Abstract
Background
There are 34 million people living with HIV worldwide and each year this number increases. Until a vaccine is discovered, the prevention of new HIV infections remains an urgent priority. Several trials studying the use of oral and topical agents for the prevention of HIV infection have already been completed. Adherence has proved to be a major challenge in achieving product efficacy.
Aim of the review
To provide the clinical pharmacist with an understanding of the oral pre-exposure prophylaxis (PrEP) and topical microbicide product pipeline whilst emphasizing the critical importance of adherence to these drugs to avert HIV infection.
Methods
PubMed/Medline and the web-based clinical trials registry (ClinTrials.gov) were searched using appropriate key words. For the time period 1992 to 2013 - all phase II and phase III safety and effectiveness studies - testing agents for prevention of HIV infection were included in the review., Efficacy estimates, adherence estimates and reported challenges with adherence were extracted.
Results
Twenty four phase II and III clinical trials were found during review. Of these, 20 trials have been completed, and six trials show effectiveness in preventing HIV infection. The majority of the successful trials were to oral PrEP and to date only one microbicide trial of a vaginal antiretroviral microbicide gel has showed effectiveness. Adherence to study product played a major role in trial outcomes and there are several reasons for non-adherence. These include high on-trial pregnancy rates, low trial retention rates, low participant perception of risk, participant characteristics such as age<25 years, single status, migratory partners and trial fatigue. Study product characteristics such as dosage form, dosing interval, as well as associated adverse events may also influence adherence.
Conclusion
Moderate to high adherence is critical to demonstrate efficacy of drugs for HIV prevention. For topical agents, intermittent use associated with coitus is more effective than daily use, particularly if sex is infrequent or partners migrant. For oral agents, daily use is effective but the motivation to use the drug and high risk perception is important. In serodiscordant couples, early initiation of HAART in the infected partner affords almost complete protection to the negative partner. Drugs need to be tailored to the population at risk and availability of multiple drug options are important.
Keywords: PrEP, HIV Prevention, Microbicides, Clinical trial, Adherence
Introduction
In 2012, thirty years since the discovery of HIV; an estimated 34 million (range: 31.4 million-35.9 million) people are reported to be living with HIV globally. UNAIDS reported that 2.5 million (range: 2.2-2.8 million) people became newly infected in 2011 and 1.7 million people died that year from AIDS related causes [1]. The majority of infections are due to sexual transmission of HIV. Women, particularly those in sub-Saharan Africa, comprise more than half of all new infections [1]. The hyper-vulnerability of women in the HIV/AIDS pandemic is recognized to be due to a complex interplay of biological and socio-economic factors [2]. In other regions of the world, such as North America, Eastern Europe and central Asia,, men who have sex with men; injection drug users and their sexual partners, demonstrate a disproportionate risk for HIV infection[1].
Effective global scale up of HIV treatment programs, prevention activities and declining annual HIV incidence are encouragingly becoming a reality [1]. Still incident HIV infections accrue each year. Therefore, in the absence of a vaccine, the need for effective HIV prevention agents remains a priority in both developed and developing countries. Over the last 20 years, several clinical trials have evaluated antiretroviral (ARV) and non-ARV drugs as either treatment, oral pre-exposure prophylaxis (PrEP) and/or topical microbicide prophylaxis in preventing HIV infection [3, 4]. Whilst some studies have been successful others have failed.
A microbicide is a product that can be applied to the vaginal or rectal mucosa with the intention of preventing sexually transmitted infections including HIV. Microbicide dosage forms developed include sponges, films, gels (vaginal and rectal) and vaginal rings for topical application at the site of HIV exposure in the genital tract. However, the majority of clinical trials have been conducted on vaginal microbicides. Oral PrEP agents are ARV drugs that are administered to HIV negative individuals either daily or intermittently to prevent HIV infection. Recently, the US Food and Drug Administration (FDA), registered Truvada® for the indication of prevention of sexually transmitted HIV infection [5].
Low adherence to drug has been described as the ‘Achilles heel’ of HIV prevention trials [6]. In clinical trials, adherence is measured either directly or indirectly where self-report, counts of returned drug and in some cases drug levels may be used alone or in combination with other measures to assess adherence [7]. Several barriers to adherence exist and these include, but are not limited to, low risk perception, partner non-disclosure, influence of partner beliefs on product use, poor comprehension of product use instructions and the influence of unknown efficacy of trial agents in placebo controlled trials [8]. Studies have also assessed the social and behavioral aspects of adherence and behavioral models that may positively influence adherence [9, 10]. Specifically, the question of how to obtain and sustain high adherence by individuals at risk for infection in order to achieve efficacy remains unanswered. Nevertheless, much progress has been made to better understand the barriers to adherence from previous clinical trial experience [7, 11].
For the purpose of this review, we aim to contribute to the adherence for HIV prevention discussion by assessing adherence issues for drugs in the development pipeline and those previously tested for drug specific characteristics (viz. dosage form, dosing interval) and its possible influence on behavior for optimal adherence. We believe that potential barriers related to using the product characteristics itself must be better understood to enable us to inform uptake of these agents and assist our patients and clinical trial participants to better adhere to drugs for HIV prevention.
Aim of the review
This review aims to provide the clinical pharmacist with an understanding of the oral PrEP and topical microbicide product pipeline with an emphasis on the critical importance of adherence for drug efficacy required to optimally avert HIV infection. Importantly, the review also demonstrates how product characteristics could influence adherence and attempts to describe the adherence challenges experienced by patients taking drugs for prevention of HIV infection.
Methods
Several sources of information were reviewed to obtain product information relevant to this review article. Selection criteria for inclusion of studies in the review were restricted to: Phase II and III clinical trials, utilizing drugs for the prevention of HIV infection, either completed or ongoing. A PubMed/Medline literature search was conducted using the key search terms: ‘PREP’, ‘HIV Prevention’, Microbicides ‘and ‘Clinical trial’ for obtaining published clinical trial data. In addition, a web-based clinical trials registry (ClinTrials.gov) (URL:http://clinicaltrials.gov/) was used to ascertain information on currently active, registered but not yet recruiting and completed trials. ClinTrials.gov was selected because it is the most frequently used and is the largest clinical trials database in the world with a current holding of registrations from over 130,000 trials from more than 170 countries. This database was also used to cross reference trials located in the Pubmed/Medline search and to search for trials that may have been missed in the Medline search or were not yet published. Information on candidate agents planned for future clinical trial testing was obtained from AVAC: Global advocacy for HIV Prevention (http://www.avac.org/) and the Microbicides Trials Network (http://www.mtnstopshiv.org/).
