Abstract
Tolerance to the neurochemical and psychoactive effects of cocaine after repeated use is a hallmark of cocaine addiction in humans. However, comprehensive studies on tolerance to the behavioral, psychoactive, and neurochemical effects of cocaine following contingent administration in rodents are lacking. We outlined the consequences of extended access cocaine self-administration as it related to tolerance to the psychomotor activating, dopamine (DA) elevating, and DA transporter (DAT) inhibiting effects of cocaine. Cocaine self-administration (1.5 mg/kg/inj; 40 inj; 5 days), which resulted in escalation of first hour intake, caused reductions in evoked DA release and reduced maximal rates of uptake through the DAT as measured by slice voltammetry in the nucleus accumbens core. Further, we report reductions in cocaine-induced uptake inhibition as measured by fast scan cyclic voltammetry, and a corresponding increase in the dose of cocaine required for 50% inhibition of DA uptake (Ki) at the DAT. Cocaine tolerance at the DAT translated to reductions in cocaine-induced DA overflow as measured by microdialysis. Additionally, cocaine-induced elevations in locomotor activity and stereotypy were reduced, while rearing behavior was enhanced in animals with a history of cocaine self-administration. Here we demonstrate both neurochemical and behavioral cocaine tolerance in an extended-access rodent model of cocaine abuse, which allows for a better understanding of the neurochemical and psychomotor tolerance that develops to cocaine in human addicts.
Keywords: Dopamine, Cocaine, Self-administration, Rat, Tolerance, Striatum
Introduction
Cocaine is a dopamine transporter (DAT) blocker that inhibits the uptake of dopamine (DA), thus increasing extracellular DA levels and augmenting postsynaptic DA receptor activation. The ability of cocaine to inhibit the DAT is essential for its rewarding effects, which is highlighted by the fact that transgenic mice with cocaine-insensitive DATs do not develop conditioned place preference for the drug (Chen et al., 2008). Further, the potency of stimulants for inhibiting the DAT is strongly correlated with self-administration behavior (Ritz et al., 1987, Roberts et al. 1977), suggesting that changes in cocaine potency at the DAT have important behavioral implications. In rodents, tolerance to the pharmacological DAT-inhibiting effects of cocaine has been reported previously following discrete trial, fixed-ratio, and progressive-ratio cocaine self-administration paradigms (Calipari et al., 2013a, b; Ferris et al., 2011, 2012, 2013; Mateo et al., 2005). Tolerance has also been reported to the DA elevating and locomotor activating effects of cocaine (Hurd et al., 1989; Lack et al., 2008). Although tolerance has been demonstrated to these different aspects of cocaine effects, the results are from a wide range of different self-administration paradigms, and a comprehensive understanding of the tolerance induced by the extended-access, defined-intake (5 days, 40 inj/day, 1.5 mg/kg/inj) model is lacking.
Tolerance to the euphorigenic and DA-elevating effects of cocaine has been reported consistently in human cocaine addicts (Dackis and O’Brien, 2001; Mendelson et al., 1998; Reed et al., 2009; Volkow et al., 1997a, b; Volkow et al., 1996), and is thought to mediate some aspects of continued drug taking. Tolerance has been suggested to modulate cocaine intake behavior, specifically escalation of intake that occurs after repeated use, which has been reported in both human addicts and rodent self-administration studies (Ahmed et al., 2002, 2003; Ahmed and Koob, 2005; Barrett et al., 2004; Dackis and O’Brien, 2001; Koob and Le Moal, 2001). Therefore, we aimed to define cocaine self-administration induced tolerance in rodents. We found that an extended-access, limited-intake self-administration model (Calipari et al., 2013a; Ferris et al., 2011, 2012) mimics important aspects of the tolerance reported in humans, which is characterized by deficits in baseline DA functioning, reduced cocaine potency, escalated self-administration behavior, and reduced cocaine-induced locomotor behaviors.
Materials and Methods
Animals
Male, Sprague-Dawley rats (375–400 g; Harlan Laboratories, Frederick, MD) were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. Because of the within subject design utilized in the current study, a total of 16 self-administration and 17 control animals were used. 8 controls and 7 self-administration animals were used for the locomotor and microdialysis experiments, and 9 controls and 9 self-administration animals were used for voltammetry experiments. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine. This manuscript adheres to the ARRIVE guidelines for reporting research.
Self-Administration
Rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), implanted with chronic indwelling jugular catheters, and trained for intravenous (i.v.) self-administration as previously described (Calipari et al., 2013b). Following surgery, animals were singly housed, and all self-administration sessions took place in the home cage. Each animal was maintained on a reversed light cycle (3:00 am lights off; 3:00 pm lights on), and all self-administration procedures occurred during the active/dark cycle. Sessions were six hours in length and were terminated at the end of the six hours or after 40 injections of drug. Animals self-administered cocaine (1.5 mg/kg/inj over 4 sec) on a fixed-ratio 1 schedule of administration. Concurrent with the start of each injection, the lever retracted and a stimulus light was activated for 20 seconds to signal a time-out period. Under these conditions, animals acquired a stable pattern of intake within 1 to 5 days. For self-administering animals, acquisition (Day 1) was counted when the animal reached 35 or more responses. Following acquisition, the animals were given access to 40 injections per day for a period of 5 consecutive days. In this study all animals took the maximum number of injections (40) on each of the self-administration days. Control animals were naïve rats housed under the same reversed light-dark light cycle for at least one week prior to neurochemical analysis.
