Abstract
Purpose
Our publications demonstrate that physiological concentrations of estrogen (E2) induce endoplasmic reticulum and oxidative stress which finally result in apoptosis in E2-deprived breast cancer cells, MCF-7:5C. c-Src is involved in the process of E2-induced stress. To mimic the clinical administration of c-Src inhibitors, we treated cells with either E2, a c-Src inhibitor PP2, or the combination for 8 weeks to further explore the apoptotic potential of the c-Src inhibitor and E2 on MCF-7:5C cells.
Methods
Protein levels of receptors and signaling pathways were examined by immunoblotting. Expression of mRNA was detected through real-time PCR. Cell cycles were analyzed by flow cytometry.
Results
Long-term treatment with PP2 alone or E2 alone decreased cell growth. In contrast, a combination of PP2 and E2 blocked apoptosis and the resulting cell line (MCF-7:PF) was unique, as they grew vigorously in culture with physiological levels of E2, which could be blocked by the pure antiestrogen ICI182,780. One major change was that PP2 collaborated with E2 to increase the level of insulin-like growth factor-1 receptor beta (IGF-1Rβ). Blockade of IGF-1Rβ completely abolished E2-stimulated growth in MCF-7:PF cells. Furthermore, combination treatment up-regulated transcription factors, Twist1 and Snail, and repressed E-cadherin expression which made MCF-7:PF cells display a characteristic phenotype of epithelial-mesenchymal transition (EMT).
Conclusions
These data illustrate the role of the c-Src inhibitor to block E2-induced apoptosis and enhance E2-stimulated growth. Caution must be exercised when considering c-Src inhibitors in clinical trials following the development of acquired resistance to aromatase inhibitors, especially in the presence of the patient’s own estrogen.
Keywords: c-Src, estrogen, apoptosis, epithelial-mesenchymal transition, breast cancer
1. Introduction
Aromatase inhibitors are the standard of care for the adjuvant treatment of postmenopausal patients with estrogen receptor (ER) positive breast cancer (1). Tamoxifen remains the adjuvant therapy of choice for ER-positive premenopausal patients (1). Both “antiestrogen” therapeutic strategies offer substantial benefits for enhancing patient survival (2). However, long-term therapy also raises the spectre of antihormone resistance. Despite the paucity of human ER- positive cell lines to study molecular mechanisms of antihormone resistance (3), remarkable progress has occurred over the past twenty five years that has not only replicated the clinical presentation of antihormone resistance, but also provided valuable clues to create novel second line therapies (4) like fulvestrant, the pure antiestrogen that destroys the tumor ER (5). Laboratory findings with the MCF-7 breast cancer cell line grown in athymic mice first described tamoxifen-stimulated growth as a new mechanism of drug resistance to a therapeutic intervention (6). However, it is the discovery that re-transplantation of tamoxifen-stimulated tumors into successive generations of athymic mice over 5 years results in the selection of a resistant tumor cell population that is killed by physiological levels of E2 (7,8) that has resulted in the new biology of E2-induced apoptosis (9). Indeed, E2-induced apoptosis has been used to explain the action of E2 replacement therapy for postmenopausal women in their 60’s having a lower incidence of breast cancer and mortality (10). A period of E2 deprivation (5–10 years) is necessary to select the cell population that will be vulnerable to E2-induced apoptosis (11). The same principle (4) has been suggested to explain the dramatic decrease in mortality observed in the decade after long-term adjuvant tamoxifen therapy is stopped (12); the woman’s own E2 causes apoptosis in the vulnerable antihormone resistant breast cancer cells.
The description of the evolution of tamoxifen resistance in vivo to trigger rapid tumor regression with physiological concentrations of E2 (7,8,13) was rapidly followed by similar reports in vitro with populations or selected clones of MCF-7 cells triggering apoptosis with physiological E2 after long-term E2 deprivation (14,15). Thus E2 deprivation produces the same selective pressure on MCF-7 cells as selective ER modulators (SERMs) (8, 16) to create selective cellular populations vulnerable to E2-induced apoptosis. All of these laboratory data with MCF-7 cells provide the scientific rationale for the subsequent finding that high dose (30mg daily) or low dose (6mg daily) E2 produces a 30% clinical benefit rate in patients failing aromatase inhibitor therapy (17).
Overall, the new biology of E2 action to trigger apoptosis translates appropriately to the responsiveness of human breast cancer in the clinical setting. As a result, we have used our cellular models to elucidate the molecular mechanisms that modulate E2-induced apoptosis through inducing endoplasmic reticulum stress and oxidative stress (18, 19). Recently, we have found that the oncogene c-Src is activated in two long-term E2-deprived breast cancer cell models (20) and is involved in the process of stress induced by E2 (19). Preclinical data in endocrine resistant models demonstrate that the crosstalk between ER and c-Src is an important resistance mechanism (21,22). Blockade of c-Src signaling pathways is an attractive strategy to circumvent the resistance to antihormone therapy in breast cancer (23,24). Here, we ask the question of what are the consequences of long-term physiological concentrations of E2 in combination with the c-Src inhibitor on the shift of adaptive populations in E2-deprived breast cancer cells?
To mimic the clinical administration of a c-Src inhibitor, we treated MCF-7:5C cells with different combinations in a long-term (8 weeks) study to further investigate the therapeutic potential of the combination of the c-Src inhibitor and E2 on the growth of MCF-7:5C cells compared with either E2 alone or PP2 alone. Contrary to our original hypothesis that the c-Src inhibitor would enhance the apoptotic effects of E2, the c-Src inhibitor prevented E2-induced apoptosis and allowed E2 to stimulate growth. One major mechanistic change that reversed the E2 response was that the c-Src inhibitor cooperated with E2 to increase IGF-1Rβ growth pathways, which was an important determinant for the signaling pathways of phosphatidylinositol-3 kinases/Akt and mitogen-activated protein kinase (MAPK). Furthermore, long-term combination treatment transcriptionally up-regulated EMT inducers, Twist1 and Snail, and disrupted E-cadherin mediated cell-cell adhesion. These data not only demonstrate the important role of c-Src in modulating E2-induced apoptosis but also have implications for the poor performance with c-Src inhibitors in ER positive antihormone resistant patients in clinical trials.
2. Materials and Methods
2.1 Materials
Estradiol was purchased from Sigma-Aldrich (St. Louis, MO); ICI 182,780 was from Tocris (Park Ellisville, MO). c-Src inhibitor PP2 and IGF-1Rβ inhibitor AG1024 were purchased from CalBiochem (San Diego, CA). Sources of antibodies for Western blot were as follows: ERα (sc-544), ERβ (sc-8974), PR (sc-810), and IGF-1Rβ (sc-713) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Total MAPK (#9102), phosphorylated MAPK (#9101), total Akt (#9272), phosphorylated Akt (#9271), phosphorylated c-Src (#2101), E-cadherin (#3195), N-cadherin (#4061), and Snail (#3879) antibodies were from Cell Signaling Technology (Beverly, MA). Total c-Src (GD11) and Twist1 (3E11) antibodies were from Millipore (Temecula, CA). Fibrinogen antibody (HPA00190) was from Sigma-Aldrich (St. Louis, MO).
