Abstract
Prior studies evaluating metabolic syndrome (MetS) and incident peripheral artery disease (PAD) have been limited by use of modified MetS criteria and restriction to clinical PAD endpoints. We investigated MetS and risk of developing a low ankle-brachial index (ABI) and clinical PAD in the Cardiovascular Health Study, a population-based cohort of adults aged ≥ 65 years. Participants with MetS met at least 3 of 5 Adult Treatment Panel (ATP) III criteria. Baseline “C-reactive protein (CRP)-MetS” or “Fibrinogen-MetS” were defined as presence of 3 out of 6 components, with elevated CRP (> 3 mg/L) or fibrinogen (> 341 mg/dL) as a sixth component. Incident low ABI, defined as ABI <0.9 and decline of ≥0.15, was assessed among a subset of 1899 individuals with two ABI measurements 6 years apart. Over a median follow-up of 13.7 years, 4632 individuals were followed for clinical PAD, defined as revascularization or diagnosed claudication. ATP III MetS was associated with both incident low ABI (RR 1.26; 95% CI: 1.00-1.58) and clinical PAD (HR 1.47; 95% CI: 1.11-1.94). Incorporating CRP or fibrinogen into ATP III criteria identified an additional 16-20% of individuals as having MetS, and both CRP-MetS and Fibrinogen-MetS were associated with incident low ABI (RR 1.36; 95% CI 1.07-1.72 & RR 1.43; 95% CI: 1.13-1.81, respectively) and clinical PAD (HR 1.56; 95% CI: 1.17-2.08 & HR 1.55; 95% CI 1.17-2.07, respectively). Among ATPIII MetS criteria, risk of PAD was most strongly associated with hypertension.
Keywords: Metabolic syndrome, inflammation, peripheral artery disease, cohort studies
Introduction
The metabolic syndrome (MetS) is defined by a combination of criteria including elevated triglycerides, reduced high-density lipoprotein, high blood pressure, impaired fasting glucose, and increased abdominal girth.1-3 Prevalence of the MetS has been consistently associated with incident coronary artery disease, stroke, and cardiovascular mortality.4-7 To our knowledge, there are only two published prospective studies evaluating associations of MetS and incident peripheral artery disease (PAD); however, both are limited by the use of clinical PAD alone as an endpoint.8, 9 These studies also used modified MetS criteria by substituting the presence of diabetes for impaired fasting glucose and BMI for increased abdominal girth. Given the moderate correlation between atherosclerosis across different vascular beds,10 it remains uncertain whether MetS is similarly associated with PAD.
Atherogenic risk factors such as impaired fibrinolysis, oxidative stress, hypoadiponectinemia, and increased thrombogenicity often cluster with the MetS.11, 12 While traditional definitions of MetS incorporate measures of insulin resistance, they do not account for measures of inflammation. Inflammatory markers such as CRP and IL-6 are elevated in MetS.6, 11, 13 Some have proposed that inflammation be included into the definition of the MetS,14 but whether inflammation provides additional information to standard MetS criteria is unclear.
The Cardiovascular Health Study (CHS) offers a unique opportunity to examine associations between MetS and incident PAD in a large, well-defined population with long-term follow-up. We investigated the association of MetS and its individual components with the risk of developing a low ankle-brachial index (ABI) as well as symptomatic clinical PAD. We also investigated how a modified MetS definition that includes inflammation is associated with incident PAD.
Methods
Study Participants
The CHS is a community-based, longitudinal observational study of adults aged 65 and older at baseline that was designed to evaluate risk factors for the development and progression of CVD. The study's primary objectives and design have been reported previously.15, 16 Briefly, participants were recruited from randomly sampled Medicare eligibility lists in Sacramento, California; Forsyth County, North Carolina; Washington County, Maryland; and Allegheny County, Pennsylvania. Eligibility also required an expectation to remain in the area for 3 years after recruitment, no active cancer treatment, and written informed consent. An initial 5,201 individuals were recruited between 1989 and 1990, and an additional 687 African Americans were recruited in 1992 and 1993. The study received approval from investigational review boards of the 4 clinical sites and the coordinating data center at the University of Washington.
