Abstract
Mitogen-activated and stress-activated kinase 1 (MSK1) is a nuclear serine/threonine protein kinase that acts downstream of both ERKs and p38 MAP kinases in response to stress or mitogenic extracellular stimuli. Increasing evidence has shown that MSK1 is closely associated with malignant transformation and cancer development. MSK1 should be an effective target for cancer chemoprevention and chemotherapy. However, very few MSK1 inhibitors, especially natural compounds, have been reported. We used virtual screening of a natural products database and the active conformation of the C-terminal kinase domain of MSK1 (PDB id 3KN) as the receptor structure to identify chrysin and its derivative, compound 69407, as inhibitors of MSK1. Compared with chrysin, compound 69407 more strongly inhibited proliferation and TPA-induced neoplastic transformation of JB6 P+ cells with lower cytotoxicity. Western blot data demonstrated that compound 69407 suppressed phosphorylation of the MSK1 downstream effector histone H3 in intact cells. Knocking down the expression of MSK1 effectively reduced the sensitivity of JB6 P+ cells to compound 69407. Moreover, topical treatment with compound 69407 prior to TPA application significantly reduced papilloma development in terms of number and size in a two-stage mouse skin carcinogenesis model. The reduction in papilloma development was accompanied by the inhibition of histone H3 phosphorylation at Ser10 in tumors extracted from mouse skin. The results indicated that compound 69407 exerts inhibitory effects on skin tumorigenesis by directly binding with MSK1 and attenuates the MSK1/histone H3 signaling pathway, which makes it an ideal chemopreventive agent against skin cancer.
Keywords: neoplastic transformation, MSK inhibitor, natural compound, chemoprevention, skin carcinogenesis
Introduction
Mitogen- and stress-activated kinase 1 (MSK1) is a nuclear serine/threonine protein kinase that acts downstream of both ERKs and p38 MAP kinases in response to growth factors, cellular stress stimuli such as UV or radioactive irradiation, and pro-inflammatory cytokines/factors. The well-established function of MSK1 involves the regulation of gene expression by phosphorylation of transcription factors including CREB (1), ATF1 (1) and the p65 subunit of NF-κB (2). MSK1 has also been reported to phosphorylate histone H3 at Ser10 and Ser28 (3). MSK1 is activated in response to tumor promoters, such as epidermal growth factor (EGF) (4), 12-O-tetradecanoylphorbol-13-acetate (TPA) (5), or UVB (6). It plays a positive role in the control of proliferation of HaCaT keratinocytes (7) and is involved in TPA- or EGF-induced neoplastic transformation of JB6 Cl41 cells (4). Stable knockdown of MSK1 caused loss of the malignant phenotype of Ras-transformed mouse fibroblasts, as shown by the absence of anchorage-independent growth in soft agar (8). Recently, in vivo evidence showed that MSK1/2 knockout mice developed significantly fewer skin tumors compared with wildtype mice (9). MSK1/2 signaling represents a novel tumor-promoting axis in skin carcinogenesis.
Skin tumor formation occurs in three stages: initiation, promotion, and progression (10). Chemical carcinogenesis in mouse skin has been used for several decades and remains a powerful model for understanding multistage carcinogenesis in humans. The most common chemical carcinogenesis regimen is a two-stage induction that includes an initiating application of DMBA, which induces an irreversible and specific mutation in mouse skin. Initiation with DMBA is followed by multiple, regular applications of the phorbol ester, TPA. Alterations in signal transduction pathways, including the aberrant activation of ERKs, were found to contribute to genesis and progression of mouse skin cancer (11). MSK1 is an important downstream effector of the stimulated ERKs pathway and plays a role in the process of carcinogenesis in mouse skin (9). Therefore, inhibiting MSK1 activity might be an effective strategy for skin cancer chemoprevention.
Here, we used virtual screening of a natural products database to identify MSK1 inhibitors. We identified compound 69407, a natural compound derivative of chrysin, as a novel MSK1 inhibitor. Our results indicated that compound 69407 is more potent and less toxic than chrysin in suppressing proliferation and TPA-induced neoplastic transformation of JB6 P+ cells. Moreover, using a two-stage skin carcinogenesis protocol with DMBA as initiator and TPA as the promoter, compound 69407 exerted a significant anti-promotion effect. Further studies revealed that compound 69407 appeared to exert its inhibitory effects on TPA-induced skin tumor promotion through direct inhibition of MSK1/histone H3 signaling. These data suggest that compound 69407 is a potential compound for chemoprevention of skin cancer.
Materials and Methods
General Materials and Methods are included as Supplementary Materials and Methods.
Anchorage-independent cell growth assay
TPA-induced neoplastic transformation was investigated in JB6 P+ cells. JB6 cells (8×103/ml) were exposed to TPA (10 ng/ml) and compound 69407 (0, 2.5, 5, 10 or 20 μM) in 1 ml of 0.33% basal medium Eagle agar containing 10% FBS. The cultures were maintained at 37 °C in a 5% CO2 incubator for 10 or 14 days and colonies were counted under a microscope. Cell transformation is presented as colony number per 8,000 seeded cells in soft agar as described by Colburn et al. (12).
In vitro kinase assay
MSK1 and MSK2 in vitro kinase assays were performed as described previously (4) with some modification. Different concentrations of compound 69407 were incubated with active recombinant MSK1 or MSK2 at 30 °C for 10 min. Then, 1 μg purified CREB or histone H3 was added and reactions were carried out in 1× kinase buffer (25 mM Tris/HCl pH 7.5, 5 mM β-glycerophosphate, 0.1 mM Na3VO4, 10 mM MgCl2, and 2 mM dithiothreitol) containing 50 μM unlabeled ATP with or without 10 μCi of [γ-32P] ATP at 30 °C for 30 min. Reactions were stopped by adding 6×SDS sample buffer. Phosphorylation of CREB was visualized by autoradiography and phosphorylation of the histone H3 protein at Ser10 or Ser28 was detected by Western blotting with specific antibodies. The RSK2 in vitro kinase assay (13) and Aurora B in vitro kinase assays (14) were performed as previously described, respectively.
Tumor induction and treatment
FVB/N mice (6 wk of age, male) were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained under “specific pathogen free” conditions and all animal studies were conducted according to guidelines approved by the KRIBB-IACUC (Korea Research Institute of Bioscience & Biotechnology-Institutional Animal Care and Use Committee). Groups of 6 mice each were used for the two-stage skin carcinogenesis studies. Mouse skin tumors were induced by the initiation-promotion regimen as previously described (15). The backs of mice were shaved prior to initiation. All mice received a topical application of a single 200 nmol dose of DMBA in 0.2 ml of acetone to the shaved dorsal skin. Two weeks after initiation, mice were treated topically twice weekly with vehicle (acetone) only or 200 nmol compound 69407 in 0.2 ml acetone followed 30 min later by promotion with 17 nmol TPA in 0.25 ml acetone. Mice were weighed, photographed, and tumors measured once a week beginning when first measurable tumors (1 mm3) were observed (week 9). Tumor volume was calculated using the following formula: tumor volume = 4π/3(l/2)(w/2)(h/2), where l is length, w is width, and h is height (15). At the end of the experiment, mice were sacrificed by cervical dislocation and the tumors and skin were removed and snap-frozen in liquid nitrogen and lysates were prepared for Western blot analysis.
