Abstract
Hyperactive β-catenin drives colorectal cancer, yet inhibiting its activity remains a formidable challenge. Interest is mounting in tankyrase inhibitors (TNKSi) which destabilize β-catenin through stabilizing Axin. Here, we confirm that TNKSi inhibit Wnt-induced transcription, similarly to carnosate which reduces the transcriptional activity of β-catenin by blocking its binding to BCL9, and attenuates intestinal tumors in ApcMin mice. By contrast, β-catenin’s activity is unresponsive to TNKSi in colorectal cancer cells, and in cells after prolonged Wnt stimulation. This TNKSi insensitivity is conferred by β-catenin’s association with LEF1 and BCL9-2/B9L, which accumulate during Wnt stimulation, thereby providing a feed-forward loop that converts transient into chronic β-catenin signaling. This limits the therapeutic value of TNKSi in colorectal carcinomas most of which express high LEF1 levels. Our study provides proof-of-concept that the successful inhibition of oncogenic β-catenin in colorectal cancer requires the targeting of its interaction with LEF1 and/or BCL9/B9L, as exemplified by carnosate.
Keywords: oncogenic β-catenin, APC tumor suppressor, carnosic acid, tankyrase inhibitor, LEF1, BCL9-2/B9L, ApcMin mouse model, colorectal cancer
INTRODUCTION
Wnt/β-catenin signaling plays pivotal roles in animal development and tissue homeostasis, and in human cancer (1). In the absence of Wnts, β-catenin is continually earmarked for proteasomal degradation by the Axin complex: Axin provides scaffolding for glycogen synthase kinase 3 (GSK3) to phosphorylate the N-terminus of β-catenin (after priming by casein kinase 1α, CK1α), thus generating a phospho-degron recognized by the ubiquitin ligase adaptor β-TrCP (2). This process relies on the Adenomatous polyposis coli (APC) tumor suppressor which promotes Axin complex assembly (3), releases phosphorylated-β-catenin (to be called PBC) from the complex (4), and/or promotes PBC recognition by β-TrCP and subsequent ubiquitylation (5). Wnt stimulation blocks the activity of the Axin complex, thereby causing accumulation of unphosphorylated β-catenin (equivalent to activated β-catenin, ABC). ABC thus binds to the TCF/LEF DNA-binding proteins to operate a transcriptional switch, recruiting various chromatin modifiers and remodelers to TCF/LEF target genes (6).
A wide range of cancers exhibit hyperactive β-catenin, either due to oncogenic mutations in its N-terminal phospho-degron, or through mutational inactivation of its negative regulators APC or Axin (1). Similarly, inactivation of Apc, or activation of β-catenin, initiates tumorigenesis in the murine intestine (7, 8), in which the normal crypt stem cell compartment depends on Wnt/β-catenin signaling (1). In mice, β-catenin is continually required for growth and progression of Apc-dependent adenomas and APC-mutant human xenografts (9), and the progressive accumulation of nuclear β-catenin in colorectal carcinomas also implies their continual reliance on oncogenic β-catenin through cancer progression (9, 10).
The case for β-catenin as a target for therapeutic intervention in colorectal cancer is thus overwhelming. However, developing β-catenin inhibitors has proven a considerable challenge (11): β-catenin is an intracellular protein whose oncogenic activity is little affected by upstream Wnt signaling components, and its inhibition therefore requires cell-permeable agents. Furthermore, its activity depends primarily on its binding to TCF/LEF factors through the same extensive molecular interface that also binds its negative regulators Axin and APC (4, 12, 13). Nevertheless, small-molecule antagonists have been reported that target the β-catenin-TCF interaction, or regulators of β-catenin’s activity or stability (Supplementary Figure 1). There has been a recent boom of interest in a highly promising group of compounds that inhibit tankyrase (TNKSi), which destabilize β-catenin by blocking the turnover of Axin (14-18).
We recently identified a natural compound (carnosate, CA) that destabilizes ABC in colorectal cancer cells, apparently by promoting its aggregation through an intrinsically labile α-helix in its N-terminus, which prevents binding to its co-factor BCL9 (19). CA is the only compound known to target ABC directly, and we thus set out to compare its efficacy to that of indirect β-catenin inhibitors. Here, we show that most of these elicit unspecific off-target effects, except for CA and TNKSi which specifically reduce β-catenin-dependent transcription in Wnt-stimulated cells. In APC-mutant colorectal cancer cells, CA proved the most effective β-catenin inhibitor, and it also attenuated the levels and transcriptional outputs of ABC in the murine intestine, and intestinal tumorigenesis in ApcMin mice. In contrast, although TNKSi stabilize Axin and thus reduce ABC to low levels in colorectal cancer cells, they fail to block its transcriptional activity. Notably, in APC-wt cells, β-catenin also becomes TNKSi-unresponsive after pre-stimulation with Wnt3a for 4-6 hours. This TNKSi-insensitivity is conferred by LEF1 and B9L (the nuclear paralog of BCL9, also called BCL9-2 (20, 21)). Both factors are Wnt-inducible, accumulating to high levels in cells with chronic Wnt pathway activity, which enables them to divert β-catenin from the Axin complex. Finally, most colorectal carcinomas express high levels of LEF1, which could render them TNKSi-insensitive. Our results highlight a key requirement for effective β-catenin inhibitors, namely their ability to block β-catenin’s association with LEF1 and B9L – a complex capable of limiting the Axin-dependent inhibition of β-catenin in cells with chronic Wnt/β-catenin pathway activity.
MATERIALS & METHODS
Plasmids, antibodies and chemicals
The following reagents were used: FLAG-β-catenin, TCF1(p45) (provided by H. Clevers, Hubrecht Institute, Utrecht); TOP-GFP/CMV-dsRFP (provided by C. Gottardi, Northwestern University Feinberg School of Medicine, Chicago); FLAG-BCL9, FLAG-BCL9L366K, FLAG-BCL9ΔC, FLAG-BCL9ΔCL366K (22); pcDNA-Myc-TCF4 (23); pcDNAHA-LEF1; SuperTOP (24); dimethyl sulphoxide (DMSO), CA, XAV939, IWR-1, pyrvinium pamoate, iCRT3 (Sigma Pharmaceuticals); PKF115-584 (Enzo Life Sciences); α-β-catenin (BD Transduction Laboratories); α-ABC, α-PBC (phospho-Ser33/37/Thr41), α-LEF1 (C18A7), α-TCF1, α-TCF4 (Cell Signaling); α-BCL9, α-B9L (R&D Systems); α-actin (Abcam); α-FLAG (Sigma-Aldrich). ICG-001 and 16k were synthesized by MRC Technology.