2.2 Cell Culture Conditions and Establishment of MCF-7:PF Cells
The ER-positive wild-type human breast cancer MCF-7 cells and long-term E2-deprived cell lines were cultured as previously described (20). In an attempt to investigate the therapeutic potential of combination E2 and the c-Src inhibitor, PP2, MCF-7:5C cells were long-term treated with E2 (10−9 mol/L) plus PP2 (5×10−6 mol/L) using the same medium as for control MCF-7:5C cells. At the same time, MCF-7:5C cells were treated with E2 alone (10−9 mol/L) and PP2 alone (5×10−6 mol/L) respectively which were set up as control groups to examine effects of E2 and PP2 on MCF-7:5C cells. Medium was changed every 2–3 days, adding fresh compounds. Eight weeks later, the cells treated with E2 plus PP2 that grew stably were named MCF-7:PF cells. The DNA fingerprinting patterns of MCF-7:PF and long-term E2 deprived cell lines were consistent with the report by the ATCC (Supplementary Fig. S1).
2.3 Cell Proliferation Assays
Cells were harvested after 7 days of treatment. The DNA content of the cells, a measure of proliferation, was determined as previously described (20), using a DNA fluorescence quantitation kit (Bio-Rad Laboratories, Hercules, CA).
2.4 Cell-cycle Analysis
Briefly, differently treated cells were harvested and gradually fixed with 75% EtOH on ice. After being stained with propidium iodide (PI), cells were analyzed using a FACSort flow cytometer (Becton Dickinson, San Jose, CA), and the data were analyzed with ModFit software.
2.5 Immunoblotting
Proteins were extracted in cell lysis buffer (Cell Signaling Technology, Beverly, MA) supplemented with Protease Inhibitor Cocktail Set I and Phosphatase Inhibitor Cocktail Set II (Calbiochem, San Diego, CA). The immunoblotting was performed as previously described (20).
2.6 Quantitative real-time RT-PCR
Total RNA, isolated with an RNeasy Micro kit (Qiagen, Valencia, CA), was converted to first-strand cDNA using a kit from Applied Biosystems (Foster City, CA). Quantitative real-time PCR assays were done with the SYBR Green PCR Master Mixes (Applied Biosystems, Foster City, CA) and a 7900HT Fast Real-time PCR System (Applied Biosystems, Foster City, CA). All primers were synthesized in Integrated DNA Technologies (San Diego, CA). All the data were normalized by 36B4.
2.7 Boyden chamber migration assay
As described in reference (25), the transwell chambers (Corning Inc., Corning, NY) with 8 micron pore size membrane were equilibrated overnight with media according to the manufacturer’s recommendation. About 300,000 cells were added to the upper chamber. The lower chamber had 10% charcoal-stripped fetal bovine serum as chemoattractant. Cells were allowed to migrate for 24 h and, thereafter, non-migrated cells on the upper surface of the membrane were cleaned with a cotton swab. The migrated cells on the lower surface of the membrane were fixed in methanol and stained with a HEMA 3 staining set from Fisher Scientific. The cells were then counted in at least four microscopic fields at 10×10 magnification, and experiments were conducted three times.
2.8 Transient Transfection Reporter Gene Assays
Transient transfection assays were performed using a dual-luciferase system (Promega, Madison, WI). To determine ER and PR transcriptional activity, cells were transfected with an estrogen response element (ERE)-regulated (pERE (5x) TA-ffLuc plus pTA-srLuc), or progesterone response element (PRE)-regulated (pPRE (5x) TA-ffLuc plus pTA-srLuc) dual-luciferase reporter gene sets (20). The cells were treated for 24 hours following the transfection. Then, the cells were harvested and processed for dual-luciferase reporter activity, in which the firefly luciferase activity was normalized by renilla luciferase activity.
2.9 Statistical Analysis
All reported values are the means ± SE. Statistical comparisons were determined with two-tailed Student’s t tests. Results were considered statistically significant if the P value was <0.05.
3. Results
3.1 The c-Src inhibitor completely blocked E2-induced apoptosis in MCF-7:5C cells
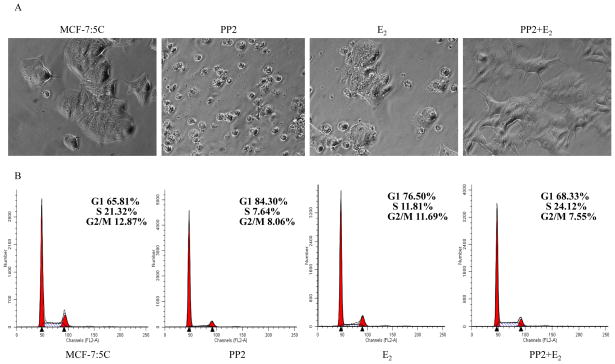
We have found that non-receptor tyrosine kinase, c-Src, is activated in E2-deprived breast cancer cell lines, MCF-7:5C and MCF-7:2A, and functions as an important transducer to mediate E2-induced stress (19, 20). Here, to mimic the clinical administration of the c-Src inhibitor, we treated MCF-7:5C cells long-term (8 weeks) with a specific c-Src inhibitor, PP2, alone or in combination with E2 to investigate the therapeutic potential in E2-deprived MCF-7:5C cells. MCF-7:5C cells exhibited a cobblestone-like epithelial phenotype (Fig. 1A). PP2 alone treated cells appeared smaller and more contracted, with decreased cell spreading (Fig. 1A). Apoptotic impairment could be observed under the microscope in cells treated with E2 alone (Fig. 1A). In contrast, combination E2 and PP2 abolished the growth inhibitory actions by E2 alone or PP2 alone and the resulting cell line (MCF-7:PF) grew vigorously, displaying a spindle-like morphology (Fig. 1A). Although E2 significantly increases S phase after short-term treatment (19), further analysis of cell cycles showed that both the c-Src inhibitor and E2 could clearly arrest cell cycles in G1 phase after long-term treatment which marks growth inhibition. However, combination PP2 and E2 was unable to arrest cell cycles in G1 phase (Fig. 1B). These data confirmed that E2-initiated apoptosis requires the c-Src tyrosine kinase pathway (19).
Figure 1. The c-Src inhibitor completely blocked E2-induced apoptosis after long-term treatment.
(A) The morphological changes after 8 weeks treatment with different combinations. MCF-7:5C cells were long-term treated with vehicle (0.1% EtOH), PP2 (5×10−6mol/L), E2 (10−9 mol/L), and E2 (10−9mol/L) plus PP2 (5×10−6mol/L) in T25 flasks, respectively. Cells were photographed under bright field illumination at (×20) magnification (Zeiss). (B) Cell-cycle changes after different treatments. Cells treated in different combinations were harvested and gradually fixed with 75% EtOH on ice. After staining with propidium iodide (PI), cells were analyzed through flow cytometry. All the data shown were representative of at least three separate experiments with similar results.
