Abstract
We previously found that human mesenchymal stem cells (MSC) or its conditioned medium restored lung protein permeability and reduced alveolar inflammation following E.coli endotoxin-induced acute lung injury (ALI) in an ex vivo perfused human lung in part through the secretion of soluble factors such as keratinocyte growth factor (KGF). Recently, MSC were found to release microvesicles (MV) that were biologically active because of the presence of mRNA or miRNA with reparative properties. MVs are circular fragments of membrane released from the endosomal compartment as exosomes or shed from the surface membranes. The current studies were designed to determine if MVs released by human bone marrow derived MSCs would be effective in restoring lung protein permeability and reducing inflammation in E.coli endotoxin-induced ALI in C57BL/6 mice. The intra-tracheal instillation of MVs improved several indices of ALI at 48 h. Compared to endotoxin-injured mice, MVs reduced extravascular lung water by 43% and reduced total protein levels in the bronchoalveolar lavage (BAL) fluid by 35%, demonstrating a reduction in pulmonary edema and lung protein permeability. MVs also reduced the influx of neutrophils and macrophage inflammatory protein-2 levels in the BAL fluid by 73% and 49% respectively, demonstrating a reduction in inflammation. KGF siRNA-pretreatment of MSC partially eliminated the therapeutic effects of MVs released by MSCs, suggesting that KGF protein expression was important for the underlying mechanism. In summary, human MSC derived microvesicles were therapeutically effective following E.coli endotoxin-induced ALI in mice in part through the expression of KGF mRNA in the injured alveolus.
Keywords: Acute Lung injury, Keratinocyte growth factor, Lipopolysaccharide, Mesenchymal stem cell, Microvesicles
INTRODUCTION
Acute lung injury (ALI) and the acute respiratory distress syndrome are major causes of acute respiratory failure in critically ill patients. Despite improvements in supportive care, mortality from ALI remains high at approximately ~40%, depending on the etiology 1,2. Recently, several studies have demonstrated that the administration of mesenchymal stem or stromal cells (MSC) improved ALI, whether from endotoxin 3-6, live E.coli bacteria 7-9 or following sepsis 10-12. However, the mechanisms underlying the therapeutic benefit of MSC remain incompletely understood 13.
In models of ALI, most of the therapeutic benefit of MSC appears to derive from the release of paracrine soluble factors, which stabilize the injured alveolar epithelium and lung endothelium, reduce inflammation, increase the absorption of pulmonary edema fluid, and have anti-microbial activity 13. Recently, MSCs have also been found to release circular membrane fragments called micro-particles or microvesicles (MV), which can be involved in cell-cell communication and the transfer of cellular material 14,15. MVs are anuclear particles, 50 nm to 1 μm in size, which contain numerous proteins, mRNAs, microRNAs, organelles and lipids similar to those present in the cells from which they originate. MVs are not apoptotic bodies. Bruno et al. demonstrated that MVs derived from adult human MSCs were renal protective following glycerol- 16, ischemia-reperfusion 17 and cisplatin-induced 18 acute kidney injury (AKI); subsequent studies suggested that the therapeutic effect of MSC derived MVs were through the transfer of mRNA and miRNA to the injured renal epithelium 18-20, leading to a decrease in apoptosis.
Currently, little is known regarding the effect of MVs released by MSC in experimental models of ALI and pulmonary edema. In this study, we hypothesized that the administration of MSC MVs may have the same therapeutic effect as the cells themselves in an E.coli endotoxin model of ALI in part through the transfer of mRNA from the MVs to the injured alveolar epithelium and lung endothelium. We were particularly interested in the expression of keratinocyte growth factor (KGF) mRNA by MSC MV because of work from our group 21 as well as other investigators who have reported that KGF can reduce lung injury in small animal models of pulmonary edema 22-26. We previously found that human MSC produced substantial quantities of KGF, and the secretion of this paracrine soluble factor mediated the restorative effect of MSC on alveolar fluid clearance (AFC) 21.
MATERIALS AND METHODS
Mesenchymal Stem Cells
Human MSCs were obtained from a NIH repository from Tulane Center for Gene Therapy. The adult stem cells met all of the criteria for MSC as defined by the International Society of Cellular Therapy 27. MSC, from 3 different human donors with the total passage number ≤ 8, were used in the experiments. Normal adult human lung fibroblasts (NHLF) were used as cellular controls (Lonza).
Isolation of MSC Microvesicles
MVs were obtained from the supernatant of MSCs and NHLFs as previously described 18. Briefly, MSCs or NHLFs were cultured until confluent in P150 flasks and then serum starved for 48 h in fresh conditioned medium (αMEM or FBM supplemented with 0.5% Bovine Albumin Fraction (MP Biomedicals, LLC)). To isolate the MVs, the conditioned medium of MSCs or NHLFs was centrifuged at 3,000 rpm for 20 minutes to remove cellular debris, then at 100,000 g (Beckman Coulter Optima L-100XP ultracentrifuge) to sediment the MVs for 1 h at 4°C, washed in phosphate buffered saline (PBS) and submitted to a second ultracentrifugation. MSC or NHLF MVs were resuspended according to the final cell count of MSCs or NHLFs after 48 h of serum starvation (10 μl per 1×106 cells) and stored at −80°C until further use. The total protein content of the MVs was also quantified.
Microvesicles with or without Keratinocyte Growth Factor siRNA Pretreatment
For siRNA experiments, MSCs were cultured in 6-well plates, 2.25×105 cells/well, pretreated with siPORT NeoFX containing 100 nM KGF siRNA (#10818 for KGF/FGF7 siRNA, Ambion) for 24 h. Pretreatment with a scrabbled siRNA (Negative Control No.1 siRNA, Ambion) was used as a siRNA control. The medium was replaced, and the subsequent conditioned medium was collected 48 h later for MV isolation 18.
RNA Isolation and RT-PCR
Total RNA was isolated from either MVs or MSCs using RNeasy Mini Kit (QIAGEN Sciences). Primers used for the RT-PCR were human angiopoietin-1 (Ang1), KGF/FGF7, CO1 & CO2 and GAPDH and were purchased from QIAGEN-SABiosciences. The RT-PCR assays were conducted following the One-Step RT-PCR protocol described by QIAGEN-SABiosciences.
E.coli Endotoxin Induced ALI in Mice
C57BL/6 male mice (10-12 weeks old, ~ 25 gms, Jackson Laboratory) were used in all experiments. All experimental protocols were approved by the Institutional Animal Care and Use Committee at UCSF. Mice were first anesthetized with Ketamine (90 mg/kg) and Xylazine (10 mg/kg) intra-peritoneal. ALI was then induced by the intra-tracheal (IT) instillation of a non-lethal dose of endotoxin from Escherichia coli O111:B4 (Sigma-Aldrich) at 4 mg/kg.
In preliminary experiments, we chose an initial dose of MSC MVs (15 μl), which is the amount of MVs released by 1.5 × 106 serum starved MSCs over 48 h or double the cell count, 750,000 MSCs, we previously used in a similar endotoxin-induced ALI model 3, to maximize the therapeutic potential. Unfortunately, 15 μl of MSC MV had only a partial effect, reducing the influx of neutrophils by 35% and protein levels by 12% in the BAL fluid compared to the endotoxin-injured mice (P>0.05). However, increasing the dose to 30 μl significantly reduced both the inflammatory response and lung protein permeability in the endotoxin-injured alveolus. Consequently, all subsequent studies were based on the primary dose of 30 μl of MSC MVs.
