently reported that BLT2 is a key regulator of androgen receptor expression in androgen-dependent cell lines and possibly a target for prostate cancer therapy (29). LTB4 also binds to the BLT1 receptor with a higher affinity than BLT2 (27). BLT1 is mainly expressed in leukocytes, where it induces signaling pathways involved in cell proliferation. BLT1 expression has been reported in ovarian, colon, and pancreatic cancer (30-32) where it has pro-proliferative effects, but has not yet been reported in prostate cancer.
We recently completed a Phase II pre-prostatectomy trial in which the primary outcome (serum IFG-1 levels) was negative, but one of the positive secondary outcomes was lower proliferation (Ki67) in radical prostatectomy specimens, of men consuming a LFFO diet as compared to a Western diet (33). The dietary intervention also resulted in lower omega-6 and higher omega-3 fatty acids in red blood cell membranes and benign and malignant prostate tissue (33). Preclinical studies suggested that fish oil may act through the COX-2/PGE-2 pathway (3) but these factors were not affected in urine, serum or prostate tissue in the LFFO group in our human trial (33). Given the association between dietary fat, omega-6 and omega-3 fatty acids, inflammation and prostate cancer (26, 34, 35), and the lack of information regarding potential mechanisms, we sought to examine the changes in serum fatty acids and pro-inflammatory eicosanoid levels in men consuming the LFFO diet compared to a Western diet. In this post-hoc analysis also investigated whether the diet-induced changes in serum pro-inflammatory eicosanoids correlate with anti-proliferative effects as measured by immunohistochemistry (Ki67) (33) and the cell cycle progression (CCP) score, a validated genetic risk score for predicting recurrence after radical prostatectomy and death from prostate cancer (36-38).
MATERIAL AND METHODS
Patients and Study design
This study is a post-hoc analysis utilizing serum and prostate tissue obtained from a previously completed Phase II randomized trial. (33). Briefly, men diagnosed with prostate cancer were randomized to a low-fat diet (20% Kcal fat) supplemented with 5 grams of fish oil per day or to a Western diet with 40% Kcal fat prior to radical prostatectomy (33). Baseline characteristics of the research subjects are described in Table 1. Subjects were ineligible if they were on insulin, 5-alpha reductase inhibitors, anti-androgens, or LHRH agonists. Compliance with the research diets was high due to utilization of the NIH-funded UCLA Clinical Research Center (CRC) chefs that prepared all meals and snacks (delivered to patients), and the UCLA CRC dietitian who closely monitored dietary intake. The study was approved by the UCLA Institutional review board and registered with clinical trial.gov # NCT00836615.
Table 1.
Research subject baseline characteristics
| Western Diet (n=21) |
Low Fat Fish Oil Diet (n=27) |
|
|---|---|---|
| Ethnicity | ||
| Caucasian (No.) | 10 | 21 |
| Black American (No.) | 9 | 6 |
| Hispanic (No.) | 2 | 0 |
| Age (years), mean ± SD | 60.4 ± 6.7 | 60.5 ± 6.3 |
| Weight (kg), mean ± SD | 91 ± 19.1 | 92.5 ± 13.1 |
| BMI (kg/m2), mean ± SD | 29 ± 4.2 | 29.8 ± 3.8 |
| Percent Body fat, mean ± SD | 25.9 ± 4.7 | 29.6 ± 3.1 |
| PSA (ng/ml), mean ± SD | 7.6 ± 5.6 | 6.9 ± 4.9 |
| Gleason sum at diagnosis (No.) | ||
| 6 | 8 | 16 |
| 7 | 11 | 9 |
| 8-9 | 1 | 2 |
Fatty acid analysis
Fatty acid analysis was performed on pre and post-intervention serum and on red blood cell membranes using gas chromatography after formation of fatty acid methyl esters (39). The method used measures phospholipids and cholesteryl esters. The intra-assay coefficients were less than 6% for all fatty acids analyzed. The inter-assay coefficients were less than 10% for all fatty acids analyzed except for palmitoleic and linolenic acids for which the inter-assay coefficient was less than 12.5%.
