Abstract
Complexity in the nervous system is established by developmental genetic programs, maintained by differential genetic profiles, and sculpted by experiential and environmental influence over gene expression. Determining how specific genes define neuronal phenotypes, shape circuit connectivity, and regulate circuit function is essential for understanding how the brain processes information, directs behavior, and adapts to changing environments. Mouse genetics has contributed greatly to current percepts of gene-circuit interfaces in behavior, but considerable work remains. Large-scale initiatives to map gene expression and connectivity in the brain, together with advanced techniques in molecular genetics, now allow detailed exploration of the genetic basis of nervous system function at the level of specific circuit connections. In this review, we highlight several key advances for defining the function of specific genes within a neural network.
Introduction
The brain is comprised of numerous inter-connected and parallel circuits essential for directing behavior. Distinct neuronal phenotypes within discrete anatomical subregions define specific nodes, or brain nuclei. Neuronal identity within nuclei is established by specific genetic profiles essential for determining cellular location, morphology, neurotransmitter phenotypes, and connectivity. A major goal of current behavioral neurobiology is to precisely define how unique genetic signatures coordinate nervous system development, maintain and modify connectivity, and facilitate information propagation to control circuit function.
Reverse genetics approaches permitting germline transmission of ectopic transgenes, targeted gene disruption through homologous recombination, and nuclease-directed genome editing, provide the means to precisely define gene function within the nervous system. These approaches, interleaved with combinatorial genetics and viral vector-based techniques, now allow for the necessity and sufficiency of specific genes to be defined not only in particular neuronal cell types, but in neurons projecting to discrete targets.
Characterization of functional anatomical connections in the brain is an essential component of gene-circuit dissection. Elucidation of the neural “connectome” has been greatly advanced by the development of tools for retrograde and anterograde tract tracing. A systematic mapping of neuronal projections of the mouse brain, discussed in detail below, provides a key resource for future experimental design. More sophisticated anatomical mapping approaches defining connections onto specific neuronal cell types have also been demonstrated (DeFalco et al., 2001; Kissa et al., 2002; Watabe-Uchida et al., 2012; Wickersham et al., 2007) and will further bolster functional identification of cell-specific connections.
In addition to emerging tools for defining cell specific anatomical connections, functional neural networks can now be tested using advanced techniques involving genetically encoded effectors for activating and inhibiting specific neuronal populations, such as light-activated channels (the channelrhodopsin family; Boyden et al., 2005), ligand-gated ion channels (Arenkiel et al., 2008; Slimko et al., 2002), and Receptors Activated Solely by Synthetic Ligands (RASSLs; Coward et al., 1998) or Designer Receptors Exclusively Activated by Designer Drugs (DREADDs; Armbruster et al., 2007). These techniques enable rapid determination of how the brain is wired and how these connections regulate behavior, thus providing a necessary platform upon which genetic control of circuit function can be explored.
In the subsequent sections, we will highlight resources for identifying gene expression profiles and brain connectivity, as well as review established and emerging technologies for targeted gene inactivation. We will outline current methods for determining gene necessity and sufficiency within specific circuit elements using conditional gene knockout and combinatorial viral vector approaches, and propose alternatives for future exploration.
Bioinformatics Tools for Directing Gene-Circuit Exploration
Identification of genes important for neural circuit function can begin with discovery-driven approaches to uncover specific expression patterns, or with hypothesis-driven designs to test the function of a single gene within a network. Traditionally, gene expression studies required individual investigators to painstakingly analyze expression profiles for a small number of genes. However, with the complete sequencing of the mouse and human genome, it has become possible to perform high-throughput mRNA in situ hybridization studies for all predicted protein encoding genes.
Large-scale gene expression atlases have been completed at the Gene Expression Nervous System Atlas (GENSAT), GenePaint, and the Allen Institute for Brain Science (AIBS) (Table 1). GENSAT has provided a broadly useful supplement to these and other in situ hybridization efforts by generating a large library of transgenic mice expressing reporter proteins, such as EGFP and the DNA recombinase Cre under the control of specific gene promoters, providing an alternative method for expression mapping and functional testing. These unbiased and systematic approaches, along with increasingly accessible web-based platforms for advanced searches of genes and brain structures, provide invaluable resources for the neuroscience community, fueling discovery-driven science.
Table 1. Bioinformatics Resources.
List of internet resources for identifying gene expression profiles, connectivity maps, and transgenic mouse lines available to the scientific community.
| Mouse Genetics | |
| Mouse Genome Informatics (MGI) | http://www.informatics.jax.org/ |
| UCSC Genome Browser | http://genome.ucsc.edu/ |
| Transgenic/Knockout Mice | |
| International Knockout Mouse Consortium (IKMC) | http://www.knockoutmouse.org/ |
| The Jackson Laboratory | http://jaxmice.jax.org/ |
| Cre-X-Mice | http://nagy.mshri.on.ca/cre_new/ |
| Mutant Mouse Regional Resource Center (MMRRC) | http://www.mmrrc.org/ |
| NIH Blueprint Cre Driver Network | http://www.credrivermice.org/ |
| Allen Institute Transgenic Mouse Project | http://connectivity.brain-map.org/transgenic |
| Gene Expression Databases | |
| Allen Mouse Brain Atlas | http://mouse.brain-map.org/ |
| Gene Expression Nervous System Atlas (GENSAT) | http://www.gensat.org |
| Gene Paint | http://www.genepaint.org/ |
| Connectome Resources | |
| Allen Mouse Brain Connectivity Atlas | http://connectivity.brain-map.org/ |
| Human Connectome Project | http://www.humanconnectomeproject.org/ |
Recent advances in mRNA isolation have also made possible the description of active transcriptomes in a given cell type. Specifically, the RiboTag (Sanz et al., 2009) and TRAP (Doyle et al., 2008; Heiman et al., 2008) methods use ribosomal subunits tagged with HA or EGFP, respectively, to immunoprecipitate polyribosomes and any accompanying mRNAs. By expressing the tagged ribosomes only in promoter-specific cell populations, a spatially and temporally selective transcriptional profile can be generated through microarray analysis, allowing for unprecedented resolution of gene expression profiles.