The treatment groups, which were given simultaneously, were: 1) PBS as carrier control, 2) 750,000 MSCs as a positive cellular control or 3) 30 μl of MSC MVs IT, KGF siRNA-pretreated MSC MVs, Neg Control siRNA-pretreated MSC MVs, MSC MVs intravenously (IV) through the external jugular vein or NHLF MVs. In separate experiments, MSC MVs were given IT at 12 h after the onset of lung injury. In additional experiments, we instilled 10 μg of goat anti-human KGF Ab (R&D Systems) with MVs IT at 0 h and a second dose at 24 h to neutralize any secreted KGF protein in the endotoxin-injured lung; control goat IgG (R&D Systems) was used as a negative control. After 48 h, both plasma and BAL fluid samples were collected from each mouse for assessment of neutrophil counts, cytokine and protein level measurements and histology as described below.
Measurement of Neutrophil Counts, Cytokine and Protein Levels in BAL Fluid and Plasma
Both BAL fluid and plasma samples were obtained from mice at 48 h after endotoxin-induced lung injury. Total cell count was determined with Z1 Coulter Particle Counter (Beckman Coulter), and a differential of the white blood cells were obtained using Hemavet HV950FS (Drew Scientific). Mouse macrophage inflammatory protein (MIP)-2 and human KGF were measured in the BAL fluid with ELISA kits (R&D Systems). Total protein concentration was measured in the BAL fluid (Pierce BCA Protein Assay Kit, Thermo Scientific).
Extravascular Lung Water
Gravimetric lung water determination was done as follows: the whole lung was excised, weighed, and then homogenized after the addition of 1 ml of double distilled H2O (ddH2O). The homogenate was centrifuged at 12,000 rpm for 10 min to obtain the supernatant, which was weighed. The hemoglobin of the supernatant was also measured. A blood sample was obtained by needle puncture of the right ventricle, and the wet weight, hemoglobin, and hematocrit of the blood sample were obtained. All samples were placed in a drying oven at 55°C for 24 h, and the dry weights were subsequently determined. The final extravascular lung water (EVLW) was calculated as described in our previous publication 3.
Histology
Lungs from endotoxin-injured with or without treatment with MSC or NHLF MVs were excised at 48 h. The lungs were gently inflated with 0.3 ml of air, and the trachea ligated. The lungs were then fixed in 4% paraformaldehyde. After fixation, lungs were embedded in paraffin, cut into 5 μm sections, and stained with H&E.
Co-culture of Endotoxin-Stimulated RAW 264.7 cells and MSC Microvesicles
Mouse RAW 264.7 cells (Sigma-Aldrich) were co-cultured either with MSC MVs (30 μl) in a standard 24-well plate (Costar, Corning) or with MSCs in a Transwell plates (250,000 cells in the upper chamber, 0.4-μm pore size, Costar, Corning) in the presence of endotoxin (500 ng/ml) at a concentration of 5×105 cells/well in DMEM without FBS for several time points (6, 12, and 24 h). The supernatants were then collected at each time point after co-incubation to assay for the levels of IL-10, TNF-α and MIP-2 with ELISA kits (R&D Systems).
Primary Cultures of Human Alveolar Epithelial Type II Cells
Human alveolar epithelial type II cells (ATII) were isolated from human lungs declined for transplantation by the Northern California Transplant Donor Network as previously described 21. Cell preparations with resistance of >1000 Ohm were used for the experiments. Alveolar epithelial permeability to protein was measured across the ATII cells on the Transwell plates under the different conditions. Type II cells were injured with 50 ng/ml of cytomix, a mixture of IL-1β, TNFα and IFNγ (R&D Systems) often used as a surrogate for ALI pulmonary edema fluid, with or without MSCs (250,000 cells in the bottom chamber) or 100 μl of MSC MVs given simultaneously in the upper chamber. Epithelial protein permeability from the apical to the basolateral membrane of the ATII monolayers were measured using radiolabeled 131I-albumin as previously described 21.
Transmission Electron Microscopy of MSC Microvesicles
Human MSC monolayers, grown on glass coverslips, or isolated MSC MVs were fixed with 3% (wt/vol) Karnovsky fixative for 1 h at 0°C. The monolayers were post fixed for 1 h in 1% veronal buffered osmic acid and then dehydrated in graded ethanols and/or propylene oxide. The cell preparations were then embedded in Epon or Araldite resins cured at 60°C. Thin sections were contrasted with saturated aqueous uranyl acetate and Reynolds lead citrate. The sections were then imaged with a JEOL 1200 EX transmission electron microscope operating at 80 kV.
Statistical Analysis
Comparisons between two groups were made using an unpaired t test. For comparisons between multiple groups, ANOVA with Bonferroni correction was used. A value of P < 0.05 was considered statistically significant. Analyses were done using SPSS software and GraphPad Prism software. Data are shown as mean ± SD.
RESULTS
Isolation and Characterization of MSC Microvesicles
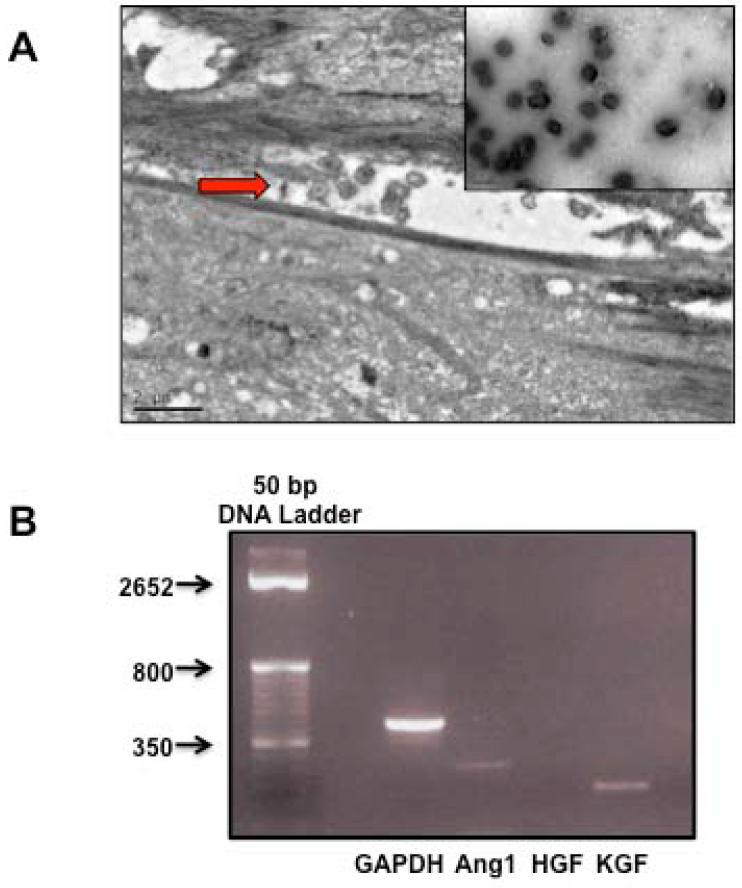
MVs were isolated from the conditioned medium of human bone marrow derived MSCs 18. For all experiments, 30 μl of the MVs was the MVs released by 3×106 serum starved MSCs over 48 h. Viability of the serum starved MSCs was >90% at 48 h prior to the isolation of the MVs. The protein concentration of the 1× dose of the MSC MV (30 μl) was 30.9 ± 17.0 μg (N = 8). Instead of using protein concentration, the dose of MVs given for the experiments was based on the final cell count of MSCs, which generated the conditioned medium, in order to compare the findings with earlier ALI experiments using whole cells. Using scanning electron microscopy, MVs, approximately 200 nM in size, were released from MSCs in culture and appeared homogeneous as spheroids (Figure 1A). By RT-PCR, MSC MVs expressed mRNA for several key MSC paracrine factors, specifically Ang1 and KGF (Figure 1B). To determine the therapeutic potential of MSC MVs compared to the cells themselves, 30 μl of MSC or NHLF MVs or 750,000 MSCs were given simultaneously with E.coli endotoxin (4 mg/kg) IT to C57BL/6 mice. The level of ALI in the mice was assessed at 48 h (Supplemental Figure 1).