Cell-based assays
Cell lines were purchased from the European Collection of Cell Cultures (HEK293T and HCT-116 in 2007, COLO320 in 2011; SW480 and DLD1 in 2013). RKO cells were kindly provided by Doug Winton (University of Cambridge; in 2012). All cell lines were authenticated by STR DNA profiling. Upon receipt, cells were frozen, and individual aliquots were taken into culture, typically for analysis within <10 passages. For SW480 and COLO320 cells, truncated APC protein was monitored by Western blot analysis (see Results). Cells were grown and transfected for Wnt reporter assays and indirect immunofluorescence as described (22). Cytotoxicity assays were done as described (19). An SW480 cell line with integrated TOP-GFP reporter (25) was isolated by negative selection and cloning of stable transfectants, and GFP was monitored by fluorescence-activated cell sorting (FACS). Standard inhibitor treatment was for 24 hours (2.5 μM XAV939, or 25 μM CA), unless specified otherwise.
qRT-PCR and Western blot analysis
cDNA was synthesized, and qRT-PCR reactions were carried out with the ABI7900 Taqman thermocycler (Applied Biosystems), with primers and gene expression assays for human Wnt target genes (22), and the following murine Wnt target genes (26): Tnfrsf12a, Mm00489103; Tbp1, Mm00446971; Bcl9l, Mm00518807; Axin2, Mm00443610; c-Myc, Mm00487804 (Applied Biosystems). Western blots were done as described (22).
Animal experiments
Animal care and procedures were done according to the standards set by the United Kingdom Home Office. Administration of single doses of CA (dissolved in British Pharmacopoeia compliant olive oil) to C57BL/6J mice by gavage, and preparation of lysates from isolated intestinal epithelia were done essentially as previously described (10, 27). ApcMin/+ control mice were fed AIN-76A, while treatment cohorts were fed AIN-76A pelleted with 0.1% carnosol or CA, or with 1% CA from weaning, as described (28). Weights were checked twice weekly, to monitor growth and food intake. Intestinal tumors were ‘blind’ scored in methacarn-fixed small intestines upon dissection, as described (10, 29). Proliferation and apoptosis was monitored by immunofluorescence using antibodies against Ki67 and cleaved caspase 3 (Asp175 and 8D5, respectively; Cell Signaling).
Tissue microarray (TMA) analysis
TMAs were processed for antibody staining as described (10, 29), except that indirect immunofluorescence was used. Scoring of protein expression levels was done blind (by A. E. K. I., an experienced histopathologist specializing in colorectal cancer), classifying LEF1 staining levels of individual sections as negative (0), weak and patchy (1), moderate and wide-spread (2) or universally strong throughout the core (3), whereby each tissue core was represented by 2-3 non-adjacent cores.
RESULTS
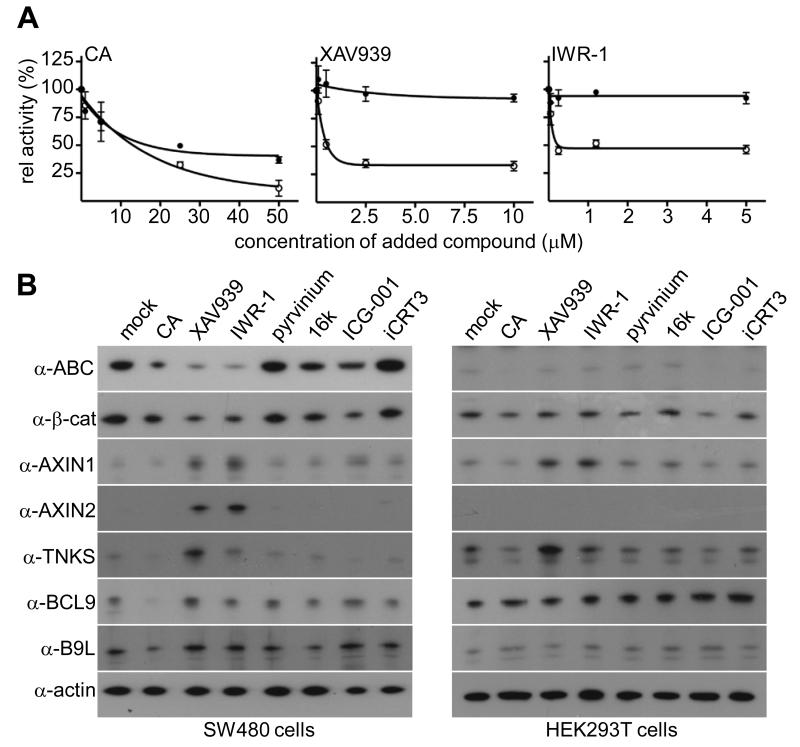
For a side-by-side comparison of previously reported β-catenin antagonists, we conducted functional tests in Wnt3a-stimulated HEK293T cells treated with inhibitors, using a TCF-specific reporter (SuperTOP) as a read-out of β-catenin-dependent transcription. The TNKSi XAV939 (15) and IWR-1 (14) inhibited SuperTOP (IC50 0.3 and 0.1 μM, respectively) more potently than CA (IC50 7 μM; Figure 1A). However, the other compounds that reduced SuperTOP (e.g. pyrvinium and ICG-001) also reduced the internal (CMV-based) control reporter and elicited pronounced cell toxicity at their IC50, indicating significant off-target effects (Supplementary Figure 2).
Figure 1. Responses to different β-catenin antagonists.
(A) SuperTOP assays in Wnt3a-stimulated HEK293T (○) or SW480 cells (●) treated with inhibitor concentrations as indicated; values are shown as % of mock-treated controls; error bars, SEM (in all figures unless otherwise specified). (B) Western blots of lysates from SW480 or HEK293T cells (as indicated), treated with inhibitors for 24 hours (25 μM CA or iCRT3, 5 μM IWR-1 or ICG-001, 2.5 μM XAV939, 1 μM 16k, 25 nM pyrvinium).
We further tested these agonists in SW480 colorectal cancer cells which express an APC truncation lacking its Axin binding sites (30), and thus accumulate high levels of ABC, as detectable by an antibody specific for this unphosphorylated form (31). Again, most inhibitors showed high cell toxicity and unspecific side-effects (Supplementary Figure 2). Of the non-toxic compounds, CA reduced SuperTOP to 40% of mock-treated SW480 cells (19), however TNKSi had very little effect (Figure 1A), even in combination with CA (Supplementary Figure 3). Notably, this was true for both XAV939 and IWR-1, which represent different classes of TNKSi (binding to the nicotinamide and adenosine pocket of TNKS, respectively; (17)), arguing that the inability of these inhibitors to reduce the β-catenin-dependent transcriptional in these cells is not limited to a single TNKSi class. We also tested TNKSi on DLD1 cells (another APC-mutant colorectal cancer cell line commonly used, e.g. (15)), which were only marginally more TNKSi-responsive than SW480 cells (Supplementary Figure 3). Our data are consistent with previous reports that TNKSi are more potent in Wnt-stimulated compared to APC-mutant cells (14-16).
