Abstract
Background
The chromodomain helicase DNA binding protein 5 (CHD5) is a family member of chromatin remodeling factors. The epigenetic silencing mechanisms of CHD5 in colorectal cancer (CRC) has not been well studied.
Methods
Here we analyzed the CHD5 methylation and mRNA expression in vitro and in clinical samples from African American patients. DNA and RNA were isolated from FFPE colon tissues. DNA was tested for methylation using methylation specific PCR and bisulfite sequencing. RNA was used for mRNA quantification using qRT-PCR. The RKO cell line was treated with 5-Aza-dC and SAHA. RKO cells were also stably transfected with a CHD5 expressing vector. The transcriptional activity was studied in the 1 kb upstream region of CHD5 promoter, using the dual reporter assay. We performed cell proliferation, migration, and invasion assays using RKO cell line.
Results
In most adenoma samples, CHD5 expression was not detected in contrast to normal tissues. In RKO cells, CHD5 silencing was associated with DNA methylation and repressive histone modifications. CHD5 expression was restored after treatment with 5-Aza-dC and SAHA. CHD5 reactivation reduced cell proliferation, migration and invasion. The reporter assay indicated that the main regulatory region of CHD5 promoter is encompassed in the −489 to −823 region with important transcriptional regulatory sites (TCF/LEF, SP1 and AP-2).
Conclusion
CHD5 gene is repressed in all types of adenoma either epigenetically or by chromosomal deletion. CHD5 activity is regulated by DNA methylation and repressive histones modifications. CHD5 likely acts as tumor suppressor gene in early colorectal carcinogenesis.
Introduction
Epigenetic changes play important roles in the initiation and progression of the tissue-specific genes expression 1. These epigenetic mechanisms result from a cross-talk between DNA methylation and histone modifications and occur through various physiologic and pathologic conditions. The combination of these dynamic interactions leads to different genome expression outcomes 2–4. Aberrant DNA methylation such as global DNA hypomethylation and regional DNA hypermethylation cause activation and/or inactivation of cancer genes 5–8. Chromatin remodeling factors such as SWI/SNF can affect gene expression by disrupting the chromatin structure in an ATP-dependent manner.
CHD5 belongs to a group of SWI/SNF proteins called chromodomain helicase DNA binding (CHD) proteins, which contains a SWI/SNF-like/ ATPase domain, and some members form a nucleosome remodeling and deacetylation (NuRD) complex that directly modifies chromatin structure and enhances the binding of transcription factors to their binding sites9. As such, CHD5 which is located on the 1p36 has an impact on gene regulation, and it was shown to be highly methylated in African American colon cancer tumors 10. It is preferentially expressed in the brain and nervous system9. CHD5 is deleted in neuroblastoma and has been demonstrated to control cell proliferation, apoptosis and senescence through the p19/p53 pathway11. CHD5 expression is regulated by promoter DNA methylation, and is silenced or down-regulated in primary tumors and cancer cell lines 11, 12. In gastric cancer, CHD5 is down regulated through promoter hypermethylation, and it is hypothesized to be a tumor suppressor gene 13,14. We recently demonstrated that CHD5 is a direct target in miR-211 regulation of posttranscription in a colon cancer cell line 15. Enforced expression of miR-211 promotes tumor cell growth at least in part by down-regulating the expression level of CHD5 gene.
The formation of adenomas are precursor lesions that generally develop into colorectal cancer (CRC) which is the third common cancer in the US. These types of polyps are the epithelial benign tumor that might become malignant and lead to adenocarcinomas. The four most common adenomas causing CRC are tubular adenoma, villous adenoma, tubulovillous adenoma and sessile serrated adenomas. In this study, we first investigated the mRNA expression level of CHD5 in different types of adenoma tissues. Next, we analyzed the epigenetic silencing mechanism by evaluating DNA methylation level of CHD5 promoter in these tissues. Finally, we investigated the molecular functions of CHD5 and its transcriptional regulation in a colon cancer cell line.
Materials and Methods
Study population, and tissue samples
A total of 24 normal and adenoma samples from African American subjects were used after the Howard University Institutional Review Board’s approval of the study. All samples were evaluated and subjected to histological diagnosis by expert pathologists. Tissues were collected and clinical data was obtained. From 24 FFPE samples, 6 samples were healthy normal colon and 18 samples were adenomas which was collected in 2002 to 2004. The adenoma samples were classified in 4 different pathological groups: tubular, tubulovillous, villous and sessile serrated adenoma.
Cell growth
We used two human colorectal carcinoma cell lines for these studies, RKO and HTC116. RKO colon cell line was selected because the CHD5 was unmethylated and HTC116 colon cell line was selected because CHD5 was methylated. CHD5 was lowly expressed in RKO and highly expressed in HCT-116 cells 12. Both cell line is well defined and they have used frequently for colorectal molecular biology studies 12. RKO cells were maintained in MEM medium (ATCC) supplemented with 10% fetal bovine serum (FBS) and Penicillin/Streptomycin (Invitrogen). HCT116 cells were maintained in the McCoy’s medium (ATCC) supplemented with 10% FBS and Penicillin/Streptomycin (Invitrogen).
Stable transfection
CHD5 expressing (sense plasmid) and non-expressing (antisense plasmid control, mock) were used 11. 10,000 cells/well were seeded 24 hr before transfection in MEM growth medium without antibiotics. The cells were transfected with 100 ng of plasmid DNA and 0.6 µl of Lipofectamine 2000 (Invitrogen) per well diluted in 100 µl of MEM medium without serum and antibiotics. After 16 hr transfection, the medium was changed with normal medium. The cells were then treated with G418 (900 µg/ml) after 48 hr of transfection to select for transfectants. The colonies were selected and isolated after two weeks.
Transient transfection
CHD5 promoter luciferase constructs in pGL3 vector were transiently co-transfected with TK-pRL plasmid (Promega) in the RKO cells. 5000 cells/well were seeded 24 hr before transfection in MEM growth medium without antibiotics. The cells were transfected with 100 ng of each plasmid DNA and 0.6 µl of Lipofectamine 2000 (Invitrogen) per well diluted in 100 µl of MEM medium without serum and antibiotics. After 24 hr of transfection, the cells were processed for reporter assay as described below.