FIGURE 1. Electron Microscopy Images of MVs Released by MSCs and MSC MV mRNA Content.
Electron microscopy demonstrates that MVs are released by MSCs in vitro following stress such as serum starvation. (A) MVs released into the inter-cellular gap separating two MSCs; bar is 2 μm. Enclosed image shows purified MVs, which appears to be a collection of homogeneous spheroids; bar is 0.5 μm. (B) RT-PCR demonstrated that MSC MV expressed the mRNAs for KGF and Ang1, two secreted proteins previously found to be involved in the therapeutic effect of MSCs in ALI.
Effect of MSC Microvesicles on Inflammatory Cell Influx into the Endotoxin-Injured Alveolus
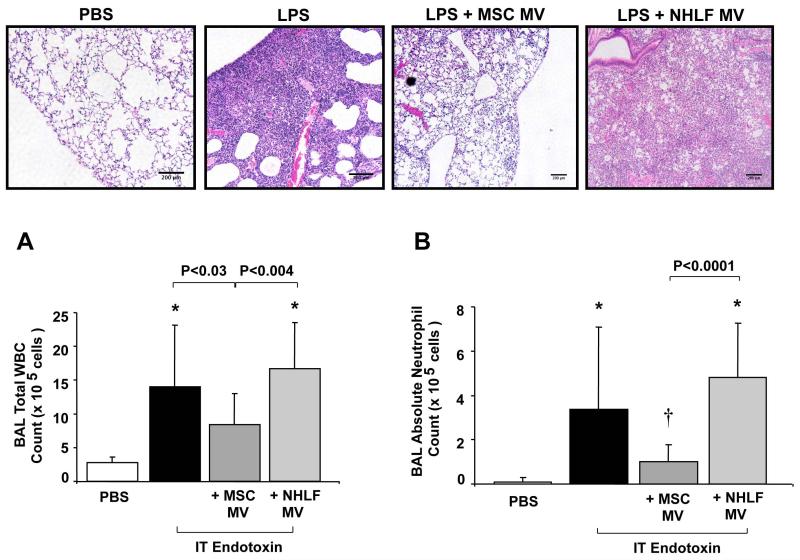
IT endotoxin produced a robust inflammatory response in the alveolus with an increase in influx of white blood cells, specifically neutrophils, an elevation of the inflammatory cytokine, MIP-2, and an increase in lung protein permeability and development of pulmonary edema at 48 h (Figure 2 & 3). Simultaneous administration of 30 μl of MSC MVs reduced the influx of white blood cells by 36%, of neutrophils by 73%, the elevation of MIP-2 by 49% and the increase in protein levels by 35% compared to the endotoxin injured lung. NHLF MVs at the same dose had no therapeutic effect. Administration of MSC MVs also reduced the weight loss of endotoxin-injured mice by 29% (N = 14-15 for PBS and MSC MV treated groups, N = 20 for endotoxin-injured, results mean ± SD gm of weight loss at 48 h, 0.9 ± 0.6 for PBS treated, 4.2 ± 0.5 for endotoxin-injured, 3.0 ± 1.4 for endotoxin + MSC MV, P < 0.007 for endotoxin vs. endotoxin + MSC MV). Administration of 30 μl of MSC MVs by the intravenous route through the external jugular vein, had a similar effect to MSC MV given IT, reducing the influx of neutrophils by 49% and reducing protein levels by 34% in the BAL fluid as compared to the endotoxin-injured mice (Supplemental Figure 2). Intravenous MSC MVs also significantly reduced the weight loss of endotoxin-injured mice by 25%. (N = 4-5, results mean ± SD gm of weight loss at 48 h, 0.2 ± 0.7 for PBS treated, 4.0 ± 0.8 for endotoxin-injured, 3.0 ± 0.4 for endotoxin + IV MSC MV, P < 0.04 for endotoxin vs. endotoxin + MSC MV).
FIGURE 2. Effect of MSC MVs on Influx of Inflammatory Cells in Endotoxin-Induced ALI in Mice.
The administration of MSC MVs reduced the influx of inflammatory cells in endotoxin-induced ALI in mice. (A) IT MSC MVs improved lung injury as assessed by histology. H&E staining of lung sections at 48 h demonstrated a reduction in inflammatory cell influx, edema, blood and thickening of the interstitium in endotoxin-injured lungs treated with MSC MVs. The MVs derived from NHLF showed no therapeutic benefit on lung injury. Scale bars, 200 μm. The IT administration of MSC MVs decreased the total white blood cells (WBC) in the BAL fluid of endotoxin-injured mice. Data is shown as mean ± SD, N = 4 for NHLF treated, N = 14 for PBS treated and N = 19-20 for endotoxin or endotoxin + MSC MV treated. *, P < 0.0003 vs. PBS treated mice by ANOVA (Bonferroni). (B) More significantly, IT administration of MSC MVs reduced the influx of neutrophils into the BAL fluid of endotoxin-injured mice. Absolute neutrophil counts are shown as mean ± SD. *, P < 0.002 vs. PBS treated and †, P < 0.002 vs. endotoxin treated mice by ANOVA (Bonferroni).
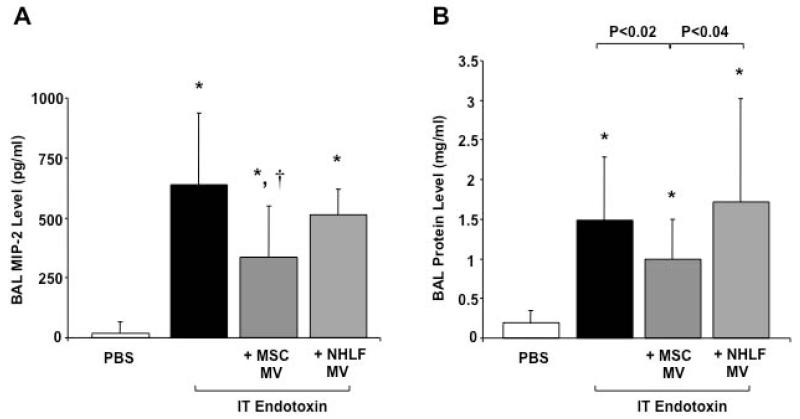
FIGURE 3. Effect of Intra-tracheal MSC MVs on Inflammation in Endotoxin-Induced ALI in Mice.