Leukotriene B4 (LTB4) and 15-S-Hydroxyeicosatetraenoic acid (15(S)-HETE) analysis
LTB4 levels were measured in duplicate by ELISA in pre and post intervention plasma following the manufacturer protocol (Assay Designs Inc., Ann Arbor, MI). Prostaglandin synthetase inhibitor, indomethacin (Sigma-Aldrich, Saint Louis, MO), was added at a concentration of 10μg/ml as recommended by the manufacturer. The intra and inter-assay coefficient of variation were 6% and 5% respectively. 15(S)-HETE levels were measured by ELISA in pre and post intervention serum following the manufacturer protocol (Enzo Life Sciences INT’L Inc., Plymouth Meeting, PA). The intra and inter-assay coefficients of variation were 9.8% and 8.9% respectively.
Cell Cycle Progression score (CCP)
The CCP score was calculated as average expression of 31 CCP genes, normalized to 15 housekeeper genes. Five paraffin embedded unstained slides and a corresponding H&E stained slide were prepared from the dominant tumor focus from the archived paraffin-embedded radical prostatectomy specimens. The mRNA extraction, RTPCR, and calculation of CCP score was performed by Myriad Genetics (Salt Lake City, UT) as previously described (36). Archived specimens with adequate malignant epithelium for CCP testing were not available for 12 patients (7 from the LFFO group and 5 from the WD group).
RNA extraction and RT-PCR
Total RNA was isolated from 22Rv1, LnCAP, PC-3, and DU145 human prostate cancer cell lines, from human peripheral blood mononuclear cell, and from frozen radical prostatectomy prostate cancer specimens from 4 patients enrolled in our study (2 LFFO patients and 2 WD patients) using trizol reagent following the manufacturer protocol (Invitrogen, Carlsbad, CA) followed by RNeasy clean up using the Qiagen RNeasy kit (Qiagen, Valencia, CA). RNA concentration was measured using a Nano Photometer Pearl (Implen, Inc., Westlake Village, CA). One microgram of collected total RNA was treated with DNAseI and reverse transcribed using Superscript III and oligo dT following the manufacturer recommendations (Invitrogen, Carlsbad, CA). Human BLT1 receptor 433bp fragment and human GAPDH 420 bp fragment were amplified by standard PCR using 5 prime hotmastermix (5 prime, Gaithersburg, MD) and specific primers. Primers for human BLT1 were 5′-CCTGAAAAGGATGCAGAAGC-3′ (forward) and 5′-AAAGGACAACACCCAGATGC-3′ (reverse). Primers for human GAPDH were 5′-GTCAGTGGTGGACCTGACCT-3′ (forward) and 5′-AGGGGTCTACATGGCAACTG-3′ (reverse). The PCR conditions were as follows: 94C for 3min; 94C 1min, 50C (BLT1) or 55C (GAPDH) 1min, 72C 1min for 35 cycles followed by 10min at 72C for final elongation using a Peltier thermal cycler PTC 200 (MJ Research, Waltham, MA). PCR products were run on a 1.5% agarose/ Ethidium Bromide gel.
Western blot analysis
Human peripheral blood mononuclear cells, human prostate cancer cell lines 22Rv1, LnCAP, PC-3, DU145, and the mouse prostate cancer cell line Myc CAP were lysed using 100μl of RIPA buffer supplemented with an EDTA-free protease inhibitor cocktail and PhosSTOP tablets as recommended by the manufacturer (Roche Applied Bioscience, Indianapolis, IN) and clarified by centrifugation. Equal amounts of protein were separated on SDS gels and electrophoretically transferred to PVDF membranes for Western blotting. The membrane was incubated overnight at 4°C with the BLT1 receptor antibody ( # 120114, Cayman Chemical, Ann Arbor, MI) at a 1/1000 dilution in 5% BSA/TBST blocking solution followed by a 1h incubation at room temperature with a 1:3000 dilution of the anti-rabbit secondary antibody covalently coupled to horseradish peroxidase (#170-6515 Bio-Rad laboratories Inc. Hercules, CA). All immune complexes in the Western blots were visualized using the West Femto Supersignal (ThermoScientific, Rockford, IL) and revealed using the Biorad Chemidoc XRS system (Bio-Rad laboratories Inc., Hercules, CA). The membrane was then stripped using Restore* Western Blot Stripping Buffer (Thermo Scientific, Hanover Park, IL) following the manufacturer recommendations. After blocking, the membranes were incubated with a rabbit anti-GAPDH antibody (Cell Signaling Technology, Inc, Danvers, MA) at a 1:5000 dilution in 5% milk overnight at 4°C.The immunocomplexes were detected as described above.