Dual-luciferase reporter assay
CHD5 transcriptional activity was tested by dual-luciferase reporter assay (Promega) in the RKO cell line, following the manufacturers’ instructions. The generic plasmid DNA (GeneScript) of 1 kb fragment of CHD5 promoter was sub-cloned into pGL3-Basic vector. Deletion constructs from the 1 kb promoter construct were made in the following series: 823 bp, 489 bp, 400 bp and 273 bp. A vector containing constitutively expressed Renilla (TK-pRL, Promega) was transiently co-transfected into RKO cell line for 24 h along with the vectors with the CHD5 promoter subclones.
Proliferation assay
Proliferation assay was performed using the Celltiter 96 Aqueous kit (Promega) following the manufacturers’ instructions. Cells were seeded in titrated cell numbers of 2000, 4000, 5000 and 10,000 in a 96 well plate. The absorbance at 490 was recorded using an ELISA plate reader.
Cell migration and invasion assay
Cell migration and invasion assays were performed using the CytoSelect 24-well. The cell migration and invasion assays (8 µm, colorimetric format) from Cell Biolabs, Inc., were performed following the manufacturers’ instructions.
Colony formation assay
Soft agar colony formation assay was performed using the colorimetric system of the 96-Well cell transformation assay kit (Cell Biolabs, Inc.) following the manufacturers’ instructions.
DNA and RNA extraction
DNA and RNA were extracted from the cell lines, fresh frozen tissues and FFPE tissue using DNA/RNA/Protein kit (Qiagen AllPrep Kit) following the manufactures’ protocol.
DNA bisulfite treatment
DNA (150 ng) conversion was performed using an EZ DNA Methylation-Gold Kit (ZYMO Research, Orange, California, USA), according to the manufacturers’ protocol. DNA was subjected to methylation specific PCR (MSP) and genomic bisulfate sequencing.
MSP and genomic bisulfite sequencing
The primers were designed as previously reported [11]. Statistical analysis was performed to detect significant changes in the frequencies of DNA methylation of CpG nucleotides between tumor and normal samples.
Quantitative real-time RT-PCR (qRT-PCR) analyses of CHD5 expression
Total RNA (1 µg) from the cell lines and primary tissue samples was isolated using the DNA/RNA kit (Qiagen). The single-stranded cDNA was subsequently synthesized using the ABI high profile cDNA synthesis Kit (Applied Biosystems). The primers were designed by Taqman gene expression assay (Applied Biosystems, CA). The qRT-PCR was performed using the ABI 7500 system. The threshold cycle number was automatically determined by ABI software version (Applied Biosystems, CA). Reactions were performed in triplicate, and the threshold cycle (Ct) numbers were averaged. The results of CHD5 expression were normalized to GAPDH (reference gene), which had minimal variation in all normal and tumor samples tested. Expression fold was calculated according to the formula ΔCt=2(Ct(test)–Ct(ref)), where Ct(ref) is the threshold cycle number for the reference gene observed in the tissue, Ct(test) is the threshold cycle number for the experimental gene observed in the tissue. RQ is the relative quantification of the ΔCt observed in the normal samples versus the ΔCt observed in the adenoma samples.
5-Aza-2'-deoxycytidine (5-Aza-dC) and N-Hydroxy-N’-phenyloctanediamide (SAHA or Vorinostat) treatment
RKO cells were seeded at a low density of 5000 cells/well in a 24 well plate. The cells were treated with different concentrations of 5-Aza-dC (Sigma-Aldrich) and SAHA (TRC: Toronto Research Chemical Inc.) in order to obtain the optimal concentrations of treatment. The cells were treated with 3 µM 5-Aza-dC for 3 days and 5 days. Every 24 hr, 5-Aza-dC was added freshly to the cells. SAHA (1 µM) was added only once, 16 hr before harvesting the cells. Total RNA and DNA were isolated and purified by the DNA/RNA AllPrep kit (Qiagen). DNA methylation levels of CHD5 in the samples before and after treatment were determined by MSP and genomic bisulfite sequencing. MRNA expression levels were determined by qRT-PCR as described above. For chromatin immunoprecipitation (ChIP) assay, chromatin from RKO and HCT 116 cells (after 5-aza-dC and SAHA treatments) was harvested following the protocol described in the assay ChIP.
ChIP assay
Chromatin immunoprecipitation was conducted as previously described16. RKO and HCT116 cell lines were fixed in 0.8% formaldehyde for 10 min at 25°C. Following lysis, samples were sonicated using a probe sonicator (Misonix) for 7 cycles (20 sec per cycle/ 1 min cooling) at a power level of 3. Chromatin sheering was optimized to a size range of 200–600 bp. Chromatin (150–200 ng) was immunoprecipitated with antibodies H3K27me3 (Upstate 07–449), H3K4me3 (Abcam ab8580), or rabbit IgG (Sigma 15006). A 5% aliquot was removed as an input fraction. Enrichment of histone marks was assayed by quantitative PCR employing SYBR green master mix (Applied Biosystems) and primers (Region 1, CHD5 H3K27me3 Forward TCTGGAAGAGAAGTCGGAGAGTGA, Reverse CTCCTTCTTGTCCTTGAGTTTCTTC; Region 2, CHD5 H3K4me3 Forward AGCGCACGGGTTAAGGCT; Reverse CTCACCTGACATCTCGTCCTCATT,) using the comparative Ct method. Control enrichment was measured by quantitative PCR using primers for RNA polymerase II large subunit (POL II Forward AGATGAAACCGTTGTCCAAAC; POL II Reverse AGGTTACGGCAGTTTGTCTCTC) for H3K4me3 and antibodies or hsSat2 (hsSat2 Forward ATCGAATGGAAATGAAAGGAGTCA; hsSat2 Reverse GACCATTGGATGATTGCAGTCA) for H3K27me3 antibodies. All experiments were conducted in three independent experimental replicates and three qPCR technical replicates.
