Abstract
The uncinate fasciculus is a major white matter tract that provides a crucial link between areas of the human brain that underlie emotion processing and regulation. Specifically, the uncinate fasciculus is the major direct fiber tract that connects the prefrontal cortex and the amygdala. The aim of the present study was to use a multi-modal imaging approach in order to simultaneously examine the relation between structural connectivity of the uncinate fasciculus and functional activation of the amygdala in a youth sample (children and adolescents). Participants were 9 to 19 years old and underwent diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI). Results indicate that greater structural connectivity of the uncinate fasciculus predicts reduced amygdala activation to sad and happy faces. This effect is moderated by age, with younger participants exhibiting a stronger relation. Further, decreased amygdala activation to sad faces predicts lower internalizing symptoms. These results provide important insights into brain structure-function relationships during adolescence, and suggest that greater structural connectivity of the uncinate fasciculus may facilitate regulation of the amygdala, particularly during early adolescence. These findings also have implications for understanding the relation between brain structure, function, and the development of emotion regulation difficulties, such as internalizing symptoms.
Keywords: fMRI, diffusion tensor imaging, emotion, development, internalizing, adolescence
1. Introduction
Successful social functioning requires the development of processes related to perceiving, interpreting, and appropriately responding to the emotional signals expressed on others' faces. Indeed, abnormal emotion processing is associated with a range of psychiatric disorders (Monk, 2008; Phillips et al., 2003; Pine, 2007). Because the neural circuitry associated with emotion processing undergoes substantial change during childhood and adolescence (Nelson et al., 2005), youth may be a time when sensitivity of this circuitry to genetic and environmental influences is increased. Understanding the structure and function of neural networks involved in emotion processing in childhood and adolescence will be an important step in understanding the development of emotion processing and how abnormalities arise (Cicchetti & Dawson, 2002; Hyde et al., 2011; Swartz & Monk, in press).
Theoretical frameworks have identified several key neural networks that play a role in emotional face processing (Burnett et al., 2011; Haxby et al., 2002; Nelson, et al., 2005; Scherf et al., 2012). The “core” face processing network, composed of the fusiform gyrus, inferior occipital cortex, and posterior superior temporal sulcus (STS), is involved in the perceptual processing of faces (e.g., recognizing a stimulus as a face). In addition, emotional face processing consistently activates regions in limbic and prefrontal areas associated with evaluating and regulating responses to emotional stimuli (sometimes referred to as “extended” face processing regions), including the amygdala, orbitofrontal cortex, ventrolateral prefrontal cortex, and anterior cingulate cortex (Fusar-Poli et al., 2009; Tahmasebi et al., 2012).
The extended face processing circuitry comprising the amygdala and prefrontal cortex is of particular interest for the development of socio-emotional function, given its role in interpreting and regulating responses to emotional faces. Ventral regions of the prefrontal cortex receive signals from the amygdala and send signals to regulate the amygdala through direct white matter pathways, including the uncinate fasciculus, one of the major white matter tracts connecting the frontal lobe with the temporal lobe and limbic system (Petrides & Pandya, 2002). Diffusion tensor imaging (DTI) measures the microstructural properties of white matter tracts (Thomason & Thompson, 2011), which we refer to as structural connectivity. One of the most frequently examined measures of structural connectivity is fractional anisotropy (FA) or the degree to which water molecules diffuse along one direction, which may relate to myelination, fiber organization, or axonal packing (Beaulieu, 2002). Higher FA is interpreted as indicating greater structural connectivity between regions.
Studies conducted in adults that have combined DTI and functional MRI (fMRI) suggest that structural connectivity of the uncinate fasciculus is related to activation as well as connectivity within prefrontal cortex-amygdala circuitry. In particular, FA within this white matter region has been shown to relate to amygdala activation to fearful faces (Kim & Whalen, 2009), and functional connectivity between the anterior cingulate cortex and amygdala during emotion processing (Tromp et al., 2012). These results suggest that, for adults, greater structural connectivity within the uncinate fasciculus may facilitate communication between the prefrontal cortex and amygdala, supporting prefrontal regulation of amygdala activity (Tromp et al., 2012). However, because brain structure and function undergo substantial changes during the periods of childhood and adolescence, it is still not known how structural connectivity relates to prefrontal cortex-amygdala function in early life.
Longitudinal and cross-sectional studies of the uncinate fasciculus generally demonstrate a pattern of increased FA with advancing age across childhood and adolescence (Lebel & Beaulieu, 2011; Lebel et al., 2008). Longitudinal studies have also demonstrated variability in individual trajectories, with some individuals demonstrating increases, decreases, or maintained levels of FA in this tract over time (Lebel & Beaulieu, 2011). Given prior observed variance in white matter integrity in youth, it is possible that developmental variation in white matter could be associated with differences in brain function or psychosocial outcomes.
Several fMRI studies have demonstrated age-related changes in neural activation associated with emotional face processing across childhood, adolescence, and adulthood. For instance, in a large cross-sectional study with participants ranging in age from 4 to 22 years old, Gee and colleagues (2013) demonstrated a linear decrease with age in amygdala activation to fearful faces. Other studies have demonstrated greater amygdala activation to emotional faces in adolescents relative to adults (Guyer et al., 2008; Hare et al., 2008; Monk et al., 2003; Passarotti et al., 2009). Research focusing strictly on child and adolescent samples has also shown changes in emotion processing associated with development. These changes include increased amygdala response to sad faces with age during early adolescence (Pfeifer et al., 2011), as well as decreased amygdala activation to neutral faces and decreased ventrolateral prefrontal cortex activity to fearful faces with pubertal development (Forbes et al., 2011). Overall, these results suggest a complex, non-linear pattern of development dependent on the nature of the emotion processing task and emotional stimuli used, with the most consistent trend indicating that amygdala activation to emotional faces decreases from adolescence to adulthood.