3.2 Inhibition of c-Src converted E2 from inducing apoptosis to stimulating growth in MCF-7:PF cells
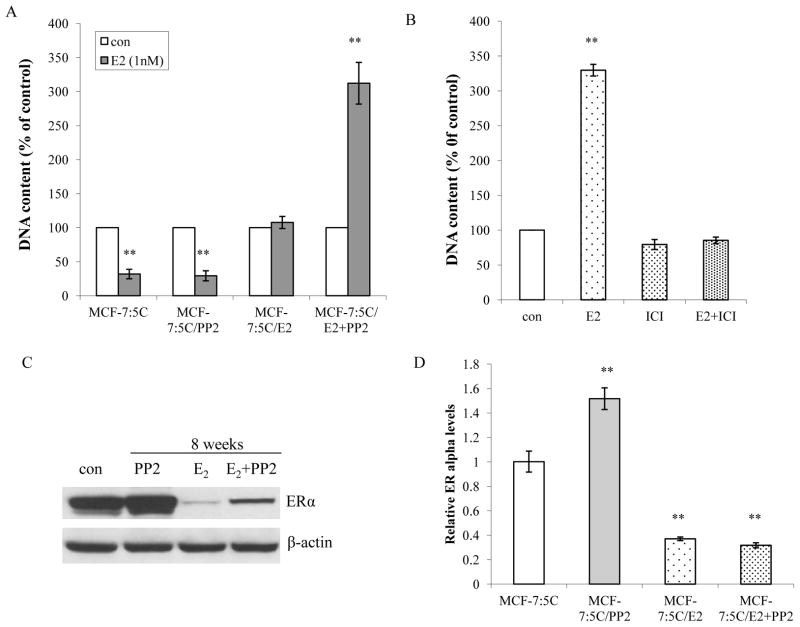
To further investigate the mechanisms underlying the c-Src inhibitor blocking the apoptosis induced by E2, the response to E2 by differently long-term treated cells was first evaluated. Physiological levels of E2 still caused growth inhibition in MCF-7:5C cells and long-term PP2 treated cells (Fig. 2A). Long-term treatment with E2 initially caused massive apoptosis, but a small fraction of surviving cells subsequently re-grew. Although apoptotic morphology could be observed under microscope at this time point (Fig. 1A), E2 did not decrease cell number compared with control cells (Fig. 2A). It implied that a balance occurred between apoptosis and cell growth caused by E2. In contrast, E2 significantly stimulated cell growth in the resulting cell line (MCF-7:PF) generated from a combination treatment of PP2 and E2 (Fig. 2A). This stimulation by E2 could be completely blocked by the pure antiestrogen ICI182,780 which demonstrated that proliferation was mediated by ERα (Fig. 2B). Similarly, the c-Src inhibitor also converted the E2 response from apoptosis to proliferation in another long-term E2-deprived, a late-apoptosis cell line MCF-7:2A (18 and Supplementary Fig. S2). It is known that blocking c-Src tyrosine kinase increases ERα expression after 24h treatment (20 and Supplementary Fig. S3A). This elevated level of ERα was stably expressed after long-term PP2 treatment (Fig. 2C). As expected, cells treated with E2 alone and MCF-7:PF cells expressed lower levels of ERα due to E2 down-regulation (Fig. 2C). The ERα protein expression was not strictly consistent with mRNA levels (Fig. 2D). There was no change in ERβ expression after long-term treatment (Supplementary data S3B). To further confirm that it was E2 which down-regulated ERα, E2 or/and PP2 were withdrawn from culture medium of MCF-7:PF cells at indicated time points. Withdrawal of E2 but not PP2, ERα expression in MCF-7:PF cells recovered to similar levels as observed in MCF-7:5C cells (Supplementary Fig. S3C). This suggested that MCF-7:PF cells have functional ERα in response to E2.
Figure 2. The c-Src inhibitor converted E2 responses from inducing apoptosis to stimulating growth.
(A) Responses to E2 in treated cells. Cells treated in different combinations were seeded in 24-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH) or E2 (10−9mol/L) without any other compounds in the medium. The cells were harvested after 7 days treatment and total DNA was determined using a DNA fluorescence quantitation kit. P<0.001, ** compared with control. (B) E2 proliferative effect was blocked by ICI182,780. MCF-7:PF cells were seeded in 24-well plates in triplicate. After one day, the cells were treated with vehicle (0.1% EtOH), E2 (10−9mol/L), ICI 182,780 (10−6mol/L), and E2 (10−9mol/L) plus ICI182,780 (10−6mol/L) respectively. The cells were harvested and total DNA was determined as above. P<0.001, ** compared with control. (C) Changes of ERα after long-term treatment. Cell lysates of different long-term treated cells were harvested. ERα was examined by immunoblotting. β-actin was detected for loading control. (D) Changes of ERα mRNA levels. The RNA of different cells was harvested in TRIzol for real-time PCR analysis. All the data shown were representative of at least three separate experiments with similar results.
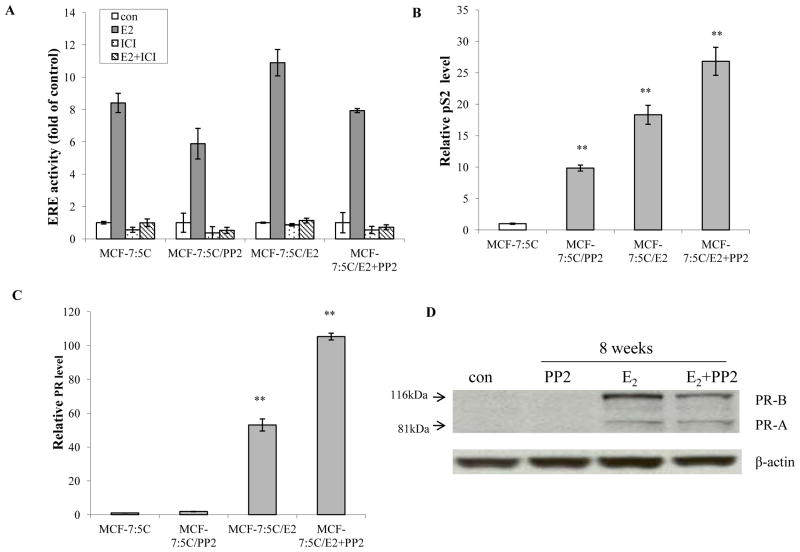
3.3 The c-Src inhibitor was additive with E2 to elevate endogenous ER target genes in MCF-7:PF cells
Although ER expression levels were quite different (Fig. 2C), the estrogen response element (ERE) activity was similar among differently treated cells (Fig. 3A). It was interesting to find that the c-Src inhibitor dramatically elevated an E2-inducible gene pS2 mRNA (Fig. 3B), although the mechanisms were unclear. Moreover, the c-Src inhibitor was additive with E2 to increase pS2 mRNA in MCF-7:PF cells (Fig. 3B). Another ER target gene, progesterone receptor (PR), was undetectable in MCF-7:5C cells compared with wild-type MCF-7 cells (Supplementary data S4A). However, adding back E2 into the medium recovered PR expression in cells treated with E2 alone and MCF-7:PF cells (Fig. 3C). The c-Src inhibitor, PP2, by itself did not regulate PR expression (Fig. 3D). Nevertheless, it synergized with E2 to up-regulate PR mRNA although without consistent high protein expression (Fig. 3D), implying the existence of a post-translational modification of PR in MCF-7:PF cells (26).
Figure 3. The c-Src inhibitor collaborated with E2 to up-regulate ER target genes.