IT administration of MSC MVs reduced the level of inflammation and protein permeability in the alveolus of endotoxin-injured mice. (A) IT MSC MVs decreased the level of MIP-2 in the BAL fluid of endotoxin-injured mice. Data are expressed as mean ± SD, N = 14-15. *, P < 0.006 vs. PBS treated and †, P < 0.002 vs. endotoxin treated mice by ANOVA (Bonferroni). (B) IT MSC MVs decreased the total protein level in the BAL fluid of endotoxin-injured mice. Data are shown as mean ± SD, N = 14-16. *, P < 0.003 vs. PBS treated by ANOVA (Bonferroni).
In separate experiments, administration of MSC MVs at 12 h after the onset of lung injury significantly reduced the influx of neutrophils by 25% (N = 3-4 for PBS or MSC MV treated group, results mean ± SD ×105 cells at 48 h, 4.92 ± 0.47 for PBS, 3.67 ± 0.41 for MSC MV treated, P < 0.007 for PBS vs. MSC MV). However, the administration of MSC MVs at 12 h only reduced the levels of MIP-2 by 13% and protein levels by 7% compared with PBS treated group.
Dose Response Effect of MSC Microvesicles on Endotoxin-Induced Acute Lung Injury
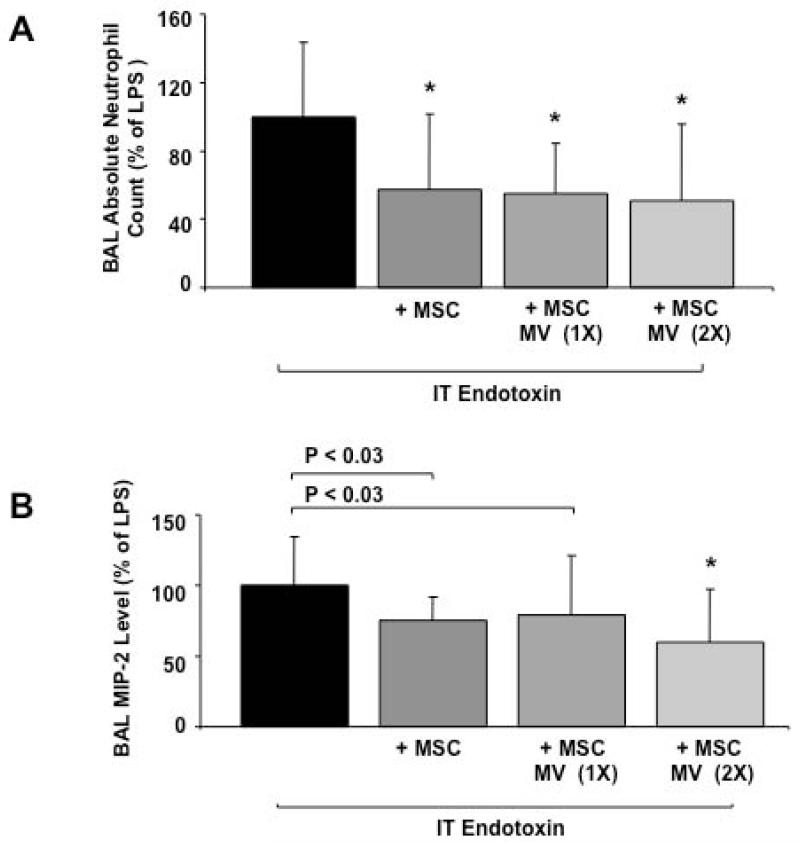
Surprisingly, doubling the IT dose of MSC MVs given (60 μl) with endotoxin had minimal additional effects in reducing the influx of neutrophils and the elevation of MIP-2 levels in the BAL fluid at 48 h. These results suggested that there may be other rate-limiting steps aside from the total amount of MVs given which require further study, such as the number of surface receptors on the MVs, i.e. CD44, required to attach to or its ligands in the injured alveolus which may limit MV uptake. However, the therapeutic effect of MSC MVs was equivalent to the IT instillation of 750,000 MSCs (Figure 4).
FIGURE 4. Dose Response of MSC MVs on Endotoxin-Induced ALI in Mice.
Doubling the dose of MSC MVs had no additional anti-inflammatory effect in endotoxin-injured mice. (A) MSC MVs, 1× or 2×, had a similar response to MSCs, the cells themselves, in reducing the influx of neutrophils into endotoxin-injured mice at 48 h. Data are shown as mean ± SD, N = 13-14 per MSC or MSC MV (2×), N = 29 for MSC MV (1×) and N = 36 for endotoxin. *, P < 0.003 vs. endotoxin treated mice by ANOVA (Bonferroni). (B) Doubling the dose of MSC MVs had no additional effect in reducing the MIP-2 level in the BAL fluid of endotoxin-injured mice. Data are shown as mean ± SD, N = 9-13 per MSC or MSC MV (2×), N = 24 for MSC MV (1×) and N = 32 for endotoxin. *, P < 0.002 vs. endotoxin treated mice by ANOVA (Bonferroni).
Effect of MSC Microvesicles on Protein Permeability Across Primary Cultures of Human Alveolar Epithelial Type II Cells Injured by An Inflammatory Insult
Exposure of human alveolar epithelial type II cells to cytomix increased albumin protein permeability by 450% over 24 h. The simultaneous administration of 100 μl of MSC MVs in the upper chamber of the Transwell plate reversed the increase in protein permeability. More importantly, the effect of MSC MVs was similar to MSCs (250,000 cells grown on the bottom chamber of the Transwell plate) on restoring cytomix-induced protein permeability (Supplemental Figure 3).
Expression of Keratinocyte Growth Factor mRNA from MSC Microvesicles
IT or IV administration of MSC MVs increased the levels of KGF protein in the BAL fluid typically by >100% as compared to endotoxin-injured lungs alone (Figure 5A), as measured using a human KGF ELISA kit (R&D Systems). Again, the level of KGF was similar to the level found in the BAL fluid of endotoxin-injured mice treated IT with 750,000 MSCs. To determine if the KGF found in the BAL fluid was derived from the expression of MV mRNA for KGF or from stored protein in the MVs itself, total KGF protein levels was measured in the MVs. Thirty μl of MVs yielded an average of 5.5 ± 1.8 pg/ml of KGF (mean ± SD pg/ml, N = 8), demonstrating that (1) there was not enough KGF protein in the MVs to account for the level found in the BAL fluid and (2) the increased in KGF level was probably mediated by the expression of the mRNA from the MSC MVs. KGF protein expression was also elevated in the medium of primary cultures of human alveolar type II cells. The presence of inflammatory injury (cytomix) was necessary to significantly increase the level of KGF (Figure 5B). Compared to NHLF MVs, the BAL fluid level of KGF following MSC MVs administration was significantly higher at both 24 h (N = 5 - 8, mean ± SD pg/ml, 22.29 ± 5.39 for MSC MV, 12.18 ± 6.89 for NHLF MV, P < 0.009 for MSC MV vs. NHLF MV) and 48 h (N = 4 - 5, mean ± SD pg/ml, 32.49 ± 2.16 for MSC MV, 18.74 ± 9.11 for NHLF MV, P < 0.007 for MSC MV vs. NHLF MV) following lung injury.