Laboratory Analyses
Please refer to supplemental methods.
Classification of Metabolic Syndrome (MetS)
MetS was defined as meeting 3 of the following 5 criteria consistent with the joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity17:(1) Large waist circumference (women ≥88 cm, men ≥102 cm), (2) elevated triglycerides (≥150 mg/dl), (3) low high-density lipoprotein (HDL-C) (men <40 mg/dl, women <50 mg/dl), (4) impaired fasting glucose (≥100 mg/dl and <126 mg/dl ), and (5) high blood pressure (≥130 and/or ≥85 mmHg or use of medications for hypertension). We categorized 8 individuals who were positive for 3 or more criteria as having MetS even if information was missing on the others; these individuals were excluded in analyses of number of positive components. Use of fibrates and nicotinic acid at baseline was rare (1.8%) in this cohort and was not included in our definition.18
Consistent with prior research evaluating an incorporated definition of inflammation and MetS, Inflammation-MetS (“CRP-MetS” or “fibrinogen-MetS”) was defined as having 3 or more of 6 components, including elevated markers of inflammation as a 6th component.19 Elevated CRP was defined as ≥3 mg/L.19 Elevated fibrinogen corresponded to a value in the highest tertile of our study population (> 341 mg/dL).
Ankle-Brachial Index (ABI)
The ABI was measured according to a standard protocol by trained technicians and has been described previously.20 After at least 5 minutes of rest and with the subject in a supine position, standard mercury sphygmomanometers and a Doppler stethoscope (8 MHz, Huntleigh Technology, Inc., Luton, United Kingdom) determined the right brachial artery and right and left leg posterior tibial artery systolic blood pressures. Repeat measurements were obtained and averaged. The correlations for each duplicate blood pressure were left leg, 0.97; right leg, 0.97; and right arm, 0.95.21 The left arm was used if the blood pressure could not be measured adequately in the right arm. The ratio of the systolic blood pressure in the leg to the arm defined the leg-specific ABI. The lower of the leg-specific ABIs was used as the patient specific ABI. When arterial flow was not abolished with the leg blood pressure cuff inflated to >300 mm Hg, the artery was deemed incompressible.
To determine the change in ABI with similar follow-up time for both enrollment cohorts, we used ABI determination in the index leg from participants who had an ABI measured in 1992-1993 and in 1998-1999. ‘ABI decline’ was defined as the difference between ABI at baseline and in the same leg in 1998-1999 (baseline minus follow-up). Incident low ABI was defined as a decline in ABI of at least 0.15 and to 0.9 or less. Requiring a decline of at least 0.15 is consistent with definitions used in prior CHS manuscripts, can help to limit regression to the mean, and avoids small, clinically insignificant changes being included in the incident low ABI definition. 22, 23
Clinical PAD
At baseline, clinical PAD was defined as either an ABI less than 0.90 at the baseline examination or both exertional leg pain relieved by rest and a physician's diagnosis of PAD. In addition, any of the following also validated a PAD diagnosis, with evidence that the test was initiated by the participant's complaint of leg pain: Doppler ultrasound showing at least a 75% reduction in the cross-sectional area of the artery or showing an ulcerated plaque; angiography showing at least a 50% reduction in the diameter or 75% reduction in the cross-sectional area of the artery or showing an ulcerated plaque; absence of a Doppler pulse in any major vessel; a positive exercise test for claudication; or bypass surgery, angioplasty, amputation, or thrombolysis for the indication of PAD. During follow-up, potential incident clinical PAD was identified by self-report of a PAD diagnosis by the participant at an annual clinic visit or during a 6-month follow-up telephone call, a PAD diagnosis found during review of medical records for other events, or as part of regular review of CMS records for the ICD-9 codes 440.2 (atherosclerosis of the native arteries of the extremities) and 443.9 (peripheral vascular disease, unspecified). After a potential PAD event was identified, medical records were then reviewed, and a final decision was adjudicated by the Morbidity Subgroups of the CHS Clinical Events Subcommittee.