There is also emerging evidence for changes in prefrontal cortex-amygdala functional connectivity from childhood to adulthood. Using a psychophysiological interaction analysis, Gee et al. (2013) demonstrated a shift in the direction of functional connectivity from childhood to adulthood with the youngest age group (4 to 9 years old) exhibiting positive amygdala-rostral anterior cingulate connectivity while viewing fearful faces whereas older participants evidenced negative functional connectivity that grew stronger with age. This shift from positive to negative connectivity was suggested to reflect increased prefrontal regulation of amygdala activation with age. Another study implementing a correlational functional connectivity analysis found that across children, adolescents, and adults, the amygdala was negatively connected with the ventral prefrontal cortex during an emotional face go/no go task and the strength of connectivity related to greater amygdala habituation (Hare, et al., 2008). However, although the amygdala and ventral prefrontal cortex showed differences in activity across the three age groups, changes in connectivity with age were not directly tested. A different study by Guyer and colleagues (2008) directly compared functional connectivity across adolescents and adults, and reported no difference in connectivity between prefrontal and amygdala regions between groups during emotion processing, indicating that these effects may be dependent on the task performed or the functional connectivity analytical approach.
All together, research that examines amygdala function and connectivity in early life has shown that structural and functional connectivity between the prefrontal cortex and amygdala increases with age and amygdala activation to emotional faces decreases with age across childhood and adolescence. Though generally examined separately, it is important to consider brain structure and function simultaneously when examining development, as it is possible that changes in brain structure constrain changes in function, or vice versa (Cicchetti & Dawson, 2002). Moreover, it is important to include behavioral measures in order to examine how changes in brain structure and function relate to emotion regulation. The only study yet to examine these relations in an adolescent sample used event-related potentials (ERPs; Taddei et al., 2012). Taddei et al. (2012) found that N400 ERP amplitudes (a response evoked by viewing emotional faces) to angry faces measured at ages 8–9 negatively predicted FA in the left uncinate fasciculus at ages 14–15. Moreover, scores on a measure of harm avoidance collected during childhood negatively predicted right uncinate fasciculus FA values in adolescence. These results demonstrate that neural activity in response to processing faces is related to structural connectivity of the uncinate fasciculus; however, because of the use of ERPs in this study, the relation between uncinate fasciculus structural connectivity and amygdala activation or functional connectivity during adolescence remains untested.
The objective of the present study was to examine the relation between structural connectivity of the uncinate fasciculus, functional activation and connectivity of prefrontal cortex-amygdala circuitry, and a measure associated with emotion regulation difficulties (internalizing symptoms) during the periods of late childhood and adolescence. Our first hypothesis was that greater structural connectivity of the uncinate fasciculus would predict reduced amygdala activation to emotional faces. Second, we hypothesized that increased structural connectivity of the uncinate fasciculus would predict greater functional connectivity between the amygdala and prefrontal cortex. Third, we hypothesized that increased functional connectivity would predict decreased amygdala activation to emotional faces. Fourth, we hypothesized that greater structural and functional connectivity, as well as decreased amygdala activation, would predict lower internalizing symptoms. Fifth, we examined whether the brain structure-function relationship was moderated by age. Because this circuitry is undergoing development during childhood and adolescence, the strength of the relationship between brain structure and function may differ across this age range.
2. Methods
2.1 Participants
Participants were recruited from the community through fliers. Parents reported that participants had no history of psychiatric diagnoses. Moreover, all participants were below the clinical cutoff score for internalizing symptoms on the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001). Participants 18 years and older provided informed consent; minor participants gave assent and their parents signed informed consent forms. A total of 79 participants between 8 and 19 years of age underwent fMRI scanning. Nineteen participants were removed from analyses due to: movement >3mm in any direction (4 participants), technical problems during scanning (2 participants), accuracy <70% on the behavioral tasks (2 participants), poor normalization or signal dropout within the amygdala or prefrontal cortex (10 participants), and showing elevated scores on a measure of autism symptoms (1 participant), leaving a total of 60 participants with valid fMRI data. Of these participants, 49 also completed DTI during the same scanning session. One participant was removed for exhibiting >3mm movement during DTI scanning and 9 participants were removed due to white pixel artifact in the DTI images. Removal of these participants resulted in a total of 39 participants (72% male) with fMRI and DTI data (Table 1). FMRI data from these participants have been reported previously (Swartz et al., 2013; Weng et al., 2011; Wiggins et al., 2012).
Table 1.
Participant Characteristics
| Measure | Mean (SD) | Min-Max |
|---|---|---|
| Age (years) | 15.3 (2.5) | 9.6–19.2 |
| CBCL Internalizing T scores | 44.8 (7.9) | 33–64 |
| Gender ID Task Accuracy | 96.6% (3.9) | 80–100% |
| Gender ID Task RT (ms) | 729.6 (133) | 536–1042.3 |
| ER Task Accuracy | 89% (6.4) | 71–98% |
| ER Task RT (ms) | 1180.1 (252) | 810.2–2104.0 |
| Handedness (percentage left-handed) | 21% |
Note. CBCL = Child Behavior Checklist; Gender ID task = gender identification task performed during scanning; RT=reaction time; ER task = emotion recognition task performed after scanning. One participant was missing data for the CBCL.
2.2 Procedure
2.2.1 Gender identification task completed during fMRI scanning
Participants underwent an implicit emotion processing task during fMRI scanning. A trial of this task consists of a fixation cross (500 ms), followed by a face (250 ms), and then a black screen (1500 ms), during which participants identify the gender of the face by pressing the thumb button for male or the index finger for female on a button box. Faces were from the NimStim set (Tottenham et al., 2009). Response times and accuracy were recorded. There were two runs of this task with 60 trials each for a total of 120 trials. There were 30 trials each of the following face expressions: fearful, happy, sad, and neutral.
2.2.2 Emotion recognition task completed after scanning
Participants completed a similar version of this task post-scanning in which they were asked to identify the emotion (rather than the gender) of the faces presented. The stimuli used and presentation times were the same as for the fMRI task. As stated above, participants that performed gender identification or emotion recognition with lower than 70% accuracy were removed from the sample.