Results
CHD5 expression in adenoma tissues
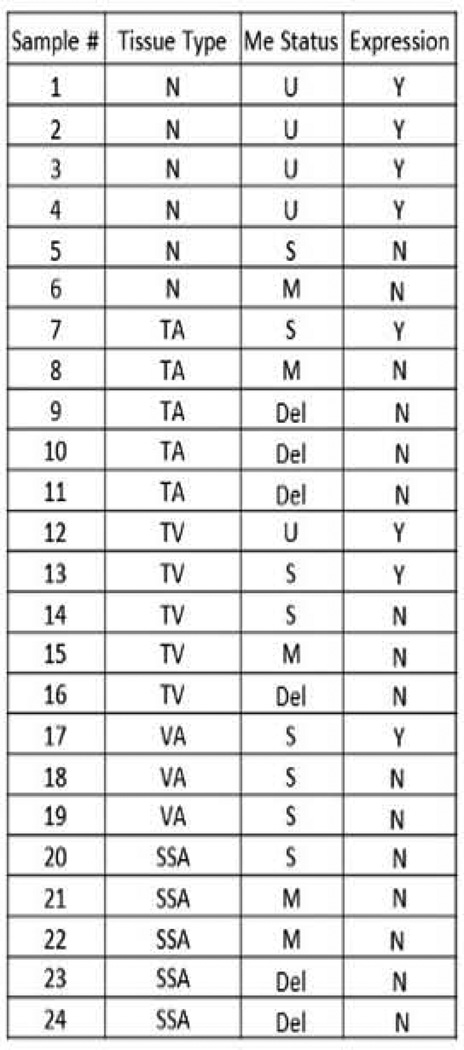
We applied MSP method in all samples, a male blood DNA was used as an unmethylated DNA control and a methylated cancer DNA sample or the IVD was used as a fully methylated DNA control. The mRNA level of CHD5 expression was analyzed by qRT-PCR. Of the 24 samples, 6 were normal tissues and the rest were adenoma polyps. Of 6 normal samples, 4 had an unmethylated CHD5 promoter (Figure 1). Only one was partially DNA methylated and one was fully methylated at the CHD5 promoter, corresponding with CHD5 silencing. In the adenoma samples, there were 5 tubular adenomas (TAs). Of these, only one TA showed partial DNA methylation, and CHD5 was expressed in this tissue. The other TA samples showed either complete DNA methylation at the CHD5 promoter region or deletion, and therefore CHD5 was silenced. Of the 5 tubulovillous adenomas (TVAs), two samples showed active CHD5, of which one had a fully methylated CHD5 promoter, and one had a variegated DNA methylation pattern. The other 3 TVA samples showed methylated CHD5 promoter and inactive gene expression. Of the 5 serrated sessile adenomas (SSAs), no sample showed CHD5 expression. Of these, 3 had a completely methylated promoter, and 2 had deleted the CHD5 region. Finally in 3 villous adenomas (VAs), only one showed active CHD5 expression, although all 3 samples possessed semi-methylated CHD5 promoter. These data suggest that deletion or aberrant DNA methylation at CHD5 promoter is common in all adenoma types from AA patients, and this is associated with transcriptional silencing of CHD5 in these samples.
Figure 1.
Summary of methylation and expression profiles of CHD5 gene in a set of adenoma (n=18) and normal colon biopsies (n=6) determined by MSP and qRT-PCR, respectively. Adenomas were of four types; TA=tubular adenoma; TV= tubulovillous adenoma; VA=villous adenoma; and SSA=sessile serrated adenoma. U=unmethylated; S=semimethylated; M= methylated; and Del = deleted. Y=Yes; N=No.
