Abstract
In 2005, the NIH consensus conference published a series of papers recommending methods to improve the conduct of clinical trials in chronic graft-versus-host disease (GVHD). Although the NIH recommendations were primarily aimed at strengthening research, several papers addressed issues relevant for clinical practice, particularly diagnosis, severity scoring, and ancillary and supportive care practices. We conducted an international survey to assess the uptake of these recommendations, identify barriers to greater use, and document the use and perceived effectiveness of available treatments. The response rate for the American survey of 1,387 practitioners was 21.8%, and it was 24.6% for 407 centers surveyed in Europe, Asia, Australia and Africa. Most respondents were familiar with the NIH consensus recommendations (94-96%) and used them in practice. Multiple barriers to greater use were reported. Besides lack of time (55-62%), unfamiliarity with the recommendations, scarcity of evidence supporting the impact of recommendations on outcomes, insufficient training/experience in chronic GVHD management, and inaccessibility of subspecialists were also endorsed. Systemic corticosteroids were reported to be the most effective treatment for chronic GVHD, but many others were perceived to have moderate or great success. Therapeutic management of steroid-refractory chronic GVHD was identified as the highest priority for research.
Keywords: chronic graft-versus-host disease, allogeneic hematopoietic cell transplantation, survey, uptake, implementation, recommendations
Introduction
Chronic graft-versus-host disease (GVHD) occurs in 10-70% of patients receiving allogeneic hematopoietic cell transplantation (HCT) depending on patient age, graft type and GVHD prophylaxis regimen. Chronic GVHD is a major cause of morbidity and mortality. It is a very heterogeneous clinical syndrome that requires specific expertise to diagnose, appropriately assess severity, and manage treatment.
There has been significant research activity and renewed interest in chronic GVHD over the last 10 years. In 2005, the National Institutes of Health (NIH) held a consensus conference and subsequently published a series of six articles summarizing recommendations to improve clinical trials in chronic GVHD.1-6 Although the focus was on the conduct of clinical trials, the group's recommendations were also relevant to clinical care, including when to initiate treatment, how to determine disease severity, and evidence supporting various ancillary and supportive care interventions.
In the eight years since the NIH conference, there is a growing body of published empiric work examining the validity and utility of the consensus recommendations.7-10 The German/Austrian/Swiss consortium on chronic GVHD recently assessed the usefulness of the NIH criteria for patient care in clinical practice and reported high rates of acceptance for definitions of chronic GVHD as well as overall and organ-specific severity scoring among the vast majority of participants.11 Nevertheless, anecdotal evidence suggests that the consensus recommendations are variably adopted in clinical practice across countries and healthcare settings, and that lack of time, training and interest may explain these variations.
The EBMT-NCI Chronic GVHD Task Force was created to facilitate collaborations to enhance use and evaluate impact of the NIH recommendations in clinical care. The Task Force met under the auspices of the NCI Center for Global Health in December 2012, and determined that there was a need to formally evaluate the current level of uptake and use of the NIH consensus recommendations, seven years following their initial development and dissemination in peer-reviewed publications. The purpose of this international survey was to document the extent to which the NIH consensus recommendations are used by practitioners, describe barriers to greater use, assess the perceived effectiveness of available treatments for chronic GVHD, and identify priorities for future studies.
Methods
Study Population
North and South America: Potential participants were identified as physicians or advanced practice professionals (nurse practitioners and physician assistants) with email addresses registered with the Center for International Blood and Marrow Transplant Research (CIBMTR). Multiple individuals per center may have participated, and center affiliation was not recorded.
Europe, Asia, Australia, and Africa: Potential participants were the physician contacts at each center that participates in the European Group for Blood and Marrow Transplantation (EBMT). Only one respondent was allowed for each center.