(A) ERE activity changes in different cells. Cells treated in different combinations were seeded in 24-well plates in triplicate and transfected with ERE firefly luciferase plasmid plus renilla luciferase plasmid as in Materials and Methods. (B) The pS2 mRNA expression. Different combinations treated cells were grown in 6-well plates in triplicate. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. (C) The PR mRNA expressed levels. The RNA of different cells was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. (D) PR protein changes after long-term treatment. Cell lysates were harvested from different treated cells. PR was examined by immunoblotting. β-actin was detected for loading control. All the data shown were representative of at least three separate experiments with similar results.
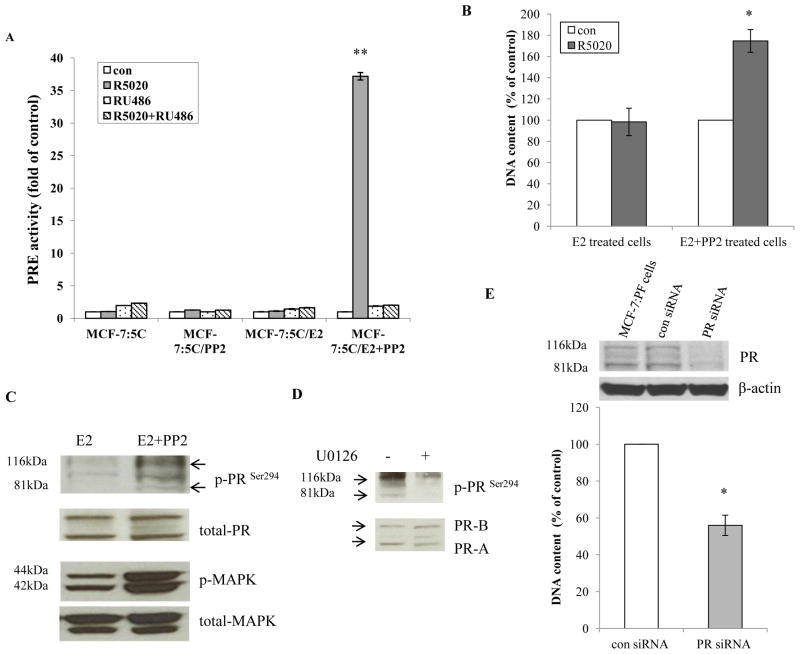
3.4 The c-Src inhibitor synergized with E2 to elevate transcriptional activity of PR in MCF-7:PF cells
As shown in Fig. 3D, E2 alone treated cells and MCF-7:PF cells had similar levels of PR protein. To further investigate the function of PR, progestin (R5020) was used to examine the activity of the progesterone response element (PRE). Interestingly, the progestin only activated PRE activity in MCF-7:PF cells which could be blocked by the anti-progestin RU486 (Fig. 4A). They also had different cell growth responses, progestin stimulated growth in MCF-7:PF cells, but not in E2 alone treated cells (Fig. 4B). Activation of MAPK results in phosphorylation of PR on Ser294, affecting transcriptional function of PR (26). In agreement with this report, we observed that phosphorylated MAPK and PR (Ser294) levels in MCF-7:PF cells were higher than that in E2 alone treated cells (Fig. 4C). Inhibition of MAPK with U0126 effectively blocked the phosphorylation of PR on Ser294 in MCF-7:PF (Fig. 4D). Although anti-progestin RU486 blocked PRE activity induced by progestin in MCF-7:PF cells (Fig. 4A), it could not inhibit cell growth activated by progestin. RU486 itself significantly promoted MCF-7:PF cell growth (Supplementary Fig. S4B). This estrogenic effect of RU486 (27) on MCF-7:PF cells could be blocked by ICI182,780 and was very similar to wild-type MCF-7 cells (Supplementary Fig. S4C and S4D). A specific siRNA was used to knock down PR that effectively inhibited MCF-7:PF cell growth (Fig. 4E). All of these results demonstrated that extracellular signal MAPK modifies PR and affects the transcriptional activity of PR.
Figure 4. The c-Src inhibitor synergized with E2 to activate PR transcriptional activity.
(A) The PRE activity in treated cells. Cells treated in different combinations were transfected with PRE firefly luciferase plasmid plus renilla luciferase plasmid as in Materials and Methods. The cells were treated with vehicle (0.1% EtOH), progestin (10−8mol/L), RU486 (10−6mol/L), and RU486 (10−6mol/L) plus progestin (10−8mol/L) in triplicate for 24 hours. P<0.001, ** compared with control. (B) Different responses to progestin between cells treated with E2 alone and MCF-7:PF cells. E2 alone treated cells and MCF-7:PF cells were plated in 24-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH) and progestin (10−8mol/L) respectively. Cells were harvested after 7 days treatment and the total DNA was determined as above. P<0.05, * compared with E2 alone treated cells. (C) MCF-7:PF cells had higher phosphorylated PR than E2 alone treated cells. Cell lysates of MCF-7:PF cells and E2 alone treated cells were harvested. Phosphorylated PR and MAPK were examined by immunoblotting. Total PR and MAPK were used as loading controls. (D) The PR was phosphorylated by MAPK in MCF-7:PF cells. MCF-7:PF cells were treated with vehicle (0.1% DMSO) and MAPK inhibitor U0126 (10−5mol/L) for 48 hours. Phosphorylated PR was examined by immunoblotting. Total PR was used as loading control. (E) Knockdown of PR by siRNA blocked cell growth. MCF-7:PF cells were transfected with control siRNA and specific PR target siRNA as manufacture’s instruction. Cell lysates were harvested after 72 hours to detect PR levels by immunoblotting. β-actin was detected for loading control. As a parallel experiment, cells were harvested after 5 days transfection for DNA growth assay as above. P<0.05, * compared with control siRNA.
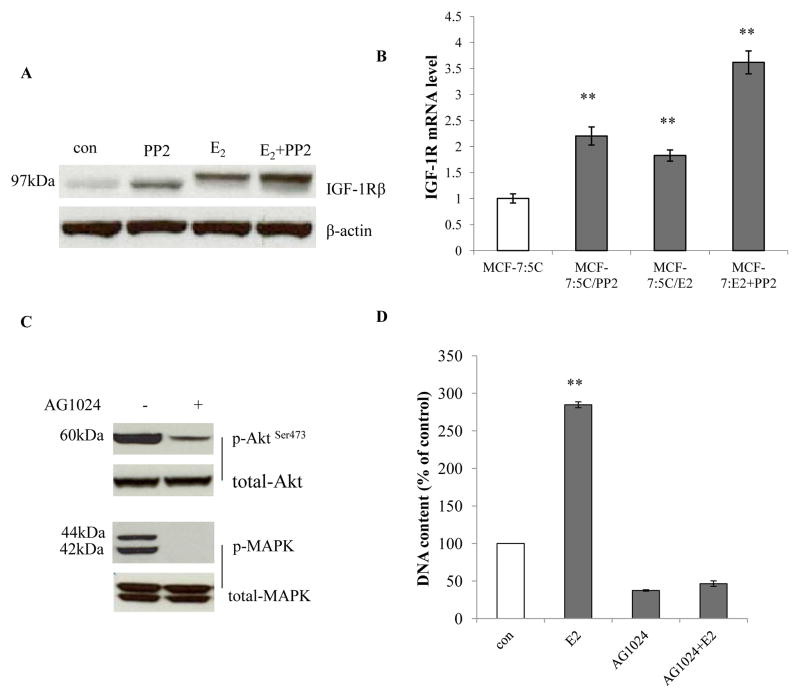
3.5 The c-Src inhibitor collaborated with E2 to enhance insulin like growth factor-1 receptor beta (IGF-1Rβ) which drove growth pathways in MCF-7:PF cells
c-Src mediates the interaction between growth factor receptors and ER in breast cancer (22,28). The c-Src inhibitor and E2 could individually increase IGF-1Rβ expression after long-term treatment. Moreover, PP2 and E2 were additive to elevate IGF-1Rβ in MCF-7:PF cells (Fig. 5A and 5B). To investigate the role of IGF-1Rβ in MCF-7:PF cells, a specific inhibitor of IGF-1Rβ, AG1024, was utilized to block receptor tyrosine kinase activity, which effectively abolished MAPK and Akt pathways (Fig. 5C) and inhibited cell growth (Fig. 5D). Importantly, AG1024 completely abolished E2 stimulation in a concentration-dependent manner in MCF-7:PF cells (Fig. 5D and Supplementary Fig. S5A). These data supported the hypothesis that IGF-1Rβ is linked tightly with the ER function in MCF-7:PF cells.