Fast Scan Cyclic Voltammetry in Brain Slices
Fast scan cyclic voltammetry was used to characterize presynaptic DA signaling in the NAc. Voltammetry experiments were conducted during the dark phase of the light cycle, 24 hours after commencement of the final self-administration session. 400 µm thick coronal brain sections containing the NAc core were cut using a vibrating tissue slicer (Leica Microsystems Inc.; Buffalo Grove, IL). Uptake, release, and cocaine-induced uptake inhibition were all determined within subject, and for each animal one slice was used. A total of 9 slices were used from cocaine self-administering animals and 9 from control animals were used in the current study. A carbon fiber electrode was placed approximately 75 µm below the surface of the slice in close proximity to a bipolar stimulating electrode in the NAc core. DA release was evoked by a single electrical pulse (300 µA, 4 msec, monophasic) applied every 5 minutes. Extracellular DA was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs. silver/silver chloride, 400 V/sec) to the electrode every 100 msec. Once the extracellular DA response was stable for three consecutive stimulations, cocaine (0.3µM–30µM) was applied cumulatively to the brain slice. Cocaine was incubated for 40 minutes at each dose, or until recordings were stable (less than 10% variability in peak height between collections). DA current was converted to concentration by electrode calibration with 3 µM DA at the end of each experiment.
Demon Voltammetry and Analysis Software was used for data acquisition and analysis (Yorgason et al., 2011). To evaluate the effects of drugs, evoked levels of DA were modeled using Michaelis–Menten kinetics, as a balance between release and uptake (Wightman et al, 1988). Michaelis–Menten modeling provides parameters that describe the amount of DA released following stimulation, the maximal rate of DA uptake (Vmax), and alterations in the ability of DA to bind to the DAT, or apparent Km. For pre-drug modeling, we followed standard voltammetric modeling procedures by setting the baseline Km parameter to 160 nM based on the affinity of DA for the DAT, whereas Vmax values were allowed to vary as the pre-drug measure of the rate of DA uptake. Following drug application, apparent Km was allowed to vary to account for changes in drug-induced DA uptake inhibition while the respective Vmax value determined for that subject at baseline was held constant. The apparent Km parameter models the amount of DA uptake inhibition following a particular dose of drug.
Calculating Ki Values
As described by Jones et al. (1995), inhibition constants (Ki) were determined by plotting the linear concentration-effect profiles and determining the slope of the linear regression. The Ki was calculated by the equation Km/slope. Ki values are reported in µM and are a measure of the drug concentration that is necessary to produce 50% uptake inhibition.
Locomotor Activity
Locomotor assessment was performed, as previously described (Lack et al. 2008.), the day following completion of cocaine self-administration (n = 7 per group). On the test day, prior to locomotor recording, animals were allowed to habituate in the testing room, in their home cages, for 60 minutes. Following habituation to the room, rats were placed in the locomotor chamber (MedAssociates; St. Albans, VT) and baseline activity recorded for 30 min. Rats then received an IP injection of saline, and activity was recorded for 90 min. Lastly, rats received an intraperitoneal (i.p.) injection of 15 mg/kg cocaine, and locomotor response was measured again. Locomotor recordings were performed in two separate groups (control and cocaine self-administration) for between-subject comparisons. Stereotopy was recorded as the total number of repetitive beam breaks without ambulation within a maximum area of 2×2 beams (within 3cm of the animal) with a minimum resting delay of 0.5 seconds.
Microdialysis
Microdialysis surgeries were performed the day following locomotor activity analysis (n = 8 for self-administration animals; 6 for naïve animals). Microdialysis guide cannulae (CMA/Microdialysis AB, Stockholm, Sweden) were stereotaxically implanted above the NAc core (anteroposterior, + 1.2 mm; lateral, 2.0 mm; ventral, 6.0 mm). Concentric microdialysis probes (2 mm membrane length, CMA/Microdialysis) were inserted the day before recording and approximately 16 h prior to the beginning of sample collection. Probes were implanted in animals the day following locomotor testing and then microdialysis experiments were conducted the following day. Thus, rats were tested ≈ 72 h following their final SA session. The probes were continuously perfused at 0.8 µl/min with artificial cerebrospinal fluid (aCSF; pH 7.4): NaCl 148 mM, KCl 2.7 mM, CaCl2 1.2 mM, MgCl2 0.85 mM. Baselines were collected for at least 2 hours and stability was defined as three collections within 10% variability. Once stable baselines were established, animals received a cocaine (15 mg/kg) i.p. challenge and DA was monitored at 15 minute intervals until DA levels returned to baseline.
High Performance Liquid Chromatography
The HPLC (ESA/Thermo Scientific, Chelmsford, MA) consisted of a syringe pump, a glassy carbon working electrode, a reference electrode, and an electrochemical detector. A 2 × 50 mm (3 µm particle) reverse-phase column (Luna, Phenomenex, Torrance, CA) was used to separate compounds. The applied potential was +650 mV as referenced to an Ag/AgCl electrode. The mobile phase (75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium salt, 100 µL/L triethylamine, 25 µM EDTA, 10% acetonitrile v/v, pH=3.0) was pumped at a rate of 170 µL/min, with a detection limit for DA of 10 pM. DA quantification was achieved by comparing dialysate samples with DA standards of known concentration.
Statistics
Graph Pad Prism (version 5, La Jolla, CA, USA) was used to statistically analyze data sets and create graphs. Self-administration data was subjected to a one-way analysis of variance (ANOVA), and differences across sessions were tested using a Tukey post hoc test. Baseline voltammetry data was compared using a two-tailed Student’s t-test. Locomotor, cocaine-induced uptake inhibition, microdialysis data, and cocaine dose-response curves were subjected to a two-way ANOVA with experimental group as the between subjects, and time (locomotor and microdialysis) or cocaine concentration (voltammetry) as the within-subject factors. Differences between groups at individual time- or concentration-points were tested using a Bonferroni post hoc test when a main effect of group was present in the ANOVA.