A crucial step in determining genetic regulation of circuit function is defining the connectivity of the nuclei expressing the gene. In addition to conventional dye and enzyme-linked tracing experiments, development of retrograde and anterograde transsynaptic viral tools has enabled more refined separation of neuronal subtypes based on their projection targets (Callaway, 2008; Song et al., 2005). Caveats to transsynaptic viral tracers include replication, which can lead to cytotoxicity, and propagation across multiple synaptic connections, which can complicate circuit analyses (Ugolini, 2010). Modifications to these viruses, making them replication incompetent and dependent on Cre-mediated recombination (DeFalco et al., 2001; Lo and Anderson, 2011; Wall et al., 2010), or co-expression of the envelope receptor protein TVA (Miyamichi et al., 2011; Wickersham et al., 2007), have significantly improved selectivity, elucidating detailed cell-specific connectivity (Watabe-Uchida et al., 2012).
Supplementing these directed efforts, the AIBS has begun a large-scale “connectomics” project to anatomically trace interconnections between the major brain regions. Anterograde tracing between large numbers of brain regions using GFP-expressing viruses has already been completed and datasets are available online describing high-resolution serial two-photon reconstruction of connections throughout the brain (Table 1).
In addition to online resources for exploring gene expression and connectivity, many resources are available to identify existing genetic tools (Table 1), including the International Knockout Mouse Consortium (IKMC), a collaboration of several regional and institutional projects. The goal of this consortium is to mutate every protein-coding gene with gene trapping or gene targeting technology. Of particular importance for studying adult neural function, conditional (i.e. floxed) gene knockouts, discussed in detail below, are being generated for all protein-coding genes (Skarnes et al., 2011). The IKMC has an online database to search for live mice, ES cell clones, and targeting vectors (Table 1). To date, they have generated over 2000 mouse lines, 36,000 ES cells clones, and 22,000 targeting vectors.
Transgenic and Gene Knockout Technologies for Cell-Specific Gene Manipulation
At the center of gene/circuit interface studies are several advances in mouse genetics: transgenesis, gene targeting through homologous recombination, and genome editing. In transgenic mice, ectopic genes (i.e. transgenes), are randomly integrated into the genome and expressed in specific cell types through the use of minimal, cell-selective promoters (Palmiter, 1998). In contrast, gene targeting through homologous recombination in cultured mouse embryonic stem (ES) cells allows for the manipulation of gene expression at specific endogenous loci (Doetschman et al., 1987; Thomas and Capecchi, 1987). These approaches are now standard practice for genetic manipulation and many transgenic and knockout mice have been generated with neuronal and behavioral phenotypes, providing a wealth of knowledge regarding the genetic regulation of neural circuit function.
More recent advances in genome editing techniques using zinc finger nucleases (ZFNs; Carbery et al., 2010), transcription activator-like effector nucleases (TALENS; Sung et al., 2013), and the clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas-associated protein (Cas) system (Wang et al., 2013) can rapidly generate targeted ES cells harboring specific genetic mutations. CRISPR/Cas-mediated genome editing, for example, can be designed for homology-directed repair-mediated editing to introduce specific base-pair substitutions, and is capable of generating multiple targeted alleles in a single mouse (Wang et al., 2013). Given the role of multiple mutations in the etiology of diseases, including psychiatric disorders (Gottesman et al., 1982) the ability to generate mice harboring multiple mutations will facilitate the elucidation of genetic interactions implicated in diseased states.
Gene targeting and genome editing technologies allow for global gene inactivation (Figure 1a), but do not permit the temporal and spatial control of gene manipulation essential for the study of gene-circuit interfaces. A major advance in genetics allowing such a level of refinement is the method of Cre-loxP recombination, described by Sternberg and Hamilton (Sternberg, 1981; Sternberg and Hamilton, 1981; Sternberg et al., 1981). Isolated from bacteriophage P1, the recombinase enzyme Cre recognizes specific palindromic DNA sequences, called loxP (locus of crossover in P1) sites, and catalyzes site-specific recombination. When loxP sites flank a section of DNA, the DNA is said to be “floxed” (Figure 1a) and Cre-mediated recombination will excise the floxed sequence (for review see: Birling et al., 2009; Branda and Dymecki, 2004; Kilby et al., 1993; Stark et al., 1992).
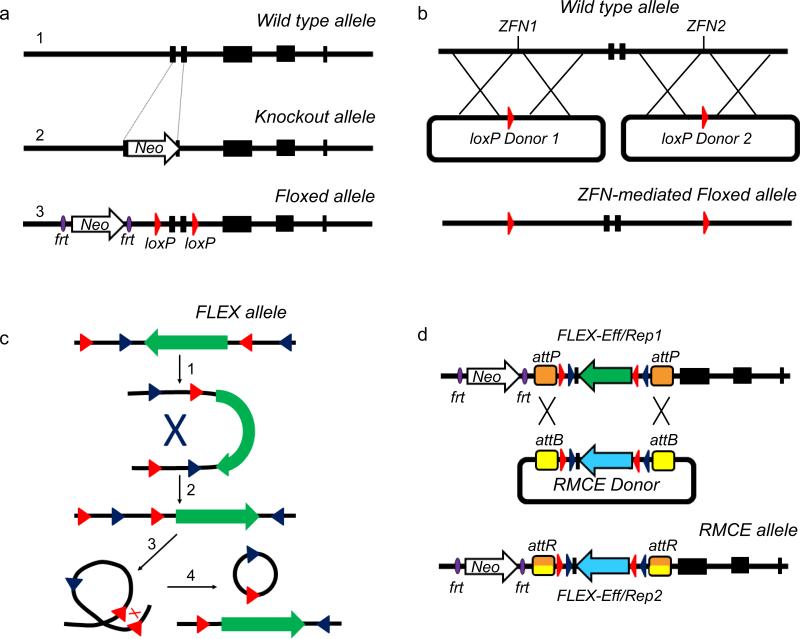
Figure 1. Approaches for generating conditional alleles.