2.2.3 fMRI data acquisition
MRI images were acquired with a 3 Tesla GE Signa Scanner. A high resolution SPGR image was collected for anatomical reference. Functional data were collected with the following parameters: T2*-weighted BOLD images collected with a reverse spiral sequence, TR=2,000 ms, TE=30 ms, 40 adjacent 3 mm axial slices, flip angle=90 degrees, FOV=22 cm, matrix = 64 × 64.
2.2.4 DTI data acquisition
DTI was conducted following fMRI scanning with the following parameters: spin-echo EPI diffusion sequence, TR=9000 ms, TE=82.3 ms, FOV=22 cm, 39 slices, thickness=3mm, skip=1mm, 15 diffusion-weighted acquisitions with b = 800 s/mm2, two averages. One non-diffusion weighted image (b=0 s/mm2) was also collected in order to transform the diffusion-weighted images to a template in MNI space.
2.2.5 Symptom measure
Internalizing symptoms were measured using T scores from the Child Behavior Checklist (CBCL) Internalizing scale (Achenbach & Rescorla, 2001). This scale was chosen as it provides a general measure of internalizing problems with a relatively broad range in a typically developing sample, whereas measures used to assess specific clinical symptoms related to disorders may not have produced a sufficient range within a non-clinical sample.
2.3 Analyses
2.3.1 fMRI data analysis
FMRI data underwent standard pre-processing including slice timing correction, realignment, co-registration of the anatomical to the functional images, normalization of the images to the SPM template in MNI space, and smoothing with an 8 mm FWHM Gaussian kernel (see Weng, et al., 2011 for further details).
Condition effects were modeled at the individual subject level with the SPM canonical hemodynamic response function and a temporal derivative. Incorrect trials were modeled as a separate condition and excluded from analyses. Group-level analyses were conducted in SPM8.
2.3.2 Functional connectivity analysis
Psychophysiological interaction (PPI) analysis was used to examine functional connectivity during emotional face processing. PPI allows for the examination of how psychological conditions modulate the connectivity between two regions (Friston et al., 1997). Time courses for amygdala activation were extracted from the left or right amygdala, defined structurally using amygdala regions of interest generated from the Wake Forest University Pickatlas (WFU Pickatlas; Maldjian et al., 2003). Regressors for the PPI included amygdala response extracted from the anatomically-defined left or right amygdala, condition effects, and the interaction between these (amygdala response x condition effects). For our primary analyses, we used the contrast of all faces > baseline as the psychological condition. Therefore, the PPI (amygdala response x all faces vs. baseline) indicates regions where connectivity with the amygdala was modulated by viewing faces. Based on the results of the first hypothesis, we also conducted secondary analyses using the emotion specific contrasts of sad > neutral and happy > neutral in order to determine modulation of connectivity based on the emotional content of faces.
Several steps were taken in order to control for movement in the PPI analyses. First, the six parameters from the realignment procedure were entered as regressors when estimating the individual models of the PPI. Second, in line with the procedure used by Van Dijk, Sabuncu, and Buckner (2012), mean framewise (volume-to-volume) displacement was calculated for each participant and entered as a regressor into second-level group analyses.
2.3.3 DTI data analysis
Diffusion-weighted images were analyzed using the FMRIB's Diffusion Toolbox (FDT) in FSL (Smith et al., 2004). First, DTI images underwent eddy current correction and linear registration to the non-diffusion weighted image in order to correct for head motion. Next, brain extraction was conducted using BET. Subsequently, the dtifit procedure in FSL was used to fit diffusion tensor models at each voxel and create an FA image for each participant. FA images were then processed using tract-based spatial statistics (TBSS) in FSL (Smith et al., 2006). FA images were realigned to the FMRIB standard-space image and transformed into MNI standard space. A mean FA skeleton was created and thresholded at .2 and each participant's FA data were projected onto the skeleton.
Regions of interest were created using the Johns Hopkins University White Matter Tractography Atlas (Mori et al., 2005). The left and right uncinate fasciculus regions were binarized and skeletonized in order to extract mean FA values from the left and right uncinate fasciculus for each participant.
2.3.4 Age-related change in uncinate fasciculus structural connectivity, amygdala activation to faces, and prefrontal cortex-amygdala connectivity
Before testing our primary hypotheses, we examined cross-sectional change for our main measures of interest. The correlation between FA and age was tested using Pearson's correlation, conducted in SPSS Software version 20. Regression analysis performed in SPM was used to measure age-related changes in amygdala activation and prefrontal cortex-amygdala functional connectivity.
For this and all subsequent analyses performed in SPM, significance was assessed at p<0.05 family-wise error (FWE) corrected using anatomically-defined ROIs created with the WFU Pickatlas. Significance for amygdala activation was tested with the bilateral amygdala ROI and prefrontal cortex-amygdala connectivity was tested with the bilateral anterior cingulate cortex (defined using the Automated Anatomical Labeling atlas), and ventromedial prefrontal cortex (defined using Brodmann's Areas 10 and 11) ROIs based on previous research (Gee, et al., 2013; Hare, et al., 2008; Tromp, et al., 2012).
For these analyses, the effects of the contrast of all faces > baseline were tested first. This approach is warranted because age-related changes in amygdala activation have been observed in response to all faces (Hare, et al., 2008), fearful faces (Gee, et al., 2013), sad faces (Pfeifer, et al., 2011), and neutral faces (Forbes, et al., 2011). Thus, hypotheses about specific a priori emotions were not selected in advance. When significant effects were observed, emotion-specific effects were examined as post-hoc analyses.
2.3.5 Hypothesis 1: Relation between uncinate fasciculus structural connectivity and amygdala activation to emotional faces
The relation between uncinate fasciculus FA and amygdala activation was examined by conducting multiple regression analyses in SPM8. First, we examined the relation of FA to amygdala activation to all faces by regressing mean FA values extracted from the left or right uncinate fasciculus onto the contrast of all faces vs. baseline. Significance was tested for the regression of left FA values with the left amygdala ROI and right FA values with the right amgydala ROI. Because this analysis involved two comparisons (left and right amygdala), Bonferroni correction was set to p<0.025. This was followed up with tests of emotion-specific effects by regressing FA onto the contrast of each emotion (fearful, happy, sad, neutral) vs. baseline.