Other covariates
Please refer to supplemental methods.
Analytic cohorts
Since diabetes is a known strong predictor of incident PAD, we excluded participants with diabetes mellitus to evaluate the separate influence of MetS on PAD incidence per se.22, 24
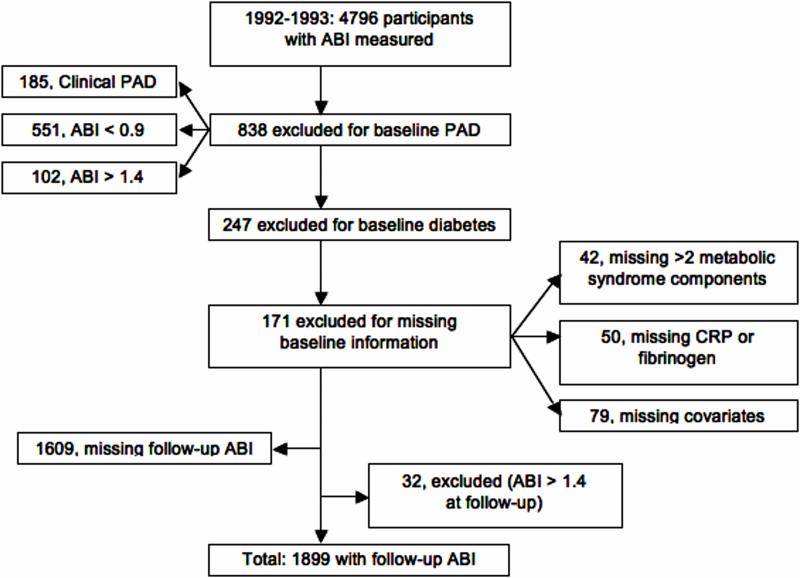
Different analytic cohorts were used for the two outcomes of incident low ABI and clinical PAD. The analysis of incident clinical PAD events used date of enrollment as baseline. Of the 5,888 participants enrolled in CHS, we excluded 151 with prevalent PAD, 50 who were missing data on more than 2 MetS components, 68 who were missing measurements on CRP or fibrinogen, 867 with diabetes, and 120 who were missing covariate information. A total of 4,632 participants were therefore available for the analysis of clinical PAD. The analysis of incident low ABI included participants who had an ABI measured in 1992-1993 and in 1998-1999, and used the 1992-93 examination as baseline (Figure 1).
Figure 1.
Flowchart of participants included in the analysis for incident low ABI*
*ABI=ankle-brachial index; PAD=peripheral artery disease
Statistical Analysis
Incident Low ABI
We used general linear models with log link, Poisson error structure, and robust standard errors to calculate relative risks for developing a low ABI according to the presence or absence of MetS, each MetS component, and each inflammatory marker. Models were adjusted for age, sex, race, clinic site, alcohol consumption, cigarette smoking (current status and pack-years of smoking), prevalent cardiovascular disease, LDL, eGFR, and physical activity levels. Prevalent CVD was defined as history of MI, angina, angioplasty, bypass surgery, or stroke. Models evaluating the presence or absence of MetS were additionally adjusted for CRP and fibrinogen. To evaluate whether the association of MetS and PAD was modified by either sex or race, we fit models that included cross-product terms and tested the statistical significance of these terms.
To evaluate whether the risk of low ABI varied according to the number of MetS components present, we categorized participants by the number of MetS components at baseline and estimated the relative risk of developing a low ABI associated with the presence of 1, 2-3 or 4-5 MetS components compared to participants with no MetS components present.