Figure 5. The c-Src inhibitor collaborated with E2 to elevate IGF-1Rβ.
(A) IGF-1Rβ changes after long-term treatment. Cell lysates of cells treated in different combinations were harvested. IGF-1Rβ was examined by immunoblotting. β-actin was detected for loading control. (B) IGF-1Rβ mRNA changes were consistent with protein levels. The RNA of differently long-term treated cells was harvested as above. P<0.001, ** compared with control. (C) Activation of Akt and MAPK pathways by IGF-1Rβ in MCF-7:PF cells. MCF-7:PF cells were treated with vehicle (0.1% DMSO) and AG1024 (10−5mol/L) for 48 hours. Cell lysates were harvested. Phosphorylated Akt and MAPK were determined by immunoblotting. Total Akt and MAPK were examined for loading controls. (D) The IGF-1R inhibitor completely blocked E2 stimulation in MCF-7:PF cells. MCF-7:PF cells were treated with vehicle (0.1% EtOH), E2 (10−9mol/L), AG1024 (10−5mol/L), and E2 (10−9mol/L) plus AG1024 (10−5mol/L) for 7 days. The cells were harvested and DNA content was determined as above. P<0.001, ** compared with control. All the data shown were representative of at least three separate experiments with similar results.
3.6 The c-Src inhibitor disrupted E-cadherin-mediated cell-cell adhesion and collaborated with E2 to increase epithelial-mesenchymal transition (EMT) in MCF-7:PF cells
Activation of c-Src kinase has been documented in E-cadherin-mediated cell-cell adhesion, which is thought to play an important role in cancer invasion and metastasis (29). Therefore, we sought to examine changes of E-cadherin associated signals after long-term treatment with the c-Src inhibitor in MCF-7:5C cells. Contrary to the effects on wild-type MCF-7 cells (29), PP2 reduced E-cadherin but increased N-cadherin and fibrinogen in MCF-7:5C cells (Fig. 6A), which are characteristic features of EMT (29). EMT is regulated by various signal transduction pathways including extracellular signal-regulated kinase (ERK) and Wnt (30). The c-Src inhibitor effectively blocked c-Src phosphorylation in both PP2 alone treated cells and MCF-7:PF cells (Fig. 6B), whereas long-term E2 treated MCF-7:5C cells still maintained the higher level of phosphorylated c-Src (Fig. 6B). Although the c-Src inhibitor blocks phosphorylated MAPK in the early stage (19,20), PP2 clearly increased MAPK but continuously blocked Akt after long-term treatment (Fig. 6B). Additionally, inducers of the EMT include several transcription factors such as Snail, Twist, as well as the secreted transforming growth factor beta (TGFβ) (31). In our cell model, the c-Src inhibitor collaborated with E2 to increase Snail and Twist1 in MCF-7:PF cells (Fig. 6C). Both PP2 alone and E2 alone increased mRNA levels of TGFβ, but combination treatment decreased TGFβ by an unclear mechanism (Supplementary Fig. S6C). Nevertheless, MCF-7:PF cells had higher migratory capacities than MCF-7:5C cells, evaluated using a Boyden chamber migration assay (Fig. 6D and 6E). All of these results suggested that multiple EMT regulators are significantly modified after long-term combination treatment.
Figure 6. The c-Src inhibitor collaborated with E2 to promote EMT and increase cell migration.
(A) Changes of EMT biomarkers after different combinations treatment. Cell lysates of different treated cells were harvested. E-cadherin, N-cadherin, and fibrinogen were examined by immunoblotting. β-actin was detected for loading control. (B) Signaling pathways changes after different combinations treatment. Cell lysates of differently treated cells were harvested. Phosphorylated c-Src, MAPK, Akt were examined by immunoblotting. Total c-Src, MAPK, and Akt were detected for loading controls. (C) Transcription factors, Twist1 and Snail, were up-regulated after long-term combination treatment. Cell lysates of different treated cells were harvested. Twist1 and Snail were examined by immunoblotting. β-actin was detected for loading control. (D) Migratory capacities of MCF-7:PF cells, compared with MCF-7:5C cells. MCF-7:5C cells and MCF-7:PF cells were loaded in Boyden chambers as in Materials and Methods. Images were taken under bright field illumination at (×10) magnification (Olympus). (E) MCF-7:PF cells had higher migratory capacities than MCF-7:5C cells. Migrated cells were stained as in Materials and Methods. Cell numbers were counted in at least four microscopic fields at (10×10) magnification. P<0.05, * compared with MCF-7:5C cells. All the data shown were representative of at least three separate experiments with similar results.
4. Discussion
Resistance to aromatase inhibitors is an important clinical problem. We have demonstrated in the laboratory that two long-term E2-deprived MCF-7 breast cancer cell lines respond to physiological concentrations of E2 by triggering apoptosis (18). This laboratory observation has clinical relevance for the prevention or treatment of E2-deprived diseases (10,17); however, only 30% of patients receive clinical benefit (17). This prompted us to investigate strategies to increase the therapeutic responsiveness in aromatase inhibitor resistant breast cancer. The oncogene c-Src is activated in E2-deprived breast cancer cell lines (20). Many observations highlight c-Src as an important therapeutic target to overcome endocrine resistance in breast cancer (21–24). We chose an eight-week treatment period in the laboratory to mimic the clinical criteria to evaluate the efficacy of endocrine therapy. Unexpectedly, the c-Src inhibitor converted the E2 response from inducing apoptosis to stimulating growth in two long-term E2 deprived breast cancer cell lines (Fig. 2A and Supplementary Fig. S2). Most importantly, we found that the c-Src inhibitor enhanced the action of E2 to up-regulate IGF-1Rβ which, in turn, promoted the MCF-7:PF cells to grow (Fig. 5A and 5D). Furthermore, the combination treatment enhanced embryonic transcription factors and repressed E-cadherin expression (Fig. 6A and 6C), a characteristic feature of EMT in the generation of invasive tumor cells.