Illustration of a traditional and conditional gene knockout approaches, showing a wild-type allele (1) and disruption of the open reading frame of the gene by insertion of a positive selection marker, such as the Neomycin resistance gene (white arrow) (2). Alternatively, a conditional allele can be through insertion of loxP sequences flanking critical exons of the gene (3), in this case the positive selection marker is flanked by frt sequences to facilitate subsequent removal by Flp recombinase. An alternative strategy for generating conditional alleles involves the use of ZFNs (b). In this case mRNAs encoding ZFNs designed to target specific DNA sequences (ZFN1 and ZFN2) and plasmids containing DNA homology to the allele flanking loxP sites are injected into fertilized eggs (adapted from Brown et al., 2013). Subsequent breeding of founders generated by this strategy allows for the establishment lines with targeted alleles allowing germline transmission. In addition to mediating gene inactivation, Cre-loxP can be utilized to turn on stable expression of transgenes using the FLEX strategy (c). In this strategy staggered non-homologous loxP pairs flank an inverted open reading frame for a transgene of interest. Initially, Cre mediates an inversion between one of the two loxP pairs (1), flipping the alternate pair in the correct orientation (2) to allow for Cre-mediated recombination (3) and excision (4), thus generating stable transgene expression. Once a desired allele has been targeted to allow transgene expression, specific effector (Eff) or reporter (Rep) cassettes can be exchanged using RMCE (d). In this case RMCE is mediated by ΦC31 recombinase which facilitates uni-directional recombination between att recognition sites.
Generating targeted ES cells harboring conditional alleles has historically been a laborious process; however the use of ZFNs to target insertion of loxP sequences into specific loci has recently been demonstrated in rat fertilized eggs (Brown et al., 2013). Utilizing an approach similar in concept to recombinase-mediated cassette exchange (RMCE, discussed below), plasmids containing donor DNA sequences flanked by specific ZFN recognition sites can be used to facilitate homologous end joining, inserting loxP sequences at specific locations (Figure 1b). Demonstrating the viability of this technique in rats opens new avenues for the exploration of gene circuit interfaces in multiple model organisms.
In addition to selective gene inactivation, the Cre/loxP system can also be used to control ectopic gene expression, either through viral-mediated conditional transgene delivery, conventional transgenesis (Brooks et al., 2000; Brooks et al., 1997), or homologous recombination of a conditional transgene into an endogenous locus, such as Rosa26 (Soriano, 1999). In these cases, either a floxed transcriptional “STOP” cassette prevents transgene expression in the absence of Cre (Lakso et al., 1992), or the transgene is inserted in an inverted orientation between staggered, non-homologous lox pairs (Schnutgen et al., 2003). The latter configuration, referred to as “FLEX” or “DIO”, takes advantage of two observations: 1) lox sequences can vary within the eight base pairs between the palindromic sequences and recombination between these non-homologous lox variants (ie loxP, lox2272, lox511) will occur with extremely low efficiency (Hoess et al., 1986); and 2) when lox sites are in the so-called head-to-head configuration, Cre mediates inversion of the DNA rather than excision (Abremski et al., 1983). Thus, with the FLEX approach, Cre initially mediates an inversion between one loxP set, placing the alternate set into the correct head-to-tail orientation allowing for Cre-mediated recombination/excision and stable transgene expression (Figure 1c). In addition to conditional expression of a single transgene, FLEX can also be used to ‘swap’ expression of cDNA cassettes, turning one gene on while the other is turned off (Schnutgen et al., 2003).
An additional means of “swapping” expression cassettes utilizes the technique of recombinase-mediated cassette exchange (RMCE; Bouhassira et al.,1997). Once a targeted allele has been generated, expression cassettes for alternate reporter or effector proteins or specific mutations with the coding region of a gene of interest can be “swapped” through targeted homologous recombination. The identification of multiple site-specific recombinases such as Cre, Flp, Dre, and ΦC31 (discussed further below), now allow for the generation of highly versatile genetically engineered alleles (Figure 1d) for rapid generation of multiple mouse lines for the manipulation of neural circuits.
Spatial and Temporal Control of Gene Expression Using Recombinase Technology
To define the role of a gene within a neural circuit component, it is essential to achieve anatomical selectivity. A large number of mouse lines have been generated expressing Cre recombinase under the control of various promoters, allowing regional and/or cell-type selective genetic manipulation (Table 2 and Figure 2a,b). One of the most common methods for generating Cre lines has been the use of non-targeted transgenics, in which a Cre expression cassette and specific upstream promoter are randomly inserted into the genome. The site of insertion and the size of the promoter can significantly affect the Cre expression pattern (Palmiter and Brinster, 1986; Wilson et al., 1990), and numerous founder lines are often required to identify a strain with the desired specificity (Tsien et al., 1996a). Variability in transgenic Cre lines can be reduced by using a bacterial artificial chromosome (BAC) to generate transgenics (Gong et al., 2007; Heintz, 2001; Yang et al., 1997). A major effort of the GENSAT project has been to generate a large library of BAC transgenic mice expressing promoter-specific Cre; nearly 250 lines are now available. BACs have sufficient capacity to include large portions of specific promoters, minimizing positional effects and yielding transgene expression more similar to that driven by an endogenous promoter of choice. However, one potential caveat of BAC transgenics that remains unresolved is the biological effects of insertion of multiple large chromosomal fragments into the genome (Ade et al., 2011; Kramer et al., 2011; Nelson et al., 2012).
Table 2.
Cre Tools Sources of Cre recombinase for use in targeted homologous recombination experiments.