To assess the individual and joint associations of MetS and inflammation markers with incident low ABI, we cross-classified participants on MetS status (presence/absence) and inflammation status (low/high). CRP and fibrinogen were considered separately and low/high categories were dichotomized at 3 mgL for CRP and the highest tertile of the distribution for fibrinogen (341 mg/dL). We calculated the relative risk of incident ABI for each category compared to the group without MetS and low inflammation levels.
Finally, adjusted relative risks were calculated incorporating CRP or fibrinogen into the MetS definition (CRP-MetS & fibrinogen-MetS) to determine if this improves risk prediction of a low ABI. A c-statistic (area under the receiver operating characteristic curve) of the modified MetS definition for a low ABI was also calculated to determine the added impact of inflammatory markers.
Incident Clinical PAD
To evaluate the association between MetS, individual MetS components, inflammatory markers and incident clinical PAD, we calculated hazard ratios and 95% CI using Cox proportional hazards models. Participants who did not develop clinical PAD were censored at the earliest of: loss to follow-up, death, or the end of follow-up for this analysis (June 2007), and time-to-event was calculated as the interval between enrollment and either incident PAD or censorship.
Results
Baseline characteristics of participants followed for clinical PAD stratified by the presence or absence of MetS are shown in Table 1.
Table 1.
Baseline characteristics of CHS participants according to presence or absence of metabolic syndrome*
| Characteristic | Metabolic Syndrome |
|
|---|---|---|
| Absent (n=2,833) | Present (n=1,799) | |
| Age, y | 72.8 (5.7) | 72.7 (5.4) |
| Male, % | 43.5 | 34.8 |
| Black, % | 14.5 | 12.5 |
| Smoking status, % | ||
| Never | 46.0 | 48.3 |
| Former | 41.5 | 40.1 |
| Current | 12.5 | 11.6 |
| Pack-years among ever smokers | 31.2 (25.9) | 35.4 (29.4) |
| Alcoholic drinks/wk, % | ||
| None | 43.5 | 51.5 |
| <7 | 39.4 | 37.3 |
| 7+ | 17.2 | 12.2 |
| Prevalent CVD, % | 16.6 | 24.4 |
| Body mass index, kg/m2 | 24.8 (3.9) | 28.6 (4.6) |
| LDL cholesterol, mg/dL | 128 (34) | 135 (36) |
| C-reactive protein, median (IQR), mg/L | 1.9 (1.0-3.6) | 3.1 (1.7-5.2) |
| Fibrinogen, mg/dL | 315.8 (64.0) | 330.0 (66.9) |
| Waist circumference, cm | 88.9 (11.8) | 100.1 (11.7) |
| Triglycerides, median (IQR), mg/dL | 100 (82-125) | 157 (119-198) |
| HDL, mg/dL | 60 (15) | 49 (13) |
| Fasting glucose, mg/dL | 96 (9) | 105 (10) |
| Systolic blood pressure, mmHg | 133 (22) | 140 (21) |
| Diastolic blood pressure, mmHg | 70 (11) | 72 (11) |
| High Blood Pressure, % | 62.2 | 90.6 |
Values given are mean (SD) unless otherwise indicated
There were 253 cases of incident low ABI among the 1899 participants with follow-up ABI measurements. There were 208 incident cases of clinical PAD among the 4632 participants followed for this outcome over a median duration of 13.7 years.
Table 2 shows the relative risk for developing a low ABI and hazard ratio for clinical PAD by baseline MetS, each MetS component and elevated CRP or fibrinogen. MetS was associated with both outcomes in the multivariate model. Additional adjustment for CRP and fibrinogen did not appreciably attenuate the association between MetS and either a low ABI (RR: 1.25; 95% CI: 0.99-1.57) or clinical PAD (HR: 1.42; 95% CI: 1.07-1.88). Additional adjustment for eGFR slightly attenuated the association between MetS and either a low ABI (RR: 1.19; 95% CI: 0.94-1.50) or clinical PAD (HR: 1.31; 95% CI: 0.96-1.77). There was evidence of effect modification by race for MetS and endpoints of low ABI (p interaction=0.05) and clinical PAD (p interaction=0.07). In analyses adjusted for age, gender, clinic site, and smoking, MetS was not associated with an increased risk of incident low ABI (RR: 0.80; 95% CI: 0.45-1.44) or clinical PAD (HR: 0.97; 95% CI: 0.46-2.06) in black participants. CRP was statistically significantly associated with developing clinical PAD but not low ABI, whereas an elevated fibrinogen level was associated with incident low ABI but not PAD.