To examine whether the relationship between amygdala activation and FA values was specific to the uncinate fasciculus, or related to white matter connectivity across the brain more broadly, we conducted control analyses in white matter regions where we did not expect to observe similar effects. That is, we selected three major white matter tracts as control regions: superior longitudinal fasciculus (SLF; linking the prefrontal cortex with the posterior parietal cortex), inferior longitudinal fasciculus (ILF; running from the occipital cortex to the temporal cortex), and corticospinal tract (CST; linking the motor cortex with the brain stem and spinal cord; Petrides & Pandya, 2002; Thomason & Thompson, 2011). We examined correlations between amygdala activation and mean FA extracted from (a) the uncinate fasciculus, (b) the SLF, (c) the ILF, and (d) the CST. Control analyses were conducted in SPSS using mean contrast values extracted from the amygdala ROI.
Finally, we conducted additional analyses in SPM using neutral faces as baseline rather than fixation to further examine the relation between amygdala activation to emotional faces and uncinate fasciculus FA. Based on the results obtained for the analyses described above, we tested the following contrasts: left amygdala activation to sad > neutral faces and left amygdala activation to happy > neutral faces. The purpose of these analyses was to investigate whether the relationship between FA and amygdala activation was specific to processing associated with the emotional content of faces, rather than face processing more generally.
2.3.6 Hypothesis 2: Relation between structural connectivity and amygdala-prefrontal cortex functional connectivity
We used a similar approach as outlined for the hypothesis above, except that, here, we tested the relation between FA and functional connectivity. Specifically, we regressed FA values onto the PPI images in SPM in order to examine regions of the prefrontal cortex where connectivity with the amygdala during face processing related to uncinate fasciculus FA.
2.3.7 Hypothesis 3: Relation between functional connectivity and amygdala activation to emotional faces
We examined the relation between amygdala activation to all faces vs. baseline and prefrontal cortex-amygdala connectivity for the PPI of all faces vs. baseline. Similar to the approach used for the first and second hypotheses, we did this by extracting mean contrast values for left or right amygdala activation to all faces vs. baseline and regressing amygdala activation onto the PPI images in SPM.
2.3.8 Additional analyses
We examined the relation between age and task performance in order to assess potential confounds (Table 2). Because gender identification RT, emotion recognition accuracy, and emotion recognition RT were related to age, any significant results for the first three hypotheses were re-examined with RT or accuracy as a covariate. Significant results were also re-examined controlling for handedness and gender, as gender differences have been shown in neural development during adolescence (Schmithorst & Yuan, 2010; Tahmasebi, et al., 2012).
Table 2.
Bivariate Correlations between Age, Task Performance, and Symptom Scores
| Age | |
|---|---|
| Gender ID Accuracy | r =.04, p = .79 |
| Gender ID RT | r = −.49, p = .002 |
| ER Accuracy | r =.35, p = .03 |
| ER RT | r = −.44, p = .005 |
| CBCL Internalizing T score | r = −.29, p = .08 |
Note. Bold signifies a significant correlation.
Gender ID=gender identification task performed during scanning; RT=reaction time; ER=emotion recognition task performed after scanning; CBCL= Child Behavior Checklist.
2.3.9 Hypothesis 4: Relation between structural connectivity, function, and internalizing symptoms
We used a similar approach as described above for testing the relation between our measures and age in order to examine the relation with internalizing symptoms. Specifically, we tested the Pearson's correlation between FA values and CBCL scores in SPSS and we used regression analyses in SPM in order to assess the relation between CBCL scores and amygdala activation and prefrontal cortex-amygdala connectivity.
2.3.10 Hypothesis 5: Moderation by age
The hypothesis that the relation between brain structure and function would be moderated by age was examined by conducting a moderation analysis using the PROCESS macro in SPSS (Preacher & Hayes, 2004). In order to examine amygdala activation in SPSS, mean contrast values were extracted from the anatomically-defined amygdala ROI. Based on the results for the first hypothesis, we used the contrasts of sad vs. neutral and happy vs. neutral for this analysis. We tested whether age moderated the relation between FA values and amygdala activation. Moderation is examined by testing whether the interaction between the predictor variable (structural connectivity) and the moderator variable (age) significantly predicts the outcome variable (amygdala activation). If the interaction is significant, then the effect of the predictor variable on the outcome differs depending on the level of the moderator. We followed up significant interactions with the Johnson-Neyman approach, which identifies the level of the moderator at which point an effect changes from significant to non-significant (Hayes & Matthes, 2009).
3. Results
3.1 Cross-Sectional Changes with Age
In line with previous research, we observed significant age-related increases in FA in the left (r =.46, p=.003) and right uncinate fasciculus (r=.42, p=.007; Figure 1). Moreover, we observed a negative relation between age and left amygdala activation to all faces vs. baseline (p=.01; Table 3; Figure 2). Post-hoc analyses demonstrated that this effect held for each emotion (Table 3). There was no significant relation with age for left or right amygdala-prefrontal cortex connectivity.
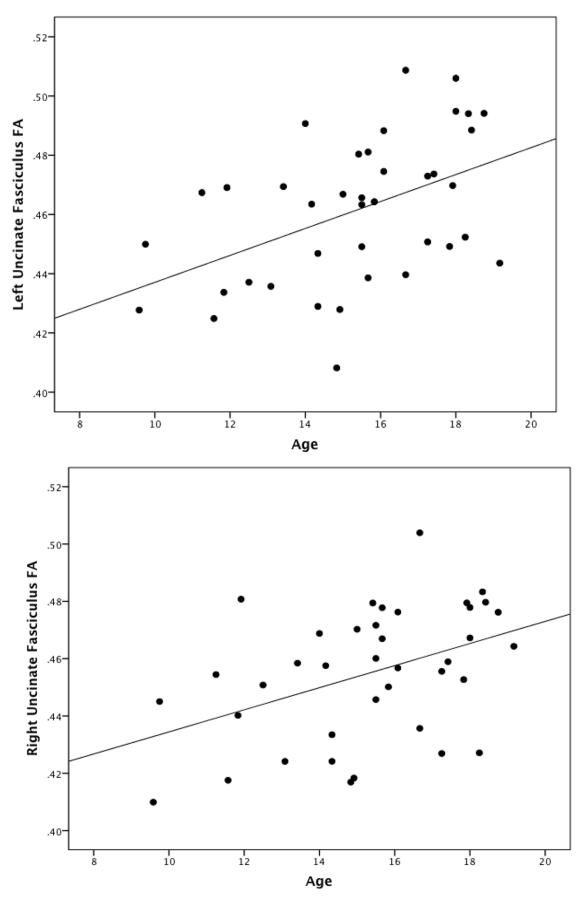
Figure 1.