Results
Cocaine self-administration results in escalation of first-hour intake over sessions
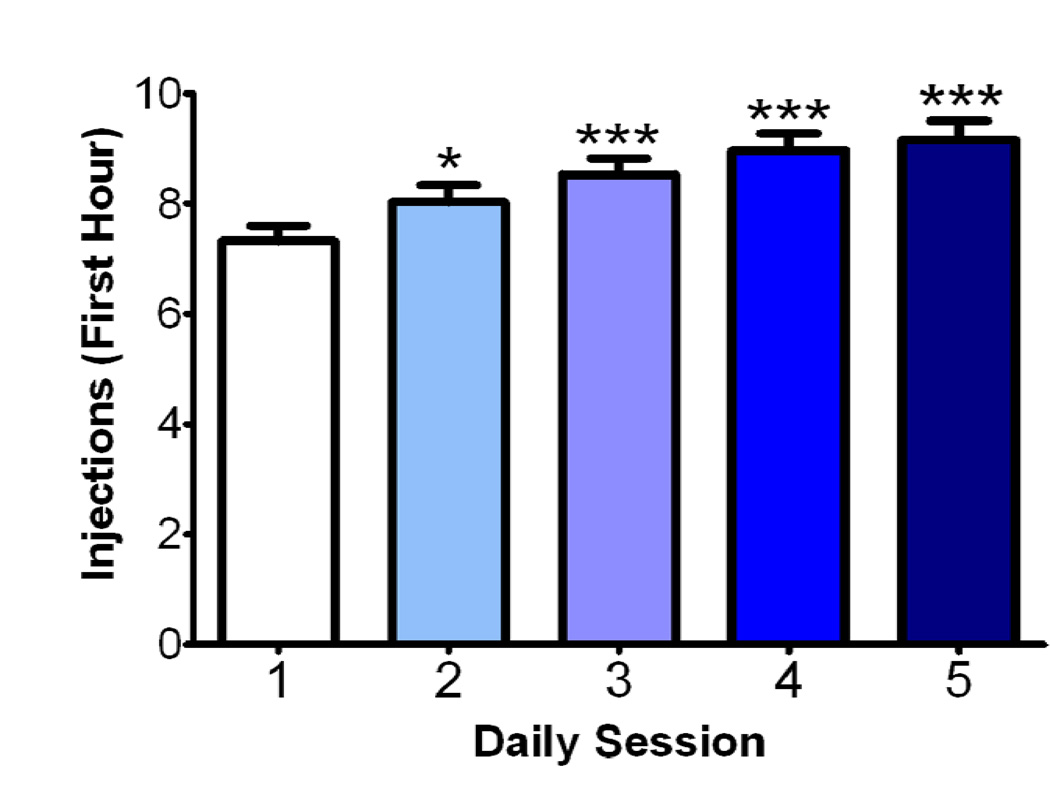
Animals self-administered cocaine (1.5 mg/kg/inj) for five consecutive days in 6 hour sessions with a maximum of 40 total injections. Injections were limited in order to ensure that animals all had the same intake over the 5 self-administration sessions, to control for total intake as a factor in differences in neurochemical effects. Because of the limit on the total number of injections, animals did not escalate in total intake over the entire session. However, traditional long-access (LgA) self-administration paradigms use increases in first-hour intake as a marker of escalation (Ahmed and Koob, 1998, 1999; Marusich et al., 2010). ANOVA revealed a main effect of session on first hour intake (F4, 15 = 19.87, p < 0.0001, Fig. 1). Tukey post hoc analysis revealed a significant increase in rate of intake on sessions 2 (p < 0.05), 3 (p < 0.001), 4 (p < 0.001), and 5 (p < 0.001) versus session 1.
Figure 1. Cocaine self-administration results in escalation of first-hour cocaine intake.
Group data presented as cocaine injections within the first hour of the self-administration session over five consecutive sessions (n = 16). The graph demonstrates that first-hour cocaine intake increases over session, indicating escalation in the amount of cocaine consumption over days. **, p < 0.01; ***, p < 0.001
Stimulated DA release and uptake are reduced following cocaine self-administration
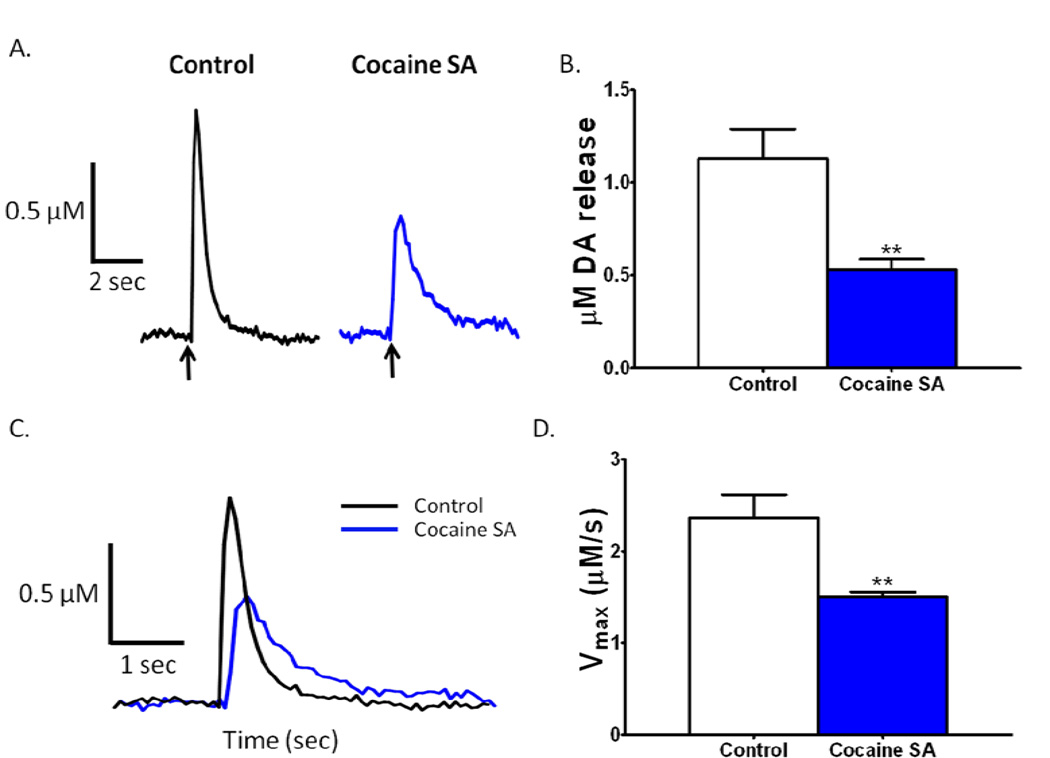
Using fast scan cyclic voltammetry, we assessed presynaptic DA signaling parameters in the NAc core to determine the effects of cocaine self-administration on DA release and uptake when drug is not present. We found significant reductions in DA release elicited by single pulse electrical stimulations after cocaine self-administration (Fig. 2A). Stimulated release was reduced by 44% versus controls (t16 = 3.575, p < 0.001; Fig. 2B). In addition to stimulated release, we also found reductions in the maximal rate of DA uptake (Vmax; Fig 2C). Vmax was reduced by 32% versus controls (t16 = 3.315, p < 0.001; Fig. 2D). These reductions in presynaptic DA measures demonstrate that DA signaling and DAT function is reduced following cocaine self-administration.