TNKSi reduce the levels but not the activity of ABC in APC-mutant colorectal cancer cells
We confirmed that CA reduces ABC levels in SW480 cells (19) (Figure 1B), explaining why it attenuates SuperTOP (see Figure 1A) and expression of endogenous AXIN2 (Figure 1B), a well-established β-catenin target gene (32). TNKSi had an even more profound effect, reducing the levels of total β-catenin, and of ABC, to <10% of mock-treated controls (Figure 1B). In contrast, the PBC levels remained high, and were even slightly increased (Supplementary Figure 3), supporting the notion that TNKSi deplete ABC by promoting its phosphorylation. Since PBC is the substrate for β-TrCP recognition and subsequent degradation (see Introduction), this explains why TNKSi reduce total β-catenin through stabilizing Axin, as previously shown (15): it is well known that overexpressed Axin promotes β-catenin degradation in SW480 cells, despite their dysfunctional APC (e.g. (3, 33)).
We also assessed the levels of β-catenin and its regulators in APC-wt cells after inhibitor treatment – namely in Wnt-stimulated HEK293T cells (Figure 1B), and in the colorectal cancer cell lines HCT116 (whose ABC is high, due to a mutation in the CK1α phosphorylation site) and RKO (whose ABC is undetectable since its Wnt pathway is inactive) (Supplementary Figure 3). XAV939 increased the levels of AXIN1 and tankyrase in these cells, but the levels of total β-catenin and ABC were essentially unaffected.
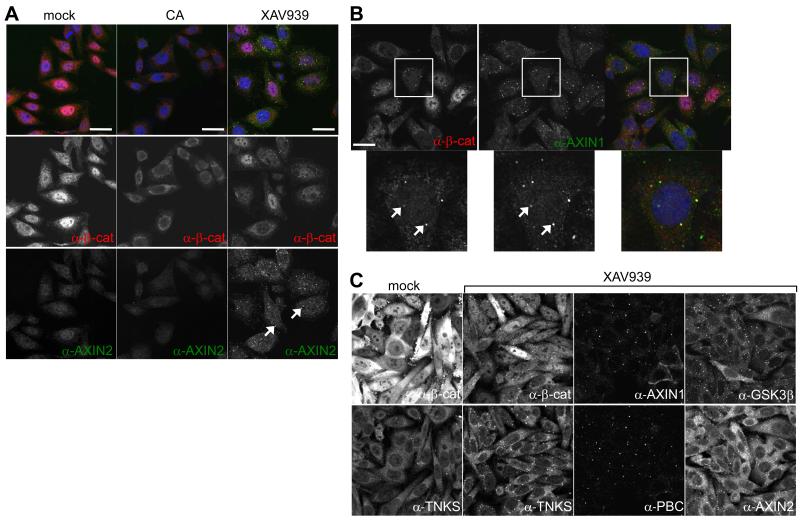
ABC is destabilized by Axin degradasomes in TNKSi-treated SW480 cells
Immunofluorescence confirmed that overall β-catenin staining was reduced in TNKSi-treated SW480 cells, consistent with our Western blots (see Figure 1B), though many cells retained substantial levels of nuclear β-catenin (Figure 2A, B), which could account for their sustained β-catenin-dependent transcription. In contrast, the nuclear β-catenin staining was reduced in CA-treated SW480 cells, which also showed less AXIN2 staining (19) (Figure 2A), reflecting reduced AXIN2 expression. Thus, the nuclear pool of β-catenin seems depleted by CA but less so by TNKSi.
Figure 2. Axin degradasomes in TNKSi-treated colorectal cancer cells.
(A, B) Confocal sections through inhibitor-treated SW480 cells, co-stained with antibodies as indicated; arrows, degradasomes containing Axin (green in merges) and β-catenin (red in merges), magnified in B; blue, 4′,6-diamidino-2-phenylindole (DAPI). (C) Confocal sections through XAV939-treated SW480 cells, stained with antibodies as indicated. Size bars, 10 μM.
We noticed discrete cytoplasmic puncta of β-catenin in TNKSi-treated SW480 cells (Figure 2B, arrows), which are neither visible in CA-treated nor in control cells. These puncta also contain Axin, and GSK3β, tankyrase (Figure 2) and APC (see below). Given that they also contain PBC (Figure 2C), they are likely to represent functional Axin degradasomes (3) that promote the phosphorylation and subsequent degradation of β-catenin. TNKSi-induced Axin degradasomes do not contain other Axin- or APC-interacting proteins such as phosphorylated LRP6 (signifying activated Wnt co-receptor (2)), nor markers for endosomes or autophagosomes (Supplementary Figure 4).
Axin degradasomes have been observed following Axin overexpression (e.g. (3, 33)), but endogenous Axin degradasomes are neither detectable in untreated SW480 cells (Figure 2A, C) nor in APC-wt cells (Supplementary Figure 4), probably because the endogenous Axin levels are low in mammalian cells (34). TNKSi thus enabled us for the first time to observe endogenous Axin degradasomes, likely because Axin is stabilized (AXIN1 3-5x, AXIN2 5-20x; Figure 1B).
We also examined COLO320 cells which express a rare APC truncation without any β-catenin and Axin binding sites (30). These cells also exhibit Axin puncta which are however negative for APC, as expected. In contrast to SW480 cells, TNKSi-treated COLO320 cells did not show reduced ABC levels (unlike CA-treated cells) but, instead, vastly increased PBC levels (Supplementary Figure S5). This indicates that the Axin degradasomes in these cells actively promote β-catenin phosphorylation (consistent with their PBC-reactivity; Supplementary Figure 5), in other words, they are fully functional with regard to scaffolding of GSK3. However, they seem unable to promote the ubiquitylation and/or proteasomal degradation of PBC, likely due to the complete lack of interaction between APC and β-catenin. They thus appear to be stalled degradasomes.
ABC activity in SW480 cells remains refractory to TNKSi even during prolonged treatment
Our immunofluorescence indicated persistence of the nuclear β-catenin pool in TNKSi-treated SW480 cells through the 24-hour treatment. We thus extended the treatment to 5 days (replenishing XAV939 daily), but found that the effects of TNKSi plateaued within 2 days, with the levels of total β-catenin and ABC no longer reducing, and those of tankyrase and Axin no longer increasing (Supplementary Figure 6). Indeed, all TNKSi-induced changes in the levels and subcellular distributions of these proteins were observed after the first day of treatment, and persisted thereafter.