Results
Several phase II and III clinical trials have been conducted in the past 21 years, investigating both ARV and non-ARV drugs in a variety of dosage forms and dosing intervals for the prevention of HIV infection. Trial results for adherence and effectiveness of oral PrEP and topical microbicides, along with potential barriers to adherence, are listed in Tables 1 and 2 respectively.
Table 1. Oral PrEP drugs for the prevention of HIV infection.
| Study, design, country and target population |
Product & Dosage Form and dosing strategy |
HIV risk reduction (Effectiveness) |
Adherence Measure |
Adherence Estimates |
Type of adherence counseling |
Potential barrier to adherence |
|---|---|---|---|---|---|---|
|
TDF2 Trial[17], Phase III RCT, Botswana Men and women 18-39 years (n=1219) |
TDF-FTC 1 tablet daily |
62.2% (95% CI: 21.5- 83.4%) |
Self-report Pill count Drug levels |
94.4% (3 day recall) 84.1% 80% |
Standard ASP | AE’s: nausea, vomiting, dizziness higher in TDF- FTC arm Highly migratory population |
|
iPrEx [21, 23, 25, 50, 51] Phase III RCT, Brazil, Ecuador, Peru, South Africa, Thailand, USA MSM and transgender women ≥18 years (n=2499) |
TDF-FTC 1 tablet daily |
44% (95% CI: 15- 63%) |
Self-report (interview and CASI) Pill count Medication Possession Ratio (MPR) Drug levels (sub- group of patients) |
95% 85-95% Drug levels: Hair : 72% TFV , 74% FTC Plasma/PBMC: 54% |
Structured ASP (Next-Steps counseling) (from Nov 2009- Feb 2010) |
Age < 25 years AE’s: nausea in first 4 weeks of PrEP Social stigma if thought to be HIV positive Unintentional disclosure of sexual orientation |
|
HPTN 052[18], Phase III RCT, Brazil, India, Kenya, Malawi, South Africa Zimbabwe ,Thailand, USA Serodiscordant couples (n=1763 couples) |
Pre-specified combination of ARV (3TC , AZT, NVP, EFV, ATV, ddI, d4T, LPV/r, TDF or TDF- FTC) administered to the HIV Infected partner early vs late Tablets - daily or twice daily depending on drug regimen selected |
96% (95% CI: 73-99%) |
Self-report Pill count |
Not reported >95% in 79% early arm >95% in 74% delayed arm |
Standard ASP | Pill burden, AE’s- ARV related side effects more frequent in the early treatment than delayed treatment group |
|
FEM PrEP[20, 24] Phase III RCT, Kenya, South Africa, Tanzania Women 18-35 years (n=1951) |
TDF-FTC 1 tablet daily |
HR: 0.94 (95%CI:0.59- 1.52) Stopped for futility |
Self-report Pill count Drug levels (pre- specified analysis in seroconverters) |
95% 88% 26% (7/26)- drug detected at beginning of infection window and 7/33 (21%) at end of window, and in 4/27 (15%) at both visits |
Standard ASP | Low risk perception |
|
Partners PrEP[12, 24] Phase III, RCT, Kenya, Uganda Heterosexual men, women serodiscordant couples >18years (n=4747 couples) |
TDF TDF-FTC 1 tablet daily |
TDF: 67% (95% CI: 44- 81%) TDF/FTC: 75% (95% CI: 55- 87%) |
Self-report Pill count Drug levels Adherence sub- study: MEMS UPC and in depth interview |
Not reported 97% , when adjusted for other factors then 92.1% 82% in random sample92% 99% |
Standard ASP - Individualized counseling as required |
AE’s- nausea and diarrhea Off study product during pregnancy and breastfeeding |
|
MTN 003 (VOICE)[13, 14] Phase IIb RCT, South Africa, Uganda, Zimbabwe Women 18-45 years (n=5029) |
TDF TDF-FTC 1 tablet daily |
TDF: HR 1.49 (95% CI: 0.97-2.29) TDF-FTC: HR 1.04 (95% CI: 0.73-1.49) |
Self- report (interview and ACASI) Pill count Drug levels in plasma (case-cohort subset) |
TDF-FTC: 91% TDF: 90% TDF-FTC: 92% TDF: 87% TDF: 28% TDF-FTC: 29% |
Structured ASP (VASP) |
Daily use of product Young and unmarried Low risk perception |
|
Pilot safety, acceptability, and adherence [19] Phase I//II RCT, Kenya MSM, FSW, 18-49 years (n=72) |
TDF-FTC 1 tablet daily or Intermittent dosing: 1 tablet Mon and Fri plus post-coital dose (not exceeding one dose a day) |
N/A | Self-report: post- coital dose MEMS Drug levels: plasma, PBMC |
100% (using timeline- follow back self-report and sexual activity data) Daily dosing: 83%, Intermittent dosing: 68% Post-coital: 26% Analysis pending |
Standard individualized ASP |
Difficulty in remembering to take intermittent doses Perception of risk Alcohol use prior to sex Disclosure of PrEP use to partner Transactional sex-may not have product accessible for post coital dose |
|
Bangkok Tenofovir Study [15, 16] Phase III RCT Bangkok IDU, men and women 20-60 years (n=2413) |
TDF 1 tablet daily |
48.9% (95%CI: 9.6-72.2) |
Pill count and patient medication diary Self –report (non- DOT) DOT- TDF adherence card Drug levels |
83.8% 100% 94.8% 70% reduction in risk if levels detectable |
Structured ASP (individualized) |
Side-effects: nausea Incarceration |
RCT= randomised controlled trial, AE’s = adverse events, ,CI= confidence interval, MSM= men who have sex with men, CASI=computer assisted self-interview, ASP= adherence support programme, MEMS-medication event monitoring system, UPC- unannounced home visit pill counts, ACASI= audio computer assisted self-interview , FSW= female sex workers, IDU= injection drug users, DOT= directly observed treatment , VASP= VOICE Adherence Strengthening Program. 3T= lamivudine , AZT=zidovudine, NVP=nevirapine, EFV= efavirenz,, ATV= atazanavir, ddI= didanosine, d4T= stavudine, LPV/r = lopinavir/ritonavir, TDF= tenofovir disoproxil fumarate or TDF-FTC = tenofovir disporoxil fumarate-emtricitabine. Unless otherwise stated all RCTs are placebo-controlled
Table 2. Topical PrEP/ Microbicides for the prevention of HIV infection.