Survey Design
Survey items were drawn from a prior study in 2001 that captured practice demographics and perceptions about the efficacy of available treatments for chronic GVHD.12 For this survey, questions were developed to address issues related to recommendations that emanated from the 2005 NIH consensus criteria, such as comfort with applying the NIH consensus recommendations, barriers to use of the NIH recommendations, interest in training materials, perceived need for specific research studies, and interest in implementing chronic GVHD clinical and research tools. Questions (n=42) were formatted primarily as single and multiple choice questions with a few more in depth, open-ended questions. Response options included Likert scales and rankings. The questions were reviewed by multiple experts in chronic GVHD, and pilot tested by the study team to refine the questions and response options. However, formal reliability and validity assessments were not conducted. Completion of the survey took approximately 8 minutes.
Survey Administration
North and South America: Human subjects approval was obtained from the Fred Hutchinson Institutional Review Board. An initial invitation email was sent as well as two reminders to non-respondents. Surveys were collected between March 11-25, 2013 using an online survey tool. A drawing was held for three cash prizes to encourage participation. A total of 302 individuals participated from 1387 potential respondents who were invited (21.8% response rate).
Europe, Asia, Australia, and Africa: The survey was administered by the EBMT office in Leiden, on behalf of the Complications and Quality of Life Working Party, so separate IRB approval was not required. Invitations to participate were emailed to the physician contact at all registered allogeneic transplant centers; two reminders were sent to non-respondents. Only one response per center was included. Surveys were collected between March 7-25, 2013 using an online survey tool. A drawing for a prize was held to encourage participation. A total of 100 centers responded from a total of 407 invited (24.6% response rate).
Analysis
As applicable, participant and transplant center demographics and responses are summarized using descriptive statistics. Statistical testing to compare the distribution of responses between America and other regions of the world was not performed, since survey responses were derived from two distinct perspectives. Participants from North and South American regions completed items based on their own individual experiences and perspectives, whereas the responses of participants from the other world regions reflected the attitudes and practices of a majority of practitioners at the respondent's transplant center. We were not able to compare responders vs. non-responders in the American survey because of limited available demographic information on non-responders. For the EBMT survey, regulations prohibit comparison of responder and non-responder centers.
Results
Table 1 shows the participant demographics and practice characteristics. Most respondents were physicians. Slightly more than half treated exclusively adult patients. More than three quarters of respondents to the EBMT survey were based in European transplant centers. There was also representation from centers in Asia, Australia and Africa. Use of all types of allogeneic donors was common. More than 80% practiced in a model where post-transplant care was directly provided by the transplant physician or transplant center, and where access to subspecialists such as ophthalmologists and dermatologists was high. Current engagement in research protocols for chronic GVHD prevention and treatment was somewhat higher among North and South American respondents than among EBMT respondents, but was still less than 50%.
Table 1. Participant and practice characteristics.
| North and South America (American group) n (%) or median (IQR)1 | Europe, Asia, Australia, Africa (EBMT group) n (%) or median (IQR) | |
|---|---|---|
| Response rate | 302/1387 (21.8%) | 100/407 (24.6%) |
| Location of transplant center |
|
|
| Type of practitioner |
|
|
| Number of allogeneic transplants performed in 2012 at center, median IQR | 75 (38-130) (n=281) | 40 (21-60) (n=87) |
| Number of patients with chronic GVHD followed by respondent, median, IQR | 15 (6-30) (n=278) | 20 (10-50) (n=85) |
Type of practice
|
|
|
Donor types2
|
|
|
Care model
|
|
|
Clinic arrangement2
|
|
|
Access to specialist care2
|
|
|
Research protocol for chronic GVHD available2
|
|
|
| Center collects and stores research biospecimens | 101 (33%) | 14 (20%) (n=71) |
The American survey allowed multiple respondents per center
more than one answer allowed
Table 2 describes the general familiarity with and fluency in using the NIH consensus recommendations for chronic GVHD and the barriers to their greater use, separately for the American and the European/Asian/African (EBMT) group. It also shows the degree of interest in materials and training about the recommendations. Overall, the characteristics and responses were very similar between the American and EBMT surveys. Ninety four to 96% were familiar with the NIH consensus criteria, and a majority was somewhat or definitely comfortable applying the consensus criteria in clinical practice. Interest in brief Fast Facts guidelines, patient education materials, and training courses was high. A little more than two thirds of all respondents (72% American and 71% EBMT) were probably or definitely interested in participating in research projects. However, significant barriers preventing the routine use of the NIH criteria were also acknowledged, with lack of time being the foremost barrier reported by both groups.