Table 2.
The relative risk of developing a low ankle-brachial index or clinical peripheral artery disease associated with the presence or absence of metabolic syndrome and its individual components.
| Participant Category | Incident Low ABI | Incident Clinical PAD | ||||
|---|---|---|---|---|---|---|
| # Cases / Total (%) | Relative Risk (95% CI) | # Cases; # Person-Years | Hazard Ratio (95% CI) | |||
| Model 1* | Model 2† | Model 1 | Model 2 | |||
| No MetS | 140 / 1183 (11.8) | 1.00 | 1.00 | 104; 34975 | 1.00 | 1.00 |
| MetS | 113 / 716 (15.8) | 1.35 (1.08-1.70) | 1.26 (1.00-1.58) | 104; 21909 | 1.69 (1.28-2.22) | 1.47 (1.11-1.94) |
| MetS components, by criteria | ||||||
| Waist circ. ≥88 (W); ≥102 (M) cm | 1.02 (0.80-1.30) | 0.96 (0.75-1.24) | 1.04 (0.78-1.39) | 0.98 (0.73-1.31) | ||
| Triglycerides ≥150 mg/dL | 1.32 (1.04-1.67) | 1.21 (0.96-1.54) | 1.14 (0.84-1.54) | 1.01 (0.74-1.37) | ||
| HDL <50 (W); <40 (M) mg/dL | 0.99 (0.76-1.29) | 0.85 (0.65-1.11) | 1.52 (1.13-2.04) | 1.29 (0.95-1.75) | ||
| High blood pressure present | 2.12 (1.53-2.93) | 2.14 (1.57-2.93) | 2.39 (1.61-3.55) | 2.27 (1.52-3.40) | ||
| Fasting glucose ≥100 mg/dL | 1.24 (0.98-1.56) | 1.19 (0.95-1.50) | 1.41 (1.07-1.86) | 1.29 (0.98-1.71) | ||
| Inflammatory Markers | ||||||
| C-reactive protein >3 mg/L | 1.27 (1.01-1.60) | 1.15 (0.92-1.45) | 2.15 (1.63-2.84) | 1.76 (1.33-2.33) | ||
| Fibrinogen >341 mg/dL | 1.57 (1.25-1.98) | 1.45 (1.15-1.81) | 1.50 (1.13-1.99) | 1.27 (0.95-1.69) | ||
Model 1 is adjusted for age, sex, race, clinic site
Model 2 is adjusted for age, sex, race, clinic site, alcohol, smoking (current status & pack-years), blocks walked, prevalent CVD, LDL
Of the individual MetS criteria, only high blood pressure was significantly associated with the development of both a low ABI and clinical PAD (Table 2), although impaired fasting glucose was nearly so. In a multivariable model including all individual MetS criteria and both inflammatory markers, hypertension remained significantly associated with the development of both a low ABI (RR: 2.07; 95% CI: 1.49-2.86) and clinical PAD (HR: 2.11, 95% CI: 1.40-3.17), CRP remained significantly associated with developing clinical PAD (HR: 1.68, 95% CI: 1.24-2.27), and fibrinogen remained significantly associated with an incident low ABI (RR: 1.41; 95% CI: 1.11-1.81). Compared with individuals with no baseline MetS criteria, the relative risk of incident low ABI increased with increasing number of MetS criteria (Table S1).