| Description | Notes | References | |
|---|---|---|---|
| Mouse Lines | |||
| Random insertion transgenics | Cre driven by a cell-type specific promoter, inserted at random into the genome | Highly subject to position effects depending on locus of integration into the genome | Tsien et al., 1996a |
| BAC transgenics | Cre driven by a cell-type specific promoter, delivered via bacterial artificial chromosome | Reduces position effects; large pieces of DNA inserted into genome | Heintz, 2001; Yang et al., 1997 |
| Targeted knock-in | Cre replaces the endogenous gene at the endogenous locus | Endogenous gene expression is disrupted | Zhuang et al., 2005 |
| Targeted IRES knock-in | A bicistronic cassette at the endogenous gene locus maintains gene expression along with Cre | Endogenous gene expression is largely maintained | Lindeberg et al., 2004 |
| Local Viruses | |||
| AAV-Cre | Adeno-associated virus expressing Cre | Stable long-term expression; possible off-target effects. | Ahmed et al., 2004; Scammell et al., 2003 |
| Lentivirus-Cre | Lentivirus expressing Cre | Stable long-term expression; possible off-target effects. | Ahmed et al., 2004 |
| Retrograde Viruses | |||
| Pseudorabies-Cre | Transsynaptic retrograde virus expressing Cre | Can be targeted to specific cell populations; toxic to cells. | Card et al., 2011 |
| Rabies-Cre | Transsynaptic retrograde virus expressing Cre | Can be targeted to specific cell populations; toxic to cells. | Osakada et al., 2011 |
| CAV-Cre | Monosynaptic retrograde virus expressing Cre | Suitable for long-term expression; no cell-selective control | Hnasko et al., 2006 |
| WGA-Cre | AAV encoding wheat-germ agglutinin (WGA)-tagged Cre | Cells infected at site of injection secrete WGA-Cre, taken up by neighboring terminals and retrogradely transported | Gradinaru et al., 2010 |
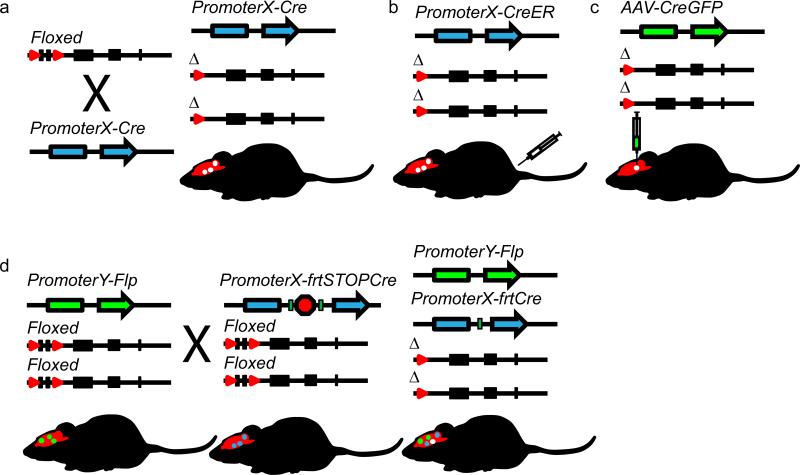
Figure 2. Mouse genetic approaches to study gene necessity in the nervous system.
Cell-selective gene inactivation (a) can be achieve by crossing a mouse with a floxed allele (top left) to a mouse with Cre expression driven by a cell-specific promoter (bottom left). Temporal regulation of gene inactivation (b) is frequently accomplished by using mice with a floxed allele on an inducible Cre background (CreER) driven by a select promoter. When injected with Tamoxifen, the CreER translocates to the nucleus to mediate gene inactivation. Regionally restricted gene inactivation in mice with a floxed allele can be achieved by site-specific viral vector delivery of Cre (c). Finally, a theoretical combinatorial recombinase-based approach (d), illustrates a mouse with a floxed allele on a Flp background, with Flp expression driven by promoter Y (left). A second mouse (middle), with the same floxed alleles, carries a Cre gene driven by promoter X and regulated by an frt-STOP cassette (frt sites are the Flp equivalent of loxP sites). Crossing these mice yields offspring (right) with inactivation of the floxed allele only in the intersectional population of cells with expression driven by both promoters X and Y.
Another strategy for generating cell-selective Cre lines is targeted ‘knock-in’ of Cre into the endogenous locus of a gene (Zhuang et al., 2005). These lines use the full endogenous promoter and regulatory elements to provide more precise transcriptional regulation without extraneous chromosomal DNA. However, they generally disrupt endogenous gene expression, which is disadvantageous if a haploinsufficiency phenotype is associated with the disruption. One solution to alteration of gene expression associated with Cre knock-in is bicistronic alleles containing an internal ribosome entry site (IRES), which enables translation of two separate proteins from a single mRNA (Lindeberg et al., 2004). One caveat of the IRES system is that the gene following the IRES sequence is often expressed at reduced levels. An alternative approach is the picornavirus “self-cleaving” 2A peptide, which encodes a translational ribosomal “skip” (Donnelly et al., 2001). Insertion of reporter or effector constructs in-frame following the 2A sequence allows for the efficient generation of independent proteins from a single transcript (Kim et al., 2011).
A major caveat to using Cre lines to selectively study gene function in specific cell types of the adult brain is the observation that some genes are transiently expressed during early development. Even if the promoter driving Cre expression is predominantly active in the adult, low levels of developmental Cre expression are sufficient to induce recombination, permanently altering expression of a given gene. For Cre lines not well-characterized, developmental expression can be tested by crossing the Cre line to a conditional reporter line. Typical reporter lines contain a transgenic fluorescent reporter, such as TdTomato (Madisen et al., 2010), or a colorimetric reporter, such LacZ (Soriano, 1999), inserted behind a floxed-STOP cassette. The reporter will be turned on permanently in a cell even if Cre is only turned on briefly, revealing the developmental expression profile of a given Cre line.
To avoid potential problems associated with developmental expression of Cre, the enzyme can be temporally regulated using a myriad of inducible systems (Figure 1c). The most widely used inducible Cre system is Cre-ER, in which the ligand-binding domain of the estrogen receptor (ER) is fused to Cre (Indra et al., 1999; Metzger et al., 1995). This fusion protein is retained in the cytosol until activated by the artificial ER ligand, tamoxifen. Upon tamoxifen binding, Cre-ER translocates to the nucleus and catalyzes recombination. Other ligand-activated Cre derivatives exist with varying degrees of temporal resolution (Bockamp et al., 2002).