Immunohistochemistry
The rabbit polyclonal BLT1 receptor (# 120114, Cayman Chemical, Ann Arbor, MI) was pre-incubated in PBS overnight at 4°C in the presence or in the absence of BLT1 receptor antibody blocking peptide (# 120112 Cayman Chemical, Ann Arbor, MI) following the manufacturer protocol. The next day, 4 μm paraffin-embedded sections of human prostate cancer adenocarcinoma were cut, paraffin was removed with xylene, and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Heat-induced antigen retrieval was carried out for all sections in 0.001M EDTA buffer, pH = 8.0 using a vegetable steamer at 95°C for 25 min. The sections were then incubated in presence of the BLT1 receptor antibody +/− blocking peptide at a 1/500 dilution for 1h at room temperature. The signal was detected using the Novolink Polymer Detection System (Leica Microsystem Inc., Buffalo Grove, IL). All sections were visualized with the diaminobenzidine reaction and counterstained with hematoxylin. Pictures were taken using a 20X objective.
Statistical analysis
Baseline subject characteristics were compared between groups using unpaired t-tests or Fisher’s exact tests. Serum and red blood cell fatty acids, LTB4, and 15(S)-HETE secondary outcomes were operationalized as the change from baseline to surgery while CCP score was measured only at surgery. Partial correlation coefficients were calculated between the tissue and serum biomarkers controlling for pathological Gleason score, weight loss, and race. Residual analysis was performed to check for deviations from models assumptions (normality, homoscedasticity). These outcomes were then compared between groups using unpaired t-tests. Kolmogorov-Smirnov tests were used to asses normality assumptions within groups for these outcomes (all p-values > 0.05). P-values <0.05 were considered significant. The data are presented as mean + SD or SEM where appropriate. Statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC) and IBM SPSS Version 19 (SPSS, Chicago, IL, USA).
RESULTS
Patients
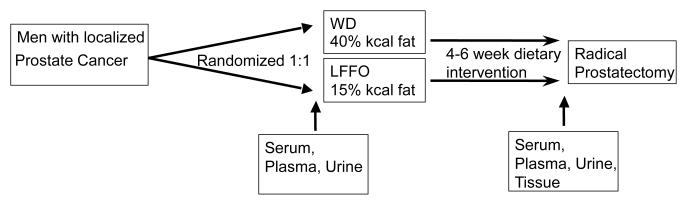
The patient characteristics were previously described (33) and are listed in Table 1. Of the 55 patients enrolled, 48 completed the trial. Twenty-one subjects were randomized to the Western diet group and 27 to the LFFO group. The majority of patients in both groups were either overweight or obese. The average duration on the diet intervention was 28 to 30 days. Patients in both groups were compliant with the diets, and patients in the LFFO group were compliant with the fish oil capsule consumption. An overview of the clinical trial design is shown in Figure 1.
Figure 1.
Schema of the previously completed prospective Phase II pre-prostatectomy trial comparing a LFFO and Western diet.
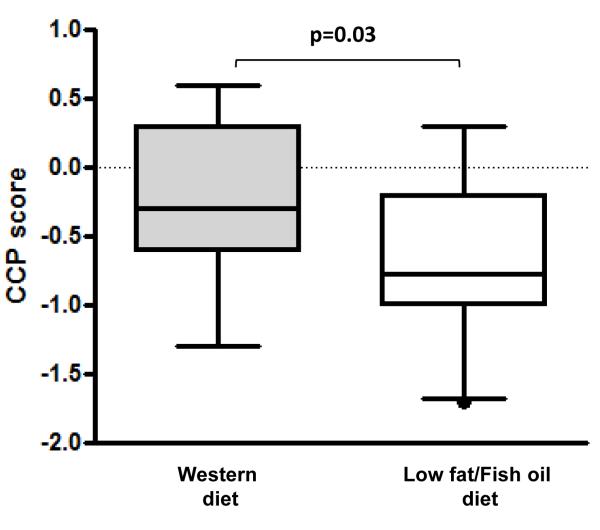
Decreased CCP score in the LFFO prostatectomy specimens
CCP score was measured in malignant radical prostatectomy tissue from 16 Western diet patients and 20 LFFO patients. As show in Figure 2, CCP score was significantly lower in the LFFO group as compared to the Western diet group (p=0.03).