| Study design, country and target population |
Product, dosage form and dosing strategy |
HIV risk reduction (effectiveness) |
Adherence measure |
Adherence estimate |
Type of adherence counseling |
Potential barrier to adherence |
|---|---|---|---|---|---|---|
|
N-9 sponge study [27] Phase II RCT Kenya FSW ≥18years (n=138) |
Nonoxynol-9, 1000mg contraceptive sponge One dose inserted before days first sex, replaced after 2- 3 partners and removed 6 hours after the last partner. Placebo – one suppository or cream applicator per day before first sex partner |
HR 1.7 (95% CI: 0.9-3.0) |
Self-report | 81% | Product use instructions - no specific adherence support mentioned |
Sex-worker cohort – inconvenient to replace sponge between clients. Placebo was not a sponge but a vaginal suppository then a cream – possible unblinding. Genital ulcers and vulvitis, higher in the sponge arm. |
|
N-9 film study[32] Phase II RCT, Cameroon FSW 18-45 years (n=1292) |
Nonoxynol-9, 70mg film One dose before each coital act |
HR 1.0 (95%CI: 0.7-1.5) |
Self-report using coital logs |
87% | Monthly product use counseling - no specific adherence support mentioned |
Genital lesions |
|
N-9 gel study [33], Phase II RCT, Kenya FSW, 18-45 years (n=278) |
Nonoxynol-9 52.5mg vaginal gel One dose before days first sex act |
HR 0.7(95%CI: 0.3-1.5) |
Self –report | 75% | Use instructions only – no specific adherence support mentioned |
Douching practices, use of other lubricants |
|
COL-1492 [34] Phase II/III RCT Benin, Cote d’lvoir, Thailand, South Africa FSW, ≥18 years (Benin, Cote d’lvoir, Thailand) ≥16 years (South Africa) (n=892) |
Nonoxynol-9 52.5mg Vaginal gel One dose before sex act and reapply if vagina cleansed after last intercourse – no set limit to gel doses per day |
HR 1.5 (95% CI:1.0-2.2) – harmful |
Self –report using pictorial coital log. Then interview incorporated because researchers noticed that coital diaries were being completed in the waiting area at study visits. |
79% | Use instructions only – no specific adherence support mentioned. |
High frequency gel use equated to a higher risk for infection. |
|
Ghana SAVVY study [22] Phase III RCT Ghana Women 18-35 years (n=2142) |
SAVVY® (1% C31G) Vaginal gel One dose 1 hour before sex. Second dose could be inserted if sex delayed more than one hour from first dose. |
Study terminated prematurely HR 0.88 (95%CI: 0.33- 2.27) |
Self-report | 75% | Use instructions only – no specific adherence support mentioned |
Lower than expected HIV incidence rates. High pregnancy rates, poor retention, choice to not use gel |
|
Nigeria SAVVY study [35] Phase III RCT Nigeria Women 18-35 years (n=2153) |
SAVVY® (1% C31G) Vaginal gel One dose before sex |
Study terminated prematurely HR 1.7 (95%CI: 0.9-3.5) |
Self-report | 78% | Use instructions only – no specific adherence support mentioned |
Lower than expected HIV incidence rates, high pregnancy rates. |
|
Nigeria CS trial[36] Phase III RCT Nigeria Women 18-35 yearsb(n=1644) |
6% Cellulose sulfate Vaginal gel One dose just before sex for each act of vaginal intercourse. Second dose could be inserted if sex delay more than 1 hour from first dose. |
Study terminated prematurely HR 0.8 (95%CI: 0.3-1.8) |
Self – report | 81% | Use instructions only – no specific adherence support mentioned |
Pregnancy, lack of supplies due to missed visits, low retention rates, vaginal douching practices |
|
CS trial [31] Phase II/III RCT Benin, India, Uganda, South Africa Women ≥ 18 years (n=1398) |
6% Cellulose sulfate Vaginal gel One dose 1 hour before sex act |
HR 1.61 (95% CI: 0.86- 3.01 ) NS but may be harmful in per protocol and interim analysis. |
In –depth interview |
87% | Use instructions only – no specific adherence support mentioned |
Pregnancy |
|
Carraguard trial [30] Phase II/III RCT South Africa, Women ≥16years (n=6202) |
Carraguard Vaginal gel One dose with each act of sex up to 1hr before sex |
HR 0.87 (95%CI: 0.69- 1.09) |
Self-report Applicator count (used and unused) Applicator staining assay |
96% - 41% |
Use instructions only, onsite gel insertion at enrolment and reminders to return product at FU visits |
Running out of supplies and forgetting to insert gel. |
|
PRO 2000 safety and acceptability study [52] Phase II RCT Uganda Women, 18-45 years (n=180) |
PRO 2000 0.5% ,2% Vaginal gel One dose inserted twice a day for 28 days |
Not powered | Structured interview Coital diary Used and unused applicator counts |
69% | No specific adherence support mentioned |
Being away from home, spotting, forgot, ill-heath |
|
MDP 301 [29] Phase III RCT South Africa, Tanzania, Uganda, Zambia Women ≥18 years (South Africa and Zambia) ≥16 year (Tanzania and Uganda) (n=9385) |
PRO 2000 0.5% ,2% Vaginal gel One dose within 1 hour prior to sex |
Study terminated for futility PRO 2000 0.5% : HR 1.05 (95% CI:0.82- 1.34) PRO 2000 2% arm discontinued in 2008 HR 1.11 (95%CI:0.82-1.51) |
Self-report Coital diaries Applicator count – used and unused In-depth interview |
89% | Social science sub-study in n=725. A Adherence measure data was triangulated but no specific adherence support mentioned |
None listed |
|
Buffergel /Pro 2000 study [28] Phase II/IIb RCT Malawi, South Africa, Zambia, Zimbabwe Women, ≥18 years (n=3101) |
Buffer gel, Pro 2000 0.5% Vaginal gel One dose 1 hour before each sex act |
HR 0.7 (95%CI:0.46-1.08) Trend |
Self-report | 81% | Difficult to dose if more than one sex act occurred per night without partner knowledge Pregnancy, difficulties applying gel, intravaginal practices |
|
|
CAPRISA 004 [37, 49] Phase IIb RCT South Africa Women 18-40 years (n=889) |
TFV 1% Vaginal gel BAT24 |
HIV: 39% (95%CI: 6-60%) HSV 2: 51% (95%CI: 22-70%) HIV: 54% (95%CI: 4-80%) if ≥80% gel adherence |
Applicator count Self-report Drug concentrations |
72% 82% >1000ng/m in CVF I associated with protection |
Structured adherence support Program (ASP), including MI counseling |
Trial fatigue Wetness of gel Post coital dose insertion if product use not disclosed to partner |
|
HPTN 059 [53, 54] Phase II RCT safety and acceptability US, India Women 18-50 years (N = 200) |
Daily: TFV 1% gel Coital use: TFV 1% gel Vaginal Gel Duration : 6 months |
N/A | Self-report | 80% :coital use within 2 hours of sex 83% : daily use |
Product use instructions and counseling as needed |
Menstruation Forgetfulness |
|
MTN 001[55, 56] Phase II RCT Adherence and PK study US, South Africa, Uganda Women 18-45 years (n=144) |
Daily TFV 1% gel Daily TDF 300mg tablet Both Duration: 6-weeks, separated by one-week washouts |