We also monitored the effects of TNKSi on β-catenin-dependent transcription over a 5-day treatment, using an integrated TCF reporter based on destabilized eGFP (enhanced green fluorescent protein) (25). SW480 cells remained unresponsive to XAV939 over 3 days, while CA reduced reporter activity after the first day, and further still by the third day of treatment. Likewise, CA reduced AXIN2 and B9L expression within 24 hours to ~20% and ~45%, respectively (19), whereas TNKSi only modestly reduced the expression of these target genes (to 75-90%), even after 5 days (Supplementary Figure 6). Thus, β-catenin remains transcriptionally active in TNKSi-treated SW480 cells during extended treatment – despite the TNKSi-induced depletion of their ABC.
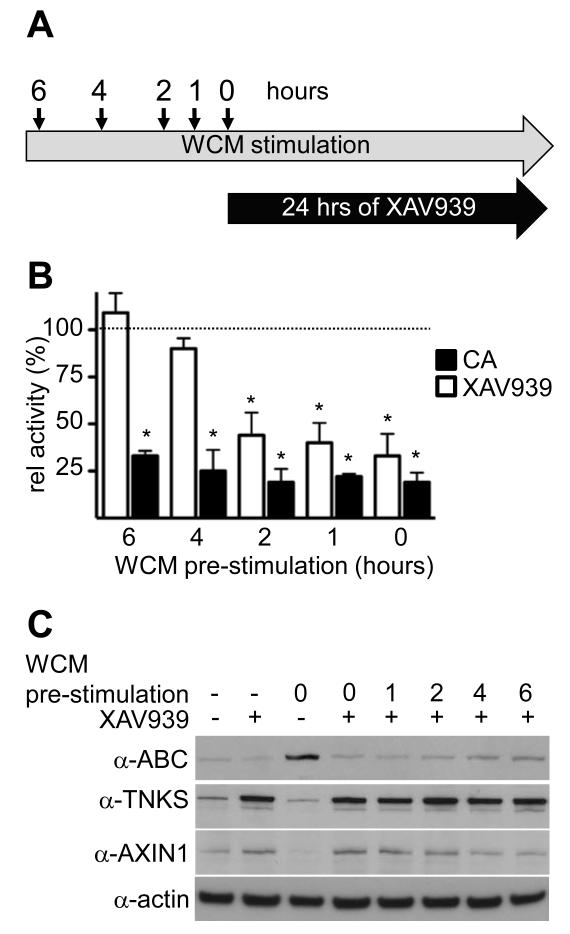
Prolonged Wnt stimulation renders β-catenin activity unresponsive to TNKSi
We asked whether β-catenin activity would also become refractory to TNKSi in APC-wt cells after prolonged Wnt stimulation. We thus stimulated HEK293T cells with Wnt3a for various periods before TI treatment (Figure 3A), and monitored their TCF-dependent transcription. As expected, SuperTOP activity was much reduced if the cells were exposed simultaneously to TNKSi and Wnt3a, but became increasingly TNKSi-insensitive with longer Wnt pre-stimulation, and was completely refractory 6 hours post-Wnt3a stimulation (Figure 3B), accompanied by a slight progressive increase of ABC and decrease of AXIN1 (Figure 3C). The same was also seen in other APC-wt cell lines such as HeLa (Supplementary Figure 7). In contrast, HEK293T cells remained fully CA-responsive, even after 6 hours of Wnt pre-stimulation (Figure 3B). Therefore, 4-6 hours of Wnt stimulation of APC-wt cells suffices to render their β-catenin activity refractory to TNKSi, mimicking the situation in APC-mutant cells.
Figure 3. Prolonged Wnt stimulation renders cells unresponsive to TNKSi.
(A) Wnt pre-treatment regime. (B) SuperTOP assays of HEK293T cells treated as outlined in (A); shown are relative activities (in %) of WCM (Wnt3a-conditioned medium)-treated controls (dotted line); P < 0.001 (*). (C) Corresponding Western blots.
LEF1- and B9L-associated β-catenin is protected from TNKSi-induced Axin degradasomes
β-catenin equilibrates rapidly between nucleus and cytoplasm (35, 36), and it is therefore unlikely that the observed TNKSi-insensitivity of the transcriptionally active β-catenin in chronically Wnt-stimulated cells is due to its insulation from the cytoplasmic pool. Indeed, we estimate that the nuclear β-catenin in unstimulated HEK293T cells turns over with a t1/2 of ~60 minutes (Supplementary Figure 6). We therefore surmised that transcriptionally active β-catenin is shielded by a factor that limits its access to Axin degradasomes. Since 6 hours of Wnt stimulation suffices to render this pool refractory to TNKSi, we further surmised that this factor would accumulate during this period, and that it would bind to β-catenin in competition with Axin. This identifies BCL9/B9L and TCF/LEF factors as potential candidates.
Examining the expression levels of these candidates in Wnt-stimulated HEK293T and HeLa cells, we found that only LEF1 and B9L are Wnt-inducible (Figure 4A). Furthermore, amongst four tested colorectal cancer cell lines with chronic Wnt pathway activity, each expressed high levels of at least one TCF/LEF and one BCL9/B9L family member, with SW480 cells expressing high levels of both LEF1 and B9L (Figure 4A). Importantly, co-immunoprecipitation revealed that TNKSi treatment reduced the β-catenin associated with TCF factors and BCL9, but not with LEF1 and B9L (Figure 4B). Thus, the LEF1- and B9L-associated β-catenin is protected from TNKSi-induced degradation in SW480 cells.
Figure 4. Overexpressed LEF1 and B9L attenuate the TNKSi response of β-catenin.
(A) Western blots of lysates from various cells as indicated above panel (+/− 24 hours WCM), probed with antibodies as indicated (arrowheads point to long and short isoforms of TCF4). (B) Western blots of lysates from inhibitor-treated SW480 cells, probed for proteins co-immunoprecipitating with β-catenin; inputs on the left. (C) SuperTOP assays of HEK293T cells, transfected for 24 hours to express proteins indicated below corresponding Western blot, treated as indicated below panel; relative activities were normalized (in %) to WCM-treated controls (dotted line).