FIGURE 5. Expression of KGF Protein from MSC MVs.
The administration of MSC MVs increased the secretion of KGF protein in the BAL fluid of endotoxin-injured mice and in the conditioned medium of human alveolar epithelial type II cells injured with cytomix. (A) IT or IV administration of MSC MVs increased the levels of KGF protein in endotoxin-injured mice at 48 h, similar to the level of MSCs. Data are expressed as mean ± SD, N = 4-9 for treatment groups and N = 12-13 for PBS or endotoxin treated mice. *, P < 0.0002 vs. PBS treated and †, P < 0.002 vs. endotoxin treated mice by ANOVA (Bonferroni). (B) KGF protein levels was also elevated in the conditioned medium of primary cultures of human alveolar type II cells injured with cytomix and exposed to 100 μl of MSC MVs in the upper chamber. Data are expressed as mean ± SD, N = 3.
Therapeutic Effect of KGF on Endotoxin-Induced ALI in Mice
To determine if recombinant human KGF would replicate most of the therapeutic effects of MSC MV, we simultaneously instilled 100 ng or 4 μg/kg IT (R&D Systems) in endotoxin-injured mice. The dose was chosen because it was within range of the KGF protein levels found in the BAL fluid of endotoxin-injured mice treated with MSCs or MSC MVs (Figure 5). The instillation of 100 ng of KGF increased BAL KGF levels to 31 ± 15 pg/ml (mean ± SD pg/ml, N = 10) at 48 h. Similar to MSC or MSC MV, the administration of KGF significantly reduced the influx of neutrophils by 29%, of MIP-2 expression by 31% and the total protein influx by 35% compared to endotoxin-injured mice at 48 h (Supplemental Figure 4A).
Effect of KGF siRNA Pretreatment of MSC on the Therapeutic Effect of Microvesicles
To determine the therapeutic effect of KGF mRNA expression in the MSC MVs, we eliminated the mRNA level using siRNA pretreatment of the MSCs prior to the collection of the conditioned medium (Figure 6A). Although viability of the cells remained > 90%, siRNA pre-treatment diminished the metabolic activity of the MSCs as suggested by the decrease in GAPDH level in the MVs. Consequently, all comparisons of the effect of KGF siRNA pretreated MSC MVs were made to MVs isolated from MSCs pretreated with a Neg control siRNA. Administration of 30 μl MVs from KGF siRNA pretreated MSCs partially eliminated the therapeutic effects of the MVs. As compared to MVs isolated from a Neg control siRNA pretreated MSCs, administration of KGF siRNA MSC MVs resulted in an increase in the influx of neutrophils by 47% and the elevation of MIP-2 levels by 56% in the BAL fluid of the endotoxin-injured lungs (Figure 6B).
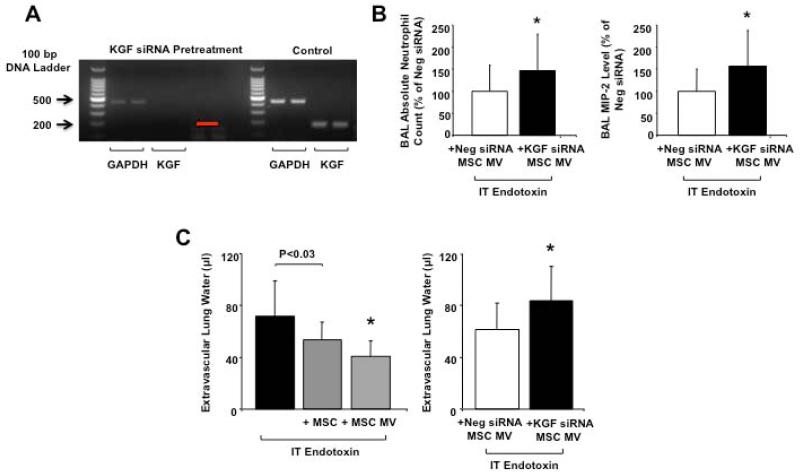
FIGURE 6. Effect of KGF siRNA Pretreatment of MSCs on the Therapeutic Effect of Secreted MSC MVs.
KGF siRNA pretreatment of MSC eliminated much of therapeutic effects of MVs released by MSC. (A) RT-PCR confirmed that KGF mRNA in MSC MVs was eliminated by KGF siRNA pretreatment of MSCs for 24 h. Red arrow, location of KGF band by size. GAPDH was used as a housekeeping gene and was expressed by both KGF siRNA-pretreated MSC MVs and control MSC MVs. (B) The IT administration of MVs released from KGF siRNA pretreated MSCs significantly increased the influx of neutrophils and the elevation of MIP-2 level in the BAL fluid of endotoxin-injured lungs compared to administration of MVs from Neg Control siRNA pretreated MSCs, demonstrating a partial loss of therapeutic effect of the MVs. Data are expressed as mean ± SD, N = 14-15 per treatment group, *, P < 0.05 vs. MV isolated from a Neg control siRNA pretreated MSC for neutrophil count; *, P < 0.02 vs. MV isolated from a Neg control siRNA pretreated MSC for MIP-2 level. (C) The administration of MSC MVs had a similar effect as the cells themselves in reducing EVLW following endotoxin-induced ALI. Pretreatment of the MSCs with KGF siRNA eliminated the therapeutic effect of the MSC MVs on EVLW as compared to Neg control siRNA-pretreated MSC MVs. Data are expressed as mean ± SD, N = 9-10 per treatment groups, N = 21 for endotoxin. *, P < 0.002 and is significant by ANOVA (Bonferroni) vs. endotoxin injured mice; N = 10, *, P < 0.03 for KGF siRNA vs. Neg Control siRNA Pretreated MSC MV.
Therapeutic Effect of MSC Microvesicles on Extravascular Lung Water
The simultaneous administration of MSC MVs IT reduced the EVLW of mice injured with endotoxin by 45% at 48 h. This effect was similar to MSCs IT (Figure 6C). Pretreatment of MSCs with KGF siRNA prior to the collection of the MVs eliminated the therapeutic effect of MVs on EVLW as compared to MVs collected from MSCs pretreated with a Neg control siRNA (Figure 6C). However, the administration of recombinant human KGF alone only reduced EVLW by 8% compared to endotoxin-injured mice (P>0.05), suggesting that additional factors such as other MV mRNA, miRNA, protein or organelle may be involved in the resolution of pulmonary edema.
Effect of Neutralization of Secreted KGF in the BAL Fluid of Endotoxin-Injured Mice Treated with MSC MVs
To determine if the KGF protein found in the BAL fluid of endotoxin-injured mice treated with MSC MVs was biologically active, we instilled 10 μg of goat anti-human KGF Ab, a neutralizing antibody, with 30 μl of MSC MV IT at time 0 h and an additional 10 μg of the antibody at 24 h to neutralize any of the secreted KGF protein in the endotoxin-injured mice. Compared to endotoxin-injured mice that received 10 μg of control goat IgG at 0 and 24 h, the administration of the anti-KGF Ab resulted in an increase in the total protein level at 48 h by 34%. Anti-KGF Ab also numerically increased the influx of neutrophils and MIP-2 levels in the BAL fluid by 140% and 31% respectively compared to control goat IgG, although these differences were not significant (Supplemental Figure 4B).