Cross-sectional change with age in left and right uncinate fasciculus fractional anisotropy (FA) values. Age is positively correlated with mean FA values extracted from the left uncinate fasciculus (r=.46, p=.003) and right uncinate fasciculus (r=.42, p=.007).
Table 3.
Left Amygdala Activation to Faces Decreases with Age
| Contrast | Negative effect of age on activation, t(37)= | P-value (FWE-corrected) | MNI coordinates (xyz) |
|---|---|---|---|
| All Faces vs. Baseline | 3.75 | .011 | −26, −2, −26 |
| Fearful vs. Baseline | 3.91 | .004 | −20, −8, −10 |
| Sad vs. Baseline | 3.14 | .023 | −20, −2, −14 |
| Happy vs. Baseline | 2.81 | .045 | −20, −8, −10 |
| Neutral vs. Baseline | 2.87 | .045 | −22, −2, −14 |
Note. FWE-correction is based on structurally-defined bilateral amygdala region of interest for contrast of all faces vs. baseline and left amygdala region of interest for emotion-specific contrasts.
FWE=family-wise error; MNI=Montreal Neurological Institute.
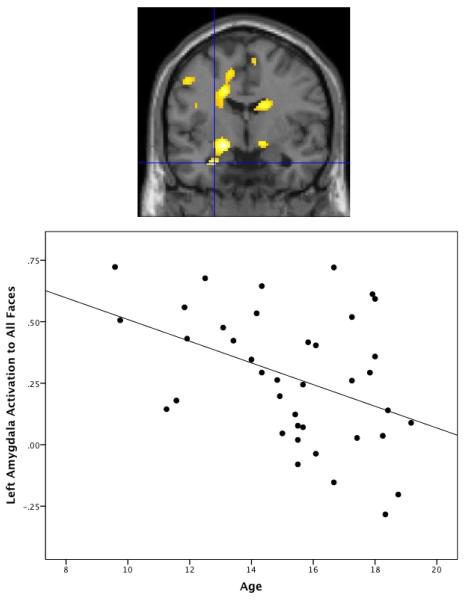
Figure 2.
Cross-sectional change with age in left amygdala activation to all faces vs. baseline. Age is negatively related to left amygdala activation to all faces, t(37)=3.75, FWE-corrected p=.011, xyz=−26, −2, −26. Figure demonstrates the negative effect of age on activation and is thresholded at p<.01 uncorrected in order to demonstrate extent of activation. Scatterplot demonstrates mean amygdala activation extracted from structurally-defined left amygdala region of interest.
3.2 Hypothesis 1: Relation between Uncinate Fasciculus FA and Amygdala Activation
In support of our first hypothesis, there was a negative relation between left uncinate fasciculus FA values and left amygdala activation to all faces vs. baseline (p=.01; Table 4). Post-hoc analyses indicated this relationship was driven by left amygdala activation to sad (p=.004) and happy faces (p=.005; Table 4; Figures 3–4; Supplementary Figure 1). These effects remained significant when controlling for gender, handedness, reaction time on the task performed during scanning, emotion recognition accuracy, and emotion recognition reaction time. Our first hypothesis was not supported for the right amygdala, although the negative relationship between right uncinate fasciculus FA values and right amygdala activation to all faces approached significance, t(37)=2.62, p=.065. Notably, when examining emotions separately, we observed a similar pattern of negative relations between right uncinate fasciculus FA values and right amygdala activation for happy, sad, and neutral faces (p's < .05), although these did not survive Bonferroni correction for multiple comparisons.
Table 4.
Relation between Left Uncinate Fasciculus Fractional Anisotropy and Left Amygdala Activation to Faces
| Contrast | Regression for negative effect of FA on activation, t(37)= | P-value (FWE-corrected) | MNI Coordinates (xyz) |
|---|---|---|---|
| All Faces vs. Baseline | 3.49 | .01 | −22, −4, −14 |
| Fearful vs. Baseline | 2.15 | .15 | −22, −2, −14 |
| Sad vs. Baseline | 3.87 | .004 | −26, 2,−18 |
| Happy vs. Baseline | 3.76 | .005 | −24, −6, −14 |
| Neutral vs. Baseline | 1.64 | .33 | −22, −2,−14 |
Note. Effects of left uncinate fasciculus fractional anisotropy values were examined with FWE-correction for the structurally defined left amygdala region of interest.
FA=fractional anisotropy; FWE=family-wise error; MNI=Montreal Neurological Institute.
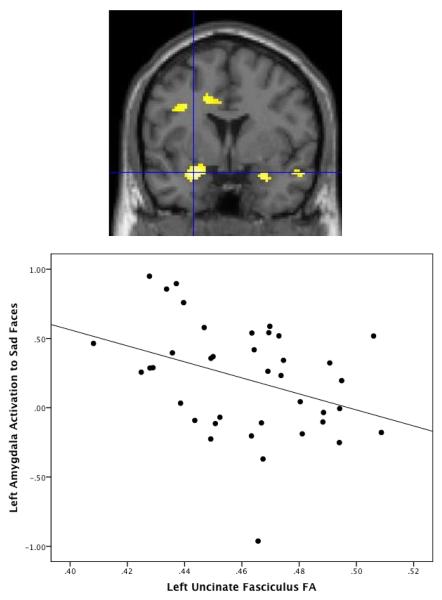
Figure 3.
