Abstract
High-resolution microendoscopy (HRME) is a low-cost, “optical biopsy” technology that allows for subcellular imaging. The purpose of this study was to determine the in vivo diagnostic accuracy of the HRME for the differentiation of neoplastic from non-neoplastic colorectal polyps and compare it to that of high-definition white-light endoscopy (WLE) with histopathology as the gold standard. Three endoscopists prospectively detected a total of 171 polyps from 94 patients that were then imaged by HRME and classified in real-time as neoplastic (adenomatous, cancer) or non-neoplastic (normal, hyperplastic, inflammatory). HRME had a significantly higher accuracy (94%), specificity (95%), and positive predictive value (87%) for the determination of neoplastic colorectal polyps compared to WLE (65%, 39%, and 55%, respectively). When looking at small colorectal polyps (less than 10 mm), HRME continued to significantly outperform WLE in terms of accuracy (95% vs. 64%), specificity (98% vs. 40%) and positive predictive value (92% vs. 55%). These trends continued when evaluating diminutive polyps (less than 5 mm) as HRME's accuracy (95%), specificity (98%), and positive predictive value (93%) were all significantly greater than their WLE counterparts (62%, 41%, and 53%, respectively). In conclusion, this in vivo study demonstrates that HRME can be a very effective modality in the differentiation of neoplastic and non-neoplastic colorectal polyps. A combination of standard white-light colonoscopy for polyp detection and HRME for polyp classification has the potential to truly allow the endoscopist to selectively determine which lesions can be left in situ, which lesions can simply be discarded, and which lesions need formal histopathologic analysis.
Keywords: Colorectal polyps, adenoma classification, microendoscopy, neoplasia, diagnostic accuracy
Introduction
Advances in colorectal screening programs and colonoscopy technology have resulted in significant decreases in colorectal cancer incidence and mortality (1-3). However, colorectal cancer remains the third most common cancer in the United States and is the second most common cause of cancer-related deaths (4, 5). Colonoscopy screening focuses on the early and accurate detection of adenomas, neoplastic polyps with malignant potential. Unfortunately, the appearance of these neoplastic adenomas is not visibly distinct compared to their non-neoplastic polyp counterparts on white light endoscopy. Therefore, the current gold standard for diagnosis entails removal of virtually all visualized polyps, followed by formal histopathologic analysis (6). Given the fact that the majority of visualized lesions are non-neoplastic and less than half of all resected polyps are neoplastic, the current gold standard results in excess polypectomies and histology costs (7, 8). Hassan et al showed reducing the number of polyps requiring formal histopathologic analysis would significantly enhance cost-effectiveness of the screening colonoscopy (9). Therefore, a tool that allowed the endoscopist to make an in vivo classification of colorectal neoplasia could significantly reduce overall costs and diminish the patient risks of unnecessary polypectomies.
Various technologies have been developed to improve the ability of white-light endoscopy at classifying neoplastic from non-neoplastic polyps and therefore, allow for a more selective biopsy approach. Dye-based chromoendoscopy showed moderate success with colorectal dysplasia detection, especially in patients with ulcerative colitis but the procedure is cumbersome (10-15). While digital chromoendoscopy such as narrow band imaging (NBI) has shown promise with sensitivities greater than 90% at diagnosing colorectal neoplasia, specificity has been much lower and overall, results have been mixed in terms of diagnostic accuracy (16-22). And while CLE platforms have been the most promising as complementary tools to white-light colonoscopy with sensitivity and specificity as high as 97%, their widespread use has been limited by their high cost and need for intravenous contrast(23-28).
High-resolution microendoscopy (HRME) is a low-cost, high resolution imaging tool consisting of a 1 millimeter diameter fiber-optic bundle that allows for subcellular imaging at 1000x magnification at 4 micrometer resolution. HRME was first described by Muldoon et al and has already been found to be effective in detecting other gastrointestinal diseases (29-33). Chang et al developed a classification system for HRME to differentiate neoplastic from non-neoplastic colorectal polyps and after viewing a brief training set, both expert and novice endoscopists could identify neoplastic polyps with high specificity and inter-observer agreement (34).
Given that HRME is both inexpensive (equipment cost is less than $2000) and can be learned quickly, it has the potential to be an effective complementary imaging tool to white-light endoscopy. The primary aim of this trial was to evaluate the in vivo diagnostic accuracy of HRME for differentiating neoplastic (adenomatous, cancer) from non-neoplastic (normal, hyperplastic, inflammatory) colorectal polyps and compare it to that of standard, high-definition white-light endoscopy with histopathology as the gold standard.