Co-culture of RAW 264.7 Cells with MSC Microvesicles Following Stimulation with Endotoxin Decreased Inflammatory Cytokine Secretion
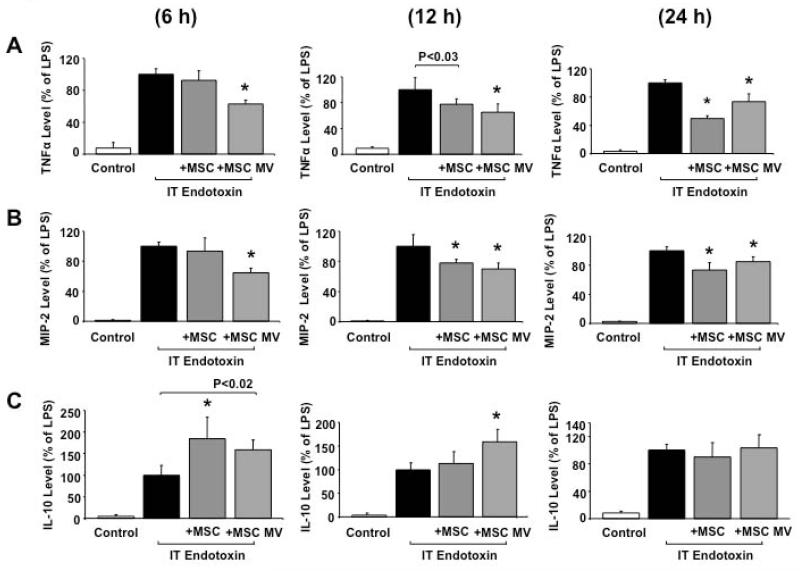
The addition of 30 μl MSC MVs to endotoxin-stimulated RAW 264.7 cells, a mouse macrophage cell line, reduced the levels of TNF-α and MIP-2 at all time points measured compared with endotoxin-stimulated mouse macrophages (Figure 7A & B). The level of reduction was similar to RAW 264.7 cells exposed to endotoxin and treated with MSCs (250,000 cells) in a Transwell plate that prevented cell contact (Figure 7A & B). Interestingly, IL-10 was significantly higher in both MSC and MSC MV treatment groups at 6 and 12 h (Figure 7C).
FIGURE 7. Effect of MSC MVs on RAW 264.7 Cells.
Co-culture of RAW 264.7 cells with MSCs or MSC MVs following exposure to endotoxin decreased inflammatory cytokine/chemokine secretion and increased the anti-inflammatory cytokine IL-10 level. (A & B) The simultaneous addition of MSCs or MSC MVs significantly reduced the levels of both TNFα and MIP-2 in the conditioned medium at all the time points (6 h, 12 h and 24 h) compared with RAW 264.7 cells exposed to endotoxin. For TNFα: Data are expressed as mean ± SD, N = 4. *, P < 0.0001 at 6 h, *, P < 0.002 at 12 h and *, P < 0.0001 at 24 h vs. endotoxin by ANOVA (Bonferroni). For MIP-2: Data are expressed as mean ± SD, N = 4. *, P < 0.0002 at 6 h, *, P < 0.005 at 12 h and *, P < 0.008 at 24 h vs. endotoxin by ANOVA (Bonferroni). (C) The simultaneous addition of MSCs or MSC MVs with RAW 264.7 cells significantly increased the level of IL-10 in the conditioned medium at 6 h and remained higher at 12 h for MSC MVs treated cells compared to cells exposed to endotoxin alone. By 24 h, IL-10 levels returned to baseline for all groups. Data are expressed as mean ± SD, N = 4. *, P < 0.002 vs. endotoxin at 6 h and *, P < 0.002 at 12 h vs. endotoxin exposed cells by ANOVA (Bonferroni).
Mitochondrial mRNA Expression in MSC Microvesicles
Based on a previous publication that suggested that mitochondrial transfer may be involved in the therapeutic effect of MSCs and/or MSC MVs 28, we measured the mRNA expression for the human mitochondrial genes, cytochrome oxidases 1 and 2 (hCO1 and hCO2), by RT-PCR. Although the size of mitochondria (0.5 – 1.0 μm) is much larger than MSC MVs (~ 200 nM), hCO1 and hCO2 were expressed in the MVs (Supplemental Figure 5).
DISCUSSION
The major findings of this study can be summarized as follows: 1) IT or IV administration of MVs derived from human MSCs decreased the influx of inflammatory cells, both total white blood cells and neutrophils, and MIP-2 cytokine and total protein levels in the alveolus of mice injured with E.coli endotoxin at 48 h (Figures 2 - 3 and Supplemental Figure 2); 2) more importantly, the administration of MVs were associated with a reduction in EVLW, a measure of pulmonary edema (Figure 6C); 3) the therapeutic effects of MSC MVs were similar to MSCs themselves (Figures 4 & 6); 4) in primary cultures of human alveolar epithelial type II cells injured with an inflammatory mixture (cytomix), MSC MVs restored protein permeability over 24 h to a similar level as MSCs (Supplemental Figure 3); 5) MVs expressed the mRNA for KGF, a MSC secreted paracrine factor previously found to restore AFC in both E.coli endotoxin and bacteria induced ALI in an ex vivo perfused human lung 9,21; 6) in both the alveolar fluid of endotoxin-injured mice treated with IT or IV MVs and in the conditioned medium of alveolar epithelial type II cells injured with cytomix treated with MVs, KGF protein levels was significantly elevated (Figure 5); 7) KGF siRNA-pretreatment of MSC partially reduced the therapeutic effect of MVs released by MSCs on both the level of inflammation and EVLW (Figure 6), suggesting that the benefit of MVs appears to be in part through KGF, perhaps by transfer of the mRNA to the injured alveolar epithelium and subsequent expression of the protein; 8) inhibition of the secreted KGF protein by a neutralizing Ab reduced the therapeutic effect of MSC MVs on protein permeability in endotoxin-injured mice, suggesting that the KGF that was measured in the BAL fluid was biologically active (Supplemental Figure 4B); and 9) in cultures of RAW 264.7 cells, a mouse macrophage cell line, MSC MVs suppressed the secretion of inflammatory cytokines and chemokines and increased the secretion of IL-10, an anti-inflammatory cytokine previously found to be important to the therapeutic effect of MSCs in mouse models of sepsis 10 (Figure 7), suggesting an immunomodulatory effect similar to the cells themselves.
Recent studies have demonstrated that the administration of mouse or human MSCs improved several parameters of ALI, whether from E.coli endotoxin 3-6, live bacteria (E.coli, Staphylococcus aureus, Pseudomonas aeruginosa) 7-9 or following sepsis 10-12. Interestingly, MSCs have been found to release MVs, which can be involved in cell-cell communication and the transfer of cellular material 14,15. Bruno et al. reported that MVs derived from adult human MSC contributed to kidney repair and survival predominantly through the transfer of microRNA, which decreased apoptosis in the injured renal epithelium. In a microarray of the MSC MVs, the authors found 239 unique transcripts for genes that were involved in cell differentiation, transcription, proliferation, and immune regulation 16. Kim et al. identified 730 proteins for cell proliferation, adhesion, migration, and morphogenesis in a proteomic analysis of MVs derived from human MSCs, potentially involved in the therapeutic effect as well 29. More recently, Lee et al. 30 observed that exosomes derived from mouse MSC conditioned medium prevented vascular remodeling and an elevation in right ventricular systolic pressure in a mouse model of hypoxic pulmonary hypertension by the prevention of pulmonary influx of macrophages and the induction of pro-inflammatory and pro-proliferative mediators. Interestingly, they found that exosomes, approximately 50 nM in size, were the biologically active component of the conditioned medium, not paracrine soluble proteins secreted by MSCs. Conditioned medium with the exosomes removed had no therapeutic effect. Despite these interesting studies, we have a limited understanding of the effects of MSC derived MVs in experimental models of ALI and pulmonary edema. In this manuscript, our primary objectives were (1) to study whether the instillation of MSC MVs had the same therapeutic effect as the cells themselves in E.coli endotoxin-induced ALI in mice and (2) to determine whether the transfer of mRNA was one of the underlying mechanisms (Supplemental Figure 1).