Results
Compound 69407, a derivative of the natural compound chrysin, inhibits MSK1 kinase activity in vitro
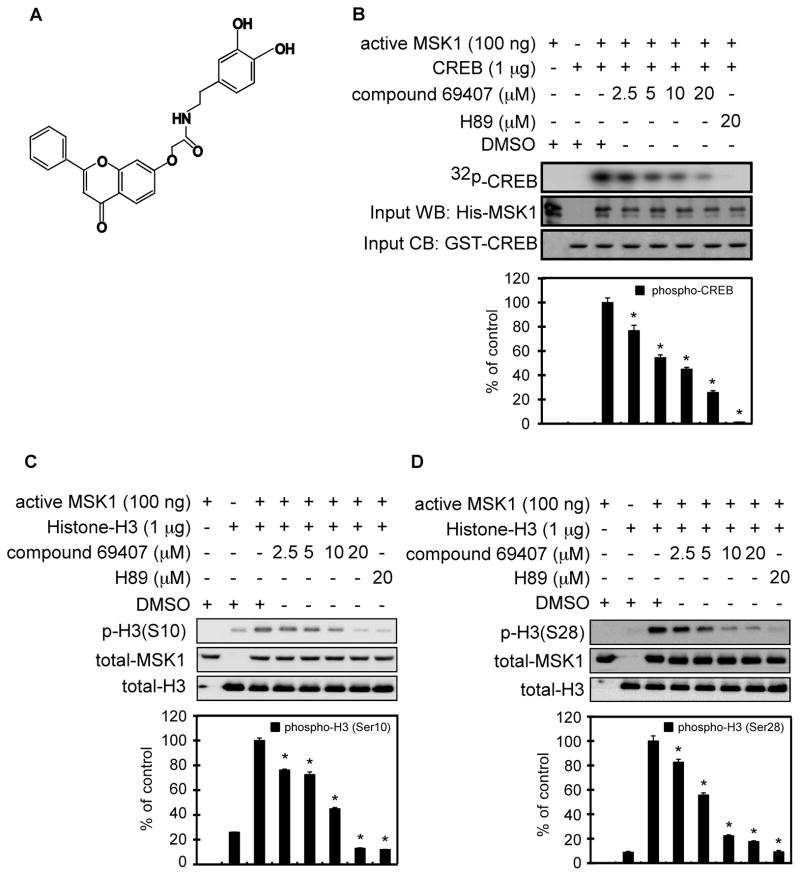
Compound 69407 (Fig. 1A) was identified by the Virtual Screening Workflow (VSW) docking screen of Natural Products Database (NPD) using the active conformation of the C-terminal kinase domain of MSK1 [PDB id 3KN (16)] as the receptor structure. To confirm the virtual screening result, the effect of compound 69407 on the kinase activity of MSK1 was first investigated using an in vitro kinase assay with GST-CREB, active MSK1, [γ-32P] ATP, and different doses of compound 69407. The autoradiography results demonstrated that compound 69407 dose-dependently inhibited the phosphorylation of CREB, indicating that the compound dose-dependently suppressed MSK1 activity in vitro (Fig. 1B). Because MSK1 is a kinase of histone H3 at both Ser10 and Ser28 (17), the effect of compound 69407 on MSK1 activity was also assessed by histone H3 in vitro kinase assays. The results indicated that compound 69407 dose-dependently inhibited the phosphorylation levels of histone H3 at Ser10 (Fig. 1C) and Ser28 (Fig. 1D). These results further confirmed that compound 69407 inhibits MSK1 activity. The effect of compound 69407 on kinases closely related to MSK1, such as MSK2 and RSK2, was assessed by in vitro kinase assays. The results indicated that compound 69407 did not inhibit MSK2 substrate CREB1 (1) phosphorylation at Ser133 (Supplementary Fig. 1A) or RSK2 substrate IκBα (13) phosphorylation at Ser32/36 (Supplementary Fig. 1B). Compound 69407 (5 μM) was also submitted to the KinaseProfiler™ assay. The results showed the extent of kinase selectivity of this compound against multiple kinases (Supplementary Table 1). Thus, this suggests that compound 69407 specifically inhibits MSK1 kinase activity.
Fig. 1. Compound 69407 inhibits MSK1 kinase activity in vitro.
(A) Chemical structure of compound 69407. (B) Compound 69407 inhibits the phosphorylation of CREB, an MSK1 substrate. The effect of compound 69407 on MSK1 activity was assessed by an in vitro kinase assay using a GST-CREB protein and [γ-32P] ATP. 32P-labeled GST-CREB was visualized by autoradiography. The level of MSK1 was determined by Western blotting with a specific antibody. Coomassie blue (CB) staining shows the loading of the GST-CREB protein. (C, D) Compound 69407 inhibits MSK1 substrate histone H3 phosphorylation at Ser10 (C) and Ser28 (D). The effect of compound 69407 on MSK1 activity was assessed by in vitro kinase assays using a histone H3 protein as substrate. The phosphorylation levels of the histone H3 protein at Ser10 (C) and Ser28 (D), total histone H3 and MSK1 were determined by Western blotting with specific antibodies. The known MSK1 inhibitor H89 was used as a positive control. Band density was measured using the NIH ImageJ software program (Version 1.41). Data are represented as means ± S.D. of 2 independent experiments. The asterisk (*, p< 0.05) indicates a significant decrease caused by compound 69407 compared with DMSO control.
Compound 69407 directly binds MSK1 kinase in vitro and ex vivo
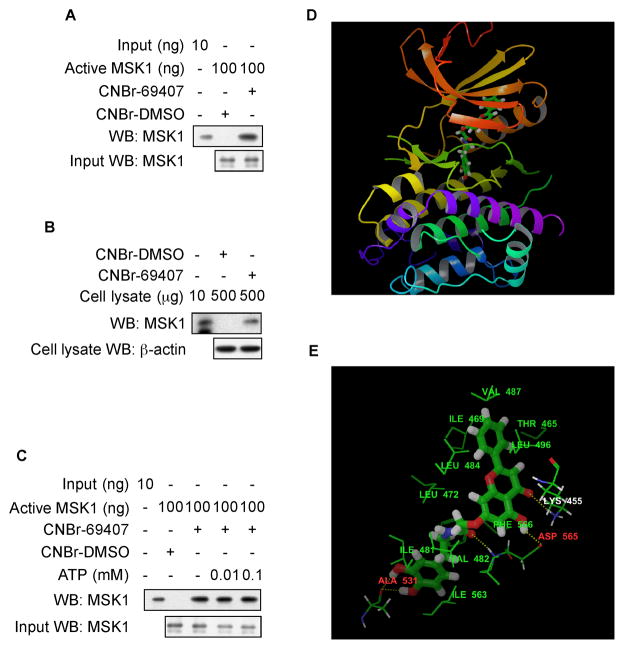
To verify direct binding of compound 69407 with MSK1, we conducted a pulldown assay with compound 69407-conjugated beads and commercially available active MSK1. The results indicated that compound 69407 bound with MSK1 (Fig. 2A). Moreover, we confirmed in cell lysates that compound 69407 bound with endogenous MSK1 (Fig. 2B). Notably, ATP did not compete with compound 69407 for binding to MSK1. In an ATP-competition assay, the binding of compound 69407 to MSK1 did not change as the concentration of ATP increased (Fig. 2C). These results suggest that compound 69407 inhibits MSK1 activity through ATP-independent binding. To predict the binding motif between compound 69407 and MSK1, we performed flexible-ligand flexible-protein docking using the IFD (Induced Fit Docking) Module (Schrödinger Suite 2011 Induced Fit Docking protocol; Glide v.5.7; Prime v.3.0). IFD has an advantage over regular Glide docking (18) because of its ability to capture the ligand-induced conformational changes in receptor active sites (19). We examined both the ATP binding site and the allosteric site of the dual kinase domains of MSK1 using the crystal structures of PDB id 1VZO (20) and 3KN5 (16), respectively, as the receptor model structures. The docking results between compound 69407 and N-terminal domain of MSK1 were not good (data not shown). However, the binding affinity between compound 69407 and the C-terminal domain as determined from IFD docking results was predicted to be better in the allosteric site (Fig. 2D), with a score of −11.73 kcal/mol, than the binding in the ATP binding pocket, with a score of −10.08 kcal/mol. Compound 69407 formed 5 hydrogen bonds with MSK1 (Fig. 2E). Three of the hydrogen bonds, including one-bifurcated, involve the backbone atoms of two residues Ala531 and Asp565, and the other two form with the side chain atoms of Asp565 and Lys455, respectively. In addition, 11 residues around the allosteric site, including Val487, Ile469, Thr465, Leu496, Leu484, Leu472, Phe566, ILE481, Val482, Ile563, and Ala531 formed hydrophobic interactions with the carbons of compound 69407 (Fig. 2E). Notably, these residues are mainly distributed into two hydrophobic clusters, one including Val487, Ile469, Thr465, Leu496, Leu484 and the other including Phe566, ILE481, Val482, Ile563, and Ala531, to form two hydrophobic pockets for accommodating the benzene ring at each side of compound 69407. Therefore, those residues should play an important role in the binding of compound 69407 with MSK1. The computational results corresponded well with the experimental results, suggesting that compound 69407, was an ATP noncompetitive inhibitor of MSK1 and located inside the allosteric binding site rather than the ATP pocket of MSK1.