Table 2.
Use of NIH consensus criteria and barriers to greater use, and interest in materials and training.
| North and South America (American group) n (%) | Europe, Asia, Australia, Africa (EBMT group) n (%) | |
|---|---|---|
| Response rate | 302/1387 (21.8%) | 100/407 (24.6%) |
Familiarity with the NIH consensus criteria
|
|
|
If familiar with the NIH consensus criteria (n=284), comfort with making the diagnosis of chronic GVHD according to NIH criteria
|
|
|
If familiar with the NIH consensus criteria (n=284), comfort with calculating the mild, moderate, or severe global severity score
|
|
|
If familiar with the NIH consensus criteria (n=284), routine use of NIH criteria for diagnosis and severity in clinical practice
|
|
|
If familiar with the NIH consensus criteria (n=284), comfort using the proposed NIH response criteria to determine patient response totreatment
|
|
|
Interest in Fast Facts
|
|
|
Interest in patient education materials
|
|
|
Interest in participating in a research project
|
|
|
Interest in training courses about chronic GVHD*
|
|
|
Has the NIH consensus conference improved our understanding of chronic GVHD*
|
|
|
Perceived barriers that prevent routine use of the NIH chronic GVHD criteria*
|
|
|
more than one answer allowed
Figures 1 and 2 show the frequency of use and perceived effectiveness of available systemic and topical/targeted treatments for chronic GVHD. Systemic corticosteroids had the highest ratings for use and perceived effectiveness. Extracorporeal photopheresis and the calcineurin inhibitors, cyclosporine and tacrolimus, were perceived as the next most effective group. Mycophenolate mofetil was reported as producing moderate or great success by 50% of EBMT respondents and 36% of American respondents. Sirolimus, rituximab and psoralen and UVA (PUVA) were also reported as having moderate or great efficacy by at least 20% of respondents from both groups. Imatinib was felt to be a moderately or greatly successful treatment by 21% of EBMT respondents compared with 15% of American ones.
Figure 1.
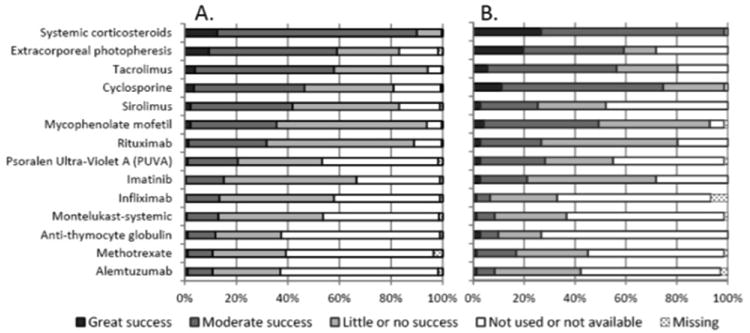
The frequency of use and perceived effectiveness of available systemic treatments for chronic GVHD in A) North and South America, and in B) Europe, Asia, Australia, Africa. The respondents were asked to express their opinion about effectiveness of treatment and the bars show the relative proportion of responses in each category.
Figure 2.