Methods
Study Design and Patient Selection
The study was approved by the Institutional Review Boards at the Icahn School of Medicine at Mount Sinai and at Rice University and all patients signed informed consent prior to enrolling. It was a prospective cohort trial conducted at one tertiary care center and the study design met the recommendations of the STARD checklist for studies of diagnostic accuracy (35). One hundred and forty-five consecutive patients undergoing routine, scheduled screening or surveillance colonoscopies were enrolled. Patients were included if they were scheduled for a colonoscopy for screening or routine polyp surveillance, willing to sign informed consent and able to complete a telephone follow-up call post-procedure. Exclusion criteria were an unhealthy or unfit patient for standard colonoscopy or one who was unwilling to sign informed consent. Recruitment into the trial was based on the presence of at least one colorectal polyp that would be resected and undergo formal histopathologic analysis. Of the 145 patients enrolled, 94 had visible polyps which were included in the analysis.
High Resolution Microendoscopy
The description and technical specifications of the high-resolution microendoscope were previously described in detail by Muldoon et al and Pierce et al (31, 36). Briefly, the HRME unit comprises of a charge-coupled camera device, a flexible imaging probe, and a topical contrast agent. The imaging probe is a 1 millimeter-diameter fiber optic bundle that is passed through the biopsy channel or accessory port of any standard colonoscope. This fiber optic imaging probe contains 30,000 optical fibers with LED illumination. The LED illumination is powered by the charge-coupled camera device and is transmitted via the imaging probe onto the mucosal surface. Once the mucosal surface has been sprayed by proflavine, a fluorescent agent, the tissue reflects fluorescence in response to the LED illumination. The reflected fluorescence is captured via the imaging probe to the charge-coupled camera device that then transmits real-time HRME images onto the attached computer. In essence, HRME functions as a battery-powered fluorescence microscope that like confocal endomicroscopy, allows visualization of the mucosa at a 1000x magnification. HRME provides a 720 micron field of view and 4.4 micron spatial resolution. The cost of the charge-coupled camera device is approximately $1500 while each probe costs approximately $300. The probe needs to be polished, and occasionally replaced, every 75-80 patients.
Training in the HRME colon classification system
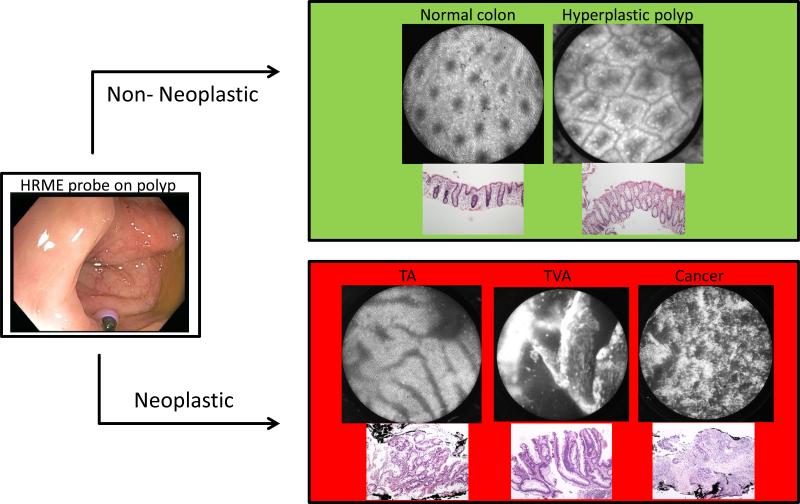
Three endoscopists (SA, BS, YY) performed both HRME and standard colonoscopy on all the patients. SA was an advanced endoscopist with expertise in endoscopic imaging including endoscopic ultrasound and confocal laser endomicroscopy. BS and YY were both general gastroenterologists. All three had no prior training in HRME for colon imaging before study initiation. Prior to patient enrollment, all three endoscopists viewed a five-minute training set that consisted of ten HRME images and explained the various features of the HRME colon classification system (34). This classification system was developed by Chang et al in conjunction with two expert gastrointestinal pathologists and is based on glandular morphology, epithelial thickness and nuclear arrangement. The HRME criteria were established based on the WHO histopathologic criteria for each category of colorectal polyps: normal colonic mucosa, hyperplastic polyp, tubular adenoma, tubulovillous adenoma, and adenocarcinoma (37). An HRME read of “non-neoplastic” was assigned to classification patterns for normal colonic mucosa, inflammatory or hyperplastic polyps (Figure 1). An HRME read of “neoplastic” included tubular adenoma, tubulovillous adenoma and adenocarcinoma. After the training set was completed, the three endoscopists imaged forty polyps in vivo using the HRME to gain competency before patient accrual began for this trial.