Fig. 2. Compound 69407 directly binds to MSK1 in vitro and ex vivo.
(A) Compound 69407 binds with MSK1 in vitro. Active MSK1 (100 ng) was subjected to a pull-down assay with compound 69407 conjugated with CNBr-Sepharose 4B beads. Compound 69407 binding of MSK1 was visualized by Western blotting (WB) with a specific MSK1 antibody. (B) Compound 69407 binds with MSK1 ex vivo. A cellular protein fraction (500 μg) of JB6 P+ cells was used for the pull-down assay with CNBr-DMSO or CNBr-compound 69407 beads. The MSK1 proteins pulled down were visualized by Western blotting with a specific MSK1 antibody. (C) Compound 69407 does not compete with ATP for binding with MSK1. Active MSK1 was incubated with ATP (0, 0.01, or 0.1 mM) and CNBr-DMSO or CNBr-compound 69407-conjugated beads in reaction buffer (500 μl). The mixtures were incubated with shaking at 4 °C overnight. After washing, the pulled down proteins were detected by Western blotting. The results shown are representative of 2 independent experiments. (D) Predicted model of the MSK1-compound 69407 complex. The binding pose of compound 69407 inside the allosteric binding site of C-terminal kinase domain of MSK1. (E) Intermolecular interactions (hydrogen bonds and hydrophobic interactions) between compound 69407 and protein residues in the allosteric site of C-terminal kinase domain of MSK1 (for clarity, oxgens, nitrogens and hydrogens of the 10 hydrophobic protein residues are not shown). Note: the α-helices are drawn as cylinders and the β-strands as arrows. Compound 69407 is shown in stick model and protein residues are shown in line model.
Compound 69407 inhibits growth of JB6 P+ cells by inducing cell cycle G2/M phase accumulation
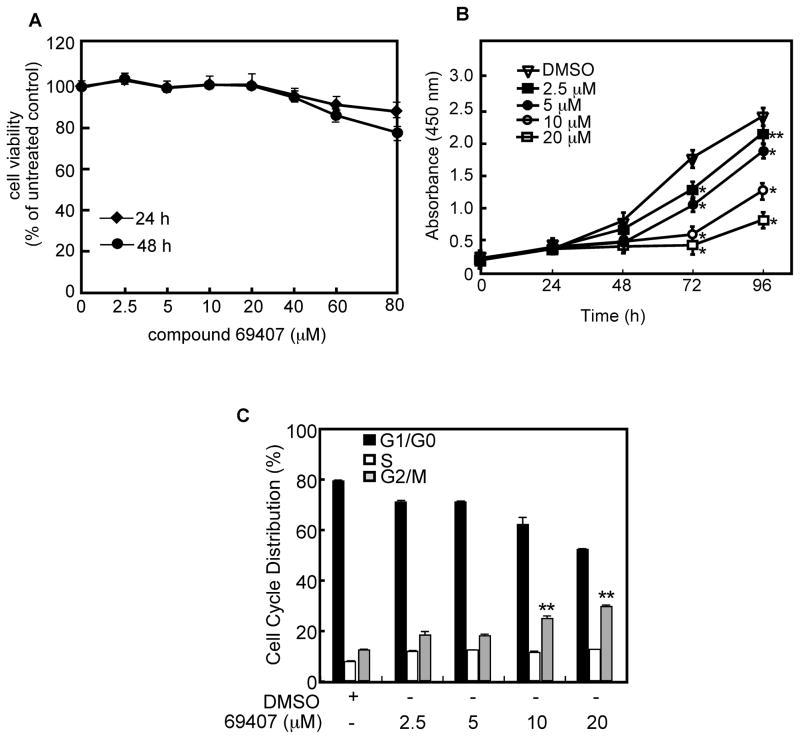
The cytotoxicity of compound 69407 was evaluated using the WST-1 assay. Concentrations of compound 69407 up to 80 μM and exposure for 48 h had a minimal effect on the viability of JB6 cells (Fig. 3A). To examine whether this compound could affect proliferation, cells were treated with different concentrations of the compound dissolved in dimethyl sulfoxide (DMSO) as vehicle for 24, 48, 72 or 96 h. Results indicated that compound 69407 strongly inhibited cell growth in a dose-dependent manner (Fig. 3B). Furthermore, cell cycle distribution analysis of asynchronously growing JB6 P+ cells treated with various doses of compound 69407 for 48 h, the FACS result indicated that G2/M arrest occurred, especially at relatively high doses (10 or 20 μM) of compound 69407 (Fig. 3C, p < 0.05). The G2/M arrest caused by compound 69407 was not due to the inhibition of Aurora B activity because compound 69407 has no effect on Aurora B kinase activity (Supplementary Fig. 2A, B). We observed that treatment with compound 69407 produced a slight blockade of S phase although this was not statistically significant. These results suggested that compound 69407 inhibits cell growth, at least in part, by inducing cell cycle G2/M arrest.
Fig. 3. Compound 69407 shows no significant cytotoxicity against JB6 P+ cells and inhibits cell proliferation by inducing G2/M accumulation.
(A) Cytotoxic effects of compound 69407 on JB6 P+ cells. Cells (1×104) were treated with DMSO or the indicated concentration of compound 69407 and determined using the WST-1 assay as described in Supplementary Material and methods. (B) Compound 69407 inhibits JB6 P+ cell proliferation. Cells (1×103) were treated with DMSO or the indicated concentration of compound 69407 and determined at the indicated timepoints using the WST-1 assay as described in Supplementary Material and methods. (C) Compound 69407 induces G2/M accumulation in JB6 P+ cells. Flow cytometry analysis (FACS) of asynchronous JB6 P+ cells treated for 48 h with DMSO or various concentrations of compound 69407 showed an increase in the percentage of cells in G2/M phase. Data are represented as means ± S.E. and the asterisk(s) indicates a significant difference (*p < 0.001 and **p < 0.05) between the DMSO-treated group and the compound 69407-treated groups.