N/A | Self-report Drug concentrations |
94% (tablet and gel) 64% (tablet use) |
Product use instructions | Menstruation Forgetfulness Being away from home Private storage Unplanned overnights Lack of privacy Gel leakage |
|
MTN 003 (VOICE) [13, 14, 57] Phase IIb RCT South Africa, Zimbabwe, Uganda Women 18-45 years (n=5029) |
TFV 1% gel Vaginal gel inserted daily (see Table 1 for oral arm) |
1% TFV gel arm stopped for futility |
Self-report Drug concentrations |
90% 22% |
VOICE Adherence Strengthening Program (VASP |
Daily use of product , even when not sexually active |
|
MTN 017 [41, 58] Phase II safety study RCT Peru, South Africa, Thailand MSM & transgender women ≥18 years old (n=186) |
Daily use low glycerin formulation TFV 1% rectal gel BAT 24 low glycerin formulation TFV 1% rectal gel Daily TDF/FTC 300/200 mg tablet |
Trial ongoing | Self-report Drug concentrations |
Trial ongoing | Product use instructions | Data unknown – trial ongoing |
|
FACTS 001 [38] PHASE III RCT South Africa Women 18-40 years (n=2900) |
TFV 1% Vaginal gel BAT 24 |
Trial ongoing | Applicator count Self-report Drug concentrations |
Trial ongoing | Structured Adherence Support Program (ASP), including MI based counselling |
Data unknown – trial ongoing |
|
MTN 020 (ASPIRE) [43] Phase III RCT Malawi, South Africa, Uganda, Zambia, Zimbabwe Women 18-45 years (n=3476) |
Dapivirine 25mg silicone elastomer vaginal matrix ring 25mg Replaced monthly |
Trial ongoing | Self-report Drug concentrations |
Trial ongoing | Adherence counselling and Educational Programme (ACE) |
Data unknown – trial ongoing |
|
IPM 027 (The Ring Study) [42] Phase III RCT Malawi, Rwanda, South Africa Women 18-45 years (n=1650) |
Dapivirine 25mg silicone elastomer vaginal matrix ring 25mg Replaced monthly |
Trial ongoing | Self-report Drug concentrations |
Trial ongoing | Product use instructions | Data unknown – trial ongoing |
|
CAPRISA 008 [39] Phase IIIb RCT South Africa (N=700) women who previously participated in the CAPRISA 004 study |
TFV 1% Gel Vaginal gel BAT 24 Intervention: Obtain product through family planning clinic services Control: Obtain product through CAPRISA clinic |
Trial ongoing | Applicator count Self-report Drug concentrations |
Trial ongoing | Structured ASP, including MI based counseling |
Data unknown – trial ongoing |
N-9 = nonoxynol 9, RCT = randomized controlled trial, FSW= female sex worker,CI= confidence interval NS = non-significant, TFV = tenofovir gel, BAT24 Regimen - within 12 hours BEFORE coitus, as soon as possible within 12 hours AFTER coitus and up to TWO doses in a 24 hour period,CVF =cervicovaginal fluid MI= motivational interviewing, VASP – VOICE adherence strengthening programme, MSM = men who have sex with men. Unless otherwise stated all RCTs are placebo-controlled, ACE – Adherence counseling and educational programme
Oral PrEP agents for the prevention of HIV infection
Table 1, provides a comprehensive summary of Phase II and III randomized control trials of oral PrEP drugs conducted between 2007 and 2012,. Seven trials evaluating the efficacy of oral PrEP have been completed. Although varied, the target populations studied included individuals known to be at high risk for HIV acquisition; young women in Africa, MSM, serodiscordant couples and injection drug users (IDUs).
The Partners PrEP trial [12] and the MTN 003 (VOICE) trial [13, 14] evaluated both tenofovir disoproxil fumarate (TDF) and tenofovir disoproxil fumarate/emtricitabine (TDF-FTC_) as oral PrEP agents. With the exception of the Bangkok Tenofovir Study [15, 16], which investigated TDF only, all other studies including the TDF2 trial [17] investigated TDF-FTC as the PrEP agent. HPTN 052 [18] differed from the other PrEP trials listed because it examined the early use of HAART in HIV infected individuals with CD4 cell counts of 350-550 cells/mm3 to prevent HIV transmission to the uninfected partner compared to delayed initiation of full treatment in the infected partner. In most studies, study product was taken daily. The exception is a pilot study on safety and adherence in Kenya [19], which tested an intermittent dosing strategy.
The TDF2, iPrEx, Partners PrEP, Bangkok Tenofovir Study and HPTN052 studies found PrEP and early initiation of antiretroviral therapy to be effective interventions in reducing risk for HIV acquisition. Efficacy estimates ranged between 44%-96%. In contrast, the FEM PrEP [20] and MTN 003 (VOICE) [13] studies did not show a reduction in risk for HIV acquisition. The FEM PrEP trial, the TDF and tenofovir gel arms of the VOICE study was stopped for futility in April 2011, September 2011 and November 2011 respectively. The VOICE trial continued with only the remaining TDF-FTC arm until its conclusion in Aug 2012. Results of the VOICE trial released in March 2013 found none of the treatment arms to be effective mainly due to suboptimal adherence [13].
All studies reviewed provided standard risk reduction counseling and sexual reproductive health services (provision of condoms and treatment of STI’s) in addition to standard adherence support counseling to participants (use and dosing instructions). Two studies developed a structured adherence support programme (ASP); “Next Step” counseling in the iPrEx trial, VOICE adherence strengthening program (VASP) was introduced in the VOICE study. The Bangkok study also included a structured interview as part of the ASP [7].
Self-report and pill counts were standard adherence measures used across all the studies and adherence calculated by these methods was typically high. Drug levels were also evaluated in seven of the studies reported in Table one [12, 13, 16, 17, 20-22]. In the FEM PrEP study adherence by self-report and pill count was 95% and 88% respectively, but drug level concentrations indicative of recent pill use were found in less than 40% of uninfected women at visits matched to the HIV infection window of seroconverters. In VOICE, overall adherence by self-report was 90% however in a sub-study, drug levels were detected in only 30% of TDF and 29% of TDF-FTC participant samples at a specific visit [13]. In the iPrEx study the Medication Possession Ratio (MPR), calculated by dividing the number of pills dispensed at a particular visit by the number of days that have elapsed from that visit to the next visit, provided a ratio of the number of days that participants would have been in ‘possession’ of study drug. MPR was found to be superior to pill count and was predictive of drug exposure[23].
