Abstract
The P3 amplitude reduction is one of the most common correlates of externalizing. However, few studies have used experimental manipulations designed to challenge different cognitive functions in order to clarify the processes that impact this reduction. To examine factors moderating P3 amplitude in trait externalizing, we administered an n-back task that manipulated cognitive control demands, working memory load, and incentives to a sample of male offenders. Offenders with high trait externalizing scores did not display a global reduction in P3 amplitude. Rather, the negative association between trait externalizing and P3 amplitude was specific to trials involving inhibition of a dominant response during infrequent stimuli, in the context of low working memory load, and incentives for performance. In addition, we discuss the potential implications of these findings for externalizing-related psychopathologies. The results complement and expand previous work on the process-level dysfunction contributing to externalizing-related deficits in P3.
Keywords: Externalizing, P3, reward, punishment, cognitive control, working memory
Externalizing represents a latent construct that is thought to underlie a broad assortment of problematic traits and behaviors, including adolescent and adult antisocial behavior, conduct disorder, substance (alcohol and drug) dependence, and low behavioral constraint. The presence of a single externalizing factor reflects the purported shared etiology and pathophysiology that exists across these otherwise categorically distinct disorders and behaviors (Krueger et al., 2002; Buckholtz et al., 2010; Gorenstein & Newman, 1980; Krueger, Markon, Patrick & Iacono, 2005; Pridmore, Chambers & McArthur, 2005; Newman & Lorenz, 2003). While the expression of each specific subcomponent or diagnosis is shaped by other (e.g., environmental) influences, the classification of this broad, highly heritable trait disposition of externalizing identifies a vulnerability to the development of any of these disorders or behaviors. From this perspective, the identification of core psychobiological processes associated with the latent construct may increase understanding of how and why externalizing disorders develop.
When studying the psychobiological processes related to externalizing, it is hard to ignore the substantial evidence that such individuals display a reliable reduction in the amplitude of the P3 event-related potential (Iacono, Malone & McGue, 2003). Specifically, the vast majority of research indicates that externalizing is negatively associated with P3 amplitude in oddball paradigms, which involves responding to target stimuli that occur infrequently and unpredictably within a series of frequent stimuli. This reduced P3 to infrequent (oddball) stimuli has been reported in community, undergraduate, and incarcerated samples (Bernat, Hall, Steffen, & Patrick, 2007; Costa, Bauer, Kuperman, Porjesz, O’Conner, Hesselbrock, et al., 2000; Patrick, Bernat, Malone, Iacono, Krueger, & McGue, 2006). Perhaps the most reliable association between reduced P3 amplitude and externalizing comes from studies of at-risk individuals defined by their familial relationships (Iacono et al., 2002; Polich, Pollock, & Bloom, 1994). Additionally, this reduction in P3 is not only associated with “at risk” individuals, but a similar pattern has been reported with externalizing-related pathologies and behaviors (e.g., aggression, childhood conduct disorder, adult antisocial personality, substance abuse) (Bauer et al. 1994ab; Gao & Raine, 2009; Kim, Kim, & Kwon, 2001; McGue & Iacono, 2005; O’Connor et al., 1994; Patrick et al., 2006; Venables, Patrick, Hall & Bernat, 2011).
The evidence supporting the externalizing-related P3 amplitude reduction is relatively consistent across individuals at risk for externalizing and those with pathological externalizing; however, the processes modulating this effect are considerably less clear. P3 is a nonspecific measure that is often described as indexing stimulus evaluation and the intensity of concomitant executive function processes (e.g., updating working memory, applying cognitive control to inhibit a dominant response, and integrating information into existing networks)(Kok, 2001; Polich, 2007). Moreover, the literature on P3 and externalizing consists primarily of studies involving the oddball task, which taps the executive function process of cognitive control, as well as other attention-related processes (Folstein & Van Petten, 2008; Huettei & McCarthy, 2004). Therefore, due to the nonspecific qualities of the P3 and the limited experimental context previous research, it is unclear what component processes influence the externalizing-related P3 amplitude reduction. If the P3 is thought to be critical for identifying and classifying externalizing individuals, it is important to understand what it represents for this population and what factors may modulate its activity.
Outside the externalizing-P3 literature, researchers have found that executive functions such as cognitive control (e.g., measured by Stroop interference and oddball tasks) and working memory (e.g., measured by go/no-go discrimination tasks and n-back) are deficient in externalizing individuals (Dolan, Bechara & Nathan, 2008; Finn et al., 2009; Morgan & Lilienfeld, 2000). Moreover, externalizing individuals appear to be hypersensitive to motivational stimuli (e.g., reward, punishment) (Bjork, Chen, Smith, & Hommer, 2009), which at times also appears to exacerbate their executive function deficits (Bernat, Nelson, Steele, Gehring & Patrick, 2011; Endres, Rickert, Bogg, Lucas & Finn, 2011; Martin & Potts, 2004). However, little work has been done to examine the impact of these various forms of executive function (e.g., working memory, different cognitive control requirements) or experimental context (e.g., motivational stimuli, non-oddball tasks) on the P3 in externalizing individuals. Thus, the extent to which the reduced P3 of externalizing individuals reflects deficient cognitive control, limitations in working memory, hypersensitivity to motivational factors, or other factors has yet to be specified.
The goal of the present study was to evaluate the influence of components of executive functioning (e.g., cognitive control, working memory) and experimental context (e.g., incentives) on trait externalizing-related P3 modulation. While it is difficult to separate out the impact of a global executive function deficit from a more specific deficit, the present study sought to clarify the factors that may additively or interactively moderate the externalizing-related deficit in P3. More specifically, using a modified version of Casbon and colleagues’ (2003) n-back task, we examined the impact of cognitive control (i.e., alternation or inhibition of dominant responses in reaction to infrequent stimuli; Casbon, Curtin, Lang, & Patrick, 2003), working memory load (1-back and 2-back), and motivational context (incentives, specifically, reward and punishment) on P3 amplitude in a sample of incarcerated male offenders. Moreover, Krueger and colleagues (2002) note that externalizing represents a common latent pathway toward a variety of pathological behaviors, such as adult antisocial behavior, conduct disorder, alcohol dependence, drug dependence, and low constraint (Krueger et al., 2002). Accordingly, in addition to exploring the trait externalzing-P3 relationship, we conducted supplemental analyses to examine the association between externalizing-related psychopathologies and the P3 response. Ultimately, by clarifying the cognitive processes that impact the P3 response in externalizing individuals, we also may be able to more precisely classify and treat these individuals.