Figure 1.
HRME Classification
Performing the colonoscopy with HRME
(31, 36). In this trial, high-definition white-light endoscopy (Olympus SIFQ80) was first performed to detect colonic polyps. Upon detection of a colonic polyp, the endoscopist would make a white-light endoscopy (WLE) diagnosis of non-neoplastic (normal, hyperplastic, or inflammatory) or neoplastic (adenoma or cancer). Once the WLE read was recorded, 1-4 ml of proflavine dye (0.01%) was sprayed onto the mucosal surface of the polyp via an endoscopic spray catheter. Proflavine is a fluorescent topical antiseptic that highlights cell nuclei and was used in the study as an investigational drug under the FDA IND 102,217. After the proflavine was applied topically, the HRME probe was inserted through the biopsy port of the colonoscope and the fiber-optic tip was positioned directly onto the surface of the polyp. Once the probe was on the polyp, images were displayed on the system laptop at a rate of 12 frames per second. The average time required to spray the proflavine dye, place the probe onto the polyp, and make the optical diagnosis ranged from 50 to 80 seconds per polyp. This time included the occasional need for multiple HRME images to make a diagnosis. Using the colon HRME classification system, the endoscopist made the in-vivo optical diagnosis of neoplastic or non-neoplastic. All WLE and HRME reads had to be categorized as neoplastic or non-neoplastic; indeterminate reads were not acceptable. The imaged polyps were then removed by forceps or snare polypectomy and sent for formal histopathology analysis as per standard of care. Prior to polypectomy, when possible, the endoscopist visually estimated the size of the polyp in millimeters as compared to the maximum jaw opening of a standard, large-capacity biopsy forceps (8.6 mm).
Reference Standard
A single expert gastrointestinal pathologist (AP) who was blinded to both the WLE and HRME diagnoses made the final diagnosis of non-neoplastic (normal, hyperplastic, inflammatory) or neoplastic (adenomatous, cancer) colorectal polyp, which served as the gold standard diagnosis for comparison.
Statistical Analysis
Log binomial regression models were used to estimate the various measures of performance, viz., sensitivity, specificity, positive predictive value, negative predictive value and accuracy(38). Accuracy in this study was defined as the percentage of time that the diagnostic test (HRME) agreed with the gold standard (histology). These models account for the correlation among multiple biopsies on the same patient (39). Interaction terms were included to assess whether the performance measures differed between the modalities of HRME and WLE. The analyses were carried out using PROC GENMOD in SAS. The sample size was calculated post hoc based on the fact that with n=171 and a postulated value for sensitivity or specificity of at least 80%, this sample size was guaranteed to estimate the accuracy parameter with a precision (95% confidence interval) of +/− 6%.
Results
Patient Characteristics
A total of 145 patients were consented for the trial from September 2010 to April 2013. Of these, 94 patients (64.8%) had at least one visible colorectal polyp and were included in the final analysis. Patient demographics are detailed in Table 1. Eighty percent of patients were undergoing a screening colonoscopy while twenty percent of patients had a history of polyps and were undergoing surveillance colonoscopy. Almost nine percent had a history of colorectal cancer. Forty-eight patients (51.1%) had 1 polyp, 28 patients (29.8%) had two polyps, 13 patients (13.8%) had three polyps, and 5 patients (5.3%) had more than three polyps. No patient reported any adverse events.
Table 1.
Patient Characteristics
| n = 94 | |
|---|---|
| Age | 59.0 +/- 10.3 |
| Gender | 55.3% Female |
| Race | 55.3% White 44.7% Non-white |
| Colonoscopy indication | 79.8% Screening 20.2% Surveillance |
| History of polyps | 19.1% |
| History of colorectal cancer | 8.5% |
| Family history of colorectal cancer | 2.1% |
| Patients with 1 polyp | 51.5% |
| Patients with 2 polyps | 29.8% |
| Patients with 3 polyps | 13.8% |
| Patients with more than 3 polyps | 5.3% |
Polyp Characteristics
A total of 171 polyps were detected by WLE, imaged by HRME, and evaluated histologically. The average size of the polyps was 7.6 mm. Forty-five percent (n = 77) were neoplastic, with the majority being tubular adenomas. Of the non-neoplastic lesions, 52% were hyperplastic. Table 2 details the polyp characteristics.