Compound 69407 suppresses TPA-induced anchorage-independent growth of JB6 P+ cells
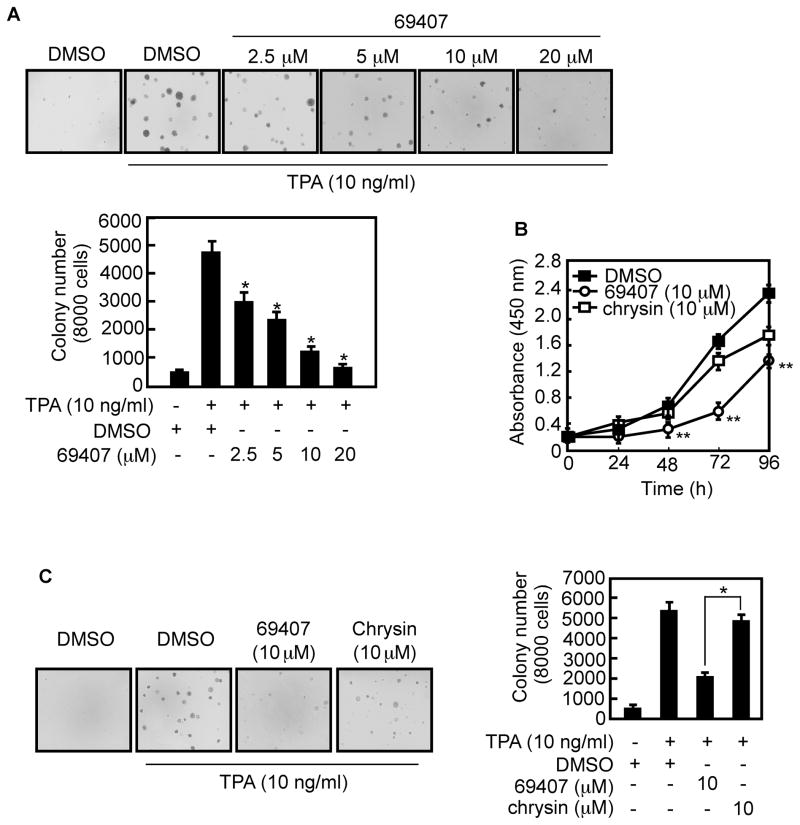
To examine the effect of compound 69407 on TPA-induced neoplastic transformation of JB6 P+ cells, soft agar assays were performed. With TPA stimulation, JB6 P+ cells readily form colonies in soft agar, exhibiting an anchorage-independent growth that is a distinctive characteristic of neoplastic transformation (21). Treatment with compound 69407 significantly inhibited TPA-promoted neoplastic transformation in a dose-dependent manner (Fig. 4A). The compound at 5, 10 or 20 μM caused a significant decrease to 49.3, 24.9 or 15.9% of the TPA-only control (Fig. 4A). The inhibition of colony formation by this compound was not caused by cytotoxicity because the effective concentration range for inhibiting cell transformation did not affect JB6 P+ cell viability (Fig. 3A). To determine whether compound 69407 is comparable with chrysin in its ability to suppress cell proliferation and transformation, we examined the effects of both compounds on proliferation and TPA-induced neoplastic transformation of JB6 P+ cells. Results showed that although either compound effectively inhibited cell proliferation and transformation, compound 69407 was much more effective (Fig. 4B, C). These data indicated that compound 69407 is more potent than chrysin at suppressing proliferation and the anchorage-independent growth capability of JB6 P+ cells.
Fig. 4. Compound 69407 inhibits TPA-induced anchorage-independent growth of JB6 P+ cells.
(A) Compound 69407 suppresses TPA-induced anchorage-independent growth of JB6 P+ cells. Soft agar assay was performed as described in Materials and methods. The cultures were incubated for 14 days and then colonies were counted. (B) Compound 69407 inhibits cell growth more strongly than chrysin. JB6 P+ cells were treated with compound 69407 or chrysin at 10 μM in 5% FBS/MEM and growth was measured at the indicated times using the WST-1 assay. (C) Compared with chrysin, compound 69407 more strongly inhibits TPA-induced transformation of JB6 P+ cells. Soft agar assay was performed as described in Materials and methods. The cultures were incubated for 14 days and then colonies were counted. Data are shown as means ± S.E. of 2 independent experiments performed in triplicate and significant differences were determined by t-test. The asterisk (*, p < 0.001) indicates a significant difference between cells treated with DMSO and TPA and cells treated with compound 69407 (A); or in cells treated with compound 69407 plus TPA compared with cells treated with chrysin plus TPA (C). The asterisks (**, p < 0.01) indicate a significant difference in cells treated with compound 69407 plus TPA and cells treated with chrysin plus TPA (B).
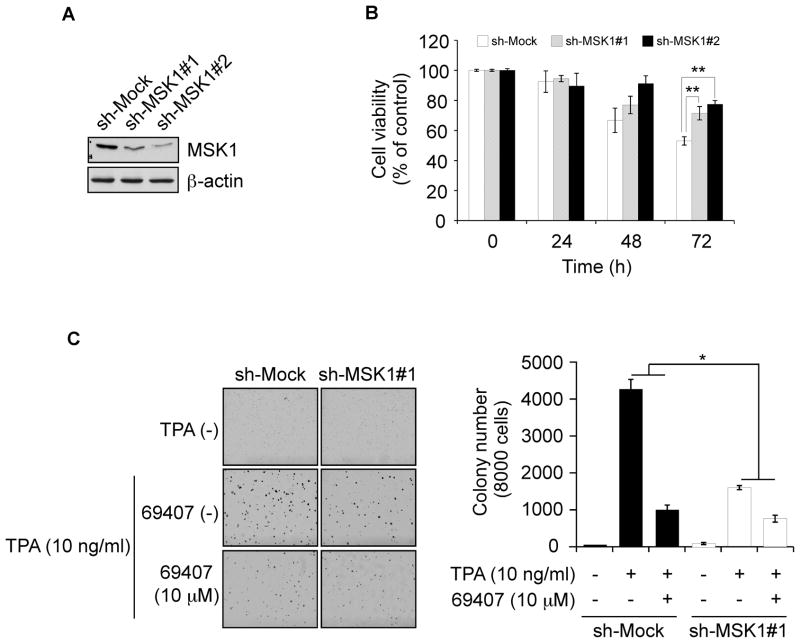
Knockdown of MSK1 decreases the sensitivity of JB6 P+ cells to compound 69407
Because compound 69407 was shown to specifically target MSK1, we then examined whether knockdown of MSK1 expression could influence the sensitivity of JB6 P+ cells to compound 69407. The efficiency of shRNA knockdown was confirmed by an obvious decrease of MSK1 protein after shRNA transfection (Fig. 5A). Results of cell viability assay indicated that JB6 P+ cells expressing sh-MSK1 were resistant to the inhibitory effect of compound 69407 on growth compared with cells expressing sh-Mock (Fig. 5B). In addition, JB6 P+ cells stably transfected with sh-Mock or sh-MSK1 were treated with vehicle or compound 69407 and subjected to a soft agar assay to examine colony formation in response to TPA stimulation. Results showed that compound 69407 (10 μM) inhibited TPA-induced anchorage-independent growth of JB6 P+ cells transfected with sh-Mock by about 75%. In contrast, the inhibition was only about 50% in JB6 P+ cells transfected with sh-MSK1 (Fig. 5C). These results suggested that MSK1 plays an important role in the sensitivity of JB6 P+ cells to the anticancer effects of compound 69407. Notably, sh-MSK cells formed fewer colonies than sh-Mock cells.