Figure 2. Reduced dopamine (DA) release and uptake following cocaine self-administration.
(A) Representative DA traces indicating that stimulated DA release is reduced following cocaine self-administration in the nucleus accumbens core. Plots are represented as DA concentration over time. (B) Grouped data showing changes in µM DA release evoked by electrical stimulation of the terminal (n = 9 per group). Cocaine self-administration results in a decrease in stimulated DA release relative to control animals. (C) Representative DA traces shifted to match for peak DA concentration, highlight differences in maximal rate of DA uptake in the nucleus accumbens core. (D) Group data (n = 9 per group) indicate that cocaine self-administration results in reduced maximal rate of uptake as compared to control animals. **, p < 0.01; SA, self-administration; Vmax, maximal rate of dopamine uptake
DAT sensitivity to cocaine is reduced following self-administration
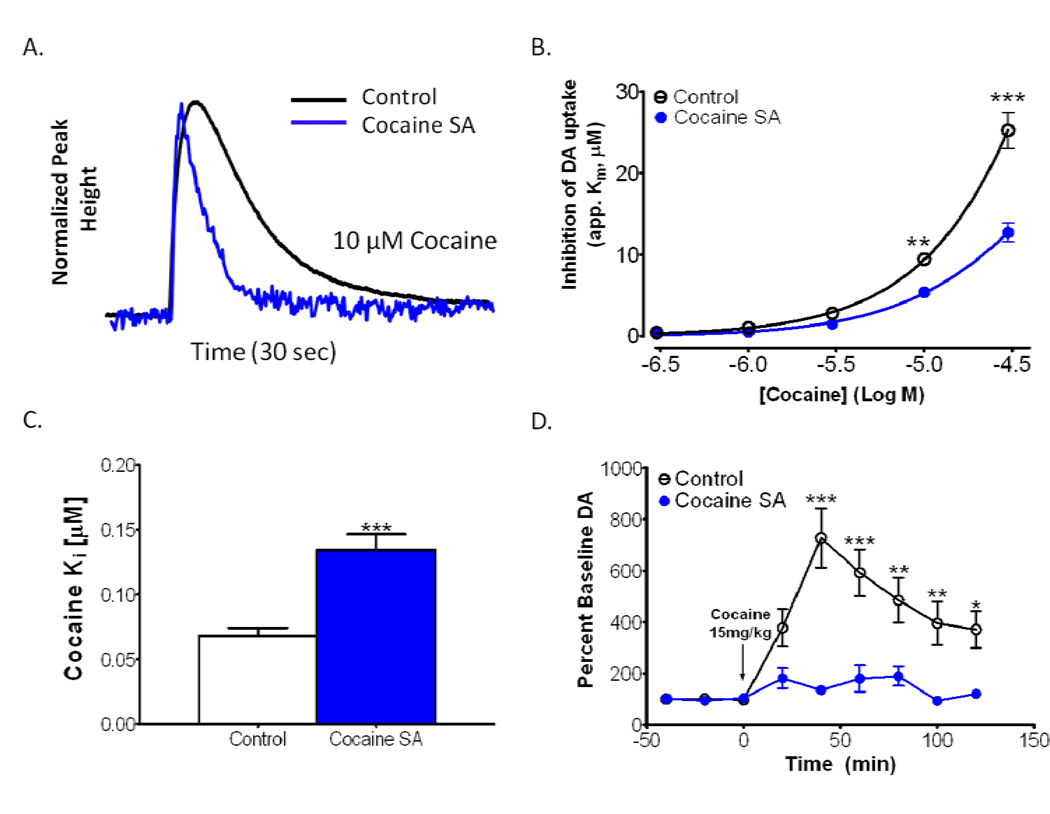
Because the DAT is the main site of action of cocaine for elevating DA, and for producing reinforcement, we aimed to determine if the effects of cocaine at the DAT were altered following extended-access, limited-intake cocaine self-administration as determined by apparent Km. We found a reduction in the potency for cocaine-induced DA uptake inhibition in the NAc core region using a number of potency measures (Fig. 3A). A two-way mixed ANOVA revealed a main effect of cocaine concentration (F4, 14= 231.3, p < 0.0001) indicating that cocaine inhibits DA uptake in a concentration dependent manner (Fig. 3B). In addition there was a main effect of group (F1, 14 = 38.35, p < 0.0001; Fig. 3B), indicating that the DAT was less sensitive to cocaine-induced DA uptake inhibition following cocaine self-administration. Bonferroni post hoc analysis revealed significant effects at the 10µM (p < 0.01) and 30 µM (p < 0.001) concentrations.