We sought to find the mechanisms by which the c-Src inhibitor blocked the apoptosis induced by E2 after long-term combination treatment. Our recent observations show that the c-Src inhibitor effectively blocks oxidative stress and extrinsic apoptotic pathways induced by E2 within 72 hours (19) since these pathways are mediated by the c-Src tyrosine kinase. Paradoxically, physiological levels of E2 still were able to induce apoptosis in long-term PP2 treated cells as in the original MCF-7:5C cells when the drug was washed out (Fig. 2A). We further found that c-Src phosphorylation was gradually recovered after withdrawal of PP2 from the medium (Supplementary Fig. S7A). Including PP2 in the medium could completely abolish E2-induced apoptosis in MCF-7:5C/PP2 treated cells (Supplementary Fig. S7B). These data confirmed that c-Src phosphorylation is required for E2-induced apoptosis (19,20) which is a critical initial protective response for cell survival. However, unlike 4-hydroxytamoxifen, the c-Src inhibitor cannot completely block apoptosis-induced by E2 during 72 hours of exposure (19). This implies that other adaptation responses can potentially occur in cell populations after long-term combination treatment. It is well known that growth factor receptors crosstalk with c-Src and the ER pathways in breast cancer cells (22,28). E2 started to increase IGF-1Rβ levels after 4 hours treatment which could be blocked by 4-hydroxytamoxifen in MCF-7:5C cells (Supplementary Fig. S5B and S5C), demonstrating an ER-dependent mechanism (32). Blockade of c-Src further enhanced levels of IGF-1Rβ (Fig. 5A). Another important growth factor receptor, epidermal growth factor receptor (EGFR), was up-regulated by both the c-Src inhibitor and E2 (Supplementary Fig. S6A). However, down-regulation of EGFR was found after combination treatment (Supplementary Fig. S6A). Importantly, blockade of EGFR had no inhibitory effects on MCF-7:PF cells, whereas inhibition of IGF-1R effectively blocked cell growth and completely abolished proliferation induced by E2 (Fig. 5D and Supplementary Fig. S6B). These results highlighted the importance of phosphorylated IGF-1R in the mediation of E2-stimulated growth. To determine whether autophosphorylation of IGF-1R by ligand IGF-1 plays an important role in the activation of receptor (33), we observed that IGF-1 levels were almost undetectable and neither E2 nor PP2 up-regulated the IGF-1 expression in our cell models through real-time PCR (data not shown). It is also necessary to note that IGF-1R is required for the activation of Akt (Fig. 5C) which is also an important pathway to suppress apoptosis (34).
In addition to up-regulation of IGF-1Rβ, expression of PR was strictly regulated by E2 (Fig. 3C and 3D). Interestingly, the function of PR was quite different between cells treated with E2 alone and MCF-7:PF cells (Fig. 4A), even though they had similar PR protein levels (Fig. 3C). Our preliminary data suggested that this may be related to PR phosphorylation on Ser294 by extracellular signaling ERK/MAPK (Fig. 4B and 4C). Consistently, other groups have reported that PR transcriptional activity is regulated by a balance between the degree of PR phosphorylation and sumoylation which can dramatically alter genetic expression (26,35). In this study, the c-Src inhibitor activated MAPK signal (Fig. 6B) which increased transcriptional activity of PR induced by E2 and finally activated the response to progestin in MCF-7:PF cells (Fig. 4A and 4B).
We addressed the question of why the c-Src inhibitor increased the extracellular signaling ERK/MAPK in MCF-7:5C cells. Our recent publication (20) shows that the c-Src inhibitor exerts different effects on two basic growth pathways, Akt and MAPK, in different breast cancer cell lines. The c-Src inhibitor continuously inhibited Akt pathway (Fig. 6B) but transiently blocked MAPK in MCF-7:5C cells (Fig. 6B) (19,20). The association of c-Src with the membrane cytoskeleton has been well documented (36). Evidence implicates (37) a role for c-Src in the regulation of the formation of focal adhesions and the extracellular matrix to affect subsequent signaling pathways. In our study, the c-Src inhibitor disrupted E-cadherin-mediated cell-cell adhesion and made the cell gain mesenchymal cell markers such as N-cadherin and fibrinogen (Fig. 6A), a characterized feature of EMT. Deposition of fibrinogen into the extracellular matrix serves as a scaffold to support binding of growth factors to activate extracelluar signaling ERK/MAPK (Fig. 6A and 6B) (38). EMT, a complex reprogramming process of epithelial cells, plays an important role in tumor invasion and metastasis (39). Current studies show that EMT is controlled by a group of embryonic transcriptional factors, such as Zeb-1/2, Twist1, and Snail, and each of these factors is capable of directly repressing E-cadherin expression (Fig. 6C) (39,40). These results suggested that the antiestrogen resistant breast cancer cell is clearly reprogrammed with regard to the variations of those signaling pathways. Therefore, further studies are required to uncover the precise interaction among these EMT inducers that may hold promise for developing novel strategies to inhibit EMT and cancer metastasis.
In summary, this study suggested that physiological levels of E2 (probably the patient’s own E2) is able to induce apoptosis in long-term E2-deprived breast cancer. However, administration with a c-Src inhibitor will cause the tumor to grow after aromatase inhibitor resistance, with a variety of signaling networks regulated by the c-Src inhibitor to promote an aggressive phenotype (Supplementary Fig. S8). These data raise a concern regarding the ubiquitous use of c-Src inhibitors in advanced aromatase inhibitor-resistant breast cancer especially when combined with E2.
Supplementary Material
Figure S1. Verification of cell line identity by DNA fingerprinting. The identity of the cell lines was verified by DNA fingerprinting using the commercially available kit, PowerPlex® 1.2 System (Promega). The following STR markers were tested: CSF1PO, TPOX, TH01, Amelogenin, vWA, D16S539, D7S820, D13S317 and D5S818. Allelic score data from the 9 polymorphic STR loci reveal a pattern almost identical among the cell lines that is very closely related to the scores reported for cells by the ATCC, and consistent with their presumptive identity. Areas of identity were highlighted in pink. Allelic loss was highlighted in green. Variant allele was highlighted in blue.
Figure S2. Inhibition of c-Src converted E2 from inducing apoptosis to stimulating growth in MCF-7:2A cells. MCF-7:2A cells were long-term (8 weeks) treated with PP2 (5×10−6 mol/L) and E2 (10−9 mol/L) as in MCF-7:5C cells. Long-term combination treated cells and MCF-7:2A cells were plated in 24-well plates in triplicate. After one day, cells were treated with different concentrations of E2 as indicated. The cells were harvested after 7 days treatment and total DNA was determined using a DNA fluorescence quantitation kit. P<0.05, * compared with MCF-7:2A cells.
Figure S3. ER changed after different treatment. A, ERα changed after short-term treatment. MCF-7:5C cells were short-term treated with vehicle (0.1% EtOH), PP2 (5×10−6 mol/L), E2 (10−9 mol/L), and E2 (10−9 mol/L) plus PP2 (5×10−6 mol/L) in 10cm dishes respectively. Cell lysates were harvested after different time points as indicated. ERα was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. B, ERβ expression levels did not change after long-term treatment. MCF-7:5C cells and differently long-term treated cells were grown in 10cm dishes respectively. Cell lysates were harvested. ERβ was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. C, ERα recovered after withdrawal of E2 in MCF-7:PF cells. E2 or/ and PP2 was withdrawn from MCF-7:PF cells culture medium as indicated. Cell lysates were harvested. ERα was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. All the data shown were representative of at least three separate experiments with similar results.