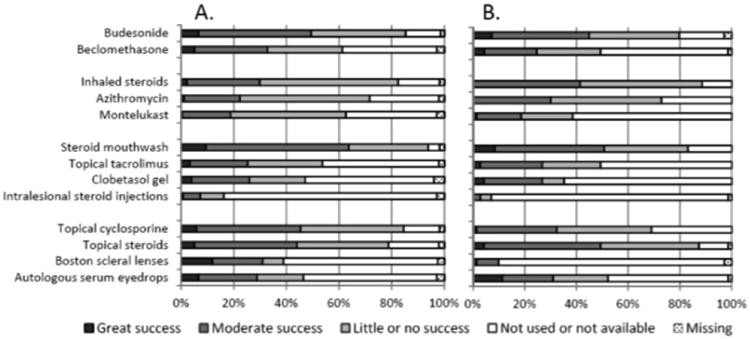
The frequency of use and perceived effectiveness of available topical or organ-directed treatments for chronic GVHD in A) North and South America, and in B) Europe, Asia, Australia, Africa. The respondents were asked to express their opinion about effectiveness of treatment and the bars show the relative proportion of responses in each category.
North and South America
One hundred eighty two (60%) of respondents used chronic GVHD resources, such as online sites, books or manuals. Of these, 144 (79%) used the website of the American Society for Blood and Marrow Transplantation (www.asbmt.org) and 86 (47%) the NIH chronic GVHD website (http://ccr.cancer.gov/resources/gvhd). Fewer accessed resources developed by the patient advocacy groups (www.bmtinfonet.org; www.nbmtlink.org; www.marrow.org), Rare Diseases Clinical Research Network (http://rarediseasesnetwork.epi.usf.edu/cGVHD) or used the EBMT Handbook or Thomas' Hematopoietic Cell Transplantation textbook.13
American respondents felt that the area of greatest priority for clinical trials was to develop effective treatments for steroid-refractory chronic GVHD, regardless of organ manifestations; this topic received a mean ranking of 1.9 on a 1-8 scale with 1 indicating the highest priority. There was moderate interest in studies of bronchiolitis obliterans syndrome (mean rank 3.1), initial therapy of chronic GVHD (mean rank 3.7), and skin sclerosis (mean rank 3.7). Respondents assigned a lower priority ranking to studies of overlap syndrome (mean rank 4.4), ocular chronic GVHD (mean rank 5.3), oral chronic GVHD (mean rank 5.4), or vaginal GVHD (mean rank 6.7).
Europe, Asia, Australia, and Africa
Respondents from 67 EBMT transplant centers (67% of centers answering this survey; 16.5% of all EBMT allogeneic transplant centers) used chronic GVHD resources, such as online sites, books or manuals. Of these, the most common source of information was the EBMT Transplant Handbook (64; 95%), international websites such as the American Society for Blood and Marrow Transplantation (31; 46%), the NIH chronic GVHD website (28; 42%), the National Marrow Donor Program (25; 37%), the German-Austrian-Swiss Chronic GVHD Consortium (20; 30%, www.gvhd.eu), or the Thomas' Hematopoietic Cell Transplantation textbook (14; 21%). Fewer used the patient advocacy groups and US Rare Diseases Clinical Research Network.
In the EBMT survey, only those respondents who indicated an interest in participating in chronic GVHD research (n=71) were asked the questions about topic priority. Of 48 evaluable responses from those indicating an interest in research, clinical trials to develop effective treatment for steroid-refractory chronic GVHD was determined to be of highest priority, with a mean rank of 2.1 on a 1-8 scale with 1 indicating the highest priority. There was also prominent interest in studies of bronchiolitis obliterans syndrome (mean rank 2.9) and skin sclerosis (mean rank 3.8). There was felt to be less need to focus research on ocular chronic GVHD (mean rank 4.7), overlap syndrome (mean rank 4.9), initial therapy of chronic GVHD (mean rank 4.9), oral chronic GVHD (mean rank 5.7) or vaginal GVHD (mean rank 6.4).
Discussion
We report the results of a global survey to investigate the status of chronic GVHD diagnosis, severity scoring and management in clinical practice throughout different world regions. Although the unit of analysis differs between the groups, with the American survey directed to individual clinicians and the EBMT survey querying centers, the results are very similar. Most respondents report being familiar with the NIH consensus criteria and feel comfortable applying them during clinical care. However, practitioners are challenged by lack of time, unfamiliarity with NIH criteria, and gaps in training opportunities, and many still question whether patient outcomes are improved when the criteria are used. A majority of respondents expressed great interest in materials and courses that would provide training, evaluation tools, clinician decision support and patient education.