Table 2.
Polyp Characteristics
| All polyps (n = 171) | |
| Non-neoplastic | 55.0% (n = 94) |
| Neoplastic | 45.0% (n = 77) |
| Non-neoplastic (n = 94) | |
| Normal colonic tissue | 26.6% (n = 25) |
| Hyperplastic polyp | 52.1% (n = 49) |
| Inflammatory polyp | 21.3% (n = 20) |
| Neoplastic ( n = 77) | |
| Tubular adenoma | 74.0% (n = 57) |
| Tubulovillous adenoma | 11.7% (n = 9) |
| Sessile/Serrated adenoma | 10.4% (n = 8) |
| Adenocarcinoma | 3.9% (n = 3) |
HRME and WLE Performance Characteristics of all Polyps
Table 3 describes the performance characteristics of HRME in differentiating neoplastic from nonneoplastic colorectal polyps compared to WLE for all 171 polyps. While sensitivity for neoplasia is not different between the two modalities, HRME has a significantly higher specificity and accuracy compared to WLE. The positive predictive value (PPV) of HRME is also significantly greater than that of WLE while the negative predictive value (NPV) is not statistically different.
Table 3.
Performance Characteristics: All Polyps
| WLE | HRME | p-value | |
|---|---|---|---|
| Sensitivity | 96.6% (92.7% - 100%) | 94.1% (88.7% - 99.8%) | 0.3286 |
| Specificity | 39.2% (28.8% - 53.2%) | 94.7% (89.8% - 99.6%) | p < 0.0001 |
| PPV | 55.0% (47.8% - 63.2%) | 86.9% (82.1% - 92.0%) | p < 0.0001 |
| NPV | 92.4% (85.2% - 100%) | 91.3% (87.0% - 95.8%) | 0.7536 |
| Accuracy | 64.8% (58.4% - 71.9%) | 94.1% (91.0% - 97.3%) | p < 0.0001 |
All values are diagnostic accuracy measures with 95% confidence interval in parenthesis.
HRME and WLE Performance Characteristics of Small and Diminutive Polyps
Tables 4 and 5 describe the performance characteristics of HRME compared to WLE for small and diminutive polyps. Small polyps were defined as polyps estimated to be less than or equal to 10 mm. Diminutive polyps were defined as polyps estimated to be less than or equal to 5 mm. While sensitivity is not different between the two modalities, HRME has a significantly higher specificity and accuracy compared to WLE for the differentiation of both small and diminutive neoplastic polyps. Similarly, PPV is significantly greater in HRME for both small and diminutive polyps while there is no statistical difference in PPV compared to WLE for either polyp size.
Table 4.
Performance Characteristics: Small Polyps
| WLE | HRME | p-value | |
|---|---|---|---|
| Sensitivity | 94.9% (88.2% - 100%) | 92.2% (81.5% - 100%) | 0.5868 |
| Specificity | 39.6% (24.4% - 64.2%) | 97.7% (93.2% - 100%) | p < 0.001 |
| PPV | 54.8% (42.2% - 71.2%) | 91.5% (85.2% - 98.3%) | p < 0.0001 |
| NPV | 90.7% (75.8% - 100%) | 91.1% (83.1% - 99.9%) | 0.9566 |
| Accuracy | 64.1% (52.4% - 78.4%) | 94.6% (88.7% - 100%) | p < 0.001 |
All values are diagnostic accuracy measures with 95% confidence interval in parenthesis.
Table 5.
Performance Characteristics: Diminutive Polyps
| WLE | HRME | p-value | |
|---|---|---|---|
| Sensitivity | 93.1% (84.9% - 100%) | 93.1% (80.6% - 100%) | 1.0 |
| Specificity | 41.4% (25.4% - 67.3%) | 97.6% (92.4% - 100%) | p < 0.001 |
| PPV | 52.6% (39.7% - 69.8%) | 92.6% (85.3% - 100%) | p < 0.0001 |
| NPV | 88.5% (73.0% - 100%) | 92.4% (83.6% - 100%) | 0.5654 |
| Accuracy | 61.7% (49.7% - 76.6%) | 95.1% (88.2% - 100%) | p < 0.001 |
All values are diagnostic accuracy measures with 95% confidence interval in parenthesis.