Method
Participants
Participants were 141 male inmates (30% African-American, 70% Caucasian) from a medium-security prison facility in Southern Wisconsin (mean age 31.04 years, SD=6.58). Individuals were recruited for participation if they met the following criteria: were between the ages of 18 to 45 years, had no history of psychosis or bipolar disorder, were not currently taking psychotropic medication, and scored 70 or higher on an estimate of IQ, the Wechsler Adult Intelligence Scale (WAIS-IQ: Wechsler, 1997). All participants provided written informed consent according to the procedures set forth by the University of Wisconsin–Madison Human Subjects Committee. Participants earned $20 for their completion of the self-report measures and the experimental task. Three outliers on the overall P3 response were identified using Studentized residuals (with Bonferroni-corrected p values <.05), and were excluded from analyses. The final sample consisted of 138 participants. Sample characteristics and descriptive statistics are presented in Table 1.
Table 1.
Means, Standard Deviations, and Ranges for Descriptive Variables
| Variable | Mean | Standard Deviation |
Range (Minimum-Maximum) |
|---|---|---|---|
| Demographic | |||
| Age | 31.04 | 6.58 | 18.00 – 45.00 |
| WAIS-IQ | 98.32 | 12.65 | 70.36 – 134.00 |
| Individual Differences | |||
| ESI Total Score (N=138) | 245.30 | 54.37 | 124.00 – 379.00 |
| Conduct Disorder Symptoms (N=138) | 4.27 | 3.26 | 0.00 – 14.00 |
| Adult Antisocial Symptoms (N=138) | 3.78 | 1.87 | 0.00 – 15.00 |
| MPQ-BF Constraint (reverse scored) (N=137) | 72.91 | 15.92 | 39.00 – 109.00 |
| DUDIT (N=131) | 16.81 | 12.72 | 0.00 – 44.00 |
| AUDIT (N=66) | 26.02 | 6.68 | 10.00 – 40.00 |
Note: WAIS-IQ=Wechsler Adult Intelligence Scale; MPQ-BF=Multidimensional Personality Questionnaire-Brief; DUDIT= Drug Use Disorders Identification Test; AUDIT=Alcohol Use Disorders Identification Test
Externalizing Spectrum Inventory (ESI; Hall, Bernat, & Patrick, 2007)
The ESI is a 100 item self-report measure that considers a range of disinhibitory and aggressive traits, along with tendencies toward substance abuse across the lifespan. This abbreviated version of the measure was developed from the original scale that consisted of 415 items (Krueger, Markon, Patrick, Benning, & Kramer, 2007). Total scores range from 100 to 400 and higher scores on the ESI-100 are shown to correlate with lower constraint and higher negative emotionality on the Multidimensional Personality Questionnaire-Brief Form (MPQ-BF; Patrick et al., 2002; Krueger et al. 1996). For this sample, the internal consistency (Cronbach’s alpha) was .97.
Conduct Disorder (CD) and Adult Antisocial Behavior (AAB)
Participants were assessed for antisocial symptoms during a semi-structured interview and file review. Following the Diagnostic Statistical Manual–IV (DSM-IV) criteria, CD scores were calculated by summing the number of endorsed childhood (15 years and younger) symptoms (of a possible 15 symptoms). AAB was calculated by summing together the number of adult (18 years and older) symptoms each participant endorsed (of a possible 7 symptoms). Fifteen participants had two raters for antisocial behaviors; interrater reliability for this subset of scores was .99 and .94 for CD and adult AAB, respectively.
Alcohol and Drug Use Disorders Identification Test (AUDIT, Saunders, Aasland, Babor, de la Fuente & Grant, 1993; DUDIT, Berman, Bergman, Palmstierna & Schlyter, 2005)
The AUDIT and DUDIT are widely used screening instruments that assess harmful and hazardous drinking and drug use, respectively. The AUDIT, a 10-item self-report measure has a total score range from 0 to 40, with a higher scores indicating problematic (i.e., dependent) drinking. The DUDIT, an 11-item self-report questionnaire, measures frequency of drug use, (drug-related problems, and drug dependence symptoms. Scores on the DUDIT range from 0 to 44, with higher scores suggestive of more severe (i.e., dependent) drug problems. These assessment measures focus on the year of heaviest drinking/drug use. Psychometrically, these instruments have demonstrated high internal consistency, test–retest reliability, convergent validity, sensitivity, and specificity (Reinert & Allen, 2007; Voluse, Gioia, Sobell, Dum, Sobell & Simco, 2012). In this sample, the internal consistency (Cronbach’s alpha) was .91 and .92, for the AUDIT and DUDIT, respectively.
Multidimensional Personality Questionnaire-Brief Form (MPQ-BF; Patrick, Curtin, & Tellegen, 2002)
The MPQ-BF is a 155-item self-report questionnaire that assessment personality traits across the lifespan. One of the three orthogonal higher-order factors, Constraint (CON), is frequently related to externalizing tendencies (Krueger et al., 2000). Individuals low on constraint (CON, reverse scored) are characterized by impulsivity, low harm avoidance, and disinhibition. Biometric analyses of externalizing routinely include CON, along with conduct disorder, adult antisocial behavior, and substance use disorders to evaluate the shared variance associated with externalizing behavior problems (e.g., Krueger et al., 2000). For this sample the internal consistency (Cronbach’s alpha) was .75.
n-Back Task
The task employed was an adaptation of the n-back working memory task developed by Casbon and colleagues (2003). During the task, participants viewed a series of letters. Participants were instructed to monitor the letters and respond with a button press if the preceding letter in the n-back position was different from the current letter (e.g., a mismatch trial). Participants were instructed to withhold their response when the preceding letter matched the current stimulus (e.g., a match trial). The majority of trials were mismatch trials (80%), whereas match trials were infrequent (occurring 20% of the time). The task also included a manipulation of working memory load. In the low load (1-back) condition, participants were instructed to determine whether the currently presented letter matched the immediately preceding letter in the sequence. In the high load (2-back) condition, participants were required to monitor and maintain the stimulus information in working memory in order to determine whether the letter stimulus 2 positions earlier matched the current letter. Finally, the task manipulated incentives on a subset of trials; in separate blocks participants received reward following a correct response or punishment following an incorrect response (see below for details).