Survey participants had substantial experience with many chronic GVHD treatment options and reported moderate to great success with several of them, although only a small minority reported great success. Use of topical and organ-directed therapies also appears common, with moderate to great success reported with various formulations of topical steroids and calcineurin inhibitors. Developing effective therapies for steroid-refractory chronic GVHD was identified as the highest research priority in both surveys, followed by testing new treatment regimens for bronchiolitis obliterans syndrome and cutaneous sclerosis.
Despite the high level of reported familiarity with the NIH criteria and generally optimistic reports about immunosuppressive and supportive treatment, there is still room for improvement. In the Chronic GVHD consortium, based in the United States, there has been a perceptible increase in routine use of the NIH consensus criteria at participating centers, but many other centers continue to apply older definitions and less reproducible approaches to evaluation and documentation. The German/Austrian/Swiss consensus group on chronic GVHD reported that the NIH diagnostic and staging criteria are very suitable for use in clinical practice11 and have published treatment strategies for first-line and salvage treatment.14, 15 Kuzmina and colleagues performed a prospective study using the NIH criteria during routine patient visits in the outpatient clinic demonstrating the feasibility of the NIH recommended diagnostic and staging approach.10 From a research perspective, routine use of the NIH criteria in clinical care would greatly facilitate observational and registry studies. Retrospective studies relying on chart review for reclassification of chronic GVHD according to NIH criteria are limited by inadequacies of medical records in documenting the presence or absence of diagnostic and distinctive NIH-defined manifestations of chronic GVHD, and differences in historical criteria used to establish the diagnosis of chronic GVHD at various transplant centers. Therefore, it is perhaps not surprising that the association between the NIH consensus criteria and indicators such as survival and non-relapse mortality differ between retrospective and prospective clinical studies using the same NIH criteria.7, 10, 16, 17 Finally, no studies have tested whether application of the NIH consensus criteria in routine practices improves the care of patients, and this evidence gap was cited as a major barrier to greater uptake of the criteria in clinical practice.
This study has a number of limitations. Although the sample size was relatively large compared to past surveys, the response rate was approximately 20 to 25%, suggesting that respondents may not be representative of all allogeneic transplant clinicians. Low response rates are a common problem with practice surveys, and a 10-20% response rate is not unusual in other transplant provider surveys.18-20 Nevertheless, results must be interpreted cautiously since it is likely that the individuals and centers most interested and experienced with chronic GVHD research and management responded preferentially to the survey. The fact that respondents reported high rates of access to specialist care in both the American as well as the EBMT survey supports the possible existence of a response bias in our sample. Thus, we believe that the high percentages of experience with and interest in the NIH consensus recommendations observed in our survey should be considered the upper boundary of attitudes and practice patterns worldwide.
Another caveat, given our assumption that respondents were more attuned to chronic GVHD than other practitioners, is a possible social desirability bias to report greater familiarity with the NIH criteria than actual experience would demonstrate. Finally, we note that the perceived efficacy of some therapeutic agents is quite high. Clinical trials often show a lower objective success rate than those reported more globally in provider surveys.
The high interest in supportive materials and additional training, even amongst those most interested in chronic GVHD, suggest a need for additional education and outreach strategies. Some well-attended training sessions have been conducted at professional meetings such as the Tandem Transplant meetings and the EBMT meetings, and these could be repeated. Training materials are available on the Internet as videos or written material, but their existence could be better advertised. Other approaches could include ongoing professional training opportunities such as webinars, more focused guidance on implementing the NIH criteria in routine practice, and incorporating chronic GVHD measures into quality metrics to encourage institutional support for these practices. Emerging data suggesting that the NIH criteria provide prognostic information may increase routine use of the criteria in both practice and research settings, and produce intensified interest from both practice and transplant centers for additional training. A better understanding of how to help centers implement the NIH consensus recommendations in their practices could help surmount many of the barriers identified in our study, and would reduce variations in diagnosis, severity grading, and clinical management. Consistent use of the NIH consensus guidelines as a common set of metrics and standards for documentation and clinical management would support data interoperability, data pooling, and comparisons across treatment approaches. The consensus guidelines also enhance rigor in the conduct of therapeutic trials, and may ultimately strengthen the quality of survivorship care offered to individuals with chronic GVHD.