An intensive adherence sub-study in Partners Prep that involved unannounced home-visits for pill counts (UPC) and Medication Event Monitoring System (MEMS) where each bottle opening was recorded, in addition to clinic self-reported adherence and pill counts, was carried out amongst 1417 of the HIV negative participants. Additional counseling measures were undertaken in participants that had <80% adherence by UPC. PrEP efficacy in the adherence sub-study was reported as 100% [24]. The Bangkok Tenofovir study allowed participants to choose between collecting medication on a monthly basis or daily clinic visits for a directly observed treatment (DOT) dose. Participants were allowed to change between the two choices at monthly visits. Overall adherence reported in the study was high. Adherence rates were found to be better amongst women and those 40 years and older. In participants with detectable tenofovir drug levels, efficacy increased to 74%. [15, 16]. A pilot study evaluating adherence to daily PrEP dosing compared to intermittent dosing (1 dose on Monday and Friday plus a post-coital dose) in MSM and FSW found daily dosing to have better adherence [19].
Potential barriers to adherence within the clinical trials included younger age, with age less than 25 years was associated with a higher incidence of HIV infection in MSM [25] and marital status (unmarried women). In the VOICE trial lower drug concentrations and higher HIV incidence was demonstrated in younger, unmarried women compared to those over 25 and married [26]. In the pilot study conducted in Kenya, reported adherence barriers to intermittent dosing included difficulty in remembering to take an intermittent dose, alcohol use before sex, transactional sex, perception of risk and non-disclosure of PrEP use to partner which could negatively impact on motivation to use a post-coital dose [19].
Perception of risk for HIV infection was also reported as a potential barrier to adherence. Over 70% of women in the FEM PrEP trial did not perceive themselves to be at risk of infection [20]. TDF2 reported young migratory populations impacting on trial completion and retention as additional potential barriers to adherence [17]. Other barriers included side effects (adverse effects) to the drugs (nausea, diarrhea, dizziness) and social constraints of non-disclosure of PrEP use to the sexual partner.
Topical PrEP/Microbicide for the prevention of HIV infection
The mechanisms of action of candidate microbicides that have undergone clinical trial testing can be divided into four distinct classes. The first class are the nonionic surfactants such as Nonoxynol-9 [27] or C31G [22] which has a non-specific mechanism of action and pathogens (like sperm and HIV) are killed by disruption of membranes. Second are the buffer agents that enhance the natural vaginal defenses by lowering the pH and potentially inactivate HIV e.g. Buffergel [28].The third class are cell entry inhibitors which prevent HIV from attaching to, and fusing with, the host cells e.g. PRO2000 [29], Carraguard [30] and Cellulose sulfate[31].The latest and most effective class is the antiretroviral agents which act by inhibiting HIV from target cell attachment and/or replicating (examples are tenofovir and maraviroc). Dosing regimens could either be coitally dependent (dose administered around coitus) or non-coitally independent (routinely dosed, usually daily).
Table 2 provides a comprehensive summary of Phase II and III randomized placebo-controlled trials of topical PrEP, conducted between 1992 and 2012, and includes ongoing trials currently in the field. There have been 13 clinical trials completed where the effectiveness of 6 agents have been tested. Three further effectiveness studies assessing 2 more agents are currently underway.
Prior to 2010, all microbicide clinical trials failed to show effectiveness and some even demonstrated a potential for harm. The field began by testing Nonoxynol-9 (N-9), previously used as a spermicide, in a variety of dosage forms: sponges, films and vaginal gels [27, 32-34] and found either no effect on HIV risk or a potential for harm (i.e. increased risk for HIV infection). Populations initially selected in these early studies were sex worker cohorts, at high risk for infection, using test product in excess of what would be used in the general population and potentially damaging vaginal mucosa. Adverse events related mostly to genital mucosal irritation and vaginal douching practices, which could have diluted or washed away the microbicide, may have contributed to adherence problems. Apart from standard product use instructions no specialized adherence interventions are described in the N-9 studies.
As the field advanced, attention turned to agents with more specific mechanisms of actions and target populations began to resemble the general population more closely. Unfortunately, none of the trials studied showed effectiveness but several lessons were learnt about the critical importance of adherence. All trials testing SAVVY® (C31G) were stopped prematurely for futility [22, 35] and all reported that considerable time off product due to high pregnancy rates and low retention which contributed to poor adherence. These factors, combined with low HIV incidence doomed these trials. The cellulose sulfate studies, PRO2000 and Buffergel trials followed suit [28-31, 36]. Again, apart from standard product use instructions no specialized adherence interventions are described. What was learnt in these studies is that self-report of adherence to product overestimated actual use, poor retention meant lack of access to supplies of product as did time off product due to pregnancy. All these studies utilized a vaginal gel in a coitally dependent dosing strategy.
In 2010, the first microbicide trial (CAPRISA 004) to demonstrate effectiveness was published [37]. The key differences in this study that may have contributed to the successful results are the use of an anti-retroviral agent (1% tenofovir gel), low pregnancy rates, high retention and the implementation of a motivational interviewing (MI) based ASP for the specific dosing instructions. “BAT 24” dosing required women to insert one dose of gel up to 12 hours before sex (pre-coitally) followed by a second dose up to 12 hours after sex (post-coitally) with no more than two doses inserted in a 24 hour period.
A subsequent trial, investigating 1% tenofovir gel dosed daily, failed to show effectiveness [13]. Based on the low plasma drug concentrations, the authors’ state that low adherence directly impacted on outcomes and that in this study, where women were predominately young and unmarried, a longer acting drug for intermittent use may have been easier to adhere to. The VOICE Adherence Strengthening Program (VASP) was used in this trial to support women with adherence issues.
Currently in the field, the FACTS 001 study [38] aims to validate the CAPRISA 004 trial results. The CAPRISA 008 study, a phase IIIb implementation trial [39] is testing a scale up strategy using family planning services to dispense study gel together with contraception and providing post-trial access of 1% tenofovir gel to participants from the CAPRISA 004 study. The development of a low glycerin formulation of 1% tenofovir gel enables the safe use of the gel in the rectum where previously the higher osmolar vaginal preparation has caused diarrhea [40]. This formulation is being tested by the Microbicide Trials Network in the MTN 017 study [41]., The dapivirine vaginal ring trials [42, 43] where the vaginal ring requires monthly replacing, hopes to provide a possible solution to previously experienced adherence challenges such as remembering to use a product daily or at the time of sex and replenishing product supply in addition to expanding the available PrEP options for women
Candidate agents of interest for future trials to prevent HIV infection
In the interests of improving adherence and efficacy, as well as expanding available options to consumers several exciting initiatives are underway to test candidate agents in pre-clinical and phase I trials for the prevention of HIV infection. These include combination products, such as combining two antiretrovirals (dapivirine/maraviroc vaginal ring), the tenofovir vaginal ring, TFV-FTC fast dissolving suppository tablets, dapivirine film/gel, injectable and other multipurpose technologies that combine contraceptives with STI treatments [44]. These candidates potentially improve on adherence challenges by focusing on product formulation characteristics, ease of dosing and synergistic mechanisms of actions.