DNA methylation and histone modifications of the CHD5 in the colon carcinoma cell line RKO
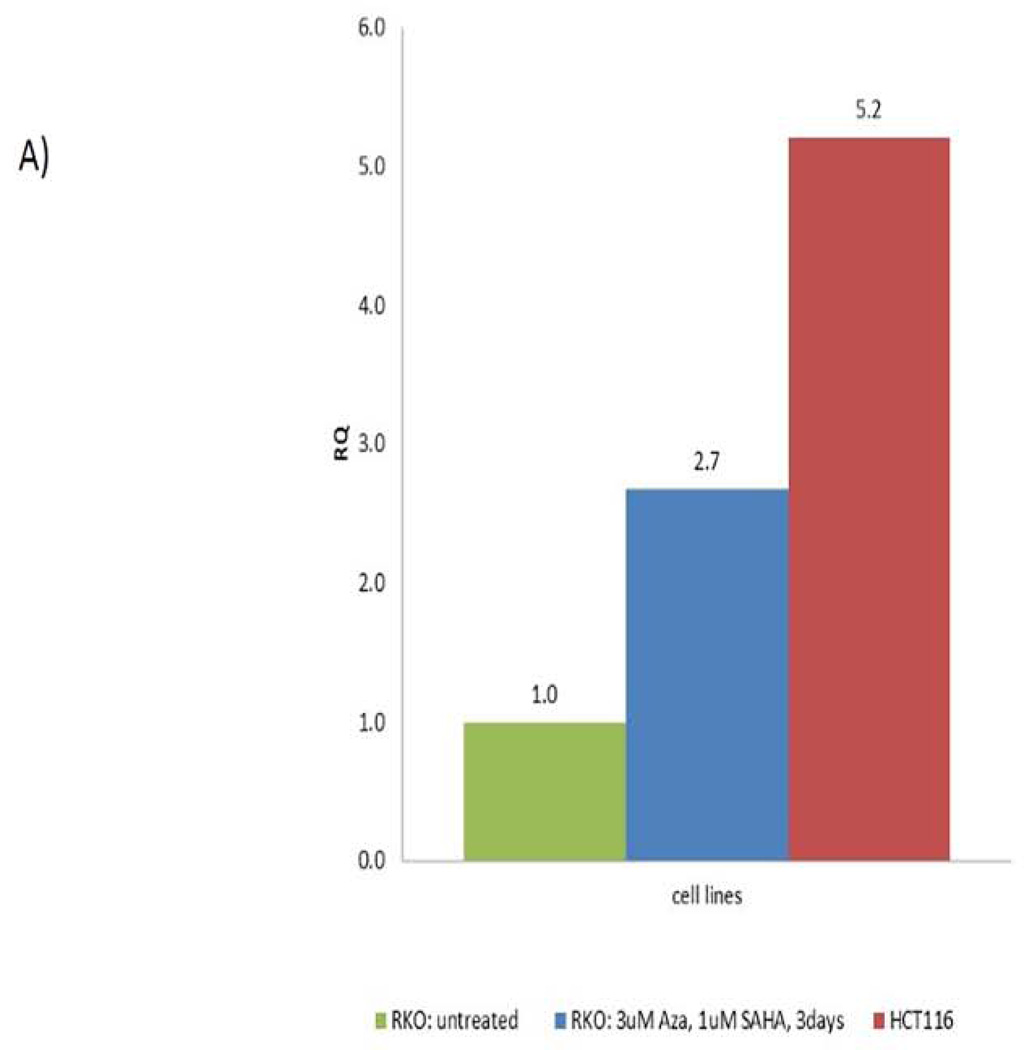
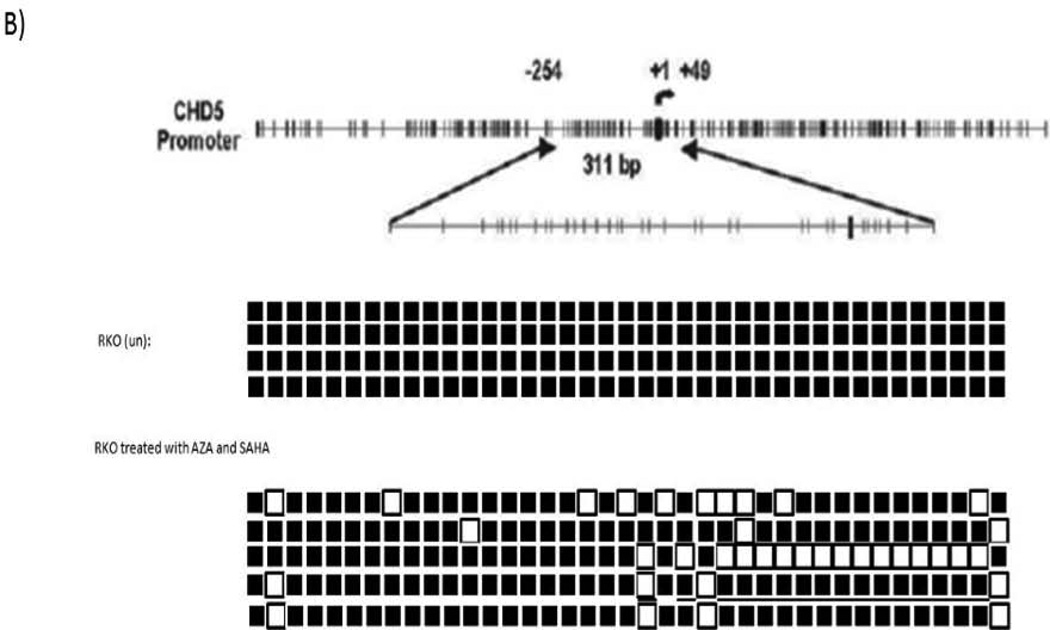
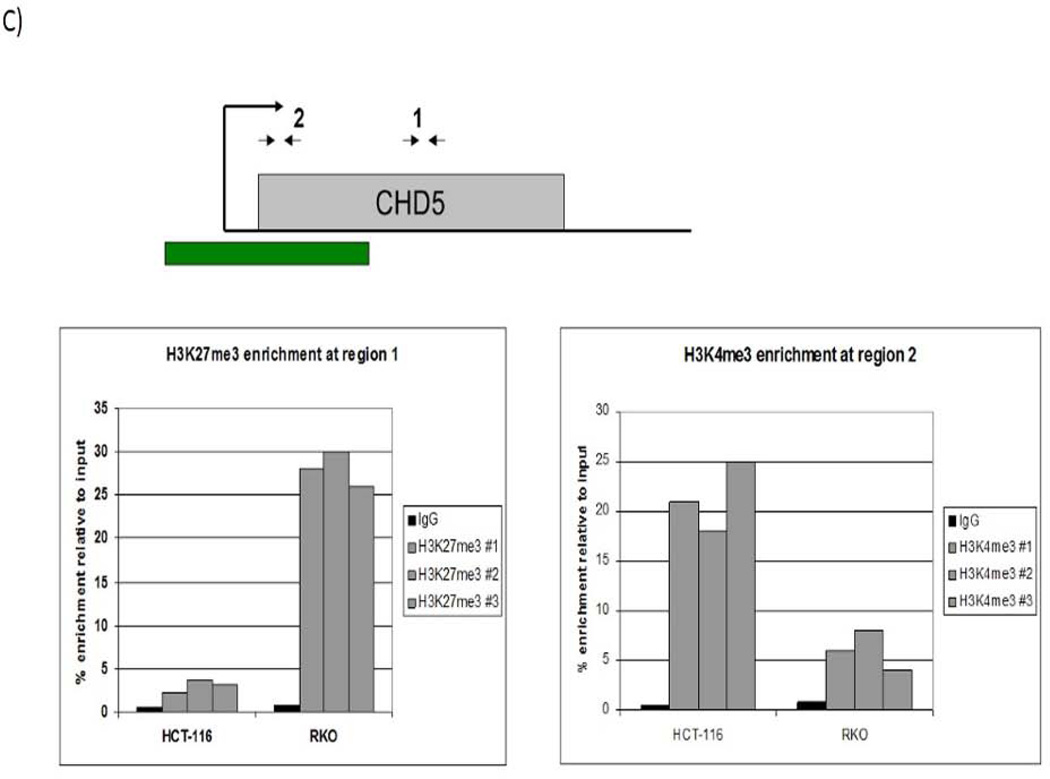
We performed genomic bisulfite sequencing to analyze the methylation status of CHD5 promoter in the RKO cell line. The cell line was then treated with 5-Aza-dC and SAHA to restore the CHD5 activity by inhibiting DNA methylation and histone deacetylation. Different concentrations and times of treatment were tested in order to reach the best nontoxic conditions of gene reactivation (supplementary data, page 22–26). Our data show that transcriptional activity of CHD5 was restored 2.7 fold with 3 µM 5-Aza-dC and 1 µM SAHA after 3 days of treatment (Fig 2-A). Demethylation of CHD5 promoter was tested by MSP and verified by sequencing (Fig 2-B). The sequencing data for the control and the treated cells is representation of ten clones (since the marks was the same for other six clones, four shown here). To check the role of histone modifications at the CHD5 regulation, a chromatin immunoprecipitation (ChIP) assay was carried out with the activating marker H3K4me3 and the inhibiting marker H3K27me3 in two different cell lines, the CHD5 expressing HCT116 cell line and RKO non-expressing cell line (Fig2-C). Our data show that the activating marker H3K4me3 is highly enriched at the transcription start of CHD5 (region 2) in the CHD5-expressing cell line HCT116, and low in the non-expressing cell line RKO. In contrast, the inhibitory marker H3K27me3 was highly enriched in the CHD5 region 1 in non-expressing cell line RKO, and low at the same region in the expressing cell line HCT116. These primers (see methods) were designed based on areas of H3K4me3 and H3K27me3 enrichment derived from ChIP-seq data generated from the ENCODE consortium. The H3K27me3 primers were located in exon 2 of the gene whereas H3K4me3 was located adjacent to the transcription start site.