Relation between fractional anisotropy (FA) and amygdala activation to sad faces. There is a negative relation between FA within the left uncinate fasciculus and left amygdala activation to sad faces vs. baseline, t(37)=3.87, FWE-corrected p=.004, xyz=−26, 2, −18. Amygdala values for scatterplot are extracted using structural left amygdala ROI.
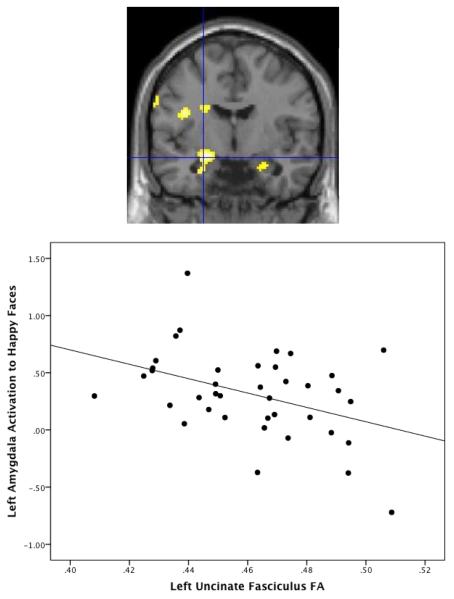
Figure 4.
Relation between fractional anisotropy (FA) and amygdala activation to happy faces. There is a negative relation between FA within the left uncinate fasciculus and left amygdala activation to happy faces vs. baseline, t(37)=3.76, FWE-corrected p=.005, xyz=−24, −6 −14. Amygdala values for scatterplot are extracted using structural left amygdala ROI.
In order to determine the specificity of the results, we examined the relation between amygdala activation and the control ROIs. We performed this control analysis with left amygdala activation and FA extracted from the left hemisphere of the control ROIs. As expected, the only significant predictor of left amygdala activation to sad or happy faces was FA within the left uncinate fasciculus; mean FA of other white matter tracts did not relate to amygdala activation (Table 5).
Table 5.
Correlations between left amygdala activation (sad, happy, fearful or neutral faces vs. baseline) and average fractional anisotropy in the left uncinate fasciculus, as well as in the left superior longitudinal fasciculus, inferior longitudinal fasciculus, and corticospinal tract, the latter 3 of which served as control white matter tracts.
| White matter tract | Correlation with left amygdala activation to sad faces vs. baseline | Correlation with left amygdala activation to happy faces vs. baseline | Correlation with left amygdala activation to fearful faces vs. baseline | Correlation with left amygdala activation to neutral vs. baseline |
|---|---|---|---|---|
| Left uncinate fasciculus | r = −.36, p = .024 | r = −.41, p = .009 | r = −.17, p = .30 | r = −.08, p = .61 |
| Left superior longitudinal fasciculus | r = −.21, p = .19 | r = −.21, p = .19 | r = −.18, p = .28 | r = −.13, p = .43 |
| Left inferior longitudinal fasciculus | r = −.18, p = .28 | r = −.11, p = .50 | r = −.10, p = .54 | r = −.05, p = .78 |
| Left corticospinal tract | r = −.23, p = .17 | r = −.09, p = .59 | r = −.05, p = .78 | r = −.07, p = .71 |
Note. Bold indicates a significant correlation.
We conducted additional analyses using neutral faces rather than fixation as baseline to determine whether the same pattern of results held when the contrast was restricted to emotional content of faces, as opposed to face processing more generally. Although significance levels were somewhat reduced, we found that the relation between left uncinate fasciculus FA and left amygdala activation held when comparing activations for sad > neutral faces, t(37)=2.76, p=.05, xyz=−26, 2, −18, and happy > neutral faces, t(37)=3.48, p=.01, xyz=−22, −8, −14. We chose to use these contrasts for testing the fourth and fifth hypotheses so that we could draw conclusions regarding amygdala activation related to the emotional content of the faces specifically.
3.3 Hypothesis 2: Relation between Structural Connectivity of the Uncinate Fasciculus and Functional Connectivity
There was no relation between uncinate fasciculus FA and left or right amygdala connectivity with the ventral prefrontal cortex for the condition of all faces vs. baseline. Based on the results of the first hypothesis, we also examined left amygdala connectivity for the conditions of sad vs. neutral and happy vs. neutral faces, but there were also no significant relations for the emotion-specific comparisons.
3.4 Hypothesis 3: Relation between functional connectivity and amygdala activation to emotional faces
We did not observe a significant relation between left or right amygdala activation to all faces vs. baseline and functional connectivity for all faces vs. baseline.
3.5 Hypothesis 4: Relation between Structural Connectivity, Function, and Internalizing Symptoms
We observed the predicted positive relationship between amygdala activation to sad vs. neutral faces and CBCL scores, left amygdala: t(36)=3.64, p=.015, xyz=−22, −10, −12 and right amygdala: t(36)=3.13, p=.049, xyz=22, −6, −14, indicating that greater amygdala activation to sad faces predicts more internalizing symptoms. There was no significant relationship between CBCL scores and amygdala activation to happy vs. neutral faces, or structural or functional connectivity.
3.6 Hypothesis 5: Moderation by Age
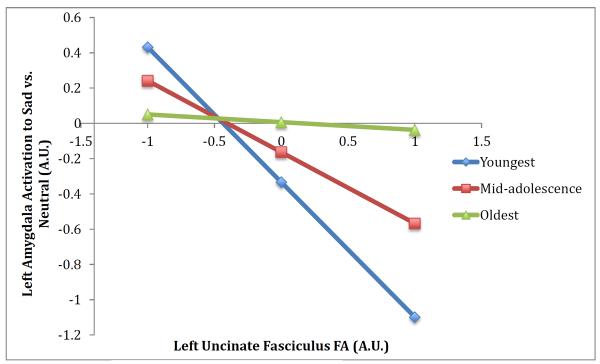
We tested whether age moderated the association between left uncinate fasciculus FA values and left amygdala activation to sad or happy faces vs. neutral faces. The regression including the moderation effect for amygdala activation to sad vs. neutral faces was significant, R2=.20, F(3, 35)=2.90, p=.049., indicating that the interaction of age x FA values predicted amygdala activation, B=.14, SE=.07, t(35)=2.07, p=.046. As shown in Figure 5, the relation between uncinate fasciculus FA values and amygdala activation to sad vs. neutral faces was only significant within younger participants; within older participants this effect was no longer present. Using the Johnson-Neyman approach, the cutoff value of 15.7 years represented the point at which this relation was no longer significant. There was no moderation effect for left uncinate fasciculus FA values and left amygdala activation to happy vs. neutral faces.