Discussion
In order for any drug to effectively prevent HIV infection it is essential that adherence to that drug be optimal. Almost all HIV prevention trials conducted to date report adherence to drug prophylaxis as a major challenge to overcome. This is not surprising given that otherwise healthy individuals are being asked to use medication to prevent a disease they may or may not be exposed to. This is an important barrier to overcome because in settings of concentrated or generalized HIV epidemics, where the population at risk of infection can be well characterized, the use of PrEP can have considerable public health benefits [45].
It is clear from several studies that adherence has a direct impact on PrEP efficacy [12, 13, 16, 20, 21, 24, 37, 46, 47]. Sub-optimal adherence to HIV prevention drugs has been attributed as a primary reason for product and ultimately trial failure [11]. Other important reasons for futility in these types of clinical trials include high on-trial pregnancy rates which reduce time on product and low HIV incidence that make reasonable size trials infeasible. Certainly studies with a high overall retention (≥; 95%) and low pregnancy rates tend to be more successful because treatment interruptions are reduced. Even in successful studies, it is interesting that adherence and ultimately product effectiveness wanes over time [21, 37]. Nonetheless, the RCT remains the ideal study design to provide the best evidence for efficacy of drugs tested in HIV prevention trials. The studies reviewed are of high quality in terms of study design, conduct and analysis – however the influence of very low adherence on trial outcome cannot be factored into this design apriori. This major limitation, found in all prevention research, is as a consequence of provision of prophylaxis in the healthy and other behavioral complexities, rather than a flaw in the RCT study design itself.
Adherence measures that have routinely been used in prevention studies are patient self – report, counts of the tablets or applicators either at the study visit or unannounced, use of coital diaries and where possible, drug concentration assays. The most subjective of these assessments are the patient self-reports which in almost all trials is an over-estimate of actual use, whereas drug levels provide the most objective method for assessing adherence. In the Partners PrEP trial [12, 48] it was reported that lower/undetectable drug levels are associated with increased risk of HIV seroconversion, similarly in the failed FEM-PREP trial drug levels were much lower in the those who seroconverted whereas in the CAPRISA 004 study vaginal tenofovir concentrations >1000ng/ml are associated with HIV protection [49].
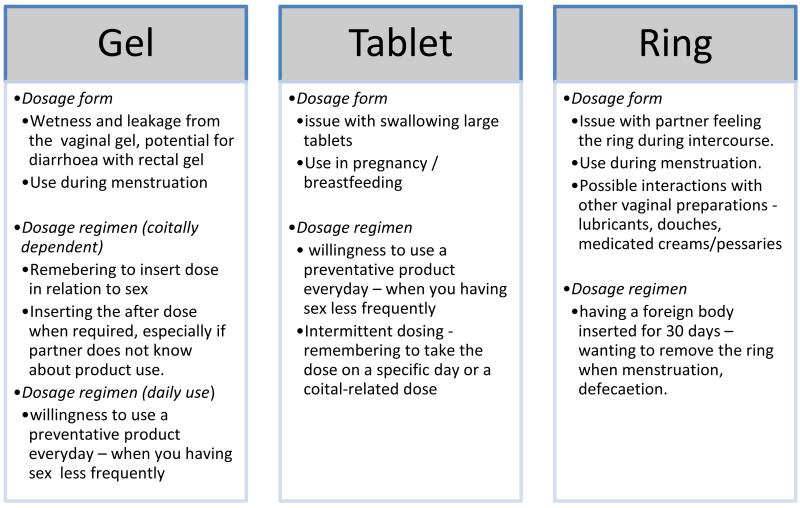
Product characteristics like dosage form and dosage strategy may also affect adherence. Figure 1 illustrates potential barriers to adherence in the three most commonly tested dosage forms: gel, tablet and ring. With the dosing interval/strategy there have been contradictory reports on the preferred intervals where some studies report daily dosing to be easier than coitally dependent whereas others report the opposite. A pilot study evaluating adherence to daily PrEP dosing compared to intermittent dosing (1 dose on Monday and Friday plus a post-coital dose) in MSM and female sex workers found daily dosing to have better adherence [19]. Reasons for this may be difficulty in remembering to take an intermittent dose or lack of opportunity to do so. In addition, almost half the participants reported alcohol use before sex which could impair adherence to the post-coital dose.
Figure 1.
Potential product dosage form characteristics and dosing regimen barriers to adherence
An interesting illustration of the contrasting outcomes of the same product, same dosage form (1% tenofovir gel) in a similar population but with different dosing strategies can be seen in the CAPRISA 004 study [37] and the VOICE study gel arm [13]. These two studies of the same agent (1% tenofovir vaginal gel) using different dosing strategies (one daily insertion vs coitally dependent BAT 24 dosing) illustrate how low to no adherence in the VOICE study vs. moderate adherence in the CAPRISA 004 study impacted trial effectiveness.
Concerns regarding partner discovery of undisclosed PrEP use may also discourage taking a post-coital dose. Side effects to prevention drugs even if mild may impact on product use and severe reactions will lead to discontinuation. Products that cannot safely be used during pregnancy and breast feeding result in time off product and also limit PrEP options in these women.
Perception of risk of HIV infection will impact on whether a participant is adherent to drug or not. In the FEM PrEP trial, when questioned, 70% of women did not perceive themselves to be at risk of infection [20]. In contrast, serodiscordant couples in stable relationships within the Partners PrEP and HPTN 052 study understood their risk for HIV acquisition as they were fully aware of their partners HIV status which may have contributed to the higher adherence rates. The frequency of contact with migratory partners that are seen intermittently may make a daily dosed prevention drug seem unnecessary and promotes chronic non-adherence. Young women less than 25 years of age and those unmarried, appear to be most likely to have difficulty adhering to HIV prevention drugs.
Issues of non-disclosure of trial participation, fears of being mistakenly identified as HIV positive or with MSMs unintended disclosure of sexual orientation, may have also influenced the behaviour of individuals where clandestine transport, storage and use of product in the absence of a partners/family knowledge and support or in some cases permission to participate may also contribute to non-adherence. Lastly, although not discussed in the trial outcomes, almost all studies were placebo controlled and unknown efficacy of the drug that one is receiving i.e. is the drug an active or a placebo may contribute to trial participant ambivalence.