Figure 2.
DNA methylation and histone modifications of CHD5 in colon carcinoma cell lines. A) Expression patterns of CHD5 methylated in RKO cell line after treating with 3 µM 5-Aza-dC and 1 µM SAHA for 3 days. HCT116 is the endogenous CHD5 expressing control cell line; CHD5 expression was normalized to GAPDH. B) Genomic bisulfite sequencing at CHD5 promoter before and after RKO cells treatment with 5-Aza-dC and SAHA. Black squares indicate the methylated CpG sites and white squares the unmethylated sites. Each line represents an allele of the 311 bp sequenced region. C) Chromatin immunoprecipitation analysis at regions 1 (with H3K27me3 antibody) and 2 (with H3K4me3 antibody) of CHD5 transcriptional locus in RKO and HCT116 cell lines.
CHD5 biological functions in CRC: Stable transfection of CHD5 inducing vector into the RKO cell line
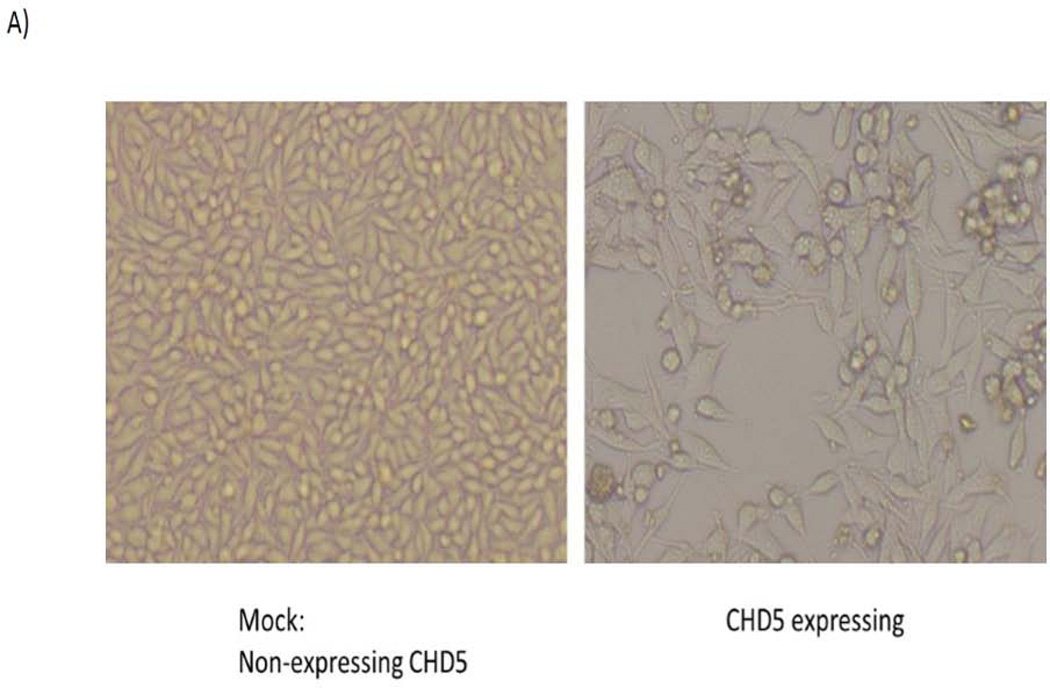
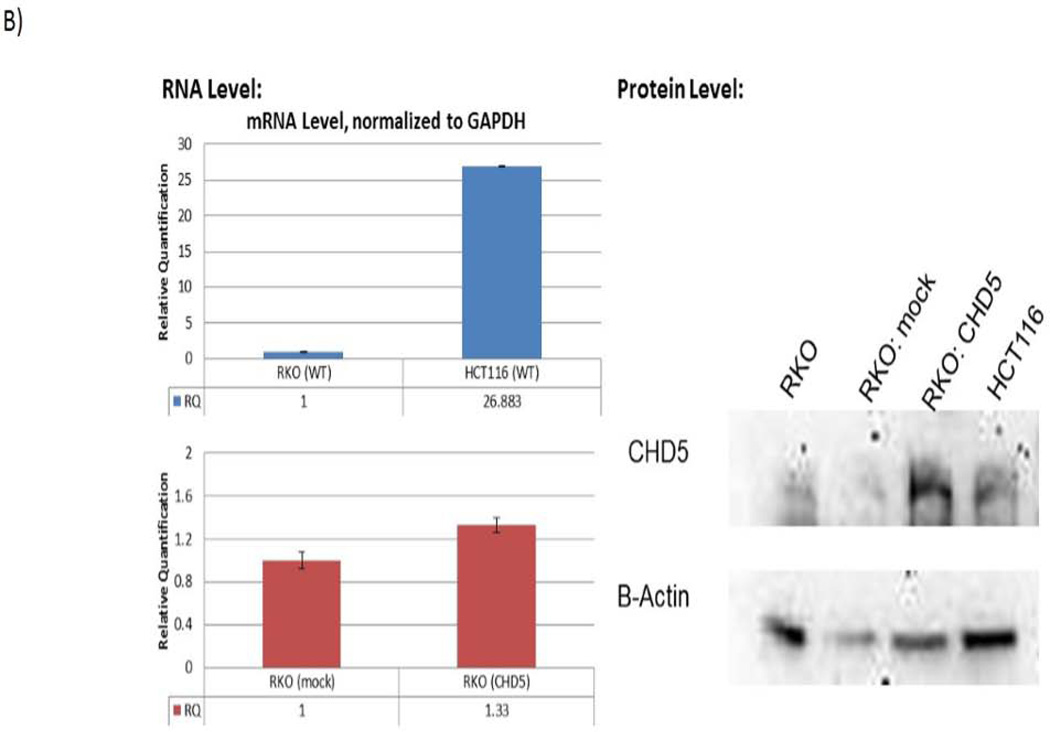
Since CHD5 expression may have an important role in the development and progression of CRC, and its role has not been well understood, we performed biological analysis to identify the consequences of CHD5 expression in CRC. In order to analyze the biological role of CHD5 in CRC, a CHD5 expression vector was stably transfected into the RKO cell line. As negative control, the antisense CHD5 vector was stably transfected into the same cell line. Fig 3A shows that the wild type RKO cells were smaller and grew as monolayer when compared to the CHD5 expressing RKO cells. RNA and protein analysis of CHD5 confirmed the expression of CHD5 in the transfected RKO cells (Fig 3B).