LEF1 and B9L confer TNKSi insensitivity on β-catenin in cells with chronic Wnt pathway activity
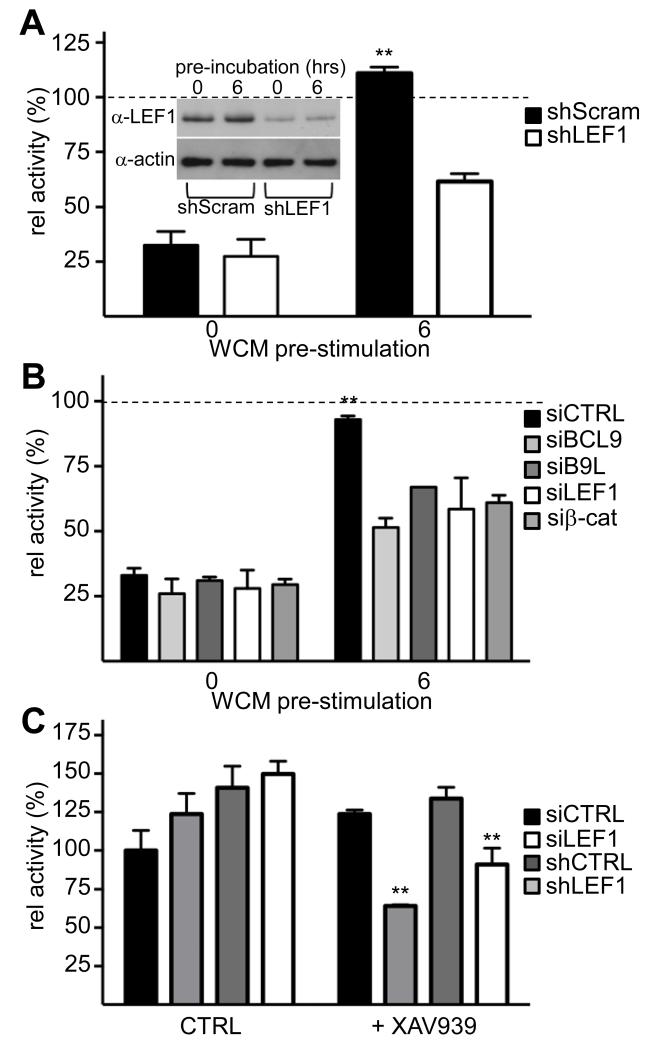
To test the ability of our candidates to confer TNKSi insensitivity on β-catenin, we overexpressed them at moderate levels in HEK293T cells (<5x over endogenous; Figure 4C) prior to stimulation with Wnt3a. Overexpression of B9L, TCF1, LEF and β-catenin increased SuperTOP activity in unstimulated and Wnt3a-treated cells (Supplementary Figure 8), but this activity was strongly reduced if the cells were simultaneously treated with TNKSi (as shown in Figure 3B), even in cells overexpressing BCL9, TCF4 or TCF1 (Figure 4C). By contrast, ~30% of the SuperTOP activity was retained in cells overexpressing B9L, but not in cells expressing a B9L mutant unable to bind β-catenin (22). Strikingly, cells overexpressing LEF1 retained >95% of their SuperTOP activity despite simultaneous exposure to TNKSi and Wnt3a (Figure 4C). Thus, LEF1 renders β-catenin completely unresponsive to TNKSi in HEK293T cells, even at moderate overexpression levels (~4x above normal) comparable to endogenous LEF1 in SW480 cells (Figure 4A).
As a further test, we asked whether reducing LEF1 levels would restore TNKSi-responsiveness in HEK293T cells pre-stimulated with Wnt3a for 6 hours. We used two different LEF1 sequences (an exon 1-specific siRNA and an exon 5-specific shRNA) both of which depleted LEF1 2-3x (Supplementary Figure 8), approximately to the levels of uninduced cells (Figure 5A). As shown in Figure 3B, pre-stimulation with Wnt3a for 6 hours rendered SuperTOP in mock-depleted HEK293T cells refractory to TNKSi, in contrast to LEF1-depleted cells which recovered a near-complete TNKSi response (Figure 5A, B). Interestingly, depletion of BCL9 and B9L also restored TNKSi sensitivity under these conditions (Figure 5B) whereas depletion of TCF1 and of TCF4 did not.
Figure 5. LEF1 depletion renders β-catenin sensitive to TNKSi.
(A, B) SuperTOP assays of HEK293T cells, treated as in Figure 3A, following transfection for 24 hours with (A) shLEF1 or shScram (control) for 24 hours (inset, corresponding Western blot), or (B) siRNAs as indicated. (C) SW480 cells treated with XAV939, following LEF1 depletion as in (A, B) as indicated; values were normalized to mock-treated siCTRL.
Finally, we tested whether LEF1 depletion in SW480 cells would render its ABC TNKSi-responsive. This was the case: LEF1 depletion reduced β-catenin-dependent transcription in TNKSi-treated SW480 cells to 50-60% compared to mock-depleted cells (Figure 5C). We conclude that their elevated LEF1 is a crucial determinant of β-catenin’s TNKSi insensitivity in these APC-mutant colorectal cancer cells.
CA reduces ABC in the normal murine intestine and the tumor numbers of ApcMin mice
Given the poor TNKSi response of cells with chronic Wnt pathway activity, there was little incentive for testing TNKSi in tissues with sustained Wnt signaling, e.g. normal intestinal crypts or Apc-mutant intestinal tumors (see Introduction). Intrinsic in vivo toxicity of some TNKS inhibitors (e.g. (16); see also Supplementary Figure S2) further argues against their use on animals. However, we decided to test CA in the ApcMin model, given its inhibitory activity in COLO320 cells whose APC mutation resembles that of the ApcMin mutation (truncating all β-catenin and Axin binding sites). Moreover, it was previously shown that orally administered CA spreads rapidly to various murine tissues including the brain, where it exhibits bio-activity (27).
We thus adopted this experimental regime (27), administering a single dose of 1 mg CA to individual mice, and monitored the transcript levels of different β-catenin target genes (22, 26) in cell lysates from intestinal epithelial preparations (Supplementary Figure 9). Indeed, the Bcl9l and Tnfrsf12a transcripts were reduced significantly throughout the monitoring period; the Axin2 transcripts were initially reduced (up to 4 hours post-carnosate), but recovered subsequently (Figure 6A). We could not detect a statistically significant effect on c-Myc transcripts, possibly because the β-catenin-dependent modulation of c-Myc transcription is subtle (37). As expected, the ABC levels were also reduced upon CA treatment, while the total β-catenin levels were not affected significantly (Figure 6B; Supplementary Figure 9), consistent with the CA effects in cell culture (19).
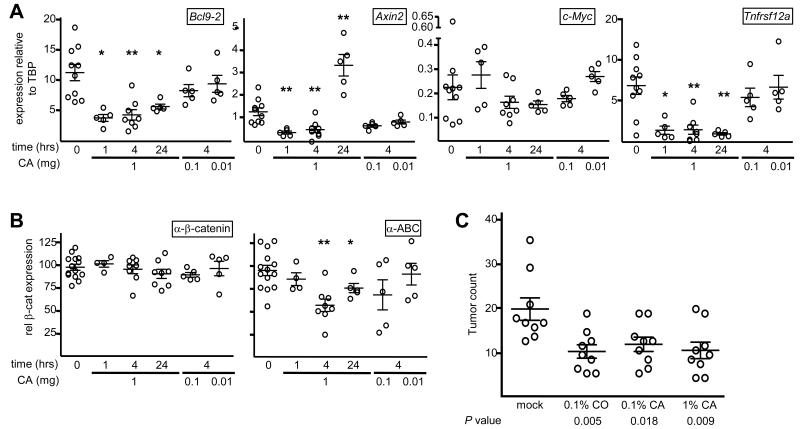
Figure 6. CA reduces β-catenin levels and outputs in the normal and neoplastic mouse intestine.