Figure S4. The expression and function of PR changed after different combinations treatment. A, PR expression levels were different between wild-type MCF-7 cells and MCF-7:5C cells. Cell lysates were harvested from MCF-7 cells and MCF-7:5C cells. PR was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. B, Anti-progestin RU486 could not block growth effects induced by progestin in MCF-7:PF cells. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% EtOH), progestin (10−8 mol/L), RU486 (10−6 mol/L), progestin (10−8 mol/L) plus RU486 (10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. P<0.01, # compared with control. C, Pure antiestrogen ICI 182,780 blocked cell growth stimulated by RU486. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% EtOH), RU486 (10−6 mol/L), ICI 182,780 (10−6 mol/L), RU486 (10−6 mol/L) plus ICI 182,780 (10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.001, ** compared with control. D, Growth response to RU486 in MCF-7, MCF-7:5C, and MCF-7:PF cell lines. Different cells were plated in 24-well plates in triplicate, respectively. After one day, cells were treated with vehicle (0.1% EtOH) and different doses of RU486 as indicated. Cells were harvested after 7 days treatment. DNA content was examined as above. All the data shown were representative of at least three separate experiments with similar results.
Figure S5. The function and expression of IGF-1Rβ changed after long-term treatment. A, MCF-7:PF cells responded to AG1024 in a dose-dependent manner. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), different doses (2×10−6 mol/L, 4×10−6 mol/L, 6×10−6 mol/L, 8×10−6 mol/L, 10×10−6 mol/L) of AG1024 plus E2 (10−9 mol/L), and AG1024 (10×10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. P<0.01, # compared with control. B, E2 quickly up-regulated IGF-1Rβ. MCF-7:5C cells were grown in six-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH) and E2 (10−9 mol/L) for different time points as indicated. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. C, E2 up-regulation of IGF-Rβ was ER-dependent. MCF-7:5C cells were grown in six-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and E2 plus 4-OHT (10−6 mol/L) for 72 hours. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S6. EGFR and TGFβ were regulated by the c-Src inhibitor and E2. A, EGFR mRNA levels changed after long-term treatment. MCF-7:5C cells and different long-term treated cells were grown in six-well plates in triplicate. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. B, Different responses to EGFR inhibitor and the IGF-1R inhibitor in MCF-7:PF cells. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), AG1024 (10−5 mol/L), and AG1478 (10−5 mol/L), respectively. Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.01, # compared with control. C, Regulation of TGFβ after long-term treatment. Different long-term treated cells were harvested in TRIzol. The mRNA levels were detected through real-time PCR. P<0.001, ** compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S7. Phosphorylation of c-Src affected the cell response to E2 in long-term PP2 treated cells. A, Changes of c-Src phosphorylation after withdrawal of PP2 in long-term PP2 treated cells. Long-term PP2 treated cells were washed with PP2 free medium for different time points as indicated, compared with MCF-7:5C cells. Cell lysates were harvested. c-Src phosphorylation was examined by immunoblotting with primary antibody. Immunoblotting for total c-Src was detected for loading control. B, Inhibition of c-Src blocked E2-induced apoptosis in long-term PP2 treated cells. MCF-7:5C/PP2 cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), PP2 (5×10−6 mol/L), and E2 (10−9 mol/L) plus PP2 (5×10−6 mol/L), respectively. Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S8. Illustrative figure of multiple factors regulated by the different treatment
Acknowledgments
Role of the funding source
Ping Fan, Fadeke A. Agboke, Russell E. McDaniel, and Elizabeth E. Sweeney’s salaries, as well as laboratory supplies supported by the following: the Department of Defense Breast Program under award number W81XWH-06-1-0590 Center of Excellence (VCJ); subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409 (VCJ); the Susan G Komen For The Cure Foundation under Award number SAC 100009 (VCJ) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008 (VCJ).
VCJ is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen For The Cure Foundation under Award number SAC100009; GHUCCTS CTSA (Grant # UL1RR031975) and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. We thank Peter Johnson for helping take cell images in the Microscopy and Imaging Shared Resource of Georgetown University.
Footnotes
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney EE, McDaniel RE, Maximov PY, Fan P, Jordan VC. Models and Mechanisms of Acquired Antihormone Resistance in Breast Cancer: Significant Clinical Progress Despite Limitations. Horm Mol Biol Clin Investig. 2012;9:143–63. doi: 10.1515/hmbci-2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–3. [PubMed] [Google Scholar]
- 5.Ariazi EA, Lewis-Wambi JS, Gill SD, et al. Emerging principles for the development of resistance to antihormonal therapy: implications for the clinical utility of fulvestrant. J Steroid Biochem Mol Biol. 2006;102:128–38. doi: 10.1016/j.jsbmb.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–7. [PubMed] [Google Scholar]
- 7.Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 9.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obiorah I, Jordan VC. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013;20:372–82. doi: 10.1097/GME.0b013e31828865a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 14.Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17 beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Lee ES, Gajdos C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–97. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 17.Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108:18879–86. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan P, Griffith OL, Agboke F, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73:4510–20. doi: 10.1158/0008-5472.CAN-12-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan P, McDaniel RE, Kim HR, Clagett D, Haddad B, Jordan VC. Modulating therapeutic effects of the c-Src inhibitor via oestrogen receptor and human epidermal growth factor receptor 2 in breast cancer cell lines. Eur J Cancer. 2012;48:3488–98. doi: 10.1016/j.ejca.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–74. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 22.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–60. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Guggisberg N, Jorda M, et al. Combined Src and aromatase inhibition impairs human breast cancer growth in vivo and bypass pathways are activated in AZD0530-resistant tumors. Clin Cancer Res. 2009;15:3396–405. doi: 10.1158/1078-0432.CCR-08-3127. [DOI] [PubMed] [Google Scholar]
- 24.Hiscox S, Jordan NJ, Smith C, et al. Dual targeting of SRC and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res Treat. 2009;115:57–67. doi: 10.1007/s10549-008-0058-6. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S, Schiff R, Katzenellenbogen BS. Post-transcriptional regulation of chemokine receptor CXCR4 by estrogen in HER2 overexpressing, estrogen receptor-positive breast cancer cells. Breast Cancer Res Treat. 2009;117:243–51. doi: 10.1007/s10549-008-0186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeng MH, Langan-Fahey SM, Jordan VC. Estrogenic actions of RU486 in hormone-responsive MCF-7 human breast cancer cells. Endocrinology. 1993;132:2622–30. doi: 10.1210/endo.132.6.8504763. [DOI] [PubMed] [Google Scholar]
- 28.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 29.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–6. [PubMed] [Google Scholar]
- 30.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–20. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107:15449–54. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genua M, Pandini G, Sisci D, et al. Role of cyclic AMP response element-binding protein in insulin-like growth factor-1 receptor up-regulation by sex steroids in prostate cancer cells. Cancer Res. 2009;69:7270–7. doi: 10.1158/0008-5472.CAN-09-0088. [DOI] [PubMed] [Google Scholar]
- 33.Hernández-Sánchez C, Blakesley V, Kalebic T, Helman L, LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem. 1995;270:29176–81. doi: 10.1074/jbc.270.49.29176. [DOI] [PubMed] [Google Scholar]
- 34.Martin MJ, Melnyk N, Pollard M, et al. The Insulin-Like Growth Factor I Receptor Is Required for Akt Activation and Suppression of Anoikis in Cells Transformed by the ETV6-NTRK3 Chimeric Tyrosine Kinase. Mol Cell Biol. 2006;26:1754–69. doi: 10.1128/MCB.26.5.1754-1769.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmstrom S, Van Antwerp ME, Iniguez-Lluhi JA. Direct and distinguishable inhibitory roles for SUMO isoforms in the control of transcriptional synergy. Proc Natl Acad Sci USA. 2003;100:15758–63. doi: 10.1073/pnas.2136933100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avizienyte E, Wyke AW, Jones RJ, et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat Cell Biol. 2002;4:632–8. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 37.Balzer EM, Whipple RA, Thompson K, et al. c-Src differentially regulates the functions of microtentacles and invadopodia. Oncogene. 2010;29:6402–8. doi: 10.1038/onc.2010.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rybarczyk BJ, Simpson-Haidaris PJ. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000;60:2033–9. [PubMed] [Google Scholar]
- 39.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 40.Li CW, Xia W, Huo L, et al. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Verification of cell line identity by DNA fingerprinting. The identity of the cell lines was verified by DNA fingerprinting using the commercially available kit, PowerPlex® 1.2 System (Promega). The following STR markers were tested: CSF1PO, TPOX, TH01, Amelogenin, vWA, D16S539, D7S820, D13S317 and D5S818. Allelic score data from the 9 polymorphic STR loci reveal a pattern almost identical among the cell lines that is very closely related to the scores reported for cells by the ATCC, and consistent with their presumptive identity. Areas of identity were highlighted in pink. Allelic loss was highlighted in green. Variant allele was highlighted in blue.