Figure 3.
Stably transfected RKO cell line with CHD5 inducing vector. Forced CHD5 expression in CHD5 silenced colonic RKO cancer cell line vs. CHD5 wild type cells. A) RKO cell culture before (left) and after (right) stable transfection with the CHD5 inducing plasmid. B) Left panel shows the level of CHD5 expression in RKO and HCT116 cell lines; and also in the stably transfected cells. Right panel shows the western blot analysis of CHD5 protein level.
CHD5 expression reduced cell proliferation in RKO cell line
We compared the proliferation of RKO wild type cells (CHD5 is methylated) with the CHD5 transfected RKO cells at different cell densities. At all cell densities, cell proliferation was reduced when CHD5 was expressed. The highest fold difference was observed at the cell density of 5000 cells/well. This result suggests that CHD5 suppresses tumor growth in these colon carcinoma cells (data not shown).
CHD5 reduced the cell migration and invasion in RKO
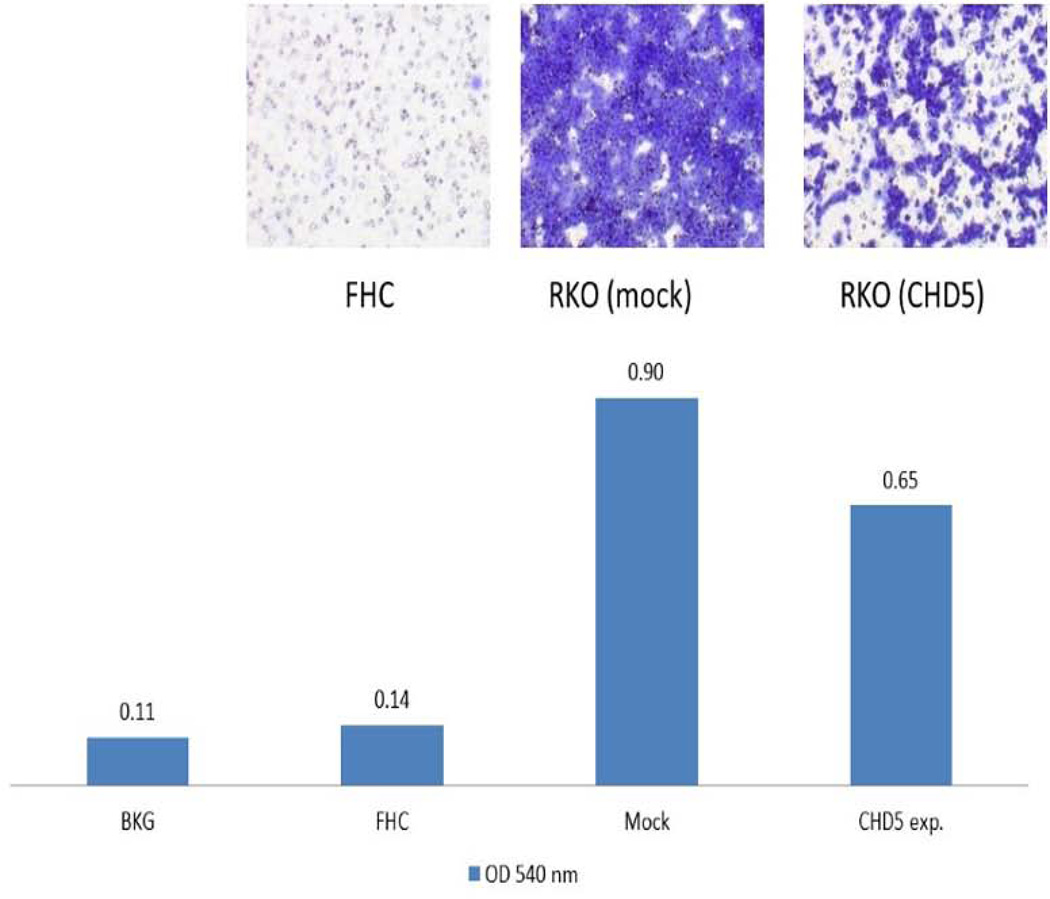
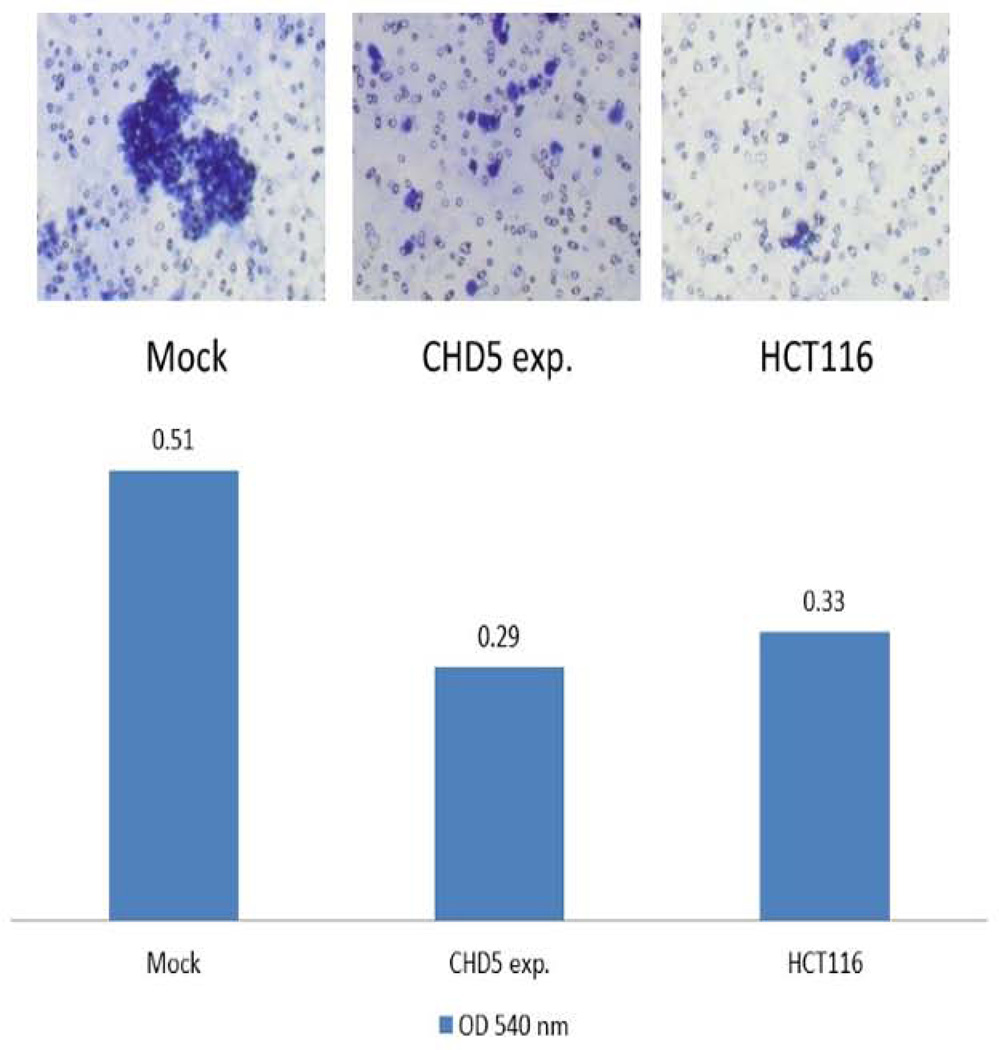
Migration and invasion assays were performed on a polycarbonate membrane (8 µm pore size) according to the manufacturers’ recommendations (Cell Biolabs, Inc., CA). The cell migration and invasion were reduced 1.4 to 1.7 fold in CHD5 overexpressing cells compared to the mock-transfected cells. As negative control, we used a normal fibroblast human colon cell line (FHC), and as positive control we used the endogenously expressing CHD5 cell line HCT116 (Fig. 4 & 5).
Figure 4.