Stimulus presentation and response collection were controlled using the Psychtoolbox extension (Brainard, 1997; Pelli, 1997; Kleiner, Brainard & Pelli, 2007) as implemented in Matlab (Mathworks). Each letter cue was presented for 250 ms, followed by a 1750 ms inter-trial-interval (ITI). Participants were given the full ITI to respond to the letter cue (e.g., maximum response time 1750 ms). The task was presented in a series of 14 blocks.
Participants initially practiced a brief version of the task, consisting of 2 blocks of 45 trials each (one block of low load and one of high load). Following practice, participants completed 12 blocks of trials with 75 trials in each block (4 blocks per condition). First, all participants completed four blocks (2 low load and 2 high load) of the task under neutral (N) conditions in which trial-by-trial performance was neither punished nor rewarded. Following the neutral blocks, participants completed the remaining task blocks under either reward (R) or punishment (P) conditions. Participants were told that they would earn or lose money during these incentive blocks; however, their performance did not actually affect how much they were paid. During these blocks, participants received auditory feedback as a function of their performance on each trial. During reward blocks, participants heard the sound of a coin drop immediately following a correct response, indicating the addition of 5 cents to their earnings. For punishment blocks, participants heard a brief blast of white noise (lasting 0.3 seconds, 96 dB) immediately following each incorrect response, indicating the loss of 5 cents to their earnings. At the end of each block participants were shown a dollar figure in green or red, for reward and punishment, respectively, representing their earnings or losses. The order of the incentive blocks was counterbalanced across participants. Of note block order did not significantly moderate the effects reported below. Prior to the start of each block, participants were given prompts about the type of block (e.g., 1-back block; reward 1-back block; punishment 2-back block) and a reminder of the task instructions (e.g. respond when the letter does not match the one n-back; do not respond when the letter matches the one n-back).
Psychophysiological Recording and Analysis
EEG was recorded throughout the experiment from Ag-AgCl electrodes mounted on an elastic cap (Electro Cap International, Eaton, OH) at four midline positions (Fz, FCz, Cz, and Pz) utilizing Neuroscan Synamps amplifiers and acquisition software (Compumedics, Charlotte, NC). Electrooculogram (EOG) was recorded above and below the left eye (VEOG) in line with the pupil. Impedance for all electrodes was kept below 10 KΩ using light abrasion on the scalp in conjunction with conductive gel at each site. Electrodes were referenced to the right mastoid. EEG signals were digitally filtered offline with a 30 Hz low-pass filter, segmented around stimulus onset (−300 to 1750 ms), and corrected to a 300 ms prestimulus baseline. Trials with EEG or EOG voltages beyond ±75 V were discarded from further analyses.
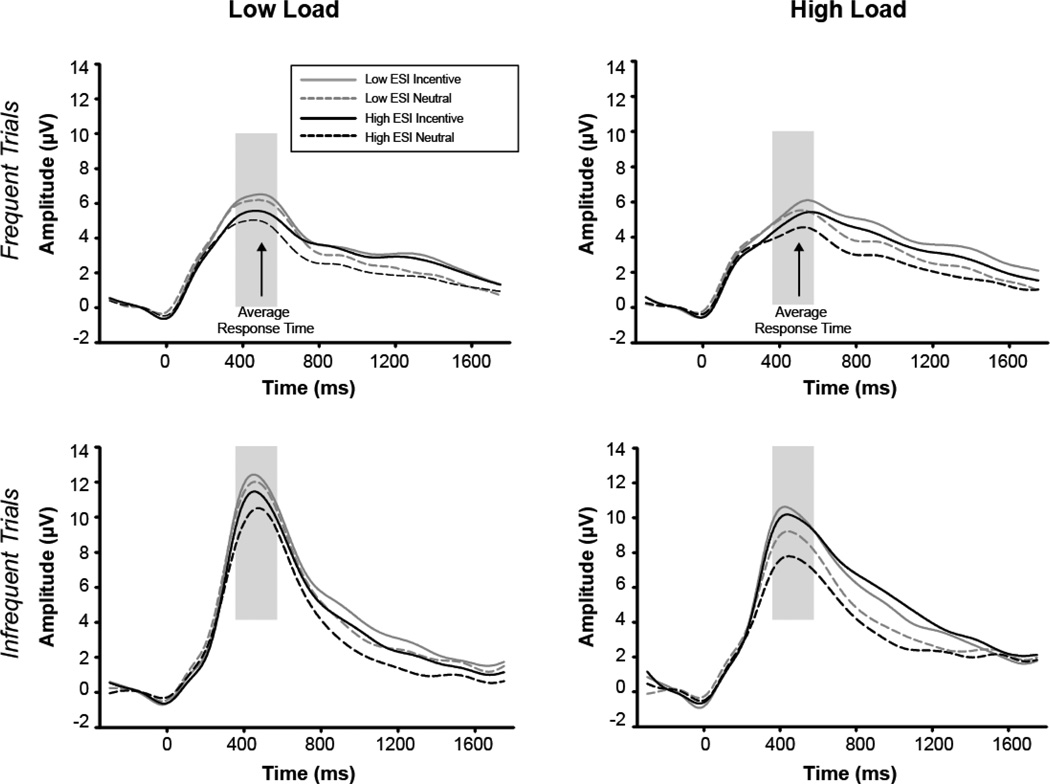
ERPs were averaged separately for all correct trials for each trial type (frequent, infrequent), condition (N, R, and P) and level of task load (low load, high load). At least 95% trials remained across conditions following artifact rejection, with an average of 940 (out of 990) trials. We focused on the P3 ERP component as it is widely examined among studies that, independently and simultaneously, investigate the externalizing spectrum and executive functioning. After inspection of the grand average ERP waveform for all participants, we determined that the P3 was maximal at Pz at 470 ms post stimulus onset (Figure 1). The magnitude of the P3 component was measured as the maximum amplitude in the window between 370–570 ms post stimulus.
Figure 1.