Figure 2.
Effect of a LFFO diet compared with a Western diet on CCP score in malignant epithelial prostate cells in radical prostatectomy specimens (WD: n= 16, LFFO: n=20). Statistical significance was assessed using unpaired t-test.
Dietary effects on serum fatty acids and circulating eicosanoids
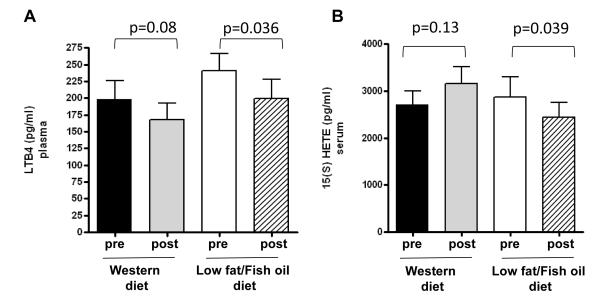
A significant decrease in mean levels of total omega-6 fatty acids, an increase in levels of total omega-3 fatty acids, and a decrease in the omega-6:omega-3 fatty acid ratio was observed in serum from subjects consuming the LFFO diet versus the Western diet (Table 2). Circulating levels of the pro-inflammatory eicosanoid 15(S)-HETE were significantly decreased in the LFFO group as compared to the Western diet group (Table 2). Post intervention 15(S)-HETE circulating levels were significantly reduced compared to pre-intervention levels in the LFFO group. Whereas LTB4 levels were not decreased in the LFFO group relative to the Western diet group (Table 2), post-intervention LTB4 levels in the LFFO group were significantly lower than pre-intervention levels. (Figure 3).
Table 2.
Changes in serum fatty acids and eicosanoids
| Fatty Acids* | WD post-intervention minus pre-intervention (n=21) (mean ± SEM ) |
LFFO post-intervention minus pre-intervention (n=27) (mean ± SEM ) |
P value |
|---|---|---|---|
|
| |||
| Palmitic | −0.8± 0.46 | −0.62±0.32 | 0.7 |
| Palmitoleic | −0.45± 0.22 | −0.28±0.1 | 0.9 |
| Stearic | −0.14±0.08 | −0.42±0.13 | 0.2 |
| Oleic | −1.17±0.63 | −2.35±0.43 | 0.2 |
| Linoleic Acid (n-6) | 2.4±0.93 | −1.68±0.63 | <0.01 |
| α-linolenic (n-3) | 0.03±0.04 | 0.009±0.05 | 0.9 |
| Eicosadienoic (n-6) | −0.005±0.009 | −0.009±0.009 | 0.6 |
| Arachidonic Acid (20:4, n-6) | 0.48± 0.27 | −0.75± 0.21 | <0.01 |
| EPA (20:5, n-3) | −0.23±0.05 | 1.97±0.14 | <0.01 |
| Docosapentaenoic (n-3) | 0.007± 0.02 | 0.16±0.03 | <0.01 |
| DHA (22:6, n-3) | −0.12±0.08 | 3.98±0.19 | <0.01 |
| Total n-6 | 2.88±1.12 | −2.44± 0.67 | <0.01 |
| Total n-3 | −0.32±0.13 | 6.12 ± 0.3 | <0.01 |
| n-6/n-3 | 1.41±0.42 | −5.76±0.42 | <0.01 |
|
| |||
| Eicosanoids** |
% change from baseline
(n=21) (mean ± SEM ) |
% change from baseline
(n=27) (mean ± SEM ) |
P value |
|
| |||
| 15(S)-HETE | 24.7±11.4 | −7.2±6.6 | 0.02 |
| LTB4 | −9.7±7.4 | −14.9±5.6 | 0.6 |
Changes in serum fatty acids are expressed as post-intervention minus pre-intervention.