Discussion
This is the first real-time, in vivo study prospectively assessing the diagnostic accuracy of HRME in differentiating neoplastic from non-neoplastic colorectal polyps. The trial demonstrated that HRME, with the use of a topical contrast agent, can effectively provide subcellular images of colorectal polyps; these images can be interpreted in real-time as neoplastic or non-neoplastic based on the colon HRME classification system. Proflavine, the topical contrast agent used, was also tolerated safely by all study patients and no adverse events were reported. With histopathology as the gold standard for diagnosis, this study showed that HRME had 94% sensitivity and 95% specificity for the differentiation of neoplastic from non-neoplastic colorectal polyps. Compared to high-definition WLE, HRME was significantly more accurate (94% vs. 65%) and demonstrated a significantly greater specificity (95% vs 39%) and positive predictive value (87% vs 55%) for colorectal neoplasia.
Given the inability of white light endoscopy to classify neoplastic from non-neoplastic colorectal polyps, multiple modalities have been developed over the past decade to assist in this differentiation. Our study demonstrates that the overall performance characteristics of HRME are at least comparable to those of these other imaging modalities (Table 6). The accuracy of HRME in differentiating neoplastic from nonneoplastic colorectal polyps was 94%, which was significantly greater than the 65% for high-definition white-light endoscopy in our study. The 94% accuracy of HRME was also an improvement over the 82% seen in NBI and the 66% to 82% seen in pCLE. Any potential modality being developed to reduce the need for polypectomies and formal histopathology must have both high accuracy and high specificity for neoplastic lesions such that the endoscopist can be confident with either leaving the non-neoplastic polyp in situ or simply discarding the lesion. The specificity of HRME for neoplasia (95%) was not only significantly greater than that of white-light (39%) in our study but also greater than the 71-78% seen in pCLE studies and the 80-85% seen in NBI studies. It was the consistently low specificity of NBI that eventually led to the development of CLE platforms and our study shows that HRME is more specific than either modality. While HRME's sensitivity for neoplastic polyps at 94% did not differ significantly from white-light at 97% in our study, it was greater than the 57-91% seen in pCLE trials and similar to that of dye-based chromoendoscopy and NBI. The endoscope-based CLE platform was the only modality to demonstrate similar performance characteristics to HRME (23, 24, 40). However, most of the eCLE trials had fewer than 50 patients and the CLE image analysis and diagnosis was not conducted in vivo. While both CLE and HRME provide subcellular imaging of the colorectal mucosa at 1000× magnification, HRME costs more than one hundred and fifty thousand dollars less, can be passed through any standard endoscope and does not require intravenous contrast.
Table 6.
Comparison of HRME to Other Imaging Modalities
Since large lesions have greater neoplastic potential and are invariably likely to be resected, the true benefit of an in vivo classification tool would be its ability to differentiate small lesions into neoplastic or non-neoplastic. More than ninety percent of lesions smaller than 10 mm carry a very low risk of malignancy (8, 41-44). Thus, a modality that could accurately classify these small lesions would allow the endoscopist to selectively determine which lesions could be left in situ, which lesions could be resected and discarded, and which lesions needed formal histopathologic analysis. Our study demonstrated accuracy of 95% for small neoplastic lesions (less than or equal to 10 mm) and diminutive neoplastic lesions (less than or equal to 5mm), both values were significantly greater than the accuracies found on white-light endoscopy. HRME's specificity for neoplasia was 98% for small polyps and 98% for diminutive polyps, again significantly higher than white-light. The negative predictive value of HRME was 91% for small polyps and 92% for diminutive polyps. Sensitivity for neoplasia was above 93% for all lesion sizes and was not significantly different than white-light. The high accuracy of HRME, along with its high specificity and high negative predictive value for neoplasia in small and diminutive polyps, may allow the endoscopist to make enhanced, confident real-time decisions regarding leaving small, nonneoplastic polyps in situ and discarding small polyps with low neoplastic potential. In 2011, the American Society of Gastrointestinal Endoscopy issued a statement that in order for a new technology to guide the decision of either resecting and discarding or not resecting and leaving in situ diminutive and suspected non-neoplastic polyps, it must demonstrate a greater than 90% negative predictive value for adenomatous histology (45). HRME fulfills this requirement and would allow the endoscopist to confidently either resect and discard diminutive polyps or leave hyperplastic polyps unresected in situ. Therefore, HRME could reduce time, number of polypectomies, and cost of histology.