Grand average ERP waveform at Pz for individuals low and high on ESI scores. Though the primary analyses were conducted using externalizing scores continuously, a median split was used here solely for depiction. ERP waveform for frequent (top) and infrequent (bottom) trials for low (left) and high (right) load conditions. Black lines represent high ESI group, grey lines represent low ESI group. Solid lines illustrate incentive conditions, dashed lines illustrate neutral condition. A digital low-pass filter was applied offline before plotting the waveforms. Gray box indicates P3 window used for analyses. Arrow indicates average response time (during frequent trials).
Data Analysis
To examine the association between the P3 ERP and externalizing, we analyzed the P3 component in a general linear model (GLM) with stimulus frequency (frequent vs. infrequent), working memory load (low load vs. high load), and incentive condition (neutral, punishment, or reward) as within-subjects categorical factors and ESI scores (standardized) as a continuous covariate. Age (standardized) was also included as a covariate in the model to control for changes in P3 across the lifespan (Jham, Shukla, Nag, Kar & Agarwal, 1995; Polich, Howard, & Starr, 1985). The assumption of homogeneity of regression slopes was met for the model and to protect against violations of the assumption of sphericity, Huynh–Feldt corrected p values are reported. Also, given that responses to incentive versus neutral stimuli engage processing in distinct ways (Baines et al., 2011), we used Helmert interaction contrasts to compare processing in neutral versus incentive trials and to contrast the processing under punishment versus reward conditions.
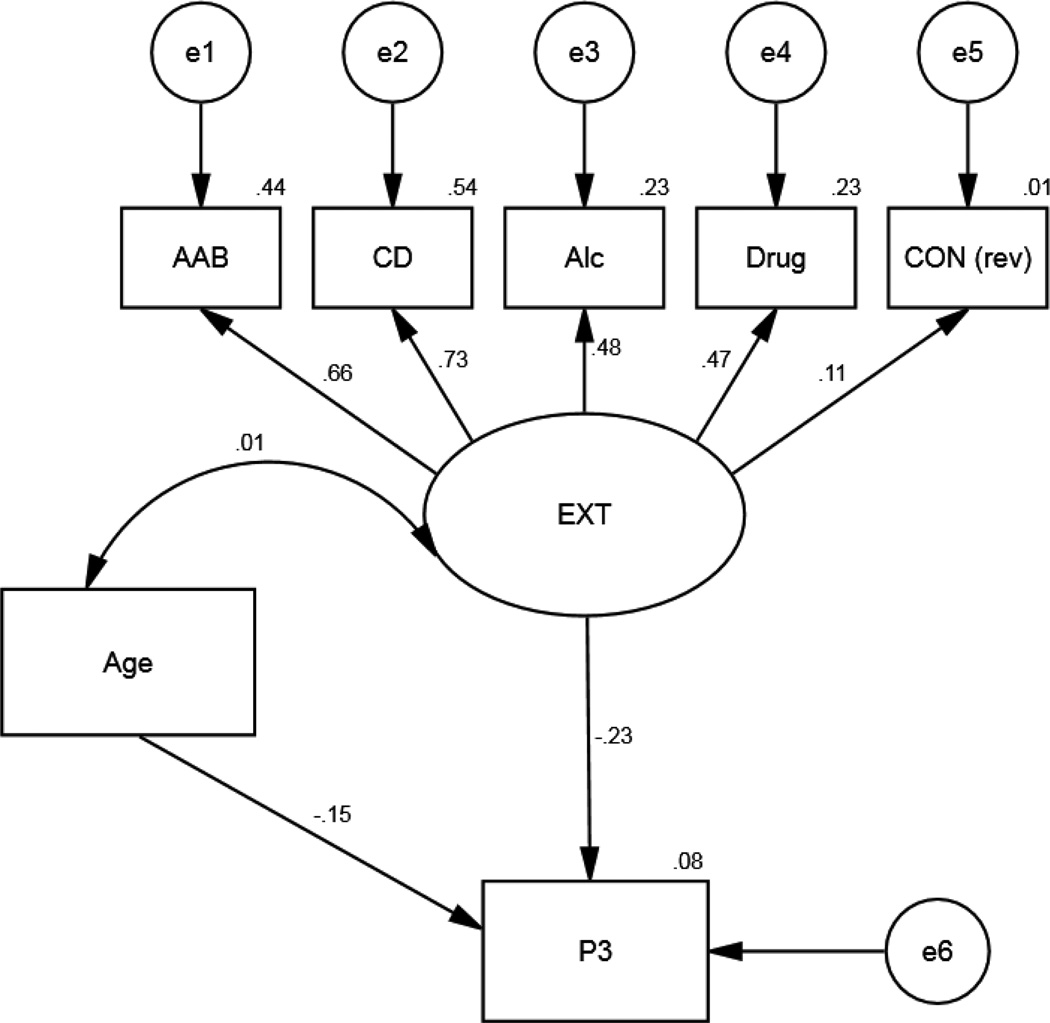
In addition, we conducted supplemental analyses to follow-up our primary analysis. The goal of these analyses was twofold; 1) to examine the relationship between latent trait externalizing, represented by externalizing-related psychopathologies, and the P3 response, and 2) to examine whether psychopathology predicted P3 beyond variance accounted for by latent trait externalizing. Using AMOS 21.0, structural equation modeling (SEM) with maximum-likelihood estimation was used to test the association between the five psychopathologies (AAB, CD, alcohol dependence, drug dependence, and reverse scored Constraint; modeled as a single latent externalizing factor) and the low load, incentive, infrequent P3 (Figure 3; Table 3 for correlations). This model also included age as a covariate with the externalizing latent factor (EXT) and as a predictor of P3.
Figure 3.
Structural equation model of the predictive paths between the latent externalizing disorders factor and the significant P3 simple effect from the primary analysis (low load, incentive, infrequent trial type). Path coefficients are standardized beta weights and are all significant at p< .05, except for the relationship between Constraint (reverse scored) and the EXT latent trait. AAB=Adult antisocial behavior, CD=Conduct Disorder, Alc=AUDIT, Drug=DUDIT, CON (rev)= MPQ-BF Constraint reverse scored, EXT=externalizing latent trait, P3=low load, incentive, infrequent trial type.
Table 3.