Figure S2. Inhibition of c-Src converted E2 from inducing apoptosis to stimulating growth in MCF-7:2A cells. MCF-7:2A cells were long-term (8 weeks) treated with PP2 (5×10−6 mol/L) and E2 (10−9 mol/L) as in MCF-7:5C cells. Long-term combination treated cells and MCF-7:2A cells were plated in 24-well plates in triplicate. After one day, cells were treated with different concentrations of E2 as indicated. The cells were harvested after 7 days treatment and total DNA was determined using a DNA fluorescence quantitation kit. P<0.05, * compared with MCF-7:2A cells.
Figure S3. ER changed after different treatment. A, ERα changed after short-term treatment. MCF-7:5C cells were short-term treated with vehicle (0.1% EtOH), PP2 (5×10−6 mol/L), E2 (10−9 mol/L), and E2 (10−9 mol/L) plus PP2 (5×10−6 mol/L) in 10cm dishes respectively. Cell lysates were harvested after different time points as indicated. ERα was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. B, ERβ expression levels did not change after long-term treatment. MCF-7:5C cells and differently long-term treated cells were grown in 10cm dishes respectively. Cell lysates were harvested. ERβ was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. C, ERα recovered after withdrawal of E2 in MCF-7:PF cells. E2 or/ and PP2 was withdrawn from MCF-7:PF cells culture medium as indicated. Cell lysates were harvested. ERα was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. All the data shown were representative of at least three separate experiments with similar results.
Figure S4. The expression and function of PR changed after different combinations treatment. A, PR expression levels were different between wild-type MCF-7 cells and MCF-7:5C cells. Cell lysates were harvested from MCF-7 cells and MCF-7:5C cells. PR was examined by immunoblotting with primary antibody. Immunoblotting for β-actin was detected for loading control. B, Anti-progestin RU486 could not block growth effects induced by progestin in MCF-7:PF cells. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% EtOH), progestin (10−8 mol/L), RU486 (10−6 mol/L), progestin (10−8 mol/L) plus RU486 (10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. P<0.01, # compared with control. C, Pure antiestrogen ICI 182,780 blocked cell growth stimulated by RU486. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% EtOH), RU486 (10−6 mol/L), ICI 182,780 (10−6 mol/L), RU486 (10−6 mol/L) plus ICI 182,780 (10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.001, ** compared with control. D, Growth response to RU486 in MCF-7, MCF-7:5C, and MCF-7:PF cell lines. Different cells were plated in 24-well plates in triplicate, respectively. After one day, cells were treated with vehicle (0.1% EtOH) and different doses of RU486 as indicated. Cells were harvested after 7 days treatment. DNA content was examined as above. All the data shown were representative of at least three separate experiments with similar results.
Figure S5. The function and expression of IGF-1Rβ changed after long-term treatment. A, MCF-7:PF cells responded to AG1024 in a dose-dependent manner. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), different doses (2×10−6 mol/L, 4×10−6 mol/L, 6×10−6 mol/L, 8×10−6 mol/L, 10×10−6 mol/L) of AG1024 plus E2 (10−9 mol/L), and AG1024 (10×10−6 mol/L). Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. P<0.01, # compared with control. B, E2 quickly up-regulated IGF-1Rβ. MCF-7:5C cells were grown in six-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH) and E2 (10−9 mol/L) for different time points as indicated. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. C, E2 up-regulation of IGF-Rβ was ER-dependent. MCF-7:5C cells were grown in six-well plates in triplicate. Cells were treated with vehicle (0.1% EtOH), E2 (10−9 mol/L), 4-OHT (10−6 mol/L), and E2 plus 4-OHT (10−6 mol/L) for 72 hours. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S6. EGFR and TGFβ were regulated by the c-Src inhibitor and E2. A, EGFR mRNA levels changed after long-term treatment. MCF-7:5C cells and different long-term treated cells were grown in six-well plates in triplicate. The RNA was harvested in TRIzol for real-time PCR analysis. P<0.001, ** compared with control. B, Different responses to EGFR inhibitor and the IGF-1R inhibitor in MCF-7:PF cells. MCF-7:PF cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), AG1024 (10−5 mol/L), and AG1478 (10−5 mol/L), respectively. Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.01, # compared with control. C, Regulation of TGFβ after long-term treatment. Different long-term treated cells were harvested in TRIzol. The mRNA levels were detected through real-time PCR. P<0.001, ** compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S7. Phosphorylation of c-Src affected the cell response to E2 in long-term PP2 treated cells. A, Changes of c-Src phosphorylation after withdrawal of PP2 in long-term PP2 treated cells. Long-term PP2 treated cells were washed with PP2 free medium for different time points as indicated, compared with MCF-7:5C cells. Cell lysates were harvested. c-Src phosphorylation was examined by immunoblotting with primary antibody. Immunoblotting for total c-Src was detected for loading control. B, Inhibition of c-Src blocked E2-induced apoptosis in long-term PP2 treated cells. MCF-7:5C/PP2 cells were plated in 24-well plates in triplicate. After one day, cells were treated with vehicle (0.1% DMSO), E2 (10−9 mol/L), PP2 (5×10−6 mol/L), and E2 (10−9 mol/L) plus PP2 (5×10−6 mol/L), respectively. Cells were harvested after 7 days treatment. DNA content was examined as above. P<0.05, * compared with control. All the data shown were representative of at least three separate experiments with similar results.
Figure S8. Illustrative figure of multiple factors regulated by the different treatment