Conclusion
The success of drugs for the prevention of HIV infection is dependent on adherence. In clinical trials moderate to high adherence is critical to demonstrate the efficacy of drugs for HIV prevention. For agents that are topically applied, intermittent dosing strategies associated with coitus are more effective than daily dosing, particularly if sex is infrequent or if partners are migrant workers. For oral agents, daily use is effective but the motivation to use the product and a high risk perception is important. Drugs need to be tailored to the population being served and several options to choose from is useful. With serodiscordant couples, early initiation of HAART in the infected partner affords almost complete protection to the negative partner. Adherence is best supported when a combination of factors related to drug choice, dosage form, dosing strategy and participant controlled factors such as perception of risk and appropriate medication taking behavior converge.
Acknowledgments
CAPRISA is supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grant no. AI51794). The authors were study personnel in the CAPRISA 004 and CAPRISA 008 Tenofovir gel trials, which was supported by the United States Agency for International Development (USAID), Family Health International (FHI),CONRAD and LIFElab, a biotechnology centre of the South African Department of Science & Technology. The Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP grant # D43TW00231) has supported Tanuja N Gengiah’s professional development. The views expressed by the authors do not necessarily reflect the views of NIH, USAID, CONRAD, FHI360 or Gilead Sciences.
List of acronyms
- AE
Adverse event
- AIDS
Acquired immune deficiency syndrome
- ARV
Antiretroviral
- ASP
Adherence support programme
- AVAC
Global advocacy for HIV Prevention
- CASI
Computer assisted self-interview
- DOT
Directly observed treatment
- FSW
Female sex worker
- HAART
Highly active antiretroviral therapy
- HIV
Human immunodeficiency virus
- HPTN
HIV prevention trials network
- IDU
Injecting drug user
- iPrEx
Pre-exposure prophylaxis Initiative
- MEMS
Medication event monitoring system
- MI
Motivational interviewing
- MPR
Medication possession ratio
- MSM
Men who have sex with men
- MTN
Microbicides trial network
- RCT
Randomized controlled trial
- STI
Sexually transmitted infection
- TDF
Tenofovir disoproxil fumarate
- TDF-FTC
Tenofovir disoproxil fumarate/emtricitabine
- TFV
Tenofovir gel
- UNAIDS
Joint United Nations programme on HIV/AIDS
- UPC
Unannounced home visit pill counts
- VASP
VOICE Adherence Strengthening Program
- VOICE
Vaginal and oral interventions to control the epidemic
Footnotes
Conflicts of interests
All authors have no conflicts of interest to declare.
References
- 1.UNAIDS . Global report: UNAIDS report on the global AIDS epidemic 2012. Joint United Nations Programme on HIV/AIDS; Geneva: [Accessed 25 March 2013]. Available from: Global report: UNAIDS report on the global AIDS epidemic 2012. [Google Scholar]
- 2.Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25. doi: 10.1097/01.aids.0000341774.86500.53. [DOI] [PubMed] [Google Scholar]
- 3.Stone A, Harrison PF. Microbicides - Ways Forward. Alliance for Microbicide Development; Silver Spring, MD, USA: 2010. [Google Scholar]
- 4.Veronese F, Anton P, Fletcher CV, DeGruttola V, McGowan I, Becker S, et al. Implications of HIV PrEP Trials Results. AIDS Res Hum Retrov. 2011;27(1):81–90. doi: 10.1089/aid.2010.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration . FDA approves first drug for reducing the risk of sexually acquired HIV infection. Silver Spring, MD: [accessed July 19, 2012]. 2012. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm. [Google Scholar]
- 6.Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What’s love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. JAIDS. 2012;59(5):463–8. doi: 10.1097/QAI.0b013e31824a060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amico KR, Mansoor LE, Corneli A, Torjesen K, van der Straten A. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17(6):2143–55. doi: 10.1007/s10461-013-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodsong C, Macqueen K, Amico KR, Friedland B, Gafos M, Mansoor L, et al. Microbicide clinical trial adherence: insights for introduction. J Int Aids Soc. 2013;16:18505. doi: 10.7448/IAS.16.1.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer RA, Morrow KM, Fisher WA, Fisher JD. Toward an information-motivation-behavioral skills model of microbicide adherence in clinical trials. AIDS Care. 2010;22(8):997–1005. doi: 10.1080/09540121003623719. [DOI] [PubMed] [Google Scholar]
- 10.Roberts ET, Matthews DD. HIV and chemoprophylaxis, the importance of considering social structures alongside biomedical and behavioral intervention. Soc Sci Med. 2012;75(9):1555–61. doi: 10.1016/j.socscimed.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muchomba FM, Gearing RE, Simoni JM, El-Bassel N. State of the science of adherence in pre-exposure prophylaxis and microbicide trials. JAIDS. 2012;61(4):490–8. doi: 10.1097/QAI.0b013e31826f9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrazzo JRG, Nair G, Palanee T, Mkhize B, Nakabiito C, Taljaard M, Piper J, Gomez Feliciano K, Chirenje M, VOICE Study Team Pre-Exposure Prophylaxis for Hiv in Women: Daily Oral Tenofovir, Oral Tenofovir/Emtricitabine, or Vaginal Tenofovir Gel in the Voice Study (MTN003); Conference of Retroviral and Opportunistic Infections; Georgia World Congress Centre, Atlanta, USA. 2013; Abstract#26LB. [Google Scholar]
- 14.Microbicide Trials Network MTN 003: Phase 2B Safety and Effectiveness Study of Tenofovir 1% Gel, Tenofovir Disoproxil Fumarate Tablet and Emtricitabine/Tenofovir Disoproxil Fumarate Tablet for the Prevention of HIV Infection in Women. 2013 Apr 23; cited. Available from: http://www.mtnstopshiv.org/node/70.