(A) RT-qPCR of transcripts in lysates from murine intestinal preparations at various times post CA administration, as indicated below panels; each symbol refers to one animal (from cohorts of 4-5 mice), from 3 independent experiments (see Supplementary Figure 9; control values are from experiments II and III); statistical significance, P < 0.025 (*) or < 0.0025 (**). (B) Quantification of Western blots (Supplementary Figure 9) by densitometry of intestinal lysates obtained as in (A); symbols and statistical significance as in (A). (C) Numbers of intestinal tumors in 105-day old ApcMin mice fed with control or supplemented diet; P values (from t tests) are relative to controls.
We also tested whether CA attenuates intestinal tumorigenesis in ApcMin mice which develop multiple intestinal neoplasms, driven by β-catenin activation following sporadic Apc loss (7). Of note, a previous study showed that carnosol, a close chemical relative of CA, reduced the tumor burden of ApcMin mice if administered in their diet (28). We thus adopted the same experimental design, administering 0.1% carnosol to ApcMin mice in their diet, or 0.1% or 1% CA (since CA is less toxic than carnosol (19)). At 105 days, control mice showed 19 ± 7.5 tumors, while all three treatment cohorts had significantly reduced tumor numbers (Figure 6C). The tumor volume was also reduced ~2x in the treatments groups compared to the control (Supplementary Figure 9). Thus, CA is as effective as carnosol in attenuating intestinal tumorigenesis in this mouse model.
LEF1 overexpression is prevalent in colorectal carcinomas
A recent analysis of LEF1 expression in colorectal carcinomas, based on immunohistochemistry, concluded that LEF1 protein is detectable only in 26% of carcinomas (38). Accordingly, most carcinomas would therefore be potentially responsive to TNKSi. However, these results contrasted with those from an earlier analysis, demonstrating that LEF1 transcripts are highly abundant in colorectal cancer cell lines and carcinomas (39). Indeed, evidence from murine models and human cancers indicates a key role of LEF1 during cancer progression (6).
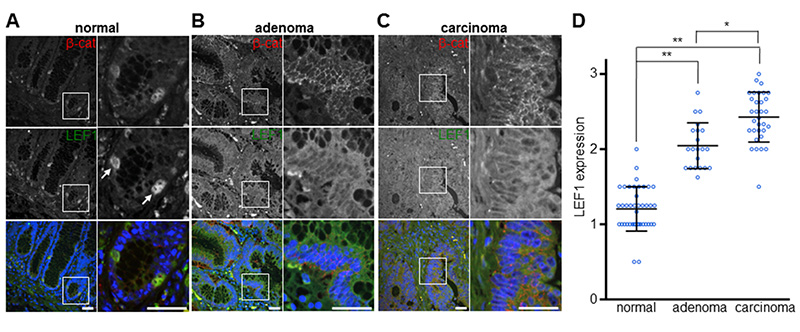
To resolve this controversy, and to examine the potential therapeutic value of TNKSi in colorectal cancer, we decided to re-examine LEF1 expression in tissue specimens from cancer patients. We thus screened a TMA containing tissue cores from normal colonic mucosa, adenomatous polyps and colon carcinomas by immunofluorescence, and using a different LEF1 antibody, the combination of which improved the sensitivity of endogenous LEF1 detection considerably (Supplementary Figure 10). Co-staining this TMA for LEF1 and β-catenin, we found that most epithelial cells of the normal mucosa were only weakly positive for both proteins, except for a small number of cells near the bottom of crypts which exhibit high levels of LEF1 and β-catenin, coinciding in each case (Figure 7A) – likely marking LGR5-positive intestinal progenitor cells (40). However, virtually all cores from adenomas (n=21) show elevated levels of both proteins, and carcinomas (n=32) showed even higher levels of LEF1 and nuclear β-catenin throughout, in each case strikingly co-inciding at the cellular level (Figure 7B, C). Semi-quantitative analysis of the immunofluorescence signal intensity of each core indicates higher levels of LEF1 in carcinomas compared to adenomas (Figure 7D), correlating with nuclear β-catenin (r = 0.78, P < 0.0001, Pearson correlation test) whose levels also increase from adenoma to carcinoma (9, 10). Our data are fully consistent with the RNA expression data (39), showing that high levels of LEF1 expression are prevalent in colorectal carcinomas.
Figure 7. Overexpression of LEF1 is prevalent and progressive in colorectal cancer.
(A-C) Immunofluorescence of representative tissue cores from normal mucosa (A), adenomas (B) and carcinomas (C), co-stained for β-catenin (red), LEF1 (green) and DAPI (blue in merge, to mark nuclei), as indicated in panels; magnifications of boxed areas are on the right. Arrows in (A) indicate putative crypt progenitor cells; size bars, 25 μM. (D) Boxplots of the TMA scoring results, indicating LEF1 expression levels (see Materials & Methods); statistical significance P < 0.001 (*) or < 0.0001 (**) (Wilcoxon rank sum tests).
DISCUSSION
While comparing the potencies of recently identified β-catenin inhibitors in cell-based assays, we encountered substantial cell toxicity and unspecific off-target effects at their IC50 for most of them, whose inhibitory activity towards β-catenin is therefore unlikely to be specific. However, CA and TNKSi behaved as specific inhibitors of β-catenin in our hands, reducing its transcriptional activity in Wnt-stimulated cells. They also destabilized ABC in APC-mutant colorectal cancer cells, but despite this, the activity of β-catenin in these cells was barely responsive to TNKSi. Importantly, β-catenin activity also became refractory to TNKSi in APC-wt cells following Wnt stimulation for 4-6 hours. We presented evidence that this TNKSi insensitivity in cells with chronic Wnt pathway activity is conferred predominantly by high levels of LEF1 and, to a lesser degree, of B9L – both products of Wnt target genes which accumulate in these cells. Our data imply that LEF1 and B9L cooperate to lock a transient burst of β-catenin-dependent signaling into a stable state of chronic Wnt/β-catenin pathway activity.
High LEF1 and B9L levels divert β-catenin from TNKSi-induced Axin degradasomes
Our experimental evidence, based on pre-expressing or depleting TCF/LEF and B9L/BCL9 factors, indicates that the TNKSi insensitivity of β-catenin in cells with chronic Wnt pathway activity is determined primarily by high levels of LEF1 and, to a lesser degree, of B9L. Both factors are unique amongst their family members in that they are Wnt-inducible and thus tend to accumulate in cells with chronic Wnt pathway activity. They also show a marked tendency to be overexpressed in colorectal carcinomas (20) (Figure 7) and in colorectal cancer cell lines (Figure 4A) albeit to varying degrees. It is possible that the observed TNKSi insensitivity of β-catenin in these cell lines is due to the cumulative expression levels of all their LEF/TCF and BCL9/B9L family members (some of which can also be overexpressed in carcinomas, e.g. BCL9 (41)).