Acknowledgments
The authors wish to thank all the participating practitioners and centers, CIBMTR for providing contact information for the American survey, and the EBMT administrative office for their help with survey distribution to EBMT centers. The Center for Global Health provided support for the initial meeting of the EBMT-NCI Chronic GVHD Task Force. This work was in part supported by the Center for Cancer Research, the intramural program of the National Cancer Institute, National Institutes of Health. The Chronic GVHD Consortium (U54 CA163438) is a part of the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Funded by CA118953, CA163438.
Footnotes
Conflicts of interest: D.W. received a research grant from Novartis and Therakos, lecture fees from Fresenius and serves as consultant for Falk-Pharma.
S.J.L. was a consultant for Allergan
The other authors report no conflicts of interest
References
- 1.Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary Therapy and Supportive Care of Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12(4):375–96. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Martin PJ, Weisdorf D, Przepiorka D, Hirschfeld S, Farrell A, Rizzo JD, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group Report. Biol Blood Marrow Transplant. 2006;12(5):491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group Report. Biol Blood Marrow Transplant. 2006;12(3):252–66. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12(2):126–37. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12(1):31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Arai S, Jagasia M, Storer B, Chai X, Pidala J, Cutler C, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118(15):4242–9. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird K, Steinberg SM, Grkovic L, Pulanic D, Cowen EW, Mitchell SA, et al. National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant. 2013;19(4):632–9. doi: 10.1016/j.bbmt.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsohn DA, Kurland BF, Pidala J, Inamoto Y, Chai X, Palmer JM, et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120(13):2545–52. doi: 10.1182/blood-2012-04-424135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuzmina Z, Eder S, Bohm A, Pernicka E, Vormittag L, Kalhs P, et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: results of a prospective study. Leukemia. 2012;26(4):746–56. doi: 10.1038/leu.2011.257. [DOI] [PubMed] [Google Scholar]
- 11.Greinix HT, Loddenkemper C, Pavletic SZ, Holler E, Socie G, Lawitschka A, et al. Diagnosis and staging of chronic graft-versus-host disease in the clinical practice. Biol Blood Marrow Transplant. 2010;17(2):167–75. doi: 10.1016/j.bbmt.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Lee SJ, Vogelsang G, Gilman A, Weisdorf DJ, Pavletic S, Antin JH, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002;8(1):32–9. doi: 10.1053/bbmt.2002.v8.pm11846354. [DOI] [PubMed] [Google Scholar]
- 13.Thomas' Hematopoietic Cell Transplantation. Fourth. Wiley-Blackwell; 2009. [Google Scholar]
- 14.Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16(12):1611–28. doi: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2011;17(1):1–17. doi: 10.1016/j.bbmt.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Arora M, Nagaraj S, Witte J, DeFor TE, MacMillan M, Burns LJ, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transplant. 2009;43(2):149–53. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 17.Jagasia M, Giglia J, Chinratanalab W, Dixon S, Chen H, Frangoul H, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207–15. doi: 10.1016/j.bbmt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Joffe S, Kim HT, Socie G, Gilman AL, Wingard JR, et al. Physicians' attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104(7):2194–200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 19.Pidala J, Craig BM, Lee SJ, Majhail N, Quinn G, Anasetti C. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(1):63–7. doi: 10.1038/bmt.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pidala J, Lee SJ, Quinn G, Jim H, Kim J, Anasetti C. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1528–36. doi: 10.1016/j.bbmt.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