Incorporating Inflammation with Metabolic Syndrome on Risk of Incident PAD
Joint associations of MetS and either fibrinogen or CRP with the development of an incident low ABI and clinical PAD are shown in tables S2 & S3 respectively. There was no evidence of synergistic associations for development of an incident low ABI. The combination of MetS and an elevated fibrinogen or CRP resulted in a higher likelihood of developing clinical PAD compared to having only one of these.
To determine whether including CRP or fibrinogen in the definition of MetS increased the predictive value of MetS for developing a low ABI, we created two modified definitions of MetS: CRP-MetS and fibrinogen-MetS (Table 3). The prevalence of CRP-MetS was 50.0% (949/1899), with 233 of 1183 (19.7%) without MetS reclassified as CRP-MetS. Participants with CRP-MetS had increased risk for an incident low ABI (RR 1.36; 95% CI 1.07–1.72 vs no CRP-MetS). The prevalence of fibrinogen-MetS was 47.6% (904/1899), with 188 of 1183 (15.9%) without MetS reclassified as fibrinogen-MetS. Participants with fibrinogen-MetS also had a significantly increased risk for an incident low ABI (RR 1.43; 95% CI 1.13-1.81) as compared with participants without fibrinogen-MetS. Compared with the c-statistic for MetS alone (0.540), the addition of fibrinogen significantly improved discrimination (c 0.565; p=0.03) but the addition of CRP did not (c 0.556; p=0.18).
Table 3.
Relative risk of incident low ABI or symptomatic PAD associated with the presence or absence of CRP-metabolic syndrome (CRP-MetS) and fibrinogen-metabolic syndrome (Fibrinogen-MetS)
| Participant Category | Low ABI | Symptomatic PAD | ||||
|---|---|---|---|---|---|---|
| # Cases / Total (%) | Relative Risk (95% CI) | # Cases; # Person-Years | Hazard Ratio (95% CI) | |||
| Model 1* | Model 2† | Model 1 | Model 2 | |||
| No MetS-CRP | 102/950 (10.7) | 1.0 | 1.0 | 78 29130 |
1.0 | 1.0 |
| MetS-CRP | 151/949 (15.9) | 1.49 (1.18-1.89) | 1.36 (1.07-1.72) | 130 27555 |
1.83 (1.38-2.43) | 1.56 (1.17-2.08) |
| No MetS-Fibrinogen | 104/995 (10.5) | 1.0 | 1.0 | 83 30514 |
1.0 | 1.0 |
| MetS-Fibrinogen | 149/904 (16.5) | 1.57 (1.24-1.98) | 1.43 (1.13-1.81) | 125 26370 |
1.81 (1.37-2.39) | 1.55 (1.17-2.07) |
Model 1 is adjusted for age, sex, race (black, non-black), clinic site
Model 2 is adjusted for age, sex, race, clinic site, alcohol, smoking (current status & pack-years), blocks walked, prevalent CVD, LDL
CRP-MetS and fibrinogen-MetS were also associated with risk of developing clinical PAD (Table 3). The hazard ratios were 1.56 (95% CI 1.17-2.08) for CRP-MetS and 1.55 (95% CI 1.17-2.07) for fibrinogen-MetS. The c-statistics for both CRP-MetS (0.566) and fibrinogen-MetS (0.567) related to the development of clinical PAD were similar to the c-statistic for MetS alone (0.556). Comparing the area under the curve for MetS versus MetS-inflammation, the p-values were 0.37 and 0.29 for MetS vs MetS-CRP and MetS vs MetS-Fib respectively.
Discussion
In a cohort of community-dwelling older adults, MetS was associated with the development of PAD, defined either by a low ABI or the development of clinically manifest PAD, after the adjustment for traditional risk factors and markers of inflammation. Hypertension was the only MetS component independently associated with incident PAD. Inflammatory markers CRP and fibrinogen were also independently associated with incident PAD.