Figure 3. Cocaine self-administration results in tolerance to the dopamine transporter (DAT)-inhibiting and dopamine (DA)-elevating effects of cocaine.
(A) Representative DA traces (normalized peak height) plotted over time highlight reduced ability of cocaine to inhibit DA uptake in the nucleus accumbens core of animals with a history of cocaine self-administration. (B) Group data showing the potency of cocaine in naïve and cocaine self-administering animals, plotted as apparent Km over cocaine concentration (n = 9 controls, 7 self-administration animals). Cocaine potency is reduced over a dose-response (0.3–30 µM) following cocaine self-administration. (C) The Ki for cocaine was significantly increased in animals with a history of cocaine self-administration (n = 9 controls, 7 self-administration animals). Increased Ki values indicate that higher concentrations of drug are needed in cocaine self-administering animals, to achieve the same degree of DA uptake inhibition in naïve animals. (D) DA overflow in the NAc was measured by microdialysis in freely-moving animals (n = 8 controls, 6 self-administration animals). Pre-drug baselines were determined, animals were injected with 15 mg/kg cocaine i.p., and cocaine-induced elevations in DA overflow were monitored. *, p < 0.05; **, p < 0.01; ***, p < 0.001; SA, self-administration; Sec, seconds; Min, minutes
Ki is the drug concentration that results in 50% uptake inhibition. This measurement can determine relative potencies between drugs in naïve animals as well as potency changes following drug self-administration. The Ki for cocaine was significantly higher in animals with a history of cocaine self-administration (t14 = 5.312, p < 0.0001; Fig. 3C), indicating that cocaine potency was reduced.
Cocaine-induced DA overflow is reduced following a history of cocaine self-administration
In order to determine if the changes in DA kinetics following cocaine self-administration translated to reductions in extracellular DA levels, we performed microdialysis in freely-moving animals approximately 72 hrs following the last self-administered cocaine infusion (Fig. 3D). There were no statistically significant differences in basal DA levels across groups [Cocaine self-administration: 2.3nM ± 1.154; Control: 1.2nM ± 0.3725]. A two-way mixed ANOVA revealed a main effect of time, indicating that cocaine challenge significantly elevated DA levels when collapsing both groups (F8, 12 = 16.39, p < 0.0001). Additionally, there was a significant main effect of treatment group, comparing cocaine self-administering animals to naïve animals (F1, 12 = 12.51, p < 0.01). Bonferroni post hoc analysis revealed significant differences at the 40 (p < 0.001), 60 (p < 0.001), 80 (p < 0.01), 100 (p < 0.01), and 120 (p < 0.05) minute time points. Finally, there was a significant Time × Treatment Group interaction (F8, 96 = 10.75, p < 0.0001). These differences indicate that extended-access cocaine self-administration results in neurochemical tolerance to the DA elevating effects of systemically administered cocaine.
Given the differential effect of cocaine challenge between these two groups across time, we conducted a repeated measures ANOVA on each treatment group to test whether each cocaine challenge significantly increased dopamine for each treatment group. Both the cocaine self-administration group (F8, 56 = 20.21, p < 0.0001), and the naïve group (F8, 40 = 2.61, p < 0.05) showed significant elevations in dopamine following cocaine challenge.
Behavioral analysis showed no differences in basal locomotor activity, but tolerance to cocaine effects following self-administration
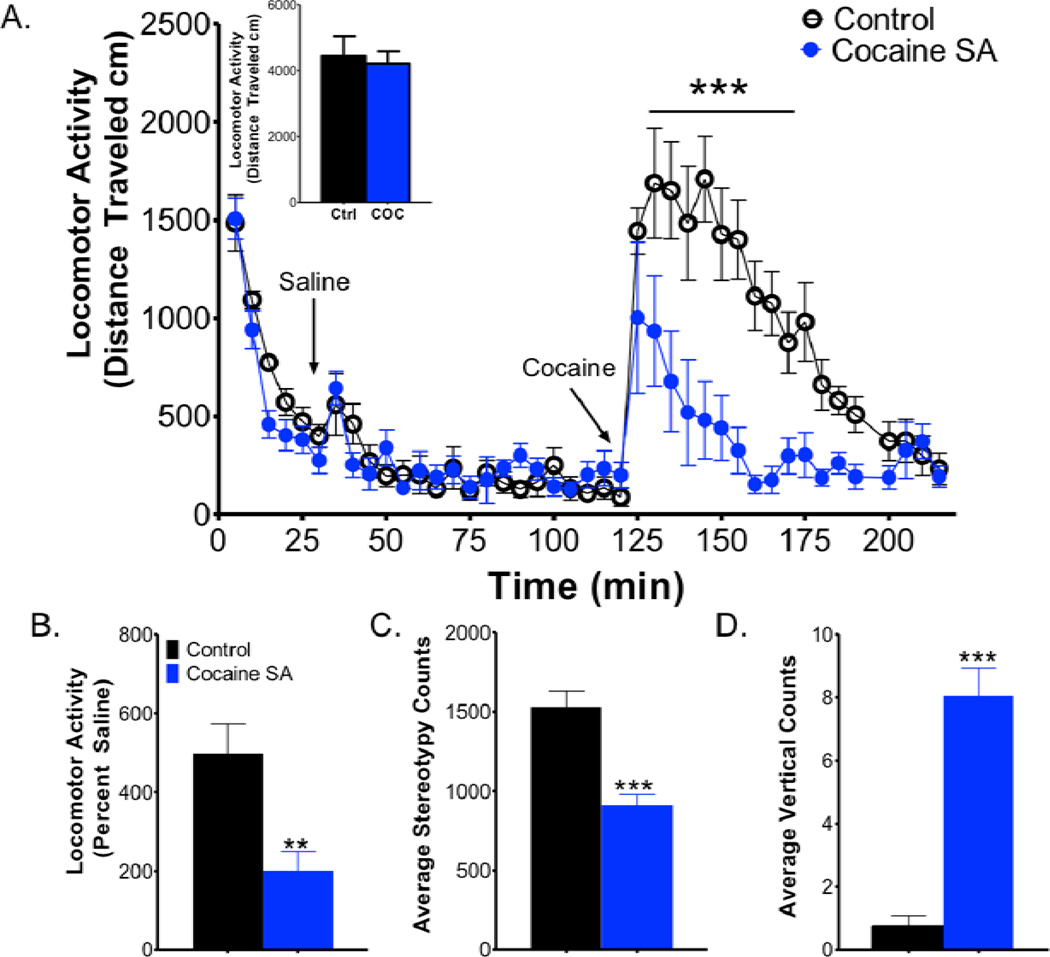
We used locomotor analysis to determine the effects of prior cocaine self-administration on the psychomotor activating effects of a cocaine challenge (15 mg/kg i.p.; Fig. 4). Figure 4A illustrates the time course of locomotor activity, with habituation to the chamber (30 min) followed by saline-induced locomotion (90min) and cocaine-induced locomotion. Saline-induced locomotion was not significantly different across groups (Fig. 4 A, inset), demonstrating that differences in cocaine-induced locomotor activity are not due to differences in response to injection. A two-way mixed ANOVA revealed a main effect of cocaine self-administration on cocaine-induced locomotor activity (F1, 12 = 27.49, p < 0.0001 Fig. 4 A, B) and a significant Time × Self-administration interaction (F17, 12 = 4.055, p < 0.0001). Bonferroni post hoc analysis revealed significant differences in cocaine-induced locomotion between cocaine self-administration and control groups at time points 130–165 minutes post-injection (Fig. 4 A). Further, locomotor activity was significantly different between groups when it was presented as total distance traveled in the first 30 min (t12 = 2.665, p < 0.05), 90 min (t12 = 1.925, p < 0.05) or as percent baseline (t12 = 3.388, p < 0.01; Fig. 5 B). Thus, cocaine is less effective at stimulating locomotor activity following cocaine self-administration, which is indicative of tolerance to the psychomotor activating effects of cocaine.