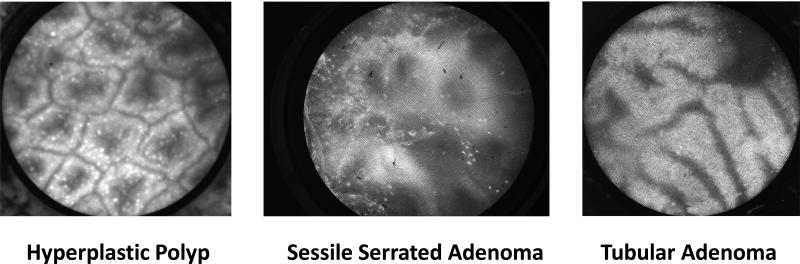
Our prospective study had several notable strengths. Most importantly, our study was a true real-time, in vivo assessment of HRME's diagnostic abilities. Prior CLE studies utilized “offline” or post-hoc analysis of CLE images acquired during the colonoscopy. When images are analyzed offline, the endoscopist has the advantage of long, careful inspection of the images, which is not feasible during an actual colonoscopy. When pCLE images were read in real-time during colonoscopy and compared to offline pCLE images, the in vivo diagnoses showed significantly lower accuracy (46). Our HRME diagnoses were made in real-time with the diagnosis of neoplastic or non-neoplastic being made as soon as the probe was placed on the polyp. Another significant advantage to our study was that we had three endoscopists with no prior HRME expertise perform the procedures. The majority of recent CLE trials have been performed by a single expert endoscopist, who in some cases had performed greater than 100 prior CLE procedures (28). In some cases, the observers who post-hoc analyzed the CLE images had previously assessed over 250 such images (26). While one of our endoscopists was an expert in advanced endoscopic imaging, the other two were general gastroenterologists. The other unique aspect to our study was the inclusion of sessile serrated adenomas (SSAs). There were seven SSAs in the 171 polyp study (4%) and the average size of these lesions was 9.6 mm. HRME had a sensitivity of 85.7% and a specificity of 100% for classifying SSAs as neoplastic polyps. Given the limited sample size, it is difficult to confidently state the specific HRME characteristics for SSAs but SSAs tended to have more distorted glands and a greater epithelial to crypt space ratio compared to hyperplastic polyps while not having the linear crypts or elongated nuclei seen typically in tubular adenomas (Figure 2). Further investigation into the HRME characteristics of SSAs is warranted and being planned.
Figure 2.
HRME Images
There are multiple potential limitations to our study. Primarily, it is important to recognize that the HRME unit is an experimental system that is not commercially available and that proflavine, the topical contrast agent, is an investigational drug only approved for research purposes. Moreover, while some of the CLE studies selected quality-controlled images for analysis, we did not assess the quality of our HRME images prior to analysis. This was due to the fact that the HRME images were acquired and analyzed in real-time and we believe that this fact further enhances our study as a true in-vivo assessment modality. Despite the potential for suboptimal images, the HRME still produced excellent performance characteristics compared to its quality-controlled predecessors. Another potential limitation of our study protocol was that the same endoscopist who made the WLE diagnosis then proceeded to make the HRME diagnosis on the same polyp. Unlike the offline CLE studies, the individual making the HRME read was not blinded to the interpretation of the WLE read. However, the pathologist making the final gold standard diagnosis was blinded to both WLE and HRME reads. While there was no blinding between the WLE and HRME reads, we believe that this study design is a more clinically relevant representation of how the two modalities could complement each other in real-time.
In conclusion, our in vivo, prospective study demonstrated that HRME can be a very effective modality in the differentiation of neoplastic from non-neoplastic colorectal polyps. HRME's high accuracy, specificity, and negative predictive value for neoplastic polyps compared not only to high-definition WLE but also to advanced modalities such as NBI and CLE , make it a very attractive complementary tool A combination of standard white-light colonoscopy for polyp detection and HRME for polyp classification has the potential to allow the endoscopist to determine which lesions can be left in situ, which lesions can be discarded, and which lesions need formal histopathologic analysis. Such a technique could decrease not only procedure times but also the number of polypectomies and the cost of histology. Future studies should include a randomized trial comparing HRME to standard of care for small and diminutive polyps as well as a trial directly comparing HMRE to confocal laser endomicroscopy in effort to further validate HRME's efficacy as a classification modality for colorectal neoplasia.
Study Highlights.
- What is current knowledge?
- Screening with colonoscopy has decreased colorectal cancer incidence and mortality
- All polyps are not malignant and do not need to be removed
- Current colonoscopy technology cannot differentiate between neoplastic and non neoplastic polyps
- What is new here?