A second inducible Cre system utilizes the tetracycline-controlled transactivator (tTA; Gossen and Bujard, 1992; Gossen et al., 1995; Kistner et al., 1996; Lindeberg et al., 2002). With this system (Tet-off), transcription of Cre (or any desired gene) is regulated by the tetO promoter, which requires binding of tTA protein. tTA is inhibited by administration of doxycycline, which binds to the protein and prevents it from activating tetO. An alternate system (Tet-on) uses a mutated version of tTA (rtTA) that only binds to the promoter in the presence, rather than absence, of doxycycline, creating a system in which Cre expression can be turned on, rather than off, by doxycycline. Tet-controlled Cre lines are available that can be crossed with mouse lines expressing tTA or rtTA under any given promoter, allowing for flexible and controlled Cre expression (Schonig et al., 2002). Though unintended “leaky” gene expression is a concern, particularly with rtTA, improved versions of the protein have been developed and can be used in combination with a tetracycline-controlled transcriptional silencer (tTS) to greatly increase the “tightness” of the system (Freundlieb et al., 1999; Sun et al., 2007; Urlinger et al., 2000). Several online resources are available for finding traditional and inducible Cre lines, including Jackson Laboratories, Cre-X-mice, GENSAT, Mutant Mouse Regional Resource Center (MMRRC), NIH Blueprint Cre Driver Network, and the AIBS Transgenic project (Table 1).
Viral Delivery of Recombinases, Transsynaptic Tools, and Combinatorial Approaches
Promoter-specific Cre mouse lines can provide cell selectivity and inducible systems can provide temporal resolution, but in many cases more restricted Cre expression is desirable. Region-restricted inactivation of a gene of interest can be achieved through viral-mediated delivery of Cre (Scammell et al., 2003; Figure 2c), and a cell-type specific promoter will refine expression further. Several types of virus are currently used for delivering genes into the brain (for review, see: Davidson and Breakefield, 2003; Mah et al., 2002; Washbourne and McAllister, 2002). Two of the most commonly used are adeno-associated virus (AAV) and lentivirus; both effectively transduce neurons in vivo and are suitable for long-term, stable gene expression. An additional advantage of viral-based methods is that their use extends to species other than mice, including rats and primates (Kordower et al., 1999; Naldini et al., 1996).
Though viral gene delivery provides regional specificity, neurons from one nucleus can project to several distinct downstream targets. Genetic isolation of neurons that project to specific targets is required for effective dissection of circuit elements. Several retrogradely transducing viral vectors, including rabies virus (Osakada et al., 2011), pseudorabies virus (PRV; Card et al., 2011), and canine adenovirus (CAV; Hnasko et al., 2006), have been engineered to deliver Cre to neurons projecting to select targets, but each has limitations. In addition to their synaptic uptake and retrograde transport, CAV and PRV can also transduce neurons at the site of injection or fibers of passage (Aston-Jones and Card, 2000; Soudais et al., 2001). Both rabies and PRV are cytotoxic, limiting their use to applications not requiring prolonged viral-mediated expression (Ugolini, 2010). CAV does not cause cell death and does not replicate or spread to upstream synaptic partners (Soudais et al., 2004), making it suitable for long-term gene expression in projection-specific neuronal populations; however, CAV is technically challenging to generate. Cytotoxity issues can also be overcome using locally transducing viral vectors expressing Cre protein tagged with transsynaptic proteins such as wheat germ agglutinin (WGA) or tetanus toxin light chain (TTC) (Gradinaru et al., 2010). These approaches currently lack the ability to limit which neurons take up the virus and express the encoded genes, though further specificity could be achieved using cell-type specific promoters or by requiring co-expression of additional factors, as described above.
Improved isolation of neurons projecting to specific targets can also be achieved using combinatorial approaches. For example, in a combinatorial viral approach, a retrograde virus containing Cre is injected into a target region of interest, and a second virus containing a conditional transgene (e.g. AAV-FLEX-Gene or AAV-floxed-STOP-Gene) is injected into the afferent of interest. However, to take full advantage of the many floxed conditional mouse lines available, additional combinatorial approaches need to be developed.
One potential approach involves the use of multiple recombinases. In addition to Cre, numerous other recombinases have been identified that recognize unique recombination sites, such as Flippase (Flp), ΦC31, and Dre (Birling et al., 2009), which can be combined for intersectional purposes. For example, conditional gene expression can be made dependent on both Cre and Flp recombinases (Dymecki et al., 2010; Kim and Dymecki, 2009). With this approach, Cre and Flp are each under the control of distinct promoters, and an intersectional population of cells expressing both recombinases is used to target a very specific group of neurons. The recombinases can be introduced via any combination of transgenic mouse lines, locally transducing viruses, and/or retrogradely transducing viruses. To date, such an intersectional approach has only been used to express marker proteins (Dymecki et al., 2010), but it could easily be adapted to the expression of any transgene or targeted allele. Alternatively, generating recombinase-inducible recombinase systems, such as Flp-inducible Cre (Figure 2d), would allow for higher resolution intersectional gene manipulation, taking advantage of the many floxed mouse lines already in existence. Thus, integrating these tools with existing transgenic, gene knock-in, and viral vector based approaches will profoundly improve circuit-level dissection of gene function.
Defining Necessity and Sufficiency of a Gene within a Circuit
Testing the necessity and sufficiency of a gene within a circuit can be achieved using a variety of techniques, often relying on a combination of the approaches outlined above. If a gene of interest is only expressed in a discrete neuronal population monosynaptically connected with another nucleus, then conventional gene inactivation may adequately establish necessity within the circuit. Unfortunately, this scenario is the exception, rather than the rule, and more specific gene manipulation is typically required.
Conditional mice are not always available for a gene of interest, but this can often be overcome using viral vector delivery systems for knockdown of gene expression with RNA interference (RNAi; Davidson and Boudreau, 2007; Dreyer, 2011). Commercially available libraries of short-hairpin RNAs (shRNA) can be used to direct initial in vitro screens, and generating small libraries of sequences for testing is relatively straightforward. Cell-selective gene expression knockdown can be achieved using floxed shRNA-based approaches. These techniques have been used effectively both in vitro and in vivo (Fritsch et al., 2004; Tiscornia et al., 2004; Ventura et al., 2004; Zhou et al., 2007), though to our knowledge this technology has not yet been utilized for highly specific gene knockdown in the nervous system.
Conditional RNAi is particularly amenable to projection-specific expression (Figure 3a). This can be achieved by combining injection of a retrogradely transported Cre virus into the projection target region and an AAV or lentivirus containing a floxed shRNA into the projection origin region. An alternate strategy would be to generate a retrograde virus containing a floxed shRNA and inject it into a Cre-expressing mouse. A major limitation to this latter approach is the laborious nature of generating CAV vectors and the toxicity of rabies and PRV vectors.