Effect of CHD5 on cell migration. Cell migration of RKO cell line after stable transfection of the CHD5 expressing plasmid as determined on a polycarbonate membrane (8 µm pore size). The bar chart shows the intensity of cell migration of each experiment. BKG: Background; FHC: Normal Fibroblast Human Colon cell line; Mock: negative control, CHD5 antisense expressing plasmid; and CHD5 exp=the RKO induced CHD5 expressing cell line.
Figure 5.
Effect of CHD5 on cell invasion. Cell invasion of RKO cell line after stable transfection of the CHD5 expressing plasmid as determined on a polycarbonate membrane (8 µm pore size). The bar chart shows the cell invasiveness of each experiment. Mock: negative control, CHD5 antisense expressing plasmid; and CHD5 expressing RKO cell line; and HCT116: the CHD5 endogenous expressing cell line, as a positive control.
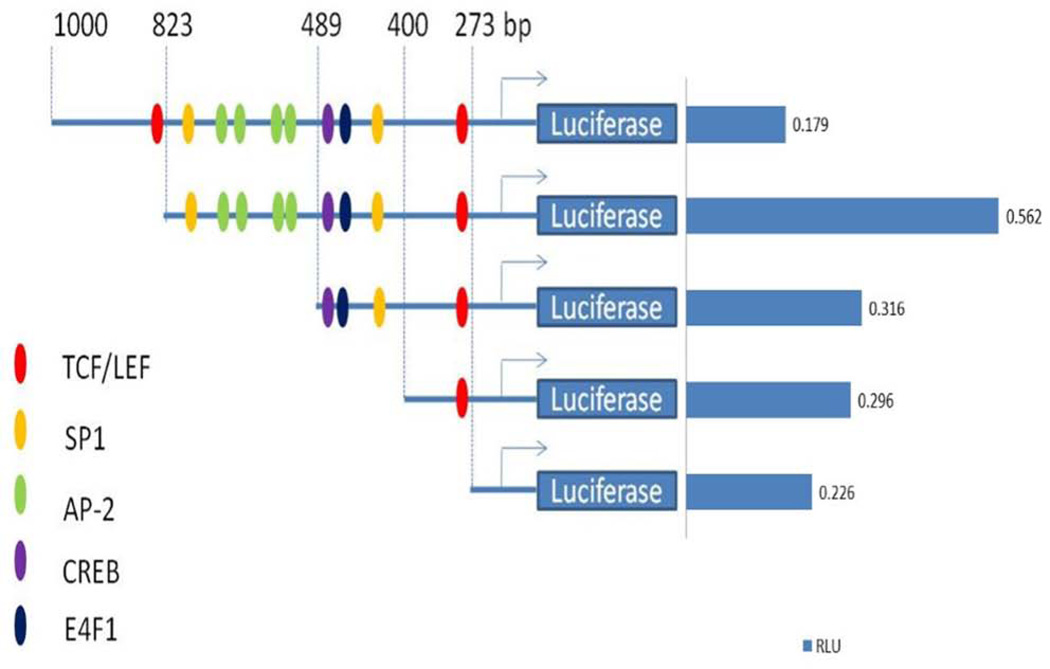
Luciferase Reporter activity of CHD5 promoter in RKO
Transcriptional activity of CHD5 promoter was analyzed in a 1 kb region upstream of the transcription start site (TSS). We performed the dual luciferase reporter assay according to similar previous study16 in RKO cells (Fig. 6). The highest reporter activity was observed at a region from −825 to +50. This activity was dropped 2 fold by deletion of 336 bp from −489 to +50. This indicates that the active region is encompassed from −825 to −489 of CHD5 promoter, where few putative binding sites of transcription factors such as SP1 and AP2 were detected using the proscan TF software.
Figure 6.