- High-resolution microendoscopy may facilitate the identification of neoplastic colorectal polyps in-vivo
- High-resolution microendoscopy is significantly less expensive (< $2,000) than other novel colonoscopic imaging tools
- A combination of standard colonoscopy and HRME may allow the endoscopist to determine which polyps can be left in situ, which lesions can be discarded, and which lesions need formal histopathologic analysis
Acknowledgments
Financial support: This work was supported by the NIH/NCI grant “Optical Systems for In Vivo Molecular Imaging of Cancer” (CA 103830-07).
Footnotes
Specific Author Contributions: N. Parikh was involved in study design, acquisition of data, analysis and interpretation of data, drafting and editing of manuscript. The author has read and approved the final draft of the manuscript submitted. D. Perl was involved in acquisition, analysis and interpretation of data. The author has read and approved the final draft of the manuscript submitted. M. Lee was involved in acquisition of data. The author has read and approved the final draft of the manuscript submitted. B. Shah was involved in performance of colonoscopy and acquisition of data. The author has read and approved the final draft of the manuscript submitted. Y. Young was involved in performance of colonoscopy and acquisition of data. The author has read and approved the final draft of the manuscript submitted. S. Chang was involved in development of the classification system and critical revision of the manuscript for intellectual content. The author has read and approved the final draft of the manuscript submitted. R. Shukla was involved in development of the classification system and critical revision of the manuscript for intellectual content. The author has read and approved the final draft of the manuscript submitted. A. Poydorides was involved in analysis and interpretation of data. The author has read and approved the final draft of the manuscript submitted. E. Moshier was involved in statistical analysis of data. The author has read and approved the final draft of the manuscript submitted. J. Godbold was involved in statistical analysis of data. The author has read and approved the final draft of the manuscript submitted. E. Zhou was involved in acquisition of data. The author has read and approved the final draft of the manuscript submitted. J. Mitcham was involved in study design and acquisition of data. The author has read and approved the final draft of the manuscript submitted. R. Richards-Kortum was involved in study conception and design, obtaining funding and critical revision of the manuscript for intellectual content. The author has read and approved the final draft of the manuscript submitted. S. Anandasabapathy was involved in study conception and design, performance of colonoscopy, acquisition of the data, obtaining funding, and critical revision of the manuscript for intellectual content. The author has read and approved the final draft of the manuscript submitted.
Guarantor of the article: Sharmila Anandasabapathy
Conflicts of Interest: N. Parikh, D. Perl, M. Lee, B. Shah, Y. Young, S. Chang, R. Shukla, A. Poydorides, E. Moshier, J. Godbold, E. Zhou, J. Mitcham, and S. Anandasabapathy have no conflicts of interest to report. Rebecca Richards-Kortum serves as an unpaid scientific advisor to Remicalm LLC, holds patents related to optical diagnostic technologies that have been licensed to Remicalm LLC, and holds minority ownership in Remicalm LLC.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002-2010. MMWR Morb Mortal Wkly Rep. 2011;60:884–9. [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–91. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–59. doi: 10.3322/canjclin.56.3.143. quiz 184-5. [DOI] [PubMed] [Google Scholar]
- 7.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011;74:135–40. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CS, O'Brien MJ, Yang S, et al. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol. 2004;99:2242–55. doi: 10.1111/j.1572-0241.2004.40131.x. [DOI] [PubMed] [Google Scholar]
- 9.Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865–9. 869, e1–3. doi: 10.1016/j.cgh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Trecca A, Gaj F, Di Lorenzo GP, et al. Improved detection of colorectal neoplasms with selective use of chromoendoscopy in 2005 consecutive patients. Tech Coloproctol. 2006;10:339–44. doi: 10.1007/s10151-006-0304-z. [DOI] [PubMed] [Google Scholar]
- 11.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. 1906, e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Kiesslich R, von Bergh M, Hahn M, et al. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001–6. doi: 10.1055/s-2001-18932. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim JW, Cho YK, et al. Detection of colorectal adenomas by routine chromoendoscopy with indigocarmine. Am J Gastroenterol. 2003;98:1284–8. doi: 10.1111/j.1572-0241.2003.07473.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874–82. doi: 10.1053/j.gastro.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–9. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 16.Pasha SF, Leighton JA, Das A, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–70. doi: 10.1038/ajg.2011.436. quiz 371. [DOI] [PubMed] [Google Scholar]