Figure 5.
The association between structural connectivity and amygdala activation to sad faces is moderated by age. Predicted outcomes for amygdala activation were estimated using the regression equation for the moderation effect at three different levels of the moderator variable: mean age of sample (mid-adolescence), 1 standard deviation below (youngest), and 1 standard deviation above (oldest) in order to visualize the interaction.
4. Discussion
This study contributes to our understanding of brain structure-function relationships in childhood and adolescence by revealing three primary findings. First, this is the first study to demonstrate that increased FA within the uncinate fasciculus predicts reduced amygdala activation to emotional faces in children and adolescents. Second, we demonstrated that increased amygdala activation to sad faces predicts greater internalizing symptoms. Finally, we observed that the relation between uncinate fasciculus FA and amygdala activation to sad faces is moderated by age, with younger participants demonstrating a stronger negative relation.
Previous research with pediatric samples had demonstrated age-related increases in uncinate fasciculus FA (Lebel & Beaulieu, 2011) and decreases in amygdala activation to emotional faces from childhood to adulthood (Gee, et al., 2013). However, until the present study, no work had demonstrated a direct relation between structural connectivity and amygdala function during these developmental periods. The finding of a negative relationship between uncinate fasciculus FA and amygdala activation suggests that increased structural connectivity of the uncinate fasciculus may improve communication between the prefrontal cortex and amygdala, allowing for more efficient regulation of amygdala activity. Therefore, it may be that increased structural connectivity with age facilitates regulation of the amygdala, leading to the declines in amygdala activation from childhood through adolescence observed in the present study and in previous research. Future research with longitudinal data will be necessary in order to test the direction of this effect, and whether structural connectivity predicts changes in amygdala activation or vice versa.
Additionally, the relation between amygdala activation to sad faces and internalizing symptoms suggests that structural connectivity may indirectly affect risk for developing internalizing problems. Specifically, reduced structural connectivity predicts greater amygdala activation to sad faces, and this in turn predicts more internalizing symptoms. Individuals with decreased structural connectivity may therefore be more likely to exhibit amygdala hyper-activation, which could then place them at risk for the development of internalizing problems.
A significant moderation effect demonstrated that uncinate fasciculus FA values were more predictive of amygdala activation to sad vs. neutral faces in younger relative to older participants. This may be due to greater variability in amygdala activation in late childhood and early adolescence, leading to a stronger relationship between structural connectivity and amygdala activation in younger participants. If confirmed in future research, this moderation effect suggests that the periods of late childhood and early adolescence may be sensitive periods during which structural connectivity of the uncinate fasciculus plays a particularly important role in emotion processing and regulation, given that amygdala activation may be more variable during these developmental stages.
We observed that the relation between uncinate fasciculus FA and amygdala activation was only significant for the left hemisphere and was specific to sad and happy faces. This relation approached significance in the right hemisphere and we observed negative relations between right uncinate fasciculus FA and right amygdala activation to sad, happy, and neutral faces; therefore, we suggest that future research with larger samples would likely produce results that survive correction for multiple comparisons in both hemispheres. Interestingly, although amygdala activation to fearful and neutral faces decreased with age, it did not relate to uncinate fasciculus FA. One potential explanation for this observation is that additional or alternative factors predict amygdala activation to fearful and neutral faces in children and adolescents, such as greater emotion recognition with age. Notably, the relation between amygdala activation and internalizing symptoms was specific to sad faces. This concurs with previous research on abnormal processing of sad faces in depression (Fritzsche et al., 2010) and suggests that amygdala activation to sad faces specifically may be predictive of internalizing problems.
Of note, we did not observe significant relations between functional connectivity and age, structural connectivity, or amygdala activation. Importantly, structural and functional connectivity measure two distinct phenomena, and it has been noted that functional connectivity is often observed between two regions known to have no direct structural connections (Buckner, Krienen, & Yeo, 2013). Therefore, it may not be the case that structural connectivity between two regions will relate to functional connectivity in a uniform fashion, particularly in younger participants, for whom modulation of connectivity by psychological context (what is measured by PPI) may still be undergoing development. Thus, although previous research in adults has shown that structural connectivity relates to prefrontal cortex-amygdala functional connectivity (Tromp et al., 2012), this effect may not be as strong in younger participants and may only be robustly observed later in development. Indeed, theoretical frameworks of adolescent development have noted that prefrontal cortex activation and connectivity may have greater individual variability during this stage because functional measures may be highly dependent on the motivational context of the task and participants' strategy use (Crone & Dahl, 2012). Further research directly comparing the relation between structural and functional connectivity in adolescents and adults would help to shed light on these issues.
These findings have several implications for future research. First, if confirmed through longitudinal research, these results suggest that development across childhood and adolescence involves increased uncinate fasciculus structural connectivity accompanied by decreased amygdala activation to emotional faces, which may serve as neural correlates associated with the development of emotion regulation. An important question for future research is whether individuals who deviate from these trajectories, such as showing slower or premature increases in structural connectivity and decreases in amygdala activation relative to same-age peers, are at heightened risk for the development of psychopathology. Indeed, decreased uncinate fasciculus FA has been associated with a range of disorders including social anxiety disorder, generalized anxiety disorder, and major depressive disorder (Cullen et al., 2010; Phan et al., 2009; Tromp et al., 2012) whereas increased uncinate fasciculus FA has been associated with conduct disorder and obsessive compulsive disorder (Passamonti et al., 2012; Sarkar et al., 2013; Zarei et al., 2011). Altered trajectories of uncinate fasciculus development could be associated with the abnormal patterns of amygdala activation observed in these disorders (Etkin & Wager, 2007; Passamonti et al., 2010; Yang et al., 2010). Given that many studies conducted in pediatric samples find higher FA values in patients relative to controls (Passamonti et al., 2012; Sarkar et al., 2013; Zarei et al., 2011), it will be important to determine whether this is specific to the disorders studied in these papers or a function of the age of the samples.