Figure 4. Cocaine self-administration results in tolerance to the locomotor activating effects of acute cocaine administration.
(A) Group data of locomotor activity as measured in cm over time plotted in five-minute bins (n = 7 per group). Data indicate that a history of cocaine self-administration results in reduced cocaine (15mg/kg; i.p.)-induced locomotor activity. (A, inset) Saline-induced locomotion was not different between controls and animals with a history of cocaine self-administration. (B) Cocaine-induced locomotor activity represented as percent of saline injection (n = 7 per group). (C) Cocaine-induced stereotypy represented as average stereotypy counts per five-minute bin over the 90-minute session (n = 7 per group). (D) Cocaine-induced vertical activity represented as average vertical counts per five-minute bin over the 90-minute session (n = 7 per group). *, p < 0.05; **, p < 0.01; ***, p < 0.001; SA, self-administration
Additionally, we assessed stereotypy behavior and vertical activity following a cocaine challenge. Student’s t-test revealed that cocaine self-administration animals had lower stereotypy counts as compared to controls (t12 = 5.206, p < 0.0001; Fig. 5 C). In addition, cocaine self-administration animals had higher vertical counts as compared to controls (t12 = 7.640, p < 0.0001; Fig. 5 D). These results highlight a change in spontaneous locomotor activity, distinct from forward activity.
Discussion
We demonstrate that a history of extended-access, defined-intake cocaine self-administration results in robust tolerance to the neurochemical and psychomotor activating effects of cocaine. DA system deficits, characterized by reductions in DA release and uptake in the NAc, were also observed in the absence of drug. The sensitivity of the DAT to cocaine-induced DA uptake inhibition was reduced, which translated to reduced cocaine-induced overflow, indicating neurochemical tolerance. The neurochemical data was supported by behavioral data that demonstrated an attenuated cocaine-induced locomotor activating response. Psychomotor activating effects of psychostimulants are hypothesized to predict the rewarding effects of the drug, suggesting that cocaine-induced reward may be reduced following cocaine self-administration (Wise and Bozarth, 1987). Because of the critical role of the DA system in reward, reinforcement, goal-reward associations, and motivated behaviors, the changes observed here could have profound behavioral effects. It is possible that reductions in DA dynamics in the NAc could have inhibitory effects on goal-directed behavior in the absence of cocaine, while increasing cocaine-related behaviors, such as cocaine seeking/intake in order to normalize DA deficits.
Because of the critical role of the NAc core in cue-reward association, assignment of motivational value, goal oriented behaviors, and the reinforcing properties of psychostimulant drugs, it was important to determine the effects of cocaine self-administration on this region (Willuhn et al., 2010). Although previous work has demonstrated reduced basal DA levels as measured by microdialysis following cocaine self-administration (Ferris et al., 2011; Hurd et al., 1989; Mateo et al., 2005; Maisonneuve et al., 1995; Weiss et al., 1992) here we found no difference in basal DA concentrations. The difference between previous work and the current study can be explained by differences in the time of microdialysis measurements relative to the final cocaine self-administration session, where measurements for the current study occurred 72 hours post self-administration as compared to 24 hours for previous work. Indeed, our previous voltammetry work showed a return to normal baseline DA function following 3 days of abstinence (Ferris et al., 2011). This suggests that changes in baseline DA may normalize rapidly after cocaine experience while behavioral and neurochemical tolerance to cocaine remain. This also suggests that differences in baseline DA functioning are not driving the cocaine tolerance, but rather they are completely dissociable.