Fig. 5. Knockdown of MSK1 attenuates the inhibitory effect of compound 69407 on JB6 P+ cells.
(A) Efficiency of sh-MSK1#1 and #2 in JB6 P+ cells. JB6 P+ cells were transfected with an sh-Mock or sh-MSK1 plasmid and stable colonies were selected with puromycin. Knockdown of MSK1 expression was analyzed by Western blotting with a specific antibody. β-Actin was used as a loading control. (B) Compound 69407 has less effect on proliferation of sh-MSK1 cells compared to sh-Mock JB6 P+ cells. Stably transfected JB6 P+ cells were treated or not treated with compound 69407 (10 μM) and proliferation was measured at 24-h intervals up to 72 h. Data are presented as a percentage of each respective vehicle-treated control. (C) Compound 69407 has less effect on anchorage-independent growth of sh-MSK1 cells compared to sh-Mock JB6 P+ cells in response to TPA stimulation. sh-Mock or sh-MSK1 stably transfected JB6 P+ cells were grown in soft agar without or with compound 69407 (10 μM) and exposure to TPA (0 or 10 ng/ml) for 10 days and colonies were counted. Data are represented as means ± S.D. of 2 separate experiments and significant differences were determined by t-test. *, p < 0.05 and **, p < 0.01, compared with sh-Mock cells.
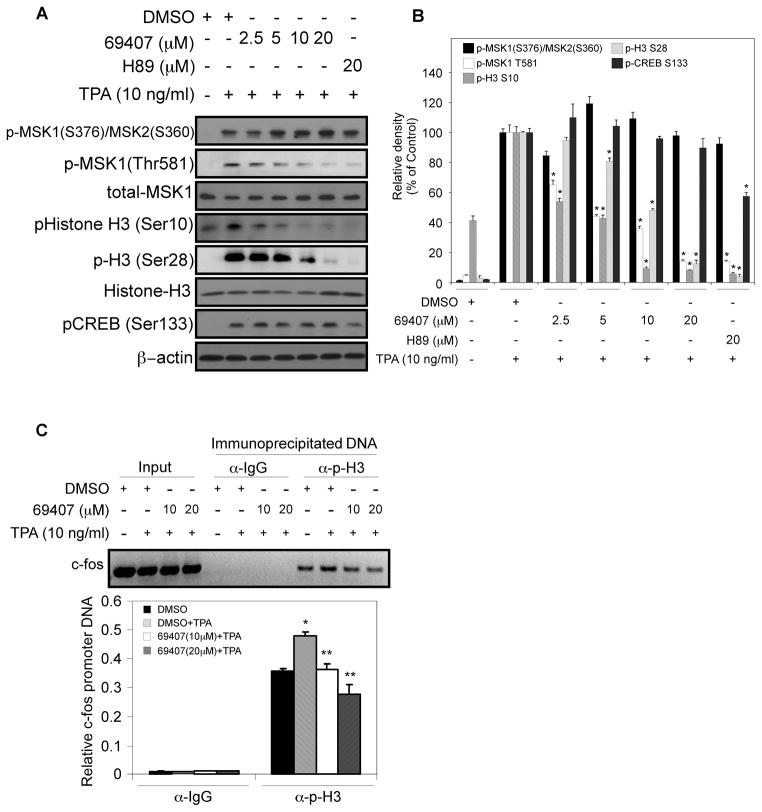
Compound 69407 inhibits MSK1-mediated association of phosphorylated histone H3 with the c-fos promoter in response to TPA in JB6 P+ cells
MSK1 is a well-known histone H3 kinase responsible for TPA-induced phosphorylation of histone H3 at Ser10 and Ser28 (5, 17). To investigate further whether compound 69407 suppresses proliferation and TPA-induced transformation by inhibiting MSK1 activity in cells, the phosphorylation status of histone H3 at Ser10 and Ser28 was assessed. In addition, because H89 is a well-characterized MSK1 inhibitor, we compared the inhibitory activity of compound 69407 with that of H89 in intact cells. Immunoblot results showed that compound 69407 dose-dependently inhibited TPA-stimulated H3 phosphorylation at both Ser10 and Ser28 in JB6 P+ cells (Fig. 6A, B). This effect was not due to the inhibition of the MSK1 upstream ERKs kinase or p38 kinase activation, because the phosphorylation of ERKs in response to TPA or the phosphorylation of p38 in response to UV radiation, a much stronger activator than TPA for p38 activation (1, 22), was unaffected by treatment of the cells with compound 69407 (Supplementary Fig. 3A, B). In contrast to the blockade of TPA-induced histone H3 phosphorylation at Ser10 and Ser28, compound 69407 had no effect on CREB phosphorylation at Ser133 elicited by TPA (Fig. 6A, B). As an MSK1 inhibitor, H89 had no effect on the phosphorylation of ERKs (Supplementary Fig. 3A), p38 (Supplementary Fig. 3B) or MSK1 at Ser376 (Fig. 6A, B), but slightly inhibited phosphorylation of CREB at Ser133 (Fig. 6A, B), which agrees with a previous report (23). However, both compound 69407 and H89 inhibited TPA-induced phosphorylation of MSK1 at Thr581 (Fig. 6A, B), a site critical for MSK1 activation (24), suggesting that compound 69407 and H89 suppress MSK1 activity by inhibiting the phosphorylation of this site. The UVC-induced phosphorylation of other MSK1 downstream effector molecules, such as the p65 subunit of NF-κB at Ser276 (25) and CREB at Ser133 (1), was not affected by compound 69407 treatment (Supplementary Fig. 3C). These data indicate that at the concentration used, compound 69407 does not have a non-specific inhibitory effect upon the activation of upstream ERKs or p38 MAP kinases. Studies have provided evidence to suggest that the phosphorylation of histone H3 at Ser10 or Ser28 following TPA stimulation plays an important role in mediating transcriptional activation of immediate-early genes such as c-fos and c-jun, which correlates with MSK1 activity (3, 23, 26). Phosphorylation events on Ser10 or Ser28 apparently do not occur together on the same promoter (26) and histone H3 at Ser10 has been shown by ChIP assay to associate with the promoter region of the IE c-fos gene upon TPA stimulation (27). We thus examined by ChIP assay whether MSK1-mediated association of phosphorylated histone H3 (Ser10) with the c-fos promoter is affected by compound 69407 treatment. The amount of c-fos promoter DNA present in immunoprecipitated chromatin fractions was then determined by PCR. Immunoprecipitation of equivalent amounts of chromatin from TPA-stimulated cells with the phospho-H3 (Ser10) antibody showed an increase in the association of phosphorylation H3 with the c-fos promoter compared with that of unstimulated cells. Treatment with compound 69407 dose-dependently reduced the enrichment of phosphorylation H3 at the c-fos gene promoter in response to TPA stimulation (Fig. 6C). These findings demonstrate that TPA-stimulated phosphorylation of histone H3 (Ser10) at the c-fos promoter is dependent on the integrity of the MSK1 signaling pathway in JB6 P+ cells. The results imply that compound 69407 acts on histone H3, a downstream substrate of MSK1, to attenuate the proliferation and transformation of JB6 P+ cells.
Fig. 6. Compound 69407 inhibits MSK1-mediated association of phosphorylated histone H3 with the c-fos promoter in response to TPA in JB6 P+ cells.