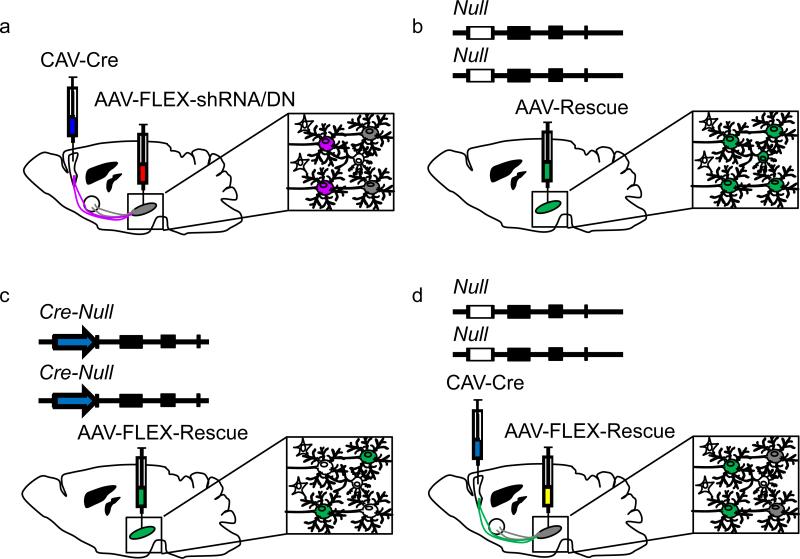
Figure 3. Combinatorial viral and genetic approaches to studying gene necessity and sufficiency in the mouse brain.
Projection-specific genetic necessity can be tested using viral delivery of shRNA or a dominant-negative (DN) version of the gene of interest (a). In this example, the retrograde transducing viral vector (CAV) containing a Cre expression cassette is injected into a target area of interest and a local transducing virus (AAV) containing either a conditional shRNA to the gene of interest or a conditional expression cassette for a DN protein is injected into the area of interest (gray). Intersectional neurons projecting to the target (purple) will express the shRNA or DN and other projection neurons (gray) will be unaffected. Genetic sufficiency can be tested in a brain nucleus of interest on a null allele background by injecting a locally transducing viral vector (b) containing a rescue cassette (AAV-Rescue), but this will be expressed in all neurons within the region injected (green). Alternatively, cell-selective gene sufficiency testing can be performed if the null allele is generated by insertion of Cre into the gene's open reading frame (c). Injection of a conditional rescue cassette (AAV-FLEX-Rescue) into a nucleus of interest restores gene expression only to the neurons endogenously expressing the gene (green). A caveat to this approach is that it will restore expression of the gene to cells projecting to multiple targets. A combined viral vector approach for testing gene sufficiency in neurons projecting to a specific target can also be performed, similar to necessity testing in (a). Here, CAV-Cre is injected into a target of interest and the conditional AAV-FLEX-Rescue virus is injected into the area of interest to express the transgene only in a specific projecting population (d).
Similar to RNAi, dominant-negative approaches can be utilized with Cre-dependent viral vectors to study necessity (Figure 2a). Numerous dominant-negative mutations exist (Herskowitz, 1987; Wells and Carter, 2001), and depending on the size of the dominant mutant form of a gene, different viral preparations can be used for packaging into conditional floxed-STOP or FLEX configurations. Resembling conditional viral-mediated RNAi approaches, conditional dominant-negatives can be combined with retrogradely transducing viral vectors or transsynaptic Cre proteins to determine gene function in specific projection neurons of the adult mouse.
Once gene necessity within a circuit has been established, it is often desirable to determine whether a gene is sufficient in a discrete cell type to mediate a given circuit function. Viral delivery of a non-conditional expression cassette can restore gene function to a particular brain region in a global knockout animal (Olson et al., 2006); however, this can lead to ectopic expression in cells that do not normally express the gene (Figure 3b). Several alternative approaches can increase specificity. First, if a floxed gene was inactivated in multiple brain areas by cell-type-specific Cre, then sufficiency within a specific area can be tested by viral-mediated delivery of a conditional expression cassette that will restore the gene of interest (Zweifel et al., 2011). To achieve even broader tests of minimal sufficiency, Cre knock-in lines can be used (Figure 3c). In this case, mice homozygous for Cre insertion are null mutants and gene expression can be restored using viral delivery of a conditional cDNA to either a large area of the brain (Quintana et al, 2012) or to specific subnuclei (Gore and Zweifel, 2013). A similar effect can be achieved using a specific Cre line crossed to a conventional global knockout.
In the rare cases in which a gene is only expressed in a specific neuronal cell type projecting to a brain region, retrograde viruses can be used to restore gene function and test for sufficiency. This is best demonstrated by selective restoration of tyrosine hydroxylase (TH) to dopamine neurons projecting to specific targets through site specific injection of CAV-Cre into a mouse with a floxed-STOP cassette disrupting the endogenous TH gene (Hnasko et al., 2006). Alternatively, CAV-Cre could be injected into a target of interest in an animal with global gene inactivation, and a conditional viral vector can be delivered to the projecting nucleus of interest (Figure 3d). In an interesting twist on the concept of necessity and sufficiency with a circuit, Parker et al. (2011) injected CAV-Cre into the ventral tegmental area (VTA) of an animal containing a floxed Grin1 gene, knocking out NMDA receptors within the VTA and its afferents. These animals were impaired in appetitive Pavlovian learning, demonstrating the necessity of this gene in those neurons. This behavior was rescued using a conditional virus to restore NMDA receptor function only in the prefrontal cortical neurons projecting to the VTA, illustrating the sufficiency of the gene in that specific afferent population.
Discussion
Fueled by both discovery- and hypothesis-driven scientific approaches, mouse genetics will continue to advance our understanding of how differential gene expression contributes to neural function during both normal cognitive processes and pathological disease states. In this post-genomic era, combining the collective knowledge emerging from large-scale initiatives to map the full mouse transcriptome and connectome will lead to a greater understanding of how differentially expressed genes function within specific circuits. These studies will likely challenge the often-held assumption that a single gene carries out the same function in disparate neuronal populations. Hinting at the rich complexity of genetic regulation of circuit function in the mammalian nervous system, it is estimated that 740 genes encode proteins in the post-synaptic density; of these, approximately one-third show a greater than five-fold difference in expression between brain regions (Hawrylycz et al., 2012).