Intercorrelations among trait externalizing, externalizing-related psychopathology, and P3.
| Age | ESI Total Score | CD | AAB | CON(rev) | DUDIT | AUDIT | |
|---|---|---|---|---|---|---|---|
| Age | -- | ||||||
| ESI Total Score | −.00 | -- | |||||
| CD Symptoms | −.01 | .53** | -- | ||||
| AAB Symptoms | .01 | .54** | .48** | -- | |||
| MPQ-BF CON (rev) | −.22* | .32** | .04 | .09 | -- | ||
| DUDIT | .04 | .49** | .31** | .36** | .14 | -- | |
| AUDIT | .09 | .42** | .38** | .27** | .24* | .25* | -- |
| P3 LL Incentive Infrequent | −.16┼ | −.18* | −.26** | −.12 | .13 | −.04 | −.04 |
Notes: ESI, Externalizing Spectrum Inventory Total Score; CD, conduct disorder, AAB, adult antisocial behavior, MPQ-BF CON (rev), Multidimensional Personality Questionnaire-Brief Constraint reverse scored; DUDIT, Drug Use Disorders Identification Test; AUDIT, Alcohol Use Disorders Identification Test; P3 LL incentive infrequent, P3 during low load, incentive, infrequent (match) trial type.
p < .001.
p < .05.
p < .10.
The fit of the model was evaluated according to several criteria: the comparative fit index (CFI), the root mean-squared error of approximation (RMSEA), and the relative chi-square index (the ratio of the chi-square statistic to the degrees of freedom). The CFI, which adjusts for the degrees of freedom, compares the fit of the model against the null model with values ranging from 0 to 1; with scores over .90 representing a good fit (Bentler, 1992). The RMSEA takes into account the error of approximation in the population and evaluates how well the model would fit the population covariance matrix with unknown but optimally chosen parameter values. A fit less than .05 is considered a good fit, and a fit less than .08 is acceptable (Arbuckle, 2008). Finally, for the ratio of the chi-square statistic to the degrees of freedom, values less than 5 indicate an acceptable model (Bollen, 1989). For the present study, fit statistics indicate that the model fit the data reasonably well (CFI = .91, RMSEA = .06, χ2/df= 20.51/13, χ2 p-value=.09). Moreover, in this sample, the internal consistency (Cronbach’s alpha) was .65 for the latent trait (EXT) (see Results for information regarding factor loading).
Results
Task-related effects on P3 amplitude
Given that our n-back task manipulated factors of stimulus frequency (trial type), working memory load, and incentives, we first sought to examine these task-related effects on P3 amplitude. The main effect of load was significant F(1,137)=74.66, p <.01, ηp2=.35, such that the low load condition elicited a larger P3 relative to the high load condition. There was also a significant main effect of trial type on the P3 response F(1,137)=556.20, p<.01, ηp2=.80, indicating that infrequent trials elicited a stronger P3 than frequent trials. Incentive also had a significant effect on P3, F(2,274)=30.38, p<.01, ηp2=.18, such that P3 during incentivized trials was larger than during neutral trials; however, there were no significant differences between reward and punishment conditions, (p=.79, LSD test). Additionally, the trial type by load interaction was significant, F(1,137)=59.22, p< .01, ηp2=.40, indicating that the effect of trial type (frequent versus infrequent trials) was greater in the low load condition. The load by incentive interaction was also significant, F(2, 274)=3.29, p= .04, ηp2=.02, indicating greater differences between incentive versus neutral trials in the low load condition; but no differences in the high load condition or between punishment and reward. Lastly, the trial type by incentive interaction was significant, F(2, 274)=27.75, p< .01, ηp2=.17, with the effect of incentive (both reward and punishment) more so than neutral conditions, relating to greater P3 responses in both frequent and infrequent trials. No other task effects were significant. Descriptive statistics for task-related P3 effects are provided in Table 2.
Table 2.
Mean and Standard deviations of P3 responses by Trial type, Load, and Incentive conditions.
| Neutral | Reward | Punishment | ||||
|---|---|---|---|---|---|---|
| Match | Mismatch | Match | Mismatch | Match | Mismatch | |
| Low Load | 13.07 (5.12) | 7.33 (3.56) | 14.02 (5.49) | 7.87 (3.72) | 14.70 (5.35) | 7.47 (3.46) |
| High Load | 10.56 (4.97) | 6.44 (2.95) | 12.04 (5.55) | 7.54 (3.12) | 12.61 (5.22) | 6.83 (2.96) |
Effects of Trait Externalizing (ESI) on P3 amplitude
The main effect of ESI was not significant, (p=.23), illustrating that the ESI total score does not relate to P3 amplitude reduction across the experimental context. Demonstrating that the P3 is influenced by a variety of task-related factors, the four-way interaction between trial type, load, incentive and ESI was significant, F(2,270)=3.93, p=.02, ηp2=.03. Specifically, examination of the Helmert interaction contrasts for the four-way interaction indicated that the incentive (punishment and reward) versus neutral contrast was significant F(1,135)=5.92, p=.02, ηp2=.04, whereas the reward versus punishment contrast was not, F(1,135)=2. 15, p=.16. Given this difference, the following analyses combine the punishment and reward trials (incentive).