Our study demonstrated a significant beneficial effect from the IT administration of MSC MVs in E.coli endotoxin-induced ALI. We found that MVs reduced lung inflammation and protein permeability, which prevented the formation of pulmonary edema as measured by the EVLW (Figures 2 - 3 and 6). Impaired AFC or the inability to absorb pulmonary edema in patients with ALI/ARDS is associated with higher morbidity and mortality 31,32. The therapeutic effects with MV administration were replicated with the IV route (Supplemental Figure 2), suggesting that MVs may home to the site of inflammation. Perhaps more significantly, the therapeutic effect of MSC MVs was similar to the cells themselves (Figures 4 and 6).
We previously found 21 that human MSCs produced substantial quantities of KGF, and the secretion of this paracrine soluble factor mediated the restorative effect of MSCs on AFC. In addition, KGF has been shown to reduce lung edema and inflammation in various ALI models with permeability edema such as injuries that are infectious in origin or directly target the lung epithelium or endothelium 22-26. Since MVs contain mRNAs present in the cells from which they originate 14,15 and MSC MVs have the capability to reduce BAL protein levels and EVLW, we hypothesized that the transfer of KGF mRNA from the MVs to the injured alveolar epithelium may contribute to the protective effect conferred with MV treatment. To determine the contribution of KGF mRNA delivery by MVs, we first demonstrated that MVs do express the mRNA for this key paracrine factor and KGF level in the BAL was also elevated at both 24 h and 48 h following MSC MV treatment (Figure 5). We then pretreated MSC with KGF siRNA, thus eliminating KGF mRNA in the MVs. Administration of MVs from KGF siRNA-pretreated MSC partially eliminated the therapeutic effect of MVs (Figure 6). These results indicated that the transfer of KGF mRNA by MVs play an important role in mediating the therapeutic effect of MVs on pulmonary edema absorption. The instillation of recombinant human KGF alone partially restored parameters of ALI following endotoxin-induced lung injury (Supplemental Figure 4A).
There are some limitations to the current study. (1) What constitutes a MSC derived MVs needs to be standardized. Bruno et al. 18 found microparticles with an average size of 200 nM as the therapeutic portion of the MSC conditioned medium, whereas Lee et al. 30 found that exosomes with an average size of 50 nM were responsible for the therapeutic effect. The current method we used to isolate MVs did not differentiate MVs by size. (2) Protein concentration is often used to quantify the MVs. However, because we believed the mRNA content of the MVs was more important in the therapeutic effect than the protein content and to compare our results to previous publications using MSCs in ALI, we dosed the MVs by total cell count. To standardize our isolation technique, we indicated that 30 μl of the MVs was the total MVs released by 3×106 serum starved MSCs over 48 h. The protein conc. of this MV dose we chose was within the range used in previous studies. (3) In addition, the method used to provoke the release of MVs from the MSCs may change the content of the MV. It is conceivable that the phenotype of the MV may differ based on whether the injury is inflammatory, hypoxic or infectious in origin. (4) Also, although the administration of recombinant KGF IT had similar results as MSC MVs in endotoxin-induced lung injury, it is unclear whether KGF acts directly on the injured alveolar epithelium or endothelium or inflammatory cells 9 or through the secretion of another factor such as GM-CSF 33. (5) Although the KGF siRNA experiments strongly suggest that the KGF protein found in the BAL fluid with MV treatment is human, it is still conceivable that the ELISA used may have measured the mouse analog of KGF. Further studies are needed. And (6) finally, the human mitochondrial genes, hCO1 and hCO2, were expressed in the MVs (Supplemental Figure 5), suggesting that other mechanisms such as organelle transfer may be involved and will need to be studied further.
CONCLUSION
In summary, we isolated, identified, and characterized MVs from human bone marrow derived MSCs and demonstrated a biologic effect that is unique to MSC MVs versus MVs derived from fibroblasts in a mouse model of endotoxin-induced ALI. MSC MVs produced similar protective effects as MSCs themselves. More significantly, the underlying mechanism of MSC MVs may be mediated in part through the transfer of KGF mRNA, probably to the injured alveolar epithelium, with subsequent expression of the protein.
Supplementary Material
SUPPLEMENTAL FIGURE 1. Potential Mechanisms Underlying the Therapeutic Effect of Microvesicles Released by Mesenchymal Stem Cells. During ALI, MSC MVs are targeted towards the injured alveolar epithelium by surface receptors, such as possibly CD44 or CD29 16 or through connexin 43 channels 28, leading to the endocytosis of its contents, which includes mRNA, miRNA, proteins and organelle. We hypothesize that one such mRNA, KGF, is subsequently expressed and secreted as a protein, and this secreted KGF is involved in suppressing inflammation and protein permeability following injury.
SUPPLEMENTAL FIGURE 2. Effect of Intravenous MSC MVs on Endotoxin-Induced ALI in Mice. The simultaneous IV administration of MSC MVs by the external jugular vein had a similar effect as MSC MVs given IT in endotoxin-induced ALI in mice. (A) IV MSC MVs reduced the influx of neutrophils into the BAL fluid of endotoxin-injured mice. Data are shown as mean ± SD, N = 4-5. *, P = 0.0002 vs. PBS treated and †, P < 0.02 vs. endotoxin treated mice by ANOVA (Bonferroni). (B) IV MSC MVs decreased the total protein level in the BAL fluid of endotoxin-injured mice. Data are expressed as mean ± SD, N = 4-5. *, P < 0.0006 vs. PBS treated and †, P < 0.02 vs. endotoxin treated mice by ANOVA (Bonferroni).
SUPPLEMENTAL FIGURE 3. Effect of MSC MVs on Protein Permeability in Primary Cultures of Human Alveolar Epithelial Type II Cells Injured by Cytomix. Similar to MSC themselves, the addition of MSC MVs restored the increase in protein permeability across primary cultures of human alveolar epithelial type II cells injured with an inflammatory insult (cytomix). Data are expressed as mean ± SD, N = 3 - 7 for treatment groups and N = 9 for cytomix injured type II cells. *, P < 0.0001 vs. control type II cells and †, P < 0.004 vs. cytomix injured type II cells by ANOVA (Bonferroni).