Though mouse genetics is an extremely powerful tool for the study of the nervous system, like any scientific tool it is not without limitations. Most genetically modified alleles are maintained on a highly inbred C57/Bl6 background strain, which exhibits some physiological and behavioral characteristics that are not shared with other strains (Belknap et al., 1993; Brase et al., 1977; Mogil et al., 1999). In addition, the small size of mice can pose challenges for some in vivo manipulations, such as electrophysiological recordings. The ongoing development of rat genetics (Schonig et al., 2012; Weber et al., 2011; Witten et al., 2011), particularly the use of ZFNs to generate targeted, conditional alleles (Brown et al., 2013), in combination with viral vectors suitable for use in multiple species, will provide greater access to genetic dissection of circuit function in a model system better suited for some investigations.
While we have outlined several methods for controlling gene expression within a circuit, there are numerous additional ways in which gene necessity and sufficiency can be tested, some of which have yet to be conceptualized. In addition, just as there is a plethora of techniques for manipulating genes, there are as many methods to interrogate circuit function in genetically modified mice. Many of these approaches directly intersect with molecular genetics, such as visualizing one or more specific cell types using fluorescent reporter mouse lines (Shuen et al., 2008), imaging dynamic changes in intracellular signaling pathways using genetically encoded indicators (Zariwala et al., 2012), or isolating a population of neurons during in vivo electrophysiology using light-activated channels (Anikeeva et al., 2012). Moving forward, it will be essential to utilize these approaches in concert and develop new techniques and novel combinations to elucidate the complex interaction between genes, neural circuits, and environmental factors.
Acknowledgments
We would like to thank members of the Zweifel laboratory for helpful discussion on this manuscript. This work was supported by the National Institutes of Health 1R01MH094536 (LSZ).
References
- Abremski K, Hoess R, Sternberg N. Studies on the properties of P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell. 1983;32:1301–1311. doi: 10.1016/0092-8674(83)90311-2. [DOI] [PubMed] [Google Scholar]
- Ade KK, Wan Y, Chen M, Gloss B, Calakos N. An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons. Front Syst Neurosci. 2011;5:32. doi: 10.3389/fnsys.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed BY, Chakravarthy S, Eggers R, Hermens WT, Zhang JY, Niclou SP, Levelt C, Sablitzky F, Anderson PN, Lieberman AR, Verhaagen J. Efficient delivery of Cre-recombinase to neurons in vivo and stable transduction of neurons using adeno-associated and lentiviral vectors. BMC Neurosci. 2004;5:4. doi: 10.1186/1471-2202-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2012;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Klein ME, Davison IG, Katz LC, Ehlers MD. Genetic control of neuronal activity in mice conditionally expressing TRPV1. Nat Methods. 2008;5:299–302. doi: 10.1038/nmeth.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Card JP. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J Neurosci Methods. 2000;103:51–61. doi: 10.1016/s0165-0270(00)00295-8. [DOI] [PubMed] [Google Scholar]
- Birling MC, Gofflot F, Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol. 2009;561:245–263. doi: 10.1007/978-1-60327-019-9_16. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- Bouhassira EE, Westerman K, Leboulch P. Transcriptional behavior of LCR enhancer elements integrated at the same chromosomal locus by recombinase-mediated cassette exchange. Blood. 1997;9:3332–3344. [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL. Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther. 1977;201:368–374. [PubMed] [Google Scholar]
- Brown AJ, Fisher DA, Kouranova E, McCoy A, Forbes K, Wu Y, Henry R, Ji D, Chambers A, Warren J, Shu W, Weinstein EJ, Cui X. Whole-rat conditional gene knockout via genome editing. Nat Methods. 2013;10:638–640. doi: 10.1038/nmeth.2516. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Cory-Slechta DA, Federoff HJ. Gene-experience interaction alters the cholinergic septohippocampal pathway of mice. Proc Natl Acad Sci U S A. 2000;97:13378–13383. doi: 10.1073/pnas.230169397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Muhkerjee B, Panahian N, Cory-Slechta D, Federoff HJ. Nerve growth factor somatic mosaicism produced by herpes virus-directed expression of cre recombinase. Nat Biotechnol. 1997;15:57–62. doi: 10.1038/nbt0197-57. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Kobiler O, Ludmir EB, Desai V, Sved AF, Enquist LW. A dual infection pseudorabies virus conditional reporter approach to identify projections to collateralized neurons in complex neural circuits. PLoS One. 2011;6:e21141. doi: 10.1371/journal.pone.0021141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H, Conklin BR. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci U S A. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbery ID, Ji D, Harrington A, Brown V, Weinstein EJ, Liaw L, Cui X. Targeted genome modification in mice using zinc-finger nucleases. Genetics. 2010;186:451–459. doi: 10.1534/genetics.110.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Boudreau RL. RNA interference: a tool for querying nervous system function and an emerging therapy. Neuron. 2007;53:781–788. doi: 10.1016/j.neuron.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S, Smithies O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature. 1987;330:576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Dam E,T, Gani D, Ryan MD. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol. 2001;82:1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions. Mol Biotechnol. 2011;47:169–187. doi: 10.1007/s12033-010-9334-x. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- Freundlieb S, Schirra-Muller C, Bujard H. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J Gene Med. 1999;1:4–12. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<4::AID-JGM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fritsch L, Martinez LA, Sekhri R, Naguibneva I, Gerard M, Vandromme M, Schaeffer L, Harel-Bellan A. Conditional gene knock-down by CRE-dependent short interfering RNAs. EMBO Rep. 2004;5:178–182. doi: 10.1038/sj.embor.7400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore BB, Zweifel LS. Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. J Neurosci. 2013;33:8640–8649. doi: 10.1523/JNEUROSCI.5532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J, Hanson DR. Schizophrenia, the epigenetic puzzle. Cambridge University Press; Cambridge ; New York: 1982. [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. BAC to the future: the use of bac transgenic mice for neuroscience research. Nat Rev Neurosci. 2001;2:861–870. doi: 10.1038/35104049. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987;329:219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PE, Kremer EJ, Palmiter RD. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess RH, Wierzbicki A, Abremski K. The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res. 1986;14:2287–2300. doi: 10.1093/nar/14.5.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indra AK, Warot X, Brocard J, Bornert JM, Xiao JH, Chambon P, Metzger D. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;27:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilby NJ, Snaith MR, Murray JA. Site-specific recombinases: tools for genome engineering. Trends Genet. 1993;9:413–421. doi: 10.1016/0168-9525(93)90104-p. [DOI] [PubMed] [Google Scholar]