Only recently was it discovered that the effects of cocaine on the DAT were reduced following extended access cocaine self-administration (Calipari et al., 2013a, b; Ferris et al., 2011, 2012, 2013). Here we replicate the reduced sensitivity of the DAT to cocaine, and demonstrate that this neurochemical tolerance at the DAT is also observed with DA overflow as measured by microdialysis, where cocaine failed to significantly increase DA levels following cocaine self-administration. This is consistent with previous work demonstrating reductions in cocaine-induced DA release as a function of cocaine experience under various cocaine self-administration paradigms (Ferris et al, 2011; Hurd et al, 1989; Mateo et al., 2005; Meil et al., 1995). The decreased DA overflow elicited by cocaine administration is likely due to the decreased ability of the compound to block the DAT. This is consistent with human studies, which have demonstrated a reduced ability of cocaine to elevate DA levels following a history of cocaine abuse (Dackis and O’Brien, 2001; Volkow et al., 1997a, b; Volkow et al., 1996). The ability of cocaine to elevate DA in the NAc is essential for its rewarding and reinforcing effects, thus reductions in cocaine-induced DA increases following this paradigm would be hypothesized to reduce the subjective effects of cocaine (Roberts et al., 1977). There are numerous human reports showing reduced positive subjective effects of cocaine in heavy users (Mendelson et al., 1998; Reed et al., 2009). In addition, human cocaine addicts often increase their intake in order to compensate for reductions in the rewarding effects of cocaine (Dackis and O’Brien, 2001). These clinical findings are consistent with the current study, where we find increased intake rates for cocaine over sessions and decreased cocaine-induced locomotor activity.
Indeed, we report here that animals escalate the rate of cocaine intake over sessions. Although this paradigm is different from traditional long-access models (LgA) due to the fixed injection maximum, animals still escalate in first hour intake, which is the same escalation that is often reported in LgA studies (Ahmed and Koob, 1998, 1999; Marusich et al., 2010). For example, although first hour injections with a traditional LgA paradigm are increased from around 14 to 20 (Ahmed & Koob, 1998), our dose is approximately two times (1.5mg/kg/inj) higher than previous work (≈ 0.75 mg/kg/inj), and animals are known to titrate lever pressing to account for differences in dose. A more comparable measure of cocaine consumption between studies using different doses is total cocaine intake. Escalation of total intake observed in the current manuscript (from ≈ 3.7 mg to ≈ 4.7 mg) is similar to previous studies using the traditional LgA paradigm (from ≈ 3.7 mg to ≈ 5.2 mg) (Ahmed & Koob, 1998). Also, it is important to note that the escalation observed with traditional LgA occurs over 14 days, while in the current manuscript similar levels of escalation occur within five days. Escalation is thought to be associated with the switch from recreational use to addiction in humans (Ahmed and Koob, 1998, 1999), and has been suggested to be due to tolerance to the rewarding effects of cocaine (Ahmed et al., 2002, 2003; Ahmed and Koob, 2005; Barrett et al., 2004; Koob and Le Moal, 2001). The observed changes, where animals consume more drug in shorter periods of time, mimic changes in the patterns of self-administration when a lower dose of cocaine is substituted for a higher one, which would be consistent with reduced cocaine effects, or tolerance (Carelli and Deadwyler, 1996). A similar escalation in cocaine dosing also commonly occurs during bingeing in humans (Dackis and O’Brien, 2001), and humans self-report tolerance to the euphorigenic effects of cocaine following repeated use (Mendelson et al., 1998; Reed et al., 2009). It is possible that the DAT tolerance, and concomitant reduction in cocaine-induced DA overflow, results in reduced rewarding effects, leading to an increase of cocaine intake to compensate. Although the tolerance could, at least in part, be driving the increase in intake over sessions, it is likely that a number of other factors converge to drive the behavioral changes induced by cocaine self-administration. Because there are substantial reductions in cocaine’s ability to inhibit the DAT and elevate DA levels, but only a small increase in first-hour intake, it is likely that tolerance is not the only factor driving escalation. For example, similar extended access paradigms show augmented drug seeking and relapse, demonstrating that some aspects of behavior are not predicted by cocaine tolerance (Koob 1996; Koob and Le Moal, 1997; Orio et al, 2009; Patterson and Markou, 2003).
Although here we find tolerance to the neurochemical effects of cocaine, both behaviorally and neurochemically, a number of other studies examining the consequences of escalation found it does not relate to shifts in dopamine function. Ahmed et al. (2003) showed that DA overflow in response to a cocaine challenge does not differ following long and short-access self-administration. Additionally, it has been shown that DAT levels are unchanged following LgA self-administration (Ben-Shahar et al., 2006;). Differences between the current model and traditional LgA models cannot be due to methodological differences such as the length of the self-administration period (5 days vs 14 days) or the limited intake in the current study, as we have previously shown that the same tolerance occurs following traditional LgA paradigms (Calipari et al., 2013b). Although it is unclear as to whether cocaine tolerance is directly responsible for the escalation in cocaine intake observed following extended-access to the compound, the two phenomena coexist in our experiments. Importantly, the tolerance to cocaine’s effects observed in the current model is congruent with the reduced ability of stimulants to elevate DA observed in cocaine-addicted individuals, confirming the translational validity of the current model (Dackis and O’Brien, 2001; Mendelson et al., 1998; Reed et al., 2009; Volkow et al., 1997a, b; Volkow et al., 1996).