Eicosanoid changes were calculated for each patient as percent change from baseline.
Figure 3.
Effect of LFFO and Western diet on plasma levels of LTB4 (A) and serum levels of 15(S)-HETE (B). 15(S)-HETE and LTB4 were measured by ELISA in duplicate (LFFO group n= 27; Western diet group n=21). Values are expressed as mean ± standard errors (SE). Statistical significance was assessed using paired t-test.
The LTB4 receptor, BLT1, is expressed in prostate cancer cell lines and prostatectomy specimens
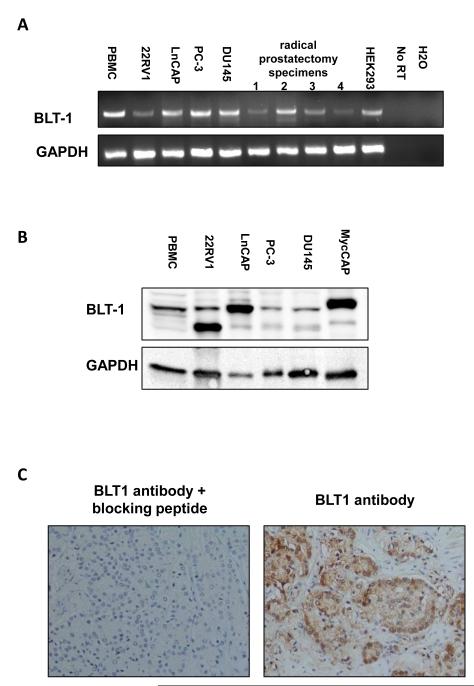
Expression of the LTB4 receptor BLT1 was assessed using RTPCR, Western blotting and immunohistochemistry (Figure 4). As shown in Figure 4A, the BLT1 gene is expressed in androgen dependent and independent prostate cancer cell lines 22Rv1, LnCAP, PC-3 and DU145, as well as in prostatectomy specimens. BLT1 expression was further confirmed by Western blot in all 4 cells lines as well as in a mouse prostate cancer cell line, MycCAP (Figure 4B). 22Rv1 expressed two forms of the receptors, the wild type form as well as a lower molecular weight form corresponding to the deglycosylated form of BLT1 (40). BLT1 protein expression was also detected in human prostate cancer specimens by immunohistochemistry (Figure 4C).
Figure 4.
Expression of the BLT1 receptor in prostate cancer cell lines and radical prostate cancer specimens from four patients. (A) BLT1 gene expression was assessed by RTPCR (in triplicate) in prostate cancer cell lines and malignant prostatectomy tissue from 4 patients. Peripheral blood mononuclear cells and HEK293 cells were used as positive control. A negative control with no reverse transcriptase was performed in each RT PCR. (B) BLT1 protein expression in prostate cancer cell lines was assessed by Western blot. Peripheral blood mononuclear cell extract was used as a positive control. (C) BLT1 receptor expression was assessed by immunohistochemistry in radical prostatectomy specimens from four patients. Preincubation of the BLT1 antibody with a blocking peptide was used to assess the BLT1 antibody specificity.
Correlation Analyses
To determine if serum 15(S)-HETE and LTB4 levels correlate with prostate cancer tissue markers of proliferation (Ki67 and CCP score), partial correlation analyses were performed controlling for Gleason score, weight loss, and race. Serum 15(S)-HETE change (pre to post-intervention) positively correlated with tissue Ki67 (r= 0.472, p<0.01), but did not correlate with CCP score (r= 0.236, p=0.2). The change in serum LTB4 levels positively correlated with CCP score (r= 0.41, p=0.02) but did not correlate with Ki67 (r= −0.147, p=0.3).