(A) JB6 P+ cells were serum-starved for 36 h followed by treatment for 1 h with DMSO, compound 69407 or H89 at the indicated concentrations. Cells were then exposed to TPA (0 or 10 ng/ml) for 10 min and harvested. Individual levels of phosphorylated and total proteins were visualized by Western blotting with specific antibodies. β-Actin was used as a loading control. (B) Band density of (A) was measured using the NIH ImageJ program (Version 1.41). The band intensities of phosphorylated proteins were normalized against their respective total proteins and phospho-CREB was normalized against β-Actin. Data are represented as means ± S.D. of 2 separate experiments and significant differences were determined by t-test. *, p < 0.05 compared with their respective DMSO plus TPA-treated control. (C) JB6 P+ cells were treated using the same protocol as described in (A) and subjected to ChIP assays with a phospho-H3(Ser10)-specific antibody. The precipitated DNA fragments were subjected to PCR analysis to test for the presence of sequences corresponding to the c-fos promoter. Input material (10%) is shown for comparison. Data are represented as means ± S.D. of 2 separate experiments and significant differences were determined by t-test. *, p < 0.05 compared with the DMSO-treated group. **, p < 0.01, compared with the DMSO plus TPA-treated group.
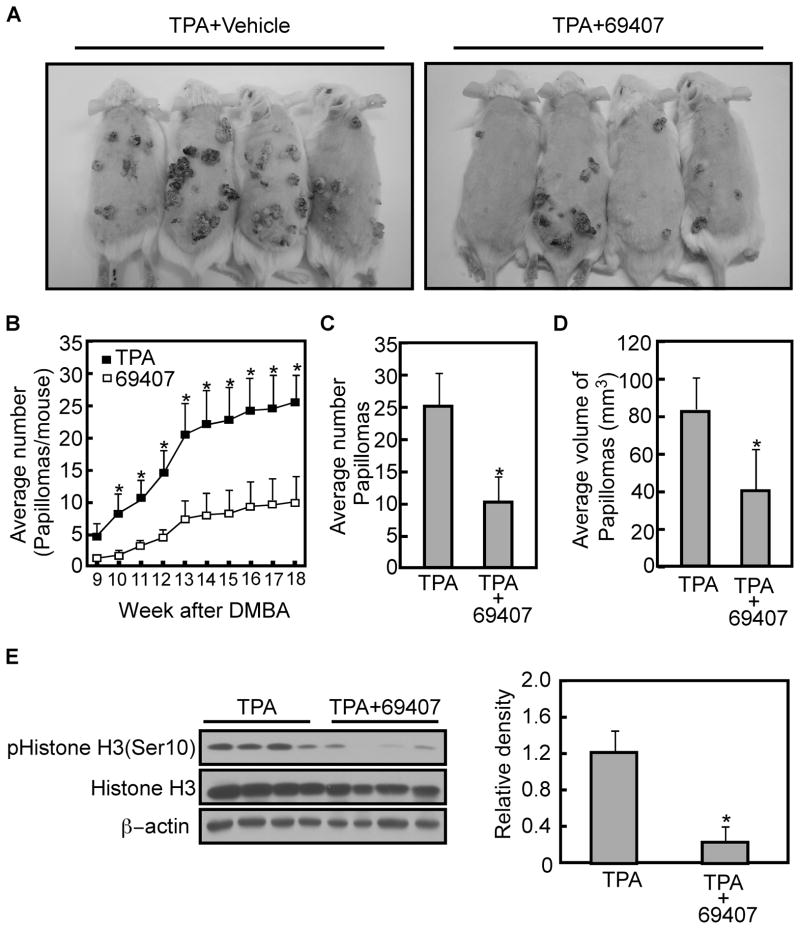
Compound 69407 attenuates TPA-promoted skin carcinogenesis in mice
Based on the data showing that compound 69407 inhibits TPA-induced transformation of JB6 P+ cells (Fig. 4A), an experiment was conducted to determine whether topical treatment with compound 69407 could inhibit tumorigenesis in vivo. Two-stage skin carcinogenesis experiments with DMBA/TPA were carried out in FVB/N mice to evaluate the effect of compound 69407 on skin tumor development and growth. Groups of FVB/N mice 6 weeks of age were initiated with 200 nmol of DMBA. Two weeks later, mice were treated topically with vehicle (acetone) or 200 nmol compound 69407 in acetone followed 30 min later by topical application of 17 nmol of TPA in acetone. The selection of 200 nmol compound 69407 for treatment was based on our prior studies, in which we administered compound 69407 at respective doses of 100, 200, or 500 nmol. At a dose of 100 nmol, the compound only exhibited a weak effect. The doses of 200 and 500 nmol were not much different in their effectiveness (Supplementary Fig. 4A, B, C). All treatments were applied twice weekly for the duration of the experiment. Tumor multiplicity (average number of papillomas per mouse) was measured weekly for each group. Results indicated that compound 69407 exerted an antitumor promoting effect (Fig. 7A, B). The group receiving topical application of 200 nmol of compound 69407 30 min prior to application of TPA had a significant reduction in papilloma development compared with the DMBA/TPA-only-treated control group (p < 0.05, Fig. 7B). In addition, mice treated with only TPA developed significantly more and larger skin tumors than mice treated with compound 69407 before TPA (Fig. 7C, D). Furthermore, topical treatment with compound 69407 had no effect on body weight in the experimental group over the course of these experiments (data not shown). To further explore the potential mechanisms by which compound 69407 inhibited TPA-induced skin tumor promotion, experiments were conducted to evaluate changes in phosphorylation of the MSK1 downstream effector histone H3 at Ser10 in tumors extracted from mouse skin. Western blot results confirmed that tumor lysates from mice treated with compound 69407 and TPA exhibited significantly lower phosphorylation levels of histone H3 (Ser10) than mice treated with TPA alone (p < 0.05, Fig. 7E). These results suggest that topically applied compound 69407 led to inhibition of TPA-induced MSK1 downstream signaling, particularly through the histone H3 protein pathway, which inhibits the formation of skin tumors.
Fig. 7. Compound 69407 attenuates TPA-promoted skin carcinogenesis in mice.
The shaved dorsal surface of each mouse was initiated by topical application of DMBA (200 nmol) in acetone. After 2 wk, mice were topically treated with compound 69407 (200 nmol) in acetone followed 30 min later with TPA (17 nmol) in acetone. (A) Representative photographs of papilloma development in TPA (left) or TPA combined with compound 69407 (right) mice. (B) Average number of papillomas developed per mouse in response to TPA or TPA combined with compound 69407. (C) Average number of papillomas in mice treated with compound 60407 and TPA or TPA only. Tumor numbers were counted every week for 18 weeks after DMBA treatment. (D) Average volume of papillomas in mice treated with compound 60407 and TPA or TPA only. Tumor diameters were measured every week for 18 weeks after DMBA treatment. (E) Treatment with TPA combined with compound 69407 attenuates histone H3 phosphorylation at Ser10. Tumors were extracted from mouse dorsal skin at week 18 after DMBA treatment and lysates were subjected to SDS-PAGE and levels of phosphorylated and total histone H3 were analyzed by Western blotting. Densitometer analysis of each band was performed using the NIH ImageJ software program (Version 1.41). The asterisk (*) indicates a significant (p < 0.05) difference.