- 15.Martin MVS, Suntharasamai P, Sangkum U, Leethochawalit M, Chiamwongpaet S, Kittimunkong S, Mock PA, Paxton L, Choopanya K. In: Bangkok Tenofovir Study Group, editor. Participant adherence in the Bangkok Tenofovir Study, an HIV pre-exposure prophylaxis trial in Bangkok; 6th IAS Conference on HIV Pathogenesis and Treatment; Rome, Italy. 2011; Abstract no TUPE350. [Google Scholar]
- 16.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 17.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and Adherence to Intermittent Pre-Exposure Prophylaxis (PrEP) for HIV-1 in African Men Who Have Sex with Men and Female Sex Workers. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0033103. DOI 10.1371/journal.pone.0033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoako M, Boakye AY, et al. SAVVY® (C31G) gel for prevention of HIV infection in women: A phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS ONE. 2007;2(12):e1312. doi: 10.1371/journal.pone.0001312. DOI: 10.71/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amico KR, Liu A, McMahan V, Anderson PL, Lama JR, Guanira J, et al., editors. Adherence Indicators and Pre-exposure Prophylaxis (PrEP) Drug Levels in the iPrEx Study; Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2011. [Google Scholar]
- 24.Haberer JE, Psaros C, Baeten J, Katibira E, Tumwesigye E, Ronald A, et al., editors. High adherence to oral PrEP is associated with lack of infections in an ancillary study of objective adherence monitoring and counseling among HIV discordant couples in Partners PrEP Study; Conference of Retroviruses and Opportunistic Infections; Seattle. 2012. [Google Scholar]
- 25.Bekker L, Glidden D, Hosek S, Brown B, Liu A, Amico R, et al., editors. Pre-exposure Prophylaxis in Young Men Who Have Sex with Men: Needs and Challenges [Paper #997]; Conference on Retroviruses and Opportunistic Infections; Georgia World Congress Centre, Atlanta. 2013. [Google Scholar]
- 26.World Health Organisation . Coverage of selected health services for HIV/AIDS prevention and care in less developed countries in 2001. World Health Organisation; Geneva, Switzerland: 2002. Available from: http://whqlibdoc.who.int/publications/9241590319.pdf. [Google Scholar]
- 27.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268(4):477–82. [PubMed] [Google Scholar]
- 28.Abdool Karim SS, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957–66. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376(9749):1329–37. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372(9654):1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 31.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, et al. Lack of Effectiveness of Cellulose Sulfate Gel for the Prevention of Vaginal HIV Transmission. New Engl J Med. 2008;359(5):463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 32.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339(8):504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 33.Richardson BA, Lavreys L, Martin HL, Jr., Stevens CE, Ngugi E, Mandaliya K, et al. Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: A randomized clinical trial. Sex Transm Dis. 2001;28(7):394–400. doi: 10.1097/00007435-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 35.Feldblum PJ, Adeiga A, Bakare R, Wevill S, Lendvay A, Obadaki F, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS ONE. 2008;3(1):e1474. doi: 10.1371/journal.pone.0001474. DOI: 10.371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halpern V, Ogunsola F, Obunge O, Wang CH, Onyejepu N, Oduyebo O, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a Phase III trial in Nigeria. PLoS ONE. 2008;3(11):e3784. doi: 10.1371/journal.pone.0003784. DOI: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.FACTS 001: Safety and Effectiveness of Tenofovir Gel in the Prevention of Human Immunodeficiency Virus (HIV-1) Infection in Young Women and the Effects of Tenofovir Gel on the Incidence of Herpes Simplex Virus (HSV-2) Infection. 2013 Apr 22; ClinicalTrials.gov. cited. Available from: http://clinicaltrials.gov/ct2/show/NCT01386294?term=facts+001&rank=1.
- 39.CAPRISA 008 :Implementation Effectiveness and Safety of Tenofovir Gel Provision Through Family Planning Services. 2013 Apr 23; ClinicalTrials.gov. cited 2013. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01691768?term=CAPRISA+008&rank=1.
- 40.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28(11):1412–21. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MicrobicideTrialsNetwork MTN-017 Backgrounder: Phase II Safety and Acceptability Study of Tenofovir Gel Reformulated for Rectal Use. 2012 cited 2013 22 April 2013. Available from: http://www.mtnstopshiv.org/news/studies/mtn017/backgrounder.
- 42.IPM 027: Safety and Efficacy Trial of a Dapivirine Vaginal Matrix Ring in Healthy HIV-Negative Women. 2013 Apr 24; ClinicalTrials.gov. cited 2013. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01539226?term=IPM+027&rank=2.
- 43.MTN 020: Phase 3 Safety and Effectiveness Trial of Dapivirine Vaginal Ring for Prevention of HIV-1 in Women (ASPIRE) 2013 Apr 24; ClinicalTrials.gov. cited 2013. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01617096?term=mtn+020&rank=1.
- 44.ARV-based prevention research and development product pipeline [database on the Internet] 2013 Apr 30; cited. Available from: http://www.avac.org/
- 45.Williams BG, Abdool Karim SS, Karim QA, Gouws E. Epidemiological impact of tenofovir gel on the HIV epidemic in South Africa. JAIDS. 2011;58(2):207–10. doi: 10.1097/QAI.0b013e3182253c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. DOI: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26(7):F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 48.Kahle E, Donnell D, James H, Thomas K, John-Stewart G, Nakku-Joloba E, et al. PrEP has high efficacy for HIV-1 prevention among higher-risk HIV-1 serodiscordant couples: a subgroup analysis from the Partners PrEP Study. J Int Aids Soc. 2012;15:138–9. [Google Scholar]
- 49.Abdool Karim SS, Kashuba A, Werner L, Abdool Karim Q. Drug concentrations following topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu A, Huang Y, Defechereux P, McMahan V, Eden C, Guanira J, et al., editors. Hair as a biological marker of daily oral pre-exposure prophylaxis (PrEP) adherence and tenofovir/emtricitabine (TFV/FTC) exposure in the Global iPrEx Study; 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. 2011; Abstract: MOLBPE037. [Google Scholar]
- 51.Tangmunkongvorakul A, Chariyalertsak S, Amico KR, Saokhieo P, Wannalak V, Sangangamsakun T, et al. Facilitators and barriers to medication adherence in an HIV prevention study among men who have sex with men in the iPrEx study in Chiang Mai, Thailand. AIDS Care. 2012 doi: 10.1080/09540121.2012.748871. DOI: 10.1080/09540121.2012.748871. [DOI] [PubMed] [Google Scholar]
- 52.Kamali A, Byomire H, Muwonge C, Bakobaki J, Rutterford C, Okong P, et al. A randomised placebo-controlled safety and acceptability trial of PRO 2000 vaginal microbicide gel in sexually active women in Uganda. Sexually transmitted infections. 2010;86(3):222–6. doi: 10.1136/sti.2009.038372. [DOI] [PubMed] [Google Scholar]
- 53.MicrobicideTrialsNetwork Press Release: Trial finds tenofovir gel safe for daily use and most women adhered to study regimens. 2008 cited 2013 23 April. Available from: http://www.mtnstopshiv.org/node/359.
- 54.Hillier S. Microbicides 2008. New Delhi, India: 2008. L. Safety and acceptability of coitally dependent use of 1% tenofovir over six months of use. Abstract No. 655. [Google Scholar]
- 55.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. DOI: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minnis AM, Gandham S, Richardson BA, Guddera V, Chen BA, Salata R, et al. Adherence and acceptability in MTN 001: a randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2013;17(2):737–47. doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MicrobicideTrialsNetwork Press Release: Daily HIV prevention approaches didn’t work for African women in VOICE study. 2013 cited 2013 22 April 2013. Available from: http://www.mtnstopshiv.org/node/4877.
- 58.MicrobicideTrialsNetwork Press Release: Reduced glycerin formulation of tenofovir vaginal gel safe for rectal use. 2012 cited 2013 22 April 2013. Available from: http://www.mtnstopshiv.org/node/4496.