DISCUSSION
A typical Western diet is high in omega-6 polyunsaturated fatty acids from corn oil and other vegetable oils, low in omega-3 fatty acids, and has a ratio of omega-6:omega-3 fatty acids of approximately 15:1 (41) while in the typical Asian diet the ratio of omega-6:omega-3 fatty acids is 4:1 (42). Whereas epidemiologic data are conflicting on the anti-cancer or cancer preventative effects of dietary omega-3 and omega-6 fatty acids (9-14), preclinical data clearly show beneficial anticancer effects of lowering omega-6 intake from corn oil and lowering the dietary ratio of omega-6:omega-3 fatty acids (3, 5). The major omega-6 fatty acid in corn oil is linoleic acid. Linoleic acid is elongated to form arachidonic acid, which is then metabolized through cyclooxygenase (COX) and lipoxygenase (LOX) pathways. The COX-2/PGE2 pathway has been implicated in prostate cancer development and progression in preclinical and clinical studies (3, 43, 44). We initially hypothesized that a LFFO diet would result in lower serum IGF-1 levels (primary outcome) as well as lower prostate tissue proliferation, lower COX-2 tissue levels, and lower tissue and serum PGE-2 levels (secondary outcomes). While a LFFO intervention resulted in lower prostate cancer proliferation (Ki-67) in our preprostatectomy Phase II trial (33), there was no effect on IGF-1 (primary outcome), COX-2 or PGE-2 levels suggesting that alternative mechanisms may be responsible for the observed anti-proliferative effects. Similarly, Lloyd et al. recently published a xenograft study demonstrating that a fish oil based diet slowed prostate cancer progression but did not affect the IGF-1 axis or the COX2/PGE2 pathway compared to olive oil, corn oil or animal fat diets (6). These results led us to hypothesize that a LFFO diet may induce a reduction in serum levels of two major products of arachidonic acid metabolism, 15(S)-HETE and LTB4, through lipoxygenase pathways.
15S-HETE is the major hydroxy derivative of arachidonic acid and is the end product of the enzyme 15-lipoxygenase while LTB4 is formed through the 5-lipoxygenase pathway (23). In the present study we found that a LFFO diet significantly reduced serum 15S-HETE levels relative to a Western diet, and that post-intervention LTB4 levels were lower than pre-intervention levels, although LTB4 levels in the LFFO group were not significantly reduced relative to the Western diet group. While involvement of the COX2/PGE2 pathway in the progression of prostate cancer has been studied extensively, there are limited reports on the role of the lipoxygenase pathway (35). Pre-clinical and clinical studies demonstrated a potential role for 5-lipoxygenase and its metabolite 5-HETE in prostate cancer progression. Gupta et al. reported that levels of 5-lipoxygenase and its product, 5-HETE, were significantly higher in malignant prostate tissue as compared to benign prostate tissue, and that inhibition of 5-lipoxygenase induces apoptosis in prostate cancer cell lines (45). In addition, in vitro studies using LNCaP and PC3 cell lines demonstrated a role for the 5-lipoxygenase pathway in proliferation and survival of prostate cancer cell lines (46, 47). The BLT2 receptor, a LTB4 receptor expressed ubiquitously, is known to bind both LTB4 and 15(S)-HETE (27) and to induce signaling pathways involved in cell proliferation (28). Recently, BLT2 was reported to be a key regulator of androgen receptor expression and possibly a target for prostate cancer therapy (29). LTB4 also binds to the BLT1 receptor which is known to be more specific for LTB4 than BLT2 (27) and mainly expressed in leukocytes where it induces signaling pathways involved in cell proliferation (28). Interestingly, herein we demonstrated the expression of BLT1 in prostate cancer specimens and in prostate cancer cell lines. To our knowledge the presence of BLT1 in prostate cancer cells has not been previously described. However, our findings are in line with other studies demonstrating the expression and the proliferative role of BLT1 receptor in other cancers such as ovarian cancer (30), colon cancer (32) and pancreatic cancer (31).