Discussion
The Ras/Raf/MEK/ERKs pathway is critical for neoplastic cell transformation and two-stage mouse skin carcinogenesis (11). MSK1 is an important downstream effector of the stimulated ERKs pathway and plays a role in the process of tumorgenesis in mouse skin (9). We found that TPA was very effective in promoting the transformation of JB6 P+ cells (Fig. 4A), corresponding with the elevation of ERKs (Supplementary Fig. 3A) and MSK1 (Fig. 6A, B) activities and, in particular, phosphorylation of histone H3 at Ser10 and Ser28 (Fig. 6A, B). Increased MSK1 activation reportedly results in elevated steady-state levels of H3 phosphorylation corresponding with tumorigenesis and metastasis in fibroblast and epidermal cells (4, 5, 27–29). Histone H3 phosphorylation at Ser10 has also been shown to be indispensable for neoplastic cell transformation because mutation of this site to alanine suppressed cell transformation promoted by EGF (29). Histone H3 phosphorylation at Ser10 clearly occurs concurrently with the transcriptional activation of several IE genes, including c-fos, c-myc, and c-jun (3, 23, 26, 27). Several lines of evidence have indicated that the aberrant expression of c-Fos and c-Jun of the AP-1 transcription factor causes neoplastic transformation and malignant progression. In addition, transactivation of c-jun and c-fos was significantly diminished in histone H3 Ser10 mutant-overexpressing JB6 Cl41 cells compared with histone H3 wildtype-overexpressing cells (29). Moreover, ChIP assay results indicated that TPA significantly increases MSK1-mediated association of phosphorylated histone H3 with theimmediate-early gene c-fos promoter in JB6 P+ cells (Fig. 6C). These results indicated that induction of c-jun and c-fos gene expression is one of the critical mechanisms for neoplastic cell transformation elicited by MSK1-mediated phosphorylation of histone H3 at Ser10. Considering that MSK1 is a link between the signaling cascade and the primary response at the gene expression level, it is an ideal candidate for cancer chemotherapy and has immense potential in the treatment of cancers.
Besides its role in malignant transformation and cancer development, MSK1-mediated H3 phosphorylation plays a role in synaptic plasticity (30) and long-term potentiation (31–33) as well as phase resetting of the circadian clock (34) in post-mitotic neurons. In the dentate gyrus of the hippocampus, MSK1-mediated H3 phosphorylation is relevant to the physiology of stress-related memory formation (35) while in the striatum, it is linked to the long-term effects of drugs of abuse and physiological reward-controlled learning (36). A recent study found a deficit of MSK1 expression in the striatum of in the R6/2 transgenic mouse model of Huntington’s disease (HD), which accounts for the absence of phosphorylation of histone H3 and is involved in transcriptional deregulation and striatal degeneration (37).
MSK1 is reportedly an efficient kinase for histone H3 in response to TPA and its activity towards H3 is uniquely sensitive to H89. It strongly inhibits TPA-stimulated histone H3 phosphorylation at Ser10, but has relatively little effect on TPA-stimulated CREB phosphorylation at Ser133 in C3H 10T1/2 cells (23). These earlier findings suggested that MSK1 is a far more efficient kinase towards histone H3 than towards CREB in response to TPA. Similarly, our results showed H89 has a relatively weak effect on TPA-stimulated phosphorylation of CREB at Ser133, whereas the same dose of H89 completely blocked TPA-induced phosphorylation of histone H3 at both Ser10 and Ser28 in JB6 P+ cells (Fig. 6A, B). In addition, compound 69407 at the same dose nearly completely blocked TPA-induced phosphorylation of histone H3 at both Ser10 and Ser28 in JB6 P+ cells (Fig. 6A, B). Moreover, our ChIP assay result indicated that compound 69407 dose-dependently inhibits the MSK1-mediated association of phosphorylated histone H3 with the immediate-early gene c-fos promoter in response to TPA stimulation in JB6 P+ cells (Fig. 6C). The results suggested that the major physiological target of compound 69407 and H89 might be MSK1/histone H3 rather than MSK1/CREB in response to TPA in JB6 P + cells. The MSK1 kinase activity towards histone H3 is inhibited by compound 69407, suggesting that inhibition of carcinogenesis in intact cells occurs because compound 69407 acts directly on MSK1, blocking its ability to phosphorylate histone H3.
We found compound 69407 strongly inhibits TPA-induced H3 phosphorylation at both Ser10 and Ser28 but has no effect on TPA-elicited CREB phosphorylation at Ser133 (Fig. 6A, B). However, as a positive control of MSK1 inhibition, H89 dramatically blocked TPA-elicited H3 phosphorylation and also had a small effect on TPA-stimulated phosphorylation of CREB at Ser133 (Fig. 6A, B), which is in agreement with an earlier report (23). In addition, phosphorylation of the p65 subunit of NF-κB at Ser276 or CREB at Ser133 elicited by UVC irradiation was not affected by compound 69407. However, both could be inhibited by H89 treatment (Supplementary Fig. 3C). These data seem contradictory to the role of compound 69407 as a MSK1 inhibitor. We hypothesized that this phenomenon might be due to different mechanisms by which compound 69407 and H89 inhibited MSK1 activity. Compound 69407 inhibits MSK1 activity in an ATP-noncompetitive manner (Fig. 2C, D and E), whereas H89 is an ATP-competitive inhibitor (38). This difference in binding to MSK1 might cause disimilar conformational changes in the MSK1 protein and might lead to a difference in the affinity MSK1’s interaction with a physiological substrate and differentially contribute to phosphate transfer. Consequently, their inhibitory effects on downstream substrate phosphorylation are likely different. A recent study, which quantitatively evaluated the specific docking domains involved in mediating interactions between ERK2 and protein substrates and defined the contributions of these interactions to phosphate transfer by using surface plasmon resonance (SPR) methodology (39), might provide some information that can further support the hypothesis.