- Kim JC, Dymecki SM. Genetic fate-mapping approaches: new means to explore the embryonic origins of the cochlear nucleus. Methods Mol Biol. 2009;493:65–85. doi: 10.1007/978-1-59745-523-7_5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS one. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K, Mordelet E, Soudais C, Kremer EJ, Demeneix BA, Brulet P, Coen L. In vivo neuronal tracing with GFP-TTC gene delivery. Mol Cell Neurosci. 2002;20:627–637. doi: 10.1006/mcne.2002.1141. [DOI] [PubMed] [Google Scholar]
- Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Bloch J, Ma SY, Chu Y, Palfi S, Roitberg BZ, Emborg M, Hantraye P, Deglon N, Aebischer P. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol. 1999;160:1–16. doi: 10.1006/exnr.1999.7178. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Sauer B, Mosinger B, Jr., Lee EJ, Manning RW, Yu SH, Mulder KL, Westphal H. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Mattsson R, Ebendal T. Timing the doxycycline yields different patterns of genomic recombination in brain neurons with a new inducible Cre transgene. J Neurosci Res. 2002;68:248–253. doi: 10.1002/jnr.10213. [DOI] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–950. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah C, Byrne BJ, Flotte TR. Virus-based gene delivery systems. Clin Pharmacokinet. 2002;41:901–911. doi: 10.2165/00003088-200241120-00001. [DOI] [PubMed] [Google Scholar]
- Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, Callaway EM, Horowitz MA, Luo L. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, Kreitzer AC. A comparison of striatal-dependent behaviors in wild-type and hemizygous Drd1a and Drd2 BAC transgenic mice. J Neurosci. 2012;32:9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–631. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Transgenic mice--the early days. Int J Dev Biol. 1998;42:847–854. [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Beutler LR, Palmiter RD. The contribution of NMDA receptor signaling in the corticobasal ganglia reward network to appetitive Pavlovian learning. J Neurosci. 2011;31:11362–11369. doi: 10.1523/JNEUROSCI.2411-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnutgen F, Doerflinger N, Calleja C, Wendling O, Chambon P, Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- Schonig K, Schwenk F, Rajewsky K, Bujard H. Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 2002;30:e134. doi: 10.1093/nar/gnf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonig K, Weber T, Frommig A, Wendler L, Pesold B, Djandji D, Bujard H, Bartsch D. Conditional gene expression systems in the transgenic rat brain. BMC Biol. 2012;10:77. doi: 10.1186/1741-7007-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, Mujica AO, Thomas M, Harrow J, Cox T, Jackson D, Severin J, Biggs P, Fu J, Nefedov M, de Jong PJ, Stewart AF, Bradley A. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Enquist LW, Bartness TJ. New developments in tracing neural circuits with herpesviruses. Virus Res. 2005;111:235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Soudais C, Laplace-Builhe C, Kissa K, Kremer EJ. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 2001;15:2283–2285. doi: 10.1096/fj.01-0321fje. [DOI] [PubMed] [Google Scholar]
- Soudais C, Skander N, Kremer EJ. Long-term in vivo transduction of neurons throughout the rat CNS using novel helper-dependent CAV-2 vectors. FASEB J. 2004;18:391–393. doi: 10.1096/fj.03-0438fje. [DOI] [PubMed] [Google Scholar]
- Stark WM, Boocock MR, Sherratt DJ. Catalysis by site-specific recombinases. Trends Genet. 1992;8:432–439. [PubMed] [Google Scholar]
- Sternberg N. Bacteriophage P1 site-specific recombination. III. Strand exchange during recombination at lox sites. J Mol Biol. 1981;150:603–608. doi: 10.1016/0022-2836(81)90384-3. [DOI] [PubMed] [Google Scholar]
- Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- Sternberg N, Hamilton D, Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between loxP and the bacterial chromosome. J Mol Biol. 1981;150:487–507. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen X, Xiao D. Tetracycline-inducible expression systems: new strategies and practices in the transgenic mouse modeling. Acta Biochim Biophys Sin (Shanghai) 2007;39:235–246. doi: 10.1111/j.1745-7270.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, Jeong D, Kim JS, Lee HW. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tiscornia G, Tergaonkar V, Galimi F, Verma IM. CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101:7347–7351. doi: 10.1073/pnas.0402107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G. Advances in viral transneuronal tracing. J Neurosci Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall NR, Wickersham IR, Cetin A, De La Parra M, Callaway EM. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A. 2010;107:21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, McAllister AK. Techniques for gene transfer into neurons. Curr Opin Neurobiol. 2002;12:566–573. doi: 10.1016/s0959-4388(02)00365-3. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Weber T, Schonig K, Tews B, Bartsch D. Inducible gene manipulations in brain serotonergic neurons of transgenic rats. PLoS One. 2011;6:e28283. doi: 10.1371/journal.pone.0028283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T, Carter DA. Genetic engineering of neural function in transgenic rodents: towards a comprehensive strategy? J Neurosci Methods. 2001;108:111–130. doi: 10.1016/s0165-0270(01)00391-0. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Annu Rev Cell Biol. 1990;6:679–714. doi: 10.1146/annurev.cb.06.110190.003335. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Falkenburger BH, Schulz JB, Tieu K, Xu Z, Xia XG. Silencing of the Pink1 gene expression by conditional RNAi does not induce dopaminergic neuron death in mice. Int J Biol Sci. 2007;3:242–250. doi: 10.7150/ijbs.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]