In the present study we also demonstrated that a LFFO intervention reduced expression of genes involved in cell cycle progression using Myriad Genetics CCP score. The CCP score has been previously shown to be an independent predictor of recurrence after radical prostatectomy and of prostate cancer death (36-38) and more recently a predictor of biochemical recurrence for patients treated with external beam radiation therapy as their primary therapy (48). The finding that CCP score was lower in the LFFO vs. the WD group provides strong support for longer term prospective randomized trials evaluating a LFFO diet in men with prostate cancer. Correlation analyses demonstrated a significant positive correlation between changes in serum 15(S)-HETE and LTB4 levels with Ki67 and CCP score respectively. Further studies are required to evaluate if 15(S)-HETE is more directly linked with proliferation as measured by Ki67, and whether LTB4 has a greater impact on the genes used to calculate the CCP score. Further preclinical and clinical studies are also warranted to determine if diet-induced changes in lipoxygenase-derived eicosanoids may impact on prostate cancer proliferation through mechanisms involving BLT1 and BLT2 receptors.
As detailed in the introduction section of this manuscript, preclinical, clinical, and epidemiologic studies have yielded conflicting results with regards to the role of omega-3 fatty acids for prostate cancer prevention and treatment. In a recent epidemiologic study, Brasky et al. reported that plasma omega-3 fatty acid levels were associated with low-grade and high-grade prostate cancer (20). On the other hand, preclinical studies suggest beneficial effects of omega-3 fatty acids for prevention and treatment of prostate cancer,(3-6) and a short-term prospective randomized clinical trial suggested potential beneficial effects of omega-3 fatty acids for prostate cancer treatment (33). In this trial, fish oil supplements were combined with a low-fat diet to achieve a reduced omega-6/omega-3 fatty acid ratio. The article by Brasky et al. did report on other components of the participants’ diets or supplement intake. Ultimately, prospective randomized trials will be required to establish dietary and supplement recommendations for prostate cancer prevention and treatment.
Limitations of the present report include that it was a post-hoc analysis and is therefore only useful for generating new hypotheses. In addition, the samples size was small (48 patients completed the trial) and malignant radical prostatectomy tissue was only available in 36 patients for measurement of the CCP score. Therefore these results will need to be validated in larger clinical trials.
In summary, a 4-6 week LFFO dietary intervention decreased serum pro-inflammatory eicosanoids and the prostate cancer tissue CCP score. We also demonstrated the expression of the LTB4 receptor BLT1 in prostate cancer cell lines and in human prostate cancer specimens. Furthermore, circulating levels of 15SHETE and LTB4 correlated with proliferation and CCP score in prostate cancer specimen suggesting a potential role for the 5- and 15- lipoxygenase pathways in the anticancer effect of a LFFO diet. Further pre-clinical and clinical studies are warranted to explore whether dietary modulation of pro-inflammatory eicosanoids impacts on prostate cancer proliferation, and if serum eicosanoids levels may function as surrogate biomarkers for efficacy of a LFFO intervention.
Acknowledgments
FUNDING: NIH P50CA92131 (W. Aronson), UCLA center grant NIH P30CA016042 (T. Grogan and D. Elashoff), CTSI NIH UL1TR000124 (T. Grogan and D. Elashoff). This manuscript is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center West Los Angeles (W. Aronson) and Myriad Genetics Laboratories.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to declare.