SUPPLEMENTAL FIGURE 4. Role of KGF in the Effect of MSC MVs in Endotoxin-Induced ALI in Mice. The administration of the recombinant KGF protein or inhibition by a neutralizing Ab demonstrated the importance of KGF protein secretion in the therapeutic response of MSC MVs. (A) The simultaneous instillation of human KGF IT decreased the influx of neutrophils and the level of the inflammatory cytokine MIP-2 and lung albumin protein levels in the BAL fluid of the endotoxin-injured mice. Data are expressed as mean ± SD, N = 8-9. *, P < 0.04 for neutrophils, *, P < 0.02 for MIP-2 levels, *, P < 0.008 for protein levels vs. endotoxin treated mice. (B) The neutralization of secreted KGF from MSC MVs diminished the therapeutic response of the MVs in endotoxin-induced ALI in mice. The administration of goat anti-human KGF Ab at time 0 and 24 h partially neutralized the effect of secreted KGF by MSC MVs on endotoxin-injured mice. Although not statistically significant, administration of anti-KGF Ab with MSC MVs increased the influx of neutrophils and MIP-2 levels in the BAL fluid by 140% and 31% respectively in endotoxin-injured mice compared to injured mice treated with control goat IgG with MSC MVs. More importantly, administration of anti-KGF Ab significantly increased protein levels by 34% compared to control goat IgG treated mice, indicating an increase in lung protein permeability. Data are expressed as mean ± SD, N = 7-10. *, P < 0.03 for BAL fluid protein levels vs. goat control IgG treated mice.
SUPPLEMENTAL FIGURE 5. MSC MV Mitochondrial mRNA Content. RT-PCR demonstrated that MSC MVs expressed the mitochondrial mRNAs, human cytochrome oxidases 1 and 2 (hCO1 and hCO2). MSCs were used as a positive control.
ACKNOWLEDGEMENTS
We thank the Northern California Transplant Donor Network for their assistance in obtaining human research lungs for the isolation of primary cultures of human alveolar TII cells.
Funding: This work was supported by the NHLBI Grant HL-51854 (M.A. Matthay), HL-093026 (J.W. Lee) & HL-113022 (J.W. Lee) and the UCSF Hamilton Endowment Funds (J.W. Lee). Some of the materials (human allogeneic MSCs) employed in this work were provided by the Tulane Center for Gene Therapy through a grant from NCRR of the NIH, Grant # P40RR017447.
Footnotes
Authors contributions: Y.G.Z. Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing. X.M.F., J.A., X.H.F, Q.H. and A.M. Collection and assembly of data. J.M.Q. and M.A.M. Conception and design, financial support, data analysis and interpretation. J.W.L. Conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST: The authors indicate no potential conflict of interest.
REFERENCES
- 1.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 5.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–481. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 7.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–539. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic Effects of Human Mesenchymal Stem Cells In Ex Vivo Human Lungs Injured with Live Bacteria. Am J Respir Crit Care Med. 2013;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 12.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 13.Lee JW, Fang X, Krasnodembskaya A, et al. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 15.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and molecular life sciences: CMLS. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 18.Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 20.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panos RJ, Bak PM, Simonet WS, et al. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest. 1995;96:2026–2033. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano T, Deterding RR, Simonet WS, et al. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15:433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 24.Sugahara K, Iyama K, Kuroda MJ, et al. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Path. 1998;186:90–98. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Viget NB, Guery BP, Ader F, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1199–1209. doi: 10.1152/ajplung.2000.279.6.L1199. [DOI] [PubMed] [Google Scholar]
- 26.Welsh DA, Summer WR, Dobard EP, et al. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med. 2000;162:1081–1086. doi: 10.1164/ajrccm.162.3.9908099. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–1257. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 32.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 33.Wu H, Suzuki T, Carey B, et al. Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J Biol Chem. 2011;286:14932–14940. doi: 10.1074/jbc.M110.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1. Potential Mechanisms Underlying the Therapeutic Effect of Microvesicles Released by Mesenchymal Stem Cells. During ALI, MSC MVs are targeted towards the injured alveolar epithelium by surface receptors, such as possibly CD44 or CD29 16 or through connexin 43 channels 28, leading to the endocytosis of its contents, which includes mRNA, miRNA, proteins and organelle. We hypothesize that one such mRNA, KGF, is subsequently expressed and secreted as a protein, and this secreted KGF is involved in suppressing inflammation and protein permeability following injury.
SUPPLEMENTAL FIGURE 2. Effect of Intravenous MSC MVs on Endotoxin-Induced ALI in Mice. The simultaneous IV administration of MSC MVs by the external jugular vein had a similar effect as MSC MVs given IT in endotoxin-induced ALI in mice. (A) IV MSC MVs reduced the influx of neutrophils into the BAL fluid of endotoxin-injured mice. Data are shown as mean ± SD, N = 4-5. *, P = 0.0002 vs. PBS treated and †, P < 0.02 vs. endotoxin treated mice by ANOVA (Bonferroni). (B) IV MSC MVs decreased the total protein level in the BAL fluid of endotoxin-injured mice. Data are expressed as mean ± SD, N = 4-5. *, P < 0.0006 vs. PBS treated and †, P < 0.02 vs. endotoxin treated mice by ANOVA (Bonferroni).
SUPPLEMENTAL FIGURE 3. Effect of MSC MVs on Protein Permeability in Primary Cultures of Human Alveolar Epithelial Type II Cells Injured by Cytomix. Similar to MSC themselves, the addition of MSC MVs restored the increase in protein permeability across primary cultures of human alveolar epithelial type II cells injured with an inflammatory insult (cytomix). Data are expressed as mean ± SD, N = 3 - 7 for treatment groups and N = 9 for cytomix injured type II cells. *, P < 0.0001 vs. control type II cells and †, P < 0.004 vs. cytomix injured type II cells by ANOVA (Bonferroni).
SUPPLEMENTAL FIGURE 4. Role of KGF in the Effect of MSC MVs in Endotoxin-Induced ALI in Mice. The administration of the recombinant KGF protein or inhibition by a neutralizing Ab demonstrated the importance of KGF protein secretion in the therapeutic response of MSC MVs. (A) The simultaneous instillation of human KGF IT decreased the influx of neutrophils and the level of the inflammatory cytokine MIP-2 and lung albumin protein levels in the BAL fluid of the endotoxin-injured mice. Data are expressed as mean ± SD, N = 8-9. *, P < 0.04 for neutrophils, *, P < 0.02 for MIP-2 levels, *, P < 0.008 for protein levels vs. endotoxin treated mice. (B) The neutralization of secreted KGF from MSC MVs diminished the therapeutic response of the MVs in endotoxin-induced ALI in mice. The administration of goat anti-human KGF Ab at time 0 and 24 h partially neutralized the effect of secreted KGF by MSC MVs on endotoxin-injured mice. Although not statistically significant, administration of anti-KGF Ab with MSC MVs increased the influx of neutrophils and MIP-2 levels in the BAL fluid by 140% and 31% respectively in endotoxin-injured mice compared to injured mice treated with control goat IgG with MSC MVs. More importantly, administration of anti-KGF Ab significantly increased protein levels by 34% compared to control goat IgG treated mice, indicating an increase in lung protein permeability. Data are expressed as mean ± SD, N = 7-10. *, P < 0.03 for BAL fluid protein levels vs. goat control IgG treated mice.
SUPPLEMENTAL FIGURE 5. MSC MV Mitochondrial mRNA Content. RT-PCR demonstrated that MSC MVs expressed the mitochondrial mRNAs, human cytochrome oxidases 1 and 2 (hCO1 and hCO2). MSCs were used as a positive control.