Our findings contrast with the two previously published studies regarding the prospective associations of MetS and incident PAD. The Edinburgh Artery Study (EAS) found no association between MetS and incident PAD.8 In the Women's Health Study, a younger cohort of females free of baseline cardiovascular disease participating in a clinical trial, MetS was associated with an increased risk of incident PAD.9 This association, however, was strongly attenuated after adjustment for inflammatory markers CRP and soluble intracellular adhesion molecule-1 and no longer significant.
The CHS cohort is comprised of older individuals with a significantly higher baseline prevalence of hypertension and may, in part, explain discrepancies between our findings and those reported in the EAS and Women's Health Study. Additionally, these two prior studies used adapted definitions of MetS and, consequently, did not capture the true prevalence of participants meeting criteria for this syndrome. Waist circumference was not available in either study and BMI was used instead, which may not capture visceral adiposity. In addition, the Women's Health Study defined glucose intolerance as the presence or absence of diabetes, and diabetes is a well-established PAD risk factor.9 Thus, overt diabetes may have driven associations in this study. Additionally, the primary endpoint for both studies was limited to symptomatic PAD. Prior studies have reported that anywhere from 20-50% of individuals with PAD are asymptomatic. 25, 26 The presence of PAD as assessed by ABI as we did here, regardless of the presence of symptoms, is strongly associated with increased cardiovascular morbidity and mortality.27 It is unclear why these findings were not consistent across race. Our null findings in Black participants may simply be due to small event numbers or chance. Further study with primary attention to racial differences is needed.
The association between inflammation and incident PAD has been demonstrated previously.28 CRP and fibrinogen both have been reported to be associated with the development of incident PAD.28, 29 Our results corroborate those of prior studies by also showing an independent association between inflammation and the development of PAD. It is unclear, however, why CRP was only significant for the development of clinical PAD and fibrinogen only significant for the development of a low ABI. As mentioned previously, the CHS cohort is different at baseline from other well-studied cohorts is terms of age and prevalence of hypertension. As a result it may be that different biomarkers are relevant in different populations. Alternatively, these results may signify that inflammation is important in development of both outcomes and the disparity found between fibrinogen and CRP is simply reflective of chance.
To our knowledge, this is the first prospective study to evaluate the incorporation of inflammation markers into a MetS definition with incident PAD. Findings from the Women's Health Study suggested that increased inflammation and endothelial activation might serve as a mechanistic link between MetS and incident PAD. Our results suggest that the relationship between MetS and incident PAD is independent of common inflammatory pathways represented by markers such as CRP and fibrinogen, but the addition of inflammation to the definition of MetS identified larger numbers of older adults at risk and, at least for MetS-Fib, significantly improved discrimination between individuals who did and did not develop a low ABI.
Hypertension was the only MetS component independently associated with incident PAD. Prior studies have documented a strong cross-sectional association between hypertension and PAD.25, 30 In CHS participants, hypertension was previously reported as a risk factor for ABI decline. 22 eGFR has been previously reported to be closely correlated to hypertension in CHS participants and may explain why findings were slightly attenuated after adjusting for this. 31 It is also possible that eGFR may be a mediator. Our findings underscore the particular role of hypertension in the development of PAD in older individuals with MetS. Current ACC/AHA guidelines recommend all individuals aged 65 or older be screened for PAD. As hypertension is extremely prevalent and a strong risk factor for PAD development in older age, our results only further justify the need to screen all individuals over the age of 65.
Our study has limitations. The CHS participants are all aged 65 years and older; therefore, our results may not be generalizable to younger aged cohorts. Follow-up ABI data was not available for many participants and this may have introduced bias to analyses involving ABI decline, although results were generally similar for analyses of incident clinical PAD that were not limited by attrition.
Perspectives
MetS is associated with the development of both a low ABI and clinical PAD. Incorporating measures of inflammation into the definition of MetS may help identify more at-risk individuals and provide additive information in predicting incident PAD. Lifestyle modification strategies such as aggressive blood pressure control, dietary changes, regular exercise, and weight loss may be useful targets to evaluate in intervention studies to determine if this reduces the risk of PAD in individuals with MetS. Considering the strong association of hypertension with incident PAD in MetS, studies are needed to determine if strict blood pressure control provides particular benefit to reduce risk of PAD.