How do LEF/TCF and BCL9/B9L factors protect the activity of ABC despite its continued conversion to PBC by the Axin complex and its consequent degradation? It seems likely that this is due to direct competitive binding: TCF/LEF factors exhibit a 20-50x higher affinity for β-catenin than (unphosphorylated) Axin and APC (42), and thus have a competitive advantage over the latter in binding to β-catenin, as previously shown (43). This advantage could be increased considerably in the ternary complex with BCL9/B9L: TCF4 and BCL9 can bind simultaneously to β-catenin, together occupying a surface on β-catenin (44) larger than that occupied by Axin or APC (4, 13), and so the combined affinity of β-catenin for LEF and B9L is likely to exceed its affinity to the Axin complex by at least two orders of magnitude. We thus propose that high levels of LEF1 and B9L bind to and divert a significant fraction of the de novo synthesized β-catenin to the nucleus, prior to its access to Axin, thereby creating a continuous pipeline that fuels the pool of transcriptionally active β-catenin.
LEF1 and B9L, despite being predominantly nuclear at steady-state (21, 45), are likely to shuttle rapidly in and out of the nucleus, like β-catenin itself (35, 36) and, on overexpression, shift cytoplasmic β-catenin into the nucleus (21, 45). Furthermore, these factors are partially cytoplasmic in APC-mutant cancer cell lines and in colorectal carcinomas (Figure 7). It is therefore plausible that they can access de novo synthesized β-catenin in the cytoplasm, in competition with Axin. Of note, the nuclear export function of APC is disabled by most APC truncations found in colorectal cancer cell lines and carcinomas (46) and, therefore, the APC-mediated conveyance of nuclear β-catenin to the cytoplasmic Axin complex is attenuated in these APC-mutant cells.
Is LEF1 unique amongst TCF factors in conferring TI insensitivity on β-catenin? Although this has not been assessed side-by-side, it appears that LEF1 has a slightly higher binding affinity to β-catenin than TCF4 (42, 47). Furthermore, LEF1 exhibits the same key (aspartic acid to glutamic acid) substitution as TCF3 that allows formation of a hairpin in its β-catenin-binding domain, thereby increasing its interface with β-catenin (12). Indeed, TCF3 was found to shield β-catenin from Axin by direct competition for binding in early Xenopus embryos (48). However, neither TCF3 nor other TCFs are likely candidates for conferring TNKSi insensitivity on β-catenin in cells with chronic Wnt pathway activity since (i) none of them accumulate in response to Wnt stimulation in APC-wt cells (Figure 4A), (ii) neither TCF1 nor TCF4 protect β-catenin from Axin-dependent degradation in cells with chronic Wnt pathway activity (Figure 4B, C), and (iii) TCF3 is not expressed in any of the APC-mutant cells we tested (Figure 4A).
TNKSi-induced destabilization of β-catenin occurs downstream of its nuclear conveyance by LEF1 and B9L
CA and TNKSi both destabilize ABC in colorectal cancer cells – TNKSi considerably more so than CA – but they achieve this by distinct mechanisms. TNKSi increase Axin degradasome activity, thus depleting ABC by converting it to PBC, the substrate for β-TrCP recognition and proteasomal degradation. In contrast, CA promotes selectively the proteasomal degradation of ABC (19), without affecting the levels of PBC or total β-catenin (Figure 1B), suggesting that this route of ABC destabilization does not involve Axin. Importantly, only the CA- but not the TNKSi-induced destabilization of ABC proved effective in reducing its transcriptional activity.
The likely reason for this is that CA blocks the binding of BCL9/B9L to ABC, apparently by altering the conformation of a structurally labile N-terminal α-helix of β-catenin (abutting its BCL9-binding site); this α-helix constitutes an ‘Achilles Heel’ which renders β-catenin aggregation-prone when disordered (19). Therefore, CA acts upstream of, or in parallel to, the nuclear conveyance of ABC by LEF1 and B9L. In contrast, the TNKSi-induced destabilization of ABC appears to occur downstream of this conveyance, following β-catenin’s nuclear exit.
CA attenuates β-catenin activity and intestinal tumorigenesis in mice
We have shown inhibitory effects of CA on transcriptional outputs of β-catenin in the normal intestine, and on intestinal tumorigenesis, confirming its bio-activity in murine tissues (27). The tumor-attenuating effects of CA in the ApcMin model are relatively modest, but they are equivalent to those of its chemical relative, carnosol (28). The latter have been attributed to reduced phosphorylation of tyrosine 142 within β-catenin’s ‘Achilles Heel’ ((28); see also (19)), broadly consistent with our own evidence that CA acts through this structurally labile α-helix of β-catenin to interfere with its binding to BCL9 (19). Targeting this interaction thus appears a promising strategy for developing inhibitors of β-catenin-driven intestinal neoplasia.
Limited application of TNKSi in β-catenin-dependent neoplasia
Our study confirms that TNKSi are highly effective in blocking β-catenin-dependent transcription in transiently Wnt-stimulated cells. However, the latter become refractory to TNKSi after 4-6 hours of pre-stimulation with Wnt, once LEF1 has accumulated sufficiently. Intriguingly, a similar lag period of ~4 hours following Wnt stimulation was observed before the Axin complex plateaued and stabilized at its inhibited state (49). It thus appears that the activity of the Axin complex is only susceptible to perturbations, such as TNKSi-induced Axin levels, during this initial period of re-equilibration after Wnt stimulation.
Our data imply that TNKSi are only effective in blocking β-catenin activity in tissues that experience transient bursts of Wnt signaling, and/or express low levels of LEF1 and B9L. They suggest that the combined levels of LEF1 and B9L overexpression in normal and cancerous tissues determine the TNKSi responsiveness of their β-catenin. However, most colorectal carcinomas express high levels of both proteins (20, 41) (Figure 7), which are expected to render their oncogenic β-catenin unresponsive to TNKSi. Therefore, the therapeutic value of TNKSi in colorectal cancer is somewhat limited, and crucially depends on identifying those carcinomas with low levels of these protective factors (e.g. those resembling DLD1 cells which exhibit a partial response to TNKSi).
Implications for targeting oncogenic β-catenin
Our study provides a proof-of-concept that oncogenic β-catenin can be targeted directly by a small inhibitory molecule not just in cell assays (19), but also in an animal model. Importantly, we have shown that merely destabilizing oncogenic β-catenin is not sufficient for inhibiting its activity. Our study highlights the importance of targeting the transcriptionally active β-catenin directly or its interface with LEF1, and/or with BCL9/B9L, as successfully achieved recently (50). These insights should guide the future development or application of small-molecule inhibitors of oncogenic β-catenin.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Cara Gottardi and Hans Clevers for plasmids, Andrew Merritt and his staff at MRC Technology for chemical synthesis, and Tracey Butcher and her staff for help with the animal experiments. This work was supported by the Medical Research Council (U105192713) and by Cancer Research UK (grant C7379/A8709 to M.B., and Clinical Fellowship to A.E.K.I.). The Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre.