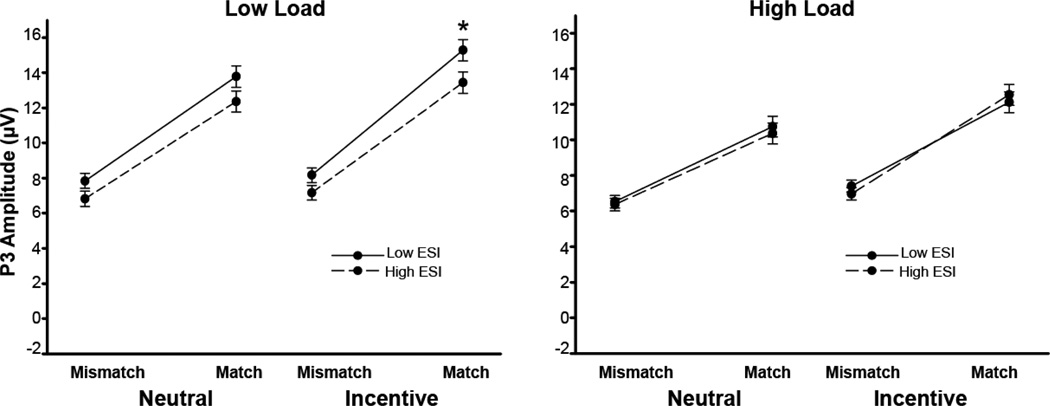
In order to unpack this significant four-way interaction, we examined the effects of the ESI total score, load, and incentive (punishment/reward and neutral) on the P3 component in each trial type, respectively. Neither the main effect of ESI nor any higher order interactions were significant in frequent trials, p’s>.32. For infrequent trials, there was a significant ESI by load by incentive interaction, F(1,135)=8.67, p <.01, ηp2=.06. Simple effects indicated that as ESI scores increased, there was a significant decrease in P3 during the low load infrequent (match) trials with incentives (B= −.93, p=.03). The simple effect of ESI was not significant in the low load neutral infrequent condition (B= −.71, p=.10) or for either of the high load trials (neutral: B= −.19, p=.64; incentive: B=.21, p=.62; Figure 2). Together, these findings indicate that the presence of incentive undermines P3 responses to the infrequent stimuli in externalizing, particularly when task demands are lower (e.g., low load).
Figure 2.
Effect of ESI scores on P3 Amplitude. The relationship between ESI scores and P3 was associated with load, trial type and incentives (load × trial type × incentive × ESI interaction). Using point estimates generated from the general linear model, the P3 means for the interaction contrast in the frequent (mismatch) and infrequent (match) trials were calculated at 1.5 SD below and above the sample mean ESI total scores, respectively. Error bars represent the standard error for the point estimates. There was noESI-related difference for mismatch trials in low load, but there was a significant difference for the low load match trials, specifically in the incentive match trials (*p < .05). There were no significant ESI-related effects in the high load condition.
Supplemental Analyses: Externalizing-Related Psychopathologies
The SEM model indicated that four of the five psychopathologies were significantly related to the latent trait of externalizing (AAB: p <.05, CD: p <.01, AUDIT: p <.01,DUDIT: p<.01, CON reverse scored: p=.28), with higher levels of each pathology related to higher levels of externalizing. Moreover, the latent trait of externalizing (EXT) was significantly (p=.03) and inversely related to the P3 estimate (i.e., low load, incentive, infrequent) (Figure 3). While age was moderately and negatively related to the P3 estimate (p=.06), it was a nonsignificant covariate in the path model (p=.89). Lastly, examination of the modification indices suggested that none of the five manifest variables predicted P3 beyond what was predicted by the latent trait. Overall, this model suggests that externalizing-related pathologies, except for low constraint, make up a latent trait of externalizing, and this latent trait is the best predictor of the distinctive P3 reported in the primary analysis above.
Discussion
The primary goal of this study was to clarify the executive function processes and experimental factors that influence the well-documented abnormal P3 response in externalizing individuals. Additionally, we were interested in expanding the examination of this phenomenon beyond the oddball task, which has dominated past research. In the present study, there was no evidence of an overall reduction in the P3 response as it related to ESI total scores. However, a complex four-way interaction emerged, highlighting several factors that interactively moderate the P3 deficit of externalizing individuals.
In essence, the four-way interaction showed that observing a P3 deficit among high ESI scoring individuals was a function of demands for cognitive control and the incentives provided for correct or incorrect responses. More specifically, when participants were required to modify a dominant response set by withholding a response on the infrequent match trials, ESI scores were significantly and negatively associated with the amplitude of the P3 response during incentivized blocks. Additionally, the significant four-way interaction indicated that this P3 deficit was observed under low working memory, but not high working memory load conditions.
In light of evidence that P3 responses tend to decrease under high load conditions (Ahmed & deFockert, 2012) and the behavior problems associated with trait externalizing are related to deficient working memory capacity (e.g., Bogg & Finn, 2010; Endres et al., 2011; Giancola, 2000), it could be expected that the externalizing-related deficit in P3 would be greater under high load conditions. However, this expectation was not supported in the present study. Moreover, in the externalizing literature, the effects of cognitive load on P3 are equivocal. Whereas studies involving the oddball task do not generally report significant effects of task difficulty on P3 (Iacono et al., 2002; Patrick et al., 2006), our 2-back manipulation resulted in significantly smaller P3 responses across all participants. This significant effect, in turn, may have limited the opportunity of observing, more specific, externalizing-related differences in P3 owing to the strength of the condition manipulation (see de Fockert & Bremner, 2011; Gazzaley, 2011; Lissek, Pine & Grillon, 2006). While recognizing the potential importance of deficient working memory capacity for the behavior problems of externalizing individuals, the current study found no evidence that increasing cognitive load was associated with the externalizing-related P3 deficit.
As in previous research using other paradigms (e.g., oddball, Go/No-Go), the present study revealed evidence of a P3 deficit among high ESI scoring individuals under low working memory load conditions. In each of these paradigms, high externalizing individuals display decreased P3 on trials that require them to revise a dominant response set in reaction to infrequent stimuli. Such requirements are strongly associated with cognitive control and thus highlight the general importance of this variable for observing externalizing-related differences in P3. In contrast to previous studies, however, the present study found that the effect of the cognitive control was moderated by incentives and cognitive load. The important contribution of incentives for observing externalizing– related differences in performance has been documented across a variety of experimental paradigms (Martin & Potts, 2004; Nelson et al., 2011). In spite of this, incentive manipulations have rarely, if ever, been used with the oddball paradigm, so clearly are not necessary for observing reduced P3 responses in externalizing individuals. Thus, closer examination of the experimental manipulations used in the oddball and n-back tasks may clarify the contribution of incentives to P3 differences in externalizing individuals, as well as their implications for cognitive control.
In the oddball task, participants must monitor the presentation of circles and alter their dominant response on infrequent trials when the circle is modified to depict a head with a nose and ear. The oddball stimuli are not only infrequent and demand response alteration, but they are also intrinsically visually salient. By contrast, in the 1-back condition of the current task, all stimuli are standard letters and thus have no intrinsic salience. Consequently, target detection relies on continuous vigilance by the participant in order to match the content of the stimuli (i.e., is the current letter the same as the previous letter?; Owen, McMillan, Laird, & Bullmore, 2005). As such, the incentive manipulation may be seen as providing a top-down or extrinsically mediated increase in the salience of infrequent events. Across paradigms, therefore, it may be that a combination of demands for cognitive control and high motivational salience (i.e. either intrinsic or extrinsic) are necessary for observing the externalizing-based P3 amplitude reduction.