- 17.Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406–12. doi: 10.1136/gut.2007.137984. [DOI] [PubMed] [Google Scholar]
- 18.Rastogi A, Early DS, Gupta N, et al. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593–602. doi: 10.1016/j.gie.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174–81. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Chiu HM, Chang CY, Chen CC, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373–9. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310–6. doi: 10.1016/j.gie.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Su MY, Hsu CM, Ho YP, et al. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711–6. doi: 10.1111/j.1572-0241.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 24.Hurlstone DP, Baraza W, Brown S, et al. In vivo real-time confocal laser scanning endomicroscopic colonoscopy for the detection and characterization of colorectal neoplasia. Br J Surg. 2008;95:636–45. doi: 10.1002/bjs.5988. [DOI] [PubMed] [Google Scholar]
- 25.Buchner AM, Shahid MW, Heckman MG, et al. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834–42. doi: 10.1053/j.gastro.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper T, van den Broek FJ, van Eeden S, et al. Feasibility and accuracy of confocal endomicroscopy in comparison with narrow-band imaging and chromoendoscopy for the differentiation of colorectal lesions. Am J Gastroenterol. 2012;107:543–50. doi: 10.1038/ajg.2012.14. [DOI] [PubMed] [Google Scholar]
- 27.Sanduleanu S, Driessen A, Gomez-Garcia E, et al. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2010;8:371–8. doi: 10.1016/j.cgh.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Shahid MW, Buchner AM, Heckman MG, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol. 2012;107:231–9. doi: 10.1038/ajg.2011.376. [DOI] [PubMed] [Google Scholar]
- 29.Vila PM, Kingsley MJ, Polydorides AD, et al. Accuracy and interrater reliability for the diagnosis of Barrett's neoplasia among users of a novel, portable high-resolution microendoscope. Dis Esophagus. 2013 doi: 10.1111/dote.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regunathan R, Woo J, Pierce MC, et al. Feasibility and preliminary accuracy of high-resolution imaging of the liver and pancreas using FNA compatible microendoscopy (with video). Gastrointest Endosc. 2012;76:293–300. doi: 10.1016/j.gie.2012.04.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muldoon TJ, Anandasabapathy S, Maru D, et al. High-resolution imaging in Barrett's esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68:737–44. doi: 10.1016/j.gie.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muldoon TJ, Pierce MC, Nida DL, et al. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15:16413–23. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MH, Vila PM, Polydorides AD, et al. Diagnostic accuracy of a novel low cost microendoscope for the detection of Barrett's neoplasia: a prospective, single-center trial. Gastroenterology. 2012;142:330–335. [Google Scholar]
- 34.Chang SS, Shukla R, Polydorides AD, et al. High resolution microendoscopy for classification of colorectal polyps. Endoscopy. 2013 doi: 10.1055/s-0032-1326502. In press. [DOI] [PubMed] [Google Scholar]
- 35.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138:W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 36.Pierce M, Yu D, Richards-Kortum R. High-resolution fiber-optic microendoscopy for in situ cellular imaging. J Vis Exp. 2011 doi: 10.3791/2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton SRBF, Boffetta P. Carcinoma of the colon and rectum. In: Bosman FTCF, Hruban RH, editors. WHO Classification of Tumours of the Digestive System. IARC Press; 2010. pp. 134–146. [Google Scholar]
- 38.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 39.Diggle PJLKY, Zeger SL. Analysis of longitudinal data. Oxford University Press; New York: 1994. [Google Scholar]
- 40.Kuiper T, Kiesslich R, Ponsioen C, et al. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc. 2012;75:1211–7. doi: 10.1016/j.gie.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 41.Butterly LF, Chase MP, Pohl H, et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343–8. doi: 10.1016/j.cgh.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Fujii T, Hasegawa RT, Saitoh Y, et al. Chromoscopy during colonoscopy. Endoscopy. 2001;33:1036–41. doi: 10.1055/s-0036-1588009. [DOI] [PubMed] [Google Scholar]
- 43.Waye JD, Lewis BS, Frankel A, et al. Small colon polyps. Am J Gastroenterol. 1988;83:120–2. [PubMed] [Google Scholar]
- 44.Lieberman D, Moravec M, Holub J, et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology. 2008;135:1100–5. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rex DK, Kahi C, O'Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–22. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 46.Shahid MW, Buchner AM, Raimondo M, et al. Accuracy of real-time vs. blinded offline diagnosis of neoplastic colorectal polyps using probe-based confocal laser endomicroscopy: a pilot study. Endoscopy. 2012;44:343–8. doi: 10.1055/s-0031-1291589. [DOI] [PubMed] [Google Scholar]