Further, locomotor analysis also revealed an altered pattern of cocaine-induced activity, with decreases in forward locomotion and stereotyped behaviors and increases in vertical activity. Elevations in DA levels mediate forward locomotion (Tilley et al., 2007), thus, decreases are consistent with the reductions in cocaine potency at the DAT as well as cocaine-induced DA overflow. Additionally, systemic administration of a selective D2, but not D1, receptor agonist is sufficient to induce stereotypy, highlighting the role of D2 receptors in mediating stereotypic behaviors (Meller et al., 1988). Thus it is possible that the reduction in cocaine-induced stereotypy following cocaine self-administration is due to reduced D2 receptor activity. Indeed, Mateo et al., (2005) demonstrated that D2 receptors are subsensitive following 10 days of cocaine self-administration. In addition, the reduction in D2 activity was observed at both one and 7 days after self-administration, indicating that some of the changes that occur following this self-administration paradigm may persist well into the withdrawal period. In addition to decreased locomotion and stereotypy, we demonstration a cocaine induced increase in vertical activity in cocaine self administering animals. Serotonin levels are inversely related to vertical activity (Brookshire and Jones, 2009); thus, it is possible that the serotonin system may also be affected by cocaine self-administration. Studies of whole brain functional activity after this self-administration paradigm have observed reduced dorsal raphe activity (Calipari et al., 2013c). This is not surprising as cocaine has direct effects on the serotonin transporter, so it is possible that tolerance to the serotonin elevating effects also develops. If cocaine’s ability to inhibit serotonin is reduced, then one would expect the increased rearing behavior demonstrated in cocaine self-administering animals.
We have shown that the development of tolerance is dependent on cocaine intake, where high sustained cocaine levels over a session result in reduced cocaine potency. Alternatively, cocaine sensitization at the DA terminal occurs following intermittent patterns of self-administration and seems to be independent of intake (Calipari et al., 2013b). Much work has been aimed at outlining the consequences and causes of neurochemical sensitization as a model of cocaine addiction (Brown et al., 2011; Cornish and Kalivas 2001; Thomas et al., 2008), and yielded important information, especially on changes in glutamate neurotransmission (Cornish and Kalivas 2001). However, sensitization of cocaine pharmacology has been difficult to demonstrate in humans. Only recently was sensitization in humans demonstrated with amphetamine administration; however, it only occurs in drug naïve individuals and is not present in heavy users (Bolieau et al., 2006). It is possible that sensitization occurs with intermittent exposure to drug during the early stages of the addiction process, but later stages, with higher and more continuous drug intake, result in tolerance. As a result, animal models which produce tolerance may have more construct validity for studying the neurochemical changes that occur within addicted individuals.
Acknowledgements
This work was funded by NIH grants R01 DA021325, R01 DA030161, P50 DA006634 (SRJ), K99 DA031791 (MJF), T32 DA007246 and F31 DA031533 (ESC).
Footnotes
Conflict of interest: The authors have no conflicts to report.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146(3):303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5(7):625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86(1):102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180(3):473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095(1):148–153. doi: 10.1016/j.brainres.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63(12):1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Brookshire BR, Jones SR. Direct and indirect 5-HT receptor agonists produce gender-specific effects on locomotor and vertical activities in C57 BL/6J mice. Pharmacol Biochem Behav. 2009;94(1):194–203. doi: 10.1016/j.pbb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schlüter OM. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31(22):8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol. 2013a doi: 10.1111/j.1369-1600.2012.00456.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DCS, Jones SR. Temporal pattern of cocaine intake determines tolerance versus sensitization of cocaine effects at the dopamine transporter. Neuropsychopharm. 2013b doi: 10.1038/npp.2013.136. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Beveridge TJR, Jones SR, Porrino LJ. Withdrawal from extended access cocaine self-administration results in dysregulated functional activity and altered locomotor activity in rats. Eur. J. Neurosci. 2013c doi: 10.1111/ejn.12381. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Ther. 1996;277(1):385–393. [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103(24):9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20(3):43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21(3):111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69(3):201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology. 2012;37(7):1708–1716. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Melchior JR, Roberts DCS, Espana RA, Jones SR. Paradoxical tolerance to cocaine after initial supersensitivity in drug-use-prone animals. Eur J Neurosci. 2013 doi: 10.1111/ejn.12266. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498(1):199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1(3):186–189. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Lack CM, Jones SR, Roberts DC. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology (Berl) 2008;195(4):517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine "binge" alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272(2):652–657. [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18(3):257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30(8):1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Meil WM, Roll JM, Grimm JW, Lynch AM, See RE. Tolerance-like attenuation to contingent and noncontingent cocaine-induced elevation of extracellular dopamine in the ventral striatum following 7 days of withdrawal from chronic treatment. Psychopharmacology (Berl) 1995 Apr;118(3):338–346. doi: 10.1007/BF02245964. 1995 Erratum in: Psychopharmacology (Berl) 121 (2): 285. [DOI] [PubMed] [Google Scholar]
- Meller E, Bordi F, Bohmaker K. Enhancement by the D1 dopamine agonist SKF 38393 of specific components of stereotypy elicited by the D2 agonists LY 171555 and RU 24213. Life Sci. 1988;42(25):2561–2567. doi: 10.1016/0024-3205(88)90324-4. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18(4):263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29(15):4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14(17):2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Reed SC, Haney M, Evans SM, Vadhan NP, Rubin E, Foltin RW. Cardiovascular and subjective effects of repeated smoked cocaine administration in experienced cocaine users. Drug Alcohol Depend. 2009;102(1–3):102–107. doi: 10.1016/j.drugalcdep.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6(6):615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154(2):327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemannn R, Gatley SJ, MacGregor RR, Wolf AP. Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology. 1996;14(3):159–168. doi: 10.1016/0893-133X(95)00073-M. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997a;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. Imaging studies of cocaine in the human brain and studies of the cocaine addict. Ann N Y Acad Sci. 1997b;820:41–54. doi: 10.1111/j.1749-6632.1997.tb46188.x. discussion 54-5. [DOI] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593(2):314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]