One interpretation of the importance of cognitive control and motivational salience is that the P3 represents the downstream effect of attention being captured by motivational salience. The manipulations using various shapes, rewards/punishments, and frequency all are designed to make a particular stimulus stand out and bring about change in behavior. To the extent that externalizing individuals are prone to over-allocate resources to these salient or motivationally significant stimuli, they have fewer cognitive resources available for executive functions. Ultimately, this particular distribution of resources undermines the quality of evaluative stimulus processing as measured by P3 amplitude (Baskin-Sommers & Newman, 2013). Although speculative, this interpretation is consistent with externalizers’ strong attentional orienting to salient cues, dysfunction in identifying T2 stimuli in the attentional blink task, difficulty classifying rare or unexpected stimuli in the oddball task, and problems shifting their focus to inhibit drug craving and violent responses (e.g., Avila & Parcet, 2001; Baskin-Sommers, Wallace, MacCoon, Curtin & Newman, 2010; Wallace & Newman, 1997; see Baskin-Sommers & Newman, 2013 for review).
In addition to focusing on trait externalizing (ESI scores), we also examined the relationship between P3 and externalizing-related psychopathologies. Following up on the significant interaction found for the ESI scores, a latent variable (made up of alcohol use, drug use, conduct disorder, and adult antisocial behavior) also was found to predict this specific P3 deficit. Although Constraint did not load onto the latent EXT variable1, there was no evidence that any single psychopathology was a better predictor of this P3 effect than the representative latent trait. The fact that latent EXT was comprised of the more pathological behavioral expressions of externalizing (e.g., substance abuse and criminal behavior) supports the importance of the reduced amplitude of P3 as a neurobiological risk factor for a cluster of diagnostic and behavioral problems (Patrick et al., 2006). Thus, general trait externalizing, whether measured through latent EXT or ESI scores, appears to represent a broad cluster of problematic disinhibitory traits and behaviors that are reliably predictive of this particular neurobiological factor.
Before concluding, we consider potential limitations of the current study. First, while it is notable that there were some similarities between this n-back task and the traditional oddball task, there were also significant disparities. Utilizing variations in motivational context, cognitive load, and the application of a response inhibition component introduced diverse demands on executive functions and evaluative processing. However, despite the variation in experimental conditions, the premise that certain processing capacities are reflected in the P3 and deficient in externalizing is consistently supported across experimental contexts. Second, the present sample consisted solely of male prisoners. Though there was variability in the level of trait externalizing and externalizing-related pathologies (see Table 1), in general, the homogeneous nature of the sample may limit its generalizability to other populations, including individuals who do not meet a specific externalizing-related diagnosis but report higher scores on trait externalizing, at-risk samples, or females with externalizing traits/behaviors.
Overall, externalizing is related to a range of behavior problems that include drug and alcohol dependence, conduct disorder, impulsivity, and adult antisocial behavior. Previous studies indicate that a reduction in P3 amplitude is associated with externalizing-related pathological traits and behaviors. Despite the reliability of this finding, little research has been done to clarify the context in which the P3 reduction is present or to identify the psychobiological processes contributing to this response. Results from the present study suggest that externalizing individuals may display a reduced P3 response because of a propensity to over-allocate attention to motivational or salient information and, as such, have limited resources to exercise cognitive control during these contexts. Additionally, demands on working memory load do not seem to be a consistent factor impacting the externalizing-based P3 deficit. In light of the fact that no other study has examined the P3-working memory-externalizing relationship, further research is needed to clarify the potential relevance of working memory for the P3 deficit of externalizing individuals. By working to identify specific factors that contribute to the externalizing-P3 relationship, it may be possible to obtain a more comprehensive understanding of the process-level dysfunction contributing to the disinhibited behavior of these individuals.
Highlights.
-
➢
Examined influences on the P3-externalizing relationship in a sample of male prisoners.
-
➢
Trait externalizing was not associated with a global reduction in P3.
-
➢
Trait externalizing was negatively related to P3 under specific experimental conditions.
-
➢
Cognitive control, incentivized context, and working memory load influenced this relationship.
Acknowledgements
This work was supported by grant 5R21DA030876 from NIDA. We thank many at the Department of Corrections for their continued support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although the Krueger and colleagues’ (2002) original model included low Constraint as one of the indicators of latent EXT, most studies have not included the personality-based predictor of low Constraint, and more often, utilize substance-related or criminal behavior indicators to model the latent trait (Finn, Martin, Rickert, Miller, Lucas, et al., 2009; Gilmore, Malone, Bernat, & Iacono, 2010). Thus, it is unclear if the lack of a personality-externalizing-psychobiological relationship in this study relates to our sample (e.g., perhaps related to the restricted range of scores in prisoners) or a more widespread phenomenon that has been overlooked.