Reporter activity of 273, 400, 489, 823, and 1000 bp regions of CHD5 promoter. A.) RKO cells were cotransfected with truncated versions of the CHD5 promoter luciferase construct and compared with untreated cells. CHD5-luciferase activity (RLU) was normalized against Rinella to determine the transfection efficiency and confirm the quality of the assay. RKO cells cultured in medium alone served as controls. Promoter regions containing putative transcription binding sites such as TCF/LEF, SP1, AP-2, CREB and E4F1 are indicated. The 3’ end constructs with lost luciferase activity correspond to the −823 to −400 region and contain the following putative elements SP1, AP-2, CREB and E4F1 binding sites.
Discussion
CHD5, a newly identified tumor suppressor gene 17,18. And its expression is reduced in many tumor types. This reduction is related to either deletion of the 1p36 region or epigenetic modification, such as the hypermethylation of its promoter 10, 13, 19. In our previous study, the epigenetic silencing of CHD5 was investigated in different CRC populations. Our findings established a high level of methylation of this gene in African Americans (AA) when compared to Caucasians 10. It is noteworthy that AA have a higher incidence of CRC and generally are diagnosed with advanced and aggressive tumors. Here, we investigated the epigenetic status of the CHD5 gene at earlier stages of colon carcinogenesis in different adenoma types, and we characterized its functions in vitro.
CHD is a super family that can be subdivided into five subfamilies based on the presence of specific protein motifs, which endow each family of proteins with a unique function. CHD5 is most similar to CHD3 and CHD4 in that it is contains plant homeodomain motifs and is frequently deleted in many types of human cancers 20, 21 and the chromatin-remodeling activity of CHD5 is required for appropriate transcriptional activation of the p19Arf/p53 pathway 17. It is clear that CHD5 deficiency is a common initiating event in human tumorigenesis. CHD5 is frequently downregulated through promoter hypermethylation in gastric, breast, ovarian, and glioma tumors, suggesting epigenetic silencing of CHD5 by methylation may contribute to tumorigenesis in these tissues11, 14.
Studies suggest that CHD5 exert its effect as tumor suppressor gene in various malignancies, however its role was not clear in colon adenoma and cancer11, 17–19, 22. The analysis of its methylation status in several adenoma types and normal tissues showed an overall high level of methylation or deletion in most tested samples, and the methylation/deletion status correlated with the lack of expression of CHD5 gene. Two of the normal tissues showed some methylation, which was likely due to some molecular field effect taking place in these histologically normal tissues adjacent to the adenomas. These findings established the prevalence of CHD5 promoter DNA methylation at earlier stages in the carcinogenic process. Genes that have the capacity to affect the expression of multiple other genes would be attractive targets in early stages of carcinogenesis 23. The CHD5 gene through its effect on chromatin structure has also the potential to alter the expression of hundreds of genes, and any deregulation of its expression could have far reaching consequences on the tissue homeostasis.
In these experiments, CHD5 expression in the RKO cell line was restored using 5-Aza-dC and SAHA. However, the restored CHD5 expression (1.7 fold) was still lower than that observed in the HCT116 cell line, in which CHD5 was not methylated (5.2 fold). These findings suggest that other regulatory processes that might be at play in the alteration of CHD5 activity and expression. Indeed, Oliver et al. 24 have pointed to the presence of multivalent recognition of histone tails by the PHD-fingers of CHD5. Our chromatin immunoprecipitation experiments using antibodies against two modified histones, namely H3K4m3 (an activating modifier) and H3K27m3 (an inhibiting modifier) revealed that the activating modification was primarily associated with the CHD5 expressing cell line HCT116 (CHD5 not methylated), whereas the inhibiting modification was associated with RKO cell line, in which the CHD5 promoter was methylated. RKO cells appear to have CGI methylation and H3K27me3 consistence with the data showing coordinate regulation between DNAme and H3K27me3, which are both involved in the establishment and maintenance of epigenetic gene silencing. However, conflicting data have been reported and have presented evidence to support the theory that PRC2 and DNAme cooperate to achieve silencing, or alternatively that H3K27me3 and DNAme act antagonistically 25. Acquisition of CGI methylation in cancer has been found to occur preferentially at genes marked by H3K27me3 in ES cells 26–28. An unknown CGI-binding factor could be responsible for recruiting PRC2 to CGIs that then trimethylates H3K27. This H3K27me3 is recognized by polycomb PRC1 complexes that act to impede transcriptional elongation, thereby silencing genes 29.
This demonstrates the complexity of epigenetic factors regulating the expression of this gene. Due to the technical difficulty, PET could not be used for the cloning and sequencing. It is worth noting that we recently demonstrated a role for miRNA-211 in the control of CHD5 expression in CRC in vitro and in vivo15, 24. When we transfected the RKO cell line with a CHD5 expressing vector, we were able to restore CHD5 mRNA and protein to levels comparable to those in CHD5 expressing cell lines. The transfected RKO cell line became less proliferative, less invasive and with a lower migration potential when compared to parental or mock transfected RKO cells. These findings established unequivocally the tumor suppressor characteristics of CHD5 in colon tissues.
A tumor suppressor role for CHD5 has been suggested in a number of other tumor types, such as lung, gastric and ovarian cancers and others11, 13, 17, 19, 22, 30–33. At this stage, CHD5 regulation seems to involve multiple epigenetic processes such as CpG island methylation at the promoter region, histones modifications and miRNA. In addition, genetic process may also be involved such as deletion, as is the case in some of the adenomas tested in this study, and many CRC tumors analyzed by aCGH previously34, 35. Through different subcloning steps and dual luciferase experiments, we located an area between −823 and −423 upstream of the TSS. This promoter region contains some very important sites for known transcriptional factors such as TCF/LEF, CREB, SP1, AP-2 and E4F1. The presence of two TCF binding sites at CHD5 promoter, one near the TSS and the other about 823 bp apart from TSS, suggests a special regulatory action of the TCFs on CHD5 activity. In fact, the TCFs at 823 apart from TSS acted as a negative regulator for the gene activity, since the 1000 bp construct of the CHD5 promoter showed less activity in luciferase assay than the truncated one. This observation is consistent with the previous studies regarding negative regulatory role of TCFs, in Wnt “off” state, where TCFs interact with Groucho transcription repressors36, 37 preventing gene transcription. When the region contains SP1 and AP-2 sites were removed, the gene activity decreased significantly, indicating their role as influential transcription factors in CHD5 activity. The presence of the TCFs, SP1, AP-2, and other transcription sites