Novelty and Significance.
- What Is New
- We examined the combined effects of two important abnormalities that lead to atherosclerosis - the constellation of increasingly common metabolic abnormalities termed metabolic syndrome and two blood tests that reflect inflammation
- We determined how each abnormality influences two measures of blockages in arteries of the legs in older adults
- What Is Relevant
- Hypertension is a key component of metabolic syndrome and very common in these older adults
- Of the metabolic syndrome components, hypertension was most strongly linked to blocked arteries
- Summary
- Both metabolic syndrome and inflammation lead to blocked arteries in the leg
- Using inflammation to define metabolic syndrome helps to identify people at risk
Acknowledgments
Sources of Funding: This research was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grants HL080295, HL094555 and K12-HL083790, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm.
Footnotes
Conflicts of Interest: None
References
- 1.Third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 4.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in united states adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy american women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 7.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 8.Wild SH, Byrne CD, Tzoulaki I, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Metabolic syndrome, haemostatic and inflammatory markers, cerebrovascular and peripheral arterial disease: The edinburgh artery study. Atherosclerosis. 2009;203:604–609. doi: 10.1016/j.atherosclerosis.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic syndrome, inflammation, and risk of symptomatic peripheral artery disease in women. A prospective study. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kathiresan S, Larson MG, Keyes MJ, Polak JF, Wolf PA, D'Agostino RB, Jaffer FA, Clouse ME, Levy D, Manning WJ, O'Donnell CJ. Assessment by cardiovascular magnetic resonance, electron beam computed tomography, and carotid ultrasonography of the distribution of subclinical atherosclerosis across framingham risk strata. Am J Cardiol. 2007;99:310–314. doi: 10.1016/j.amjcard.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. The metabolic syndrome and insulin resistance: Relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181:101–108. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Wilson PW, Grundy SM. Should c-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, MD, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The cardiovascular health study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the cardiovascular health study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Katz R, Jenny NS, Zakai NA, LeWinter MM, Barzilay JI, Cushman M. Metabolic syndrome, inflammation, and incident heart failure in the elderly: The cardiovascular health study. Circ Heart Fail. 2008;1:242–248. doi: 10.1161/CIRCHEARTFAILURE.108.785485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the american heart association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The cardiovascular health study group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Sutton-Tyrrell K, Vogt MT, Kuller LH. Morbidity and mortality in hypertensive adults with a low ankle/arm blood pressure index. JAMA. 1993;270:487–489. [PubMed] [Google Scholar]
- 22.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF, Burke GL, Enright P, Cushman M. Risk factors for declining ankle-brachial index in men and women 65 years or older: The cardiovascular health study. Arch Intern Med. 2005;165:1896–1902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 23.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ix JH, Katz R, De Boer IH, Kestenbaum BR, Allison MA, Siscovick DS, Newman AB, Sarnak MJ, Shlipak MG, Criqui MH. Association of chronic kidney disease with the spectrum of ankle brachial index the chs (cardiovascular health study). J Am Coll Cardiol. 2009;54:1176–1184. doi: 10.1016/j.jacc.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 26.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 27.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of c-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA : the journal of the American Medical Association. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 29.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. European Heart Journal. 2007;28:354–362. doi: 10.1093/eurheartj/ehl441. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Cardiovascular heart study (chs) collaborative research group. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 31.Odden MC, Tager IB, Gansevoort RT, Bakker SJ, Fried LF, Newman AB, Katz R, Satterfield S, Harris TB, Sarnak MJ, Siscovick D, Shlipak MG. Hypertension and low HDL cholesterol were associated with reduced kidney function across the age spectrum: a collaborative study. Ann Epidemiol. 2013;23:106–111. doi: 10.1016/j.annepidem.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]