Footnotes
Conflict of interest: None declared
REFERENCES
- 1.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza-Topaz C, Mieszczanek J, Bienz M. The Adenomatous polyposis coli tumour suppressor is essential for Axin complex assembly and function and opposes Axin’s interaction with Dishevelled. Open Biol. 2011;1:110013. doi: 10.1098/rsob.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol Cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, et al. APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol Cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 7.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 8.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholer-Dahirel A, Schlabach MR, Loo A, Bagdasarian L, Meyer R, Guo R, et al. Maintenance of adenomatous polyposis coli (APC)-mutant colorectal cancer is dependent on Wnt/β-catenin signaling. Proc Natl Acad Sci USA. 2011;108:17135–17140. doi: 10.1073/pnas.1104182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe C, Ibrahim AE, Graeb M, de la Roche M, Schwarz-Romond T, Fiedler M, et al. Dvl2 promotes intestinal length and neoplasia in the ApcMin mouse model for colorectal cancer. Cancer Res. 2010;70:6629–6638. doi: 10.1158/0008-5472.CAN-10-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31:2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a β-catenin/Tcf complex. Cell. 2000;103:885–896. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 13.Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a β-catenin/axin complex suggests a mechanism for the β-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 16.Lau T, Chan E, Callow M, Waaler J, Boggs J, Blake RA, et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 17.Lehtio L, Chi NW, Krauss S. Tankyrases as Drug Targets. FEBS J. 2013;280:3576–3593. doi: 10.1111/febs.12320. [DOI] [PubMed] [Google Scholar]
- 18.Shultz MD, Cheung AK, Kirby CA, Firestone B, Fan J, Chen CH, et al. Identification of NVP-TNKS656: the use of structure-efficiency relationships to generate a highly potent, selective, and orally active tankyrase inhibitor. J Med Chem. 2013;56:6495–6511. doi: 10.1021/jm400807n. [DOI] [PubMed] [Google Scholar]
- 19.de la Roche M, Rutherford TJ, Gupta D, Veprintsev DB, Saxty B, Freund SM, et al. An intrinsically labile α-helix abutting the BCL9-binding site of β-catenin is required for its inhibition by carnosic acid. Nat Commun. 2012;3:680. doi: 10.1038/ncomms1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi S, Jigami T, Yasui T, Nakano T, Ohwada S, Omori Y, et al. Role of a BCL9-related β-catenin-binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res. 2004;64:8496–8501. doi: 10.1158/0008-5472.CAN-04-2254. [DOI] [PubMed] [Google Scholar]
- 21.Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between β-catenin’s adhesive and transcriptional functions. Genes Dev. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Roche M, Worm J, Bienz M. The function of BCL9 in Wnt/β-catenin signaling and colorectal cancer cells. BMC Cancer. 2008;8:199. doi: 10.1186/1471-2407-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, et al. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 24.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 25.Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the β-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 27.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits β-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 2005;65:1097–1104. [PubMed] [Google Scholar]
- 29.Sansom OJ, Berger J, Bishop SM, Hendrich B, Bird A, Clarke AR. Deficiency of Mbd2 suppresses intestinal tumorigenesis. Nat Genet. 2003;34:145–147. doi: 10.1038/ng1155. [DOI] [PubMed] [Google Scholar]
- 30.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, et al. APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”. Proc Natl Acad Sci USA. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated β-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faux MC, Coates JL, Catimel B, Cody S, Clayton AH, Layton MJ, et al. Recruitment of adenomatous polyposis coli and β-catenin to axin-puncta. Oncogene. 2008;27:5808–5820. doi: 10.1038/onc.2008.205. [DOI] [PubMed] [Google Scholar]
- 34.Tan CW, Gardiner BS, Hirokawa Y, Layton MJ, Smith DW, Burgess AW. Wnt signalling pathway parameters for mammalian cells. PLoS One. 2012;7:e31882. doi: 10.1371/journal.pone.0031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 36.Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J Cell Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- 37.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 38.Kriegl L, Horst D, Reiche JA, Engel J, Kirchner T, Jung A. LEF-1 and TCF4 expression correlate inversely with survival in colorectal cancer. J Transl Med. 2010;8:123. doi: 10.1186/1479-5876-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, et al. β-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 40.Fan XS, Wu HY, Yu HP, Zhou Q, Zhang YF, Huang Q. Expression of Lgr5 in human colorectal carcinogenesis and its potential correlation with β-catenin. Int J Colorectal Dis. 2010;25:583–590. doi: 10.1007/s00384-010-0903-z. [DOI] [PubMed] [Google Scholar]
- 41.Mani M, Carrasco DE, Zhang Y, Takada K, Gatt ME, Dutta-Simmons J, et al. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009;69:7577–7586. doi: 10.1158/0008-5472.CAN-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi HJ, Huber AH, Weis WI. Thermodynamics of β-catenin-ligand interactions: the roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem. 2006;281:1027–1038. doi: 10.1074/jbc.M511338200. [DOI] [PubMed] [Google Scholar]
- 43.von Kries JP, Winbeck G, Asbrand C, Schwarz-Romond T, Sochnikova N, Dell’Oro A, et al. Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat Struct Biol. 2000;7:800–807. doi: 10.1038/79039. [DOI] [PubMed] [Google Scholar]
- 44.Sampietro J, Dahlberg CL, Cho US, Hinds TR, Kimelman D, Xu W. Crystal structure of a β-catenin/BCL9/Tcf4 complex. Mol Cell. 2006;24:293–300. doi: 10.1016/j.molcel.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 46.Rosin-Arbesfeld R, Cliffe A, Brabletz T, Bienz M. Nuclear export of the APC tumour suppressor controls β-catenin function in transcription. EMBO J. 2003;22:1101–1113. doi: 10.1093/emboj/cdg105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knapp S, Zamai M, Volpi D, Nardese V, Avanzi N, Breton J, et al. Thermodynamics of the high-affinity interaction of TCF4 with β-catenin. J Mol Biol. 2001;306:1179–1189. doi: 10.1006/jmbi.2001.4463. [DOI] [PubMed] [Google Scholar]
- 48.Lee E, Salic A, Kirschner MW. Physiological regulation of β-catenin stability by Tcf3 and CK1ε. J Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernandez AR, Klein AM, Kirschner MW. Kinetic responses of β-catenin specify the sites of Wnt control. Science. 2012;338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 50.Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, et al. Targeted disruption of the BCL9/β-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra17. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.