Chemoprevention, the use of drugs or natural substances to inhibit carcinogenesis, is an important and rapidly evolving aspect of cancer research that is providing a practical approach to identify potentially useful inhibitors of cancer development. Chrysin (5,7-dihydroxyflavone), a natural flavonoid widely distributed in many plant extracts, honey and propolis, has been reported to be a potential cancer preventive agent (40, 41). Interest is increasing in the pharmacological activity of chrysin and its derivitives (42–44). Herein we report the chemopreventive potential of a derivative of chrysin, compound 69407. The results from in vitro and in vivo studies support compound 69407 as a novel compound with activity against cell proliferation and TPA-induced neoplastic transformation. Moreover, topical application of compound 69407 prior to TPA application inhibits tumorigenesis in a two-stage mouse skin model. In addition, compound 69407 displays much lower cytotoxicity compared with H89. More than 90% of JB6 P+ cells died when treated with H89 at 40 μM for only 24 h (data not shown). However, compound 69407, at concentrations up to 80 μM and exposure times up to 48 h, had little effect on the viability of cells (Fig. 3A). The mechanism of action involved in the anticarcinogenesis effects of compound 69407 was also investigated. The results indicated that compound 69407 exerts inhibitory effects on skin tumorigenesis by directly binding with MSK1 and inhibiting MSK1/histone H3 signaling. In conclusion, compound 69407 could be an ideal chemopreventive agent against skin cancer.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0029233); or Leap Research Program (No. 2010-0029233); -WCI: World Class Institute Program founded by the Korea Research Foundation, Ministry of Education, Science and Technology. or WCI 2009-002. The work was also supported by The Hormel Foundation and National Institutes of Health grants CA120388, R37 CA08164, CA1666011, CA172457, and ES016548. The work was further supported by National Natural Science Foundation of China (No. 31170676).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–81. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, et al. Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. Embo J. 2008;27:1682–93. doi: 10.1038/emboj.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. Embo J. 2003;22:2788–97. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HG, Lee KW, Cho YY, Kang NJ, Oh SM, Bode AM, et al. Mitogen- and stress-activated kinase 1-mediated histone H3 phosphorylation is crucial for cell transformation. Cancer Res. 2008;68:2538–47. doi: 10.1158/0008-5472.CAN-07-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strelkov IS, Davie JR. Ser-10 phosphorylation of histone H3 and immediate early gene expression in oncogene-transformed mouse fibroblasts. Cancer Res. 2002;62:75–8. [PubMed] [Google Scholar]
- 6.Zhong S, Jansen C, She QB, Goto H, Inagaki M, Bode AM, et al. Ultraviolet B-induced phosphorylation of histone H3 at serine 28 is mediated by MSK1. J Biol Chem. 2001;276:33213–9. doi: 10.1074/jbc.M103973200. [DOI] [PubMed] [Google Scholar]
- 7.Schiller M, Bohm M, Dennler S, Ehrchen JM, Mauviel A. Mitogen- and stress-activated protein kinase 1 is critical for interleukin-1-induced, CREB-mediated, c-fos gene expression in keratinocytes. Oncogene. 2006;25:4449–57. doi: 10.1038/sj.onc.1209479. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Cadahia B, Drobic B, Espino PS, He S, Mandal S, Healy S, et al. Role of MSK1 in the malignant phenotype of Ras-transformed mouse fibroblasts. J Biol Chem. 2011;286:42–9. doi: 10.1074/jbc.M110.156687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang S, Iversen L, Kragballe K, Arthur JS, Johansen C. Mice lacking MSK1 and MSK2 show reduced skin tumor development in a two-stage chemical carcinogenesis model. Cancer Invest. 2011;29:240–5. doi: 10.3109/07357907.2010.550594. [DOI] [PubMed] [Google Scholar]
- 10.Bachelor MA, Bowden GT. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–8. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Zoumpourlis V, Solakidi S, Papathoma A, Papaevangeliou D. Alterations in signal transduction pathways implicated in tumour progression during multistage mouse skin carcinogenesis. Carcinogenesis. 2003;24:1159–65. doi: 10.1093/carcin/bgg067. [DOI] [PubMed] [Google Scholar]
- 12.Colburn NH, Wendel EJ, Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981;78:6912–6. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng C, Cho YY, Zhu F, Xu YM, Wen W, Ma WY, et al. RSK2 mediates NF-{kappa}B activity through the phosphorylation of IkappaBalpha in the TNF-R1 pathway. Faseb J. 2010;24:3490–9. doi: 10.1096/fj.09-151290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H, Lee MH, Zhu F, Reddy K, Peng C, Li Y, et al. Identification of an Aurora kinase inhibitor specific for the Aurora B isoform. Cancer Res. 2013;73:716–24. doi: 10.1158/0008-5472.CAN-12-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.She QB, Chen N, Bode AM, Flavell RA, Dong Z. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343–8. [PubMed] [Google Scholar]
- 16.Malakhova M, D’Angelo I, Kim HG, Kurinov I, Bode AM, Dong Z. The crystal structure of the active form of the C-terminal kinase domain of mitogen- and stress-activated protein kinase 1. J Mol Biol. 2010;399:41–52. doi: 10.1016/j.jmb.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode AM, Dong Z. Inducible covalent posttranslational modification of histone H3. Sci STKE. 2005;2005:re4. doi: 10.1126/stke.2812005re4. [DOI] [PubMed] [Google Scholar]
- 18.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 19.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49:534–53. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 20.Smith KJ, Carter PS, Bridges A, Horrocks P, Lewis C, Pettman G, et al. The structure of MSK1 reveals a novel autoinhibitory conformation for a dual kinase protein. Structure. 2004;12:1067–77. doi: 10.1016/j.str.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Egan SE, McClarty GA, Jarolim L, Wright JA, Spiro I, Hager G, et al. Expression of H-ras correlates with metastatic potential: evidence for direct regulation of the metastatic phenotype in 10T1/2 and NIH 3T3 cells. Mol Cell Biol. 1987;7:830–7. doi: 10.1128/mcb.7.2.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.She QB, Chen N, Dong Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem. 2000;275:20444–9. doi: 10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- 23.Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. Embo J. 1999;18:4779–93. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy CE, Campbell DG, Deak M, Bloomberg GB, Arthur JS. MSK1 activity is controlled by multiple phosphorylation sites. Biochem J. 2005;387:507–17. doi: 10.1042/BJ20041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacks KA, Koch CA. Differential regulation of mitogen- and stress-activated protein kinase-1 and -2 (MSK1 and MSK2) by CK2 following UV radiation. J Biol Chem. 2010;285:1661–70. doi: 10.1074/jbc.M109.083808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drobic B, Perez-Cadahia B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38:3196–208. doi: 10.1093/nar/gkq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, et al. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J Biol Chem. 1999;274:24914–20. doi: 10.1074/jbc.274.35.24914. [DOI] [PubMed] [Google Scholar]
- 28.Drobic B, Espino PS, Davie JR. Mitogen- and stress-activated protein kinase 1 activity and histone h3 phosphorylation in oncogene-transformed mouse fibroblasts. Cancer Res. 2004;64:9076–9. doi: 10.1158/0008-5472.CAN-04-2369. [DOI] [PubMed] [Google Scholar]
- 29.Choi HS, Choi BY, Cho YY, Mizuno H, Kang BS, Bode AM, et al. Phosphorylation of histone H3 at serine 10 is indispensable for neoplastic cell transformation. Cancer Res. 2005;65:5818–27. doi: 10.1158/0008-5472.CAN-05-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–35. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- 31.Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–54. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–8. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–42. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci. 2000;3:1241–7. doi: 10.1038/81767. [DOI] [PubMed] [Google Scholar]
- 35.Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27:2701–13. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- 36.Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn JH, Maroteaux M, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–84. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roze E, Betuing S, Deyts C, Marcon E, Brami-Cherrier K, Pages C, et al. Mitogen- and stress-activated protein kinase-1 deficiency is involved in expanded-huntingtin-induced transcriptional dysregulation and striatal death. Faseb J. 2008;22:1083–93. doi: 10.1096/fj.07-9814. [DOI] [PubMed] [Google Scholar]
- 38.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–72. [PubMed] [Google Scholar]
- 39.Burkhard KA, Chen F, Shapiro P. Quantitative analysis of ERK2 interactions with substrate proteins: roles for kinase docking domains and activity in determining binding affinity. J Biol Chem. 2011;286:2477–85. doi: 10.1074/jbc.M110.177899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg Med Chem. 2004;12:6097–105. doi: 10.1016/j.bmc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Woo KJ, Jeong YJ, Inoue H, Park JW, Kwon TK. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Lett. 2005;579:705–11. doi: 10.1016/j.febslet.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 42.Zheng X, Zhao FF, Liu YM, Yao X, Zheng ZT, Luo X, et al. Synthesis and preliminary biological evaluation of chrysin derivatives as potential anticancer drugs. Med Chem. 2010;6:6–8. doi: 10.2174/157340610791208763. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X, Meng WD, Xu YY, Cao JG, Qing FL. Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett. 2003;13:881–4. doi: 10.1016/s0960-894x(02)01081-8. [DOI] [PubMed] [Google Scholar]
- 44.Lv PC, Wang KR, Li QS, Chen J, Sun J, Zhu HL. Design, synthesis and biological evaluation of chrysin long-chain derivatives as potential anticancer agents. Bioorg Med Chem. 2010;18:1117–23. doi: 10.1016/j.bmc.2009.12.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.