Moreover, these findings suggest that individual differences in uncinate fasciculus structural connectivity are predictive of amygdala activation to emotional faces. Structural connectivity of the uncinate fasciculus has been associated with both genetic influences (Pacheco et al., 2009) and environmental risk factors (Eluvathingal et al., 2006). Therefore, uncinate fasciculus structural connectivity and age-related changes in amygdala activation (Wiggins, et al., 2012) may serve as neural mediators for identifying the influence of genetic and environmental factors on psychological development (Hariri & Weinberger, 2003; Hyde, et al., 2011).
Finally, the effect of age on moderating the relation between structural connectivity and amygdala activation suggests that uncinate fasciculus development may play a particularly important role in influencing amygdala activation during late childhood and early adolescence. If future research confirms this moderation effect, then these developmental stages may represent sensitive periods and could be informative regarding the periods when intervention may be most effective.
The limitations of this study warrant mention. First, because of the multi-modal imaging approach used, a greater number of participants had to be removed from the sample due to unusable fMRI or DTI data than if only one imaging modality had been selected. Nevertheless, our sample size for this study was larger than previous research combining fMRI and DTI data in adults (20 participants in Kim & Whalen, 2009) and is comparable to cross-sectional studies conducted in children and adolescents using only fMRI (45 participants in Gee et al., 2013; 38 participants in Pfeifer et al., 2011). Second, our sample contained a higher proportion of male than female participants. Given that internalizing symptoms tend to increase for girls more than boys during adolescence, the sample composition could have decreased our ability to observe relations between the neuroimaging measures and internalizing symptoms. Future research using samples with a higher proportion of girls may find effects even stronger than the ones found in the present sample. Third, because of the cross-sectional design, we are unable to draw conclusions regarding within-person trajectories in brain structure-function relations over this period. Future research will be necessary in order to test whether the cross-sectional trajectories observed in this study are also observed longitudinally and to better examine the direction of effects between these variables.
4.1 Conclusions
In conclusion, structural connectivity of a major white matter pathway predicts amygdala activation to emotional faces during the periods of childhood and adolescence. By using brain structure and function as neural mediators, future research may help shed light on the trajectories of typical and atypical development of emotion processing and how genetic and environmental factors interact to shape individual differences in these trajectories.
Supplementary Material
Highlights
We examined relation between frontolimbic white matter tract and amygdala activity.
Greater white matter connectivity predicts less amygdala activation in adolescents.
Moderation analysis shows that relation is strongest in youngest participants.
White matter connectivity may facilitate regulation of the amygdala.
Acknowledgments
This project was supported by an Autism Speaks award to C.S.M., a Michigan Institute for Clinical Health Research (MICHR) Pre-doctoral Fellowship to J.R.S. (supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number 2UL1TR000433), a MICHR Pre-doctoral Fellowship to J.L.W. (UL1RR024986), an Autism Speaks Fellowship to JLW, and a NARSAD award to M.E.T. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No author has a conflict of interest to report.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Unviersity of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nature Neuroscience. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev. 2011;35(8):1654–1664. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Dawson G. Multiple levels of analysis. Dev Psychopathol. 2002;14:417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Lim KO. Altered white matter microstructure in adolescents with major depression: A preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):173–183. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36(4):429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychphysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fritzsche A, Dahme B, Gotlib IH, Joormann J, Magnussen H, Watz H, von Leupoldt A. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol Med. 2010;40(5):815–826. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze SA, Politi P. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler A, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Functional neuroimaging of genetic variation in serotonergic neurotransmision. Genes, Brain and Behavior. 2003;2:341–349. doi: 10.1046/j.1601-1848.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene-environment interactions. Trends Cogn Sci. 2011;15(9):417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20:1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher L, van Zijl PCM. MRI Atlas of Human White Matter. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymoprhism. J Neurosci. 2009;29(19):6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, Calder AJ. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67(7):729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, Calder AJ. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PloS One. 2012;7(11):e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci. 2009;4(4):387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2002. pp. 31–50. [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell'Acqua F, Fahy T, Deeley Q, Murphy DGM. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: A diffuson tensor imaging study. Psychological Medicine. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Dahl RE. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Dev Cogn Neurosci. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72(1):16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich M, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Monk CS. Functional magnetic resonance imaging in developmental psychopathology: Using neural function as a window into the development and treatment of psychopathology. In: Lewis M, Rudolph K, editors. Handbook of Developmental Psychopathology. 3rd ed. in press. [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei M, Tettamanti M, Zanoni A, Cappa S, Battaglia M. Brain white matter organisation in adolescence is related to childhood cerebral responses to facial expressions and harm avoidance. Neuroimage. 2012;61(4):1394–1401. doi: 10.1016/j.neuroimage.2012.03.062. [DOI] [PubMed] [Google Scholar]
- Tahmasebi AM, Artiges E, Banaschewski T, Barker GJ, Bruehl R, Buchel C, Paus T. Creating probabilistic maps of the face network in the adolescent brain: A multicentre functional MRI study. Hum Brain Mapp. 2012;33(4):938–957. doi: 10.1002/hbm.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp DPM, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TRA, Nitschke JB. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry. 2012;69(9):925–934. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, Monk CS. Neural activation to emotional faces in adolescents with autism spectrum disorders. J Child Psychol Psychiatry. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Bedoyan JK, Carrasco M, Swartz JR, Martin DM, Monk CS. Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Hum Brain Mapp, epub ahead of print. 2012 doi: 10.1002/hbm.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Paulus MP. Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, James A. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. 2011;70:1083–1090. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.