References
- Ahmed L, De Fockert JW. Focusing on attention: The effects of working memory capacity and load on selective attention. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle J. AMOS 16.0 User’s Guide. Spring House, PA: Amos Development Corporation; 2008. [Google Scholar]
- Ávila C, Parcet MA. Personality and inhibitory deficits in the stop-signal task: The mediating role of Gray’s anxiety and impulsivity. Personality and Individual Differences. 2001;29:975–986. [Google Scholar]
- Baines S, Ruz M, Rao A, Denison R, Nobre AC. Modulation of neural activity by motivational and spatial biases. Neuropsychologia. 2011;49:2489–2497. doi: 10.1016/j.neuropsychologia.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Newman JP. Differentiating the Cognition-Emotion Interactions that Characterize Psychopathy versus Externalizing Disorders. In: Robinson M, Harmon-Jones E, Watkins E, editors. Cognition and Emotion. New York: Guilford Press; 2013. pp. 501–520. [Google Scholar]
- Baskin-Sommers AR, Wolf RC, Buckholtz JW, Warren CM, Newman JP. Exaggerated Attention Blink Response in Prisoners with Externalizing Traits. Journal of Research in Personality. 2012;46:688–693. doi: 10.1016/j.jrp.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: Implications for substance abuse risk and brain development. Biological Psychiatry. 1999;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM, O’Connor S, Roberts L. P300 differences between nonalcoholic young men at average and above average risk for alcoholism: Effects of distraction and task modality. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1994a;18:263–277. doi: 10.1016/0278-5846(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994b;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Bentler P. On the fit of models to covariances and methodology to the bulletin. Psychological Bulletin. 1992;112:400–404. doi: 10.1037/0033-2909.112.3.400. [DOI] [PubMed] [Google Scholar]
- Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice detoxification settings and in a Swedish population sample. European Addiction Research. 2005;11:22–31. doi: 10.1159/000081413. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Hall JR, Steffen BV, Patrick CJ. Violent offending predicts P300 amplitude. International Journal of Psychophysiology. 2007;66:161–167. doi: 10.1016/j.ijpsycho.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele V, Gehring WJ, Patrick CJ. Externalizing psychopathology and gain/loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents: Reward processing and externalizing symptomatology. Journal of Child Psychology and Psychiatry. 2009;51(7):827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Finn PR. A Self-Regulatory Model of Behavioral Disinhibition in Late Adolescence: Integrating Personality Traits, Externalizing Psychopathology, and Cognitive Capacity. Journal of personality. 2010;78(2):441–470. doi: 10.1111/j.1467-6494.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen K. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Rui L, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Cole D, Kessler RM, Zald DH. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AM, Botvinick M, Ross LL, Stenger A, Noll D, et al. Parsing executive processes: Strategic versus evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: Diminished cognitive control and its behavioral consequences. Journal of Abnormal Psychology. 2003;112:476–487. doi: 10.1037/0021-843x.112.3.476. [DOI] [PubMed] [Google Scholar]
- Combs LA, Polich J. P3a from auditory white noise stimuli. Clinical Neurophysiology. 2006;117:1106–1112. doi: 10.1016/j.clinph.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Cragg L, Fox A, Nation K, Reid C, Anderson M. Neural correlates of successful and partial inhibitions in children: An ERP study. Developmental Psychobiology. 2009;51:533–543. doi: 10.1002/dev.20391. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Bremner AJ. Release of inattentional blindness by high working memory load: elucidating the relationship between working memory and selective attention. Cognition. 2011;121:400–408. doi: 10.1016/j.cognition.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behavioral Sciences and the Law. 2008;26:799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!…. Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Endres MJ, Rickert ME, Bogg T, Lucas J, Finn PR. Externalizing psychopathology and behavioral disinhibition: working memory mediates signal discriminability and reinforcement moderates response bias in approach-avoidance learning. Journal of Abnormal Psychology. 2011;120:336–351. doi: 10.1037/a0022501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petter C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: A meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;94:1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning: A conceptual framework for alcohol-related aggression. Experimental and Clinical Psychopharmacology. 2000;8:576–597. doi: 10.1037//1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychological Science. 1999;10:203–205. [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87:301–315. [PubMed] [Google Scholar]
- Hall RJ, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huetti SA, McCarthy G. What is odd in the oddball task?: Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia. 2004;42:379–386. doi: 10.1016/j.neuropsychologia.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li SC, Muller V, Lindenberger U. An electrophysiological study of response conflict processing across the lifespan: assessing the roles of conflict monitoring, cue utilization, response anticipation, and response suppression. Neuropsychologia. 2010;48:3305–3316. doi: 10.1016/j.neuropsychologia.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Archives of General Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral Disinhibition and the Development of Early-Onset Addiction: Common and Specific Influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jha S, Shukla R, Nag D, Kar AM, Agarwal A. Effect of age on P300 wave. Journal of the Association of Physicians of India. 1995;43:21–23. [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry and Human Development. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What’s New in Psychtoolbox-3? Perception. 2007;36:1–1. [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Strayer D. Assessing the development of automatic processing: An application of dual-task and event-related brain potential methodologies. Biological Psychology. 1988;26:231–267. doi: 10.1016/0301-0511(88)90022-1. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: The unifying role of personality in population-based research on problem behaviors. Journal of Personality. 2000;68:967–998. doi: 10.1111/1467-6494.00123. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R. Personality traits are differentially linked to mental disorders: A multitrait-multidiagnosis study of an adolescent birth cohort. Journal of Abnormal Psychology. 1996;105:299–312. doi: 10.1037//0021-843x.105.3.299. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15:1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. The American Journal of Psychiatry. 2005;162:1118–1124. doi: 10.1176/appi.ajp.162.6.1118. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2011;48:64–72. doi: 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Lorenz AR. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson R, Scherer JK, Goldsmith HH, editors. Handbook of Affective Sciences. Oxford University Press; 2003. pp. 904–929. [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue MK. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Comerchero MD. P3a from visual stimuli: Typicality, task, and topography. Brain Topography. 2003;15:141–152. doi: 10.1023/a:1022637732495. [DOI] [PubMed] [Google Scholar]
- Polich J, Howard L, Starr A. Effects of age on the P300 component of the event-related potential from auditory stimuli: peak definition, variation, and measurement. The Journal of Gerontology. 1985;40:721–726. doi: 10.1093/geronj/40.6.721. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Pridmore S, Chambers A, McArthur M. Neuroimaging in psychopathy. Australian and New Zealand Journal of Psychiatry. 2005;39:856–865. doi: 10.1080/j.1440-1614.2005.01679.x. [DOI] [PubMed] [Google Scholar]
- Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: An update of research findings. Alcoholism: Clinical and Experimental Research. 2007;31:185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- Ryan R, Lopez S, Werth T. Development and preliminary validation of a Satz-Mogel short form of the WAIS-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience. 1999;98:131–140. doi: 10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- Saunders J, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, Bernat EM. Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology. 2011;86:279–288. doi: 10.1016/j.biopsycho.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voluse AC, Gioia CJ, Sobell LC, Dum M, Sobell MB, Simco ER. Psychometric properties of the drug use and disorders identification test (DUDIT) with substance abusers in outpatient and residential treatment. Addictive Behaviors. 2012;37:36–41. doi: 10.1016/j.addbeh.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1997. [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]