REFERENCES
- 1.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, et al. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662–70. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–43. [PubMed] [Google Scholar]
- 5.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–75. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, et al. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013 doi: 10.1038/pcan.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fradet Y, Meyer F, Bairati I, Shadmani R, Moore L. Dietary fat and prostate cancer progression and survival. Eur Urol. 1999;35:388–91. doi: 10.1159/000019913. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 9.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–16. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 10.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–6. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 11.Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU, et al. Dietary fat intake and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;87:1405–13. doi: 10.1093/ajcn/87.5.1405. [DOI] [PubMed] [Google Scholar]
- 12.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–6. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 13.Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta-analysis. Am J Clin Nutr. 2010;92:1223–33. doi: 10.3945/ajcn.2010.29530. [DOI] [PubMed] [Google Scholar]
- 14.Chavarro JE, Stampfer MJ, Hall MN, Sesso HD, Ma J. A 22-y prospective study of fish intake in relation to prostate cancer incidence and mortality. Am J Clin Nutr. 2008;88:1297–303. doi: 10.3945/ajcn.2008.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norrish AE, Skeaff CM, Arribas GL, Sharpe SJ, Jackson RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Br J Cancer. 1999;81:1238–42. doi: 10.1038/sj.bjc.6690835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, et al. Serum Phospholipid Fatty Acids and Prostate Cancer Risk: Results From the Prostate Cancer Prevention Trial. Am J Epidemiol. 2011;173:1429–39. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowe FL, Allen NE, Appleby PN, Overvad K, Aardestrup IV, Johnsen NF, et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–63. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 18.Dahm CC, Gorst-Rasmussen A, Crowe FL, Roswall N, Tjonneland A, Drogan D, et al. Fatty acid patterns and risk of prostate cancer in a case-control study nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2012;96:1354–61. doi: 10.3945/ajcn.112.034157. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Wilkens LR, Henning SM, Le Marchand L, Gao K, Goodman MT, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20:211–23. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J Natl Cancer Inst. 2013;105:1132–41. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183:345–50. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009 doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831–52. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 25.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–34. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 28.Back M, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, et al. International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacological reviews. 2011;63:539–84. doi: 10.1124/pr.110.004184. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Kim GY, Kim JH. Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression. Biochem Biophys Res Commun. 2012;420:428–33. doi: 10.1016/j.bbrc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Rocconi RP, Kirby TO, Seitz RS, Beck R, Straughn JM, Jr., Alvarez RD, et al. Lipoxygenase pathway receptor expression in ovarian cancer. Reprod Sci. 2008;15:321–6. doi: 10.1177/1933719108316390. [DOI] [PubMed] [Google Scholar]
- 31.Tong WG, Ding XZ, Hennig R, Witt RC, Standop J, Pour PM, et al. Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2002;8:3232–42. [PubMed] [Google Scholar]
- 32.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 33.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, et al. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011;4:2062–71. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berquin IM, Edwards IJ, Kridel SJ, Chen YQ. Polyunsaturated fatty acid metabolism in prostate cancer. Cancer Metastasis Rev. 2011 doi: 10.1007/s10555-011-9299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel MI, Kurek C, Dong Q. The arachidonic acid pathway and its role in prostate cancer development and progression. J Urol. 2008;179:1668–75. doi: 10.1016/j.juro.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Cuzick J, Berney DM, Fisher G, Mesher D, Moller H, Reid JE, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer. 2012;106:1095–9. doi: 10.1038/bjc.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooperberg MR, Simko JP, Cowan JE, Reid JE, Djalilvand A, Bhatnagar S, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31:1428–34. doi: 10.1200/JCO.2012.46.4396. [DOI] [PubMed] [Google Scholar]
- 38.Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12:245–55. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology. 2001;58:283–8. doi: 10.1016/s0090-4295(01)01116-5. [DOI] [PubMed] [Google Scholar]
- 40.Hori T, Sato Y, Takahashi N, Takio K, Yokomizo T, Nakamura M, et al. Expression, purification and characterization of leukotriene B(4) receptor, BLT1 in Pichia pastoris. Protein Expr Purif. 2010;72:66–74. doi: 10.1016/j.pep.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 42.Sugano M, Hirahara F. Polyunsaturated fatty acids in the food chain in Japan. Am J Clin Nutr. 2000;71:189S–96S. doi: 10.1093/ajcn/71.1.189S. [DOI] [PubMed] [Google Scholar]
- 43.Jain S, Chakraborty G, Raja R, Kale S, Kundu GC. Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res. 2008;68:7750–9. doi: 10.1158/0008-5472.CAN-07-6689. [DOI] [PubMed] [Google Scholar]
- 44.Swami S, Krishnan AV, Moreno J, Bhattacharyya RS, Gardner C, Brooks JD, et al. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int J Cancer. 2009;124:2050–9. doi: 10.1002/ijc.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737–43. doi: 10.1002/1097-0142(20010215)91:4<737::aid-cncr1059>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–7. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh J, Myers CE., Jr Arachidonic acid metabolism and cancer of the prostate. Nutrition. 1998;14:48–9. doi: 10.1016/s0899-9007(97)00392-4. [DOI] [PubMed] [Google Scholar]
- 48.Freedland SJ, Gerber L, Reid J, Welbourn W, Tikishvili E, Park J, et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:848–53. doi: 10.1016/j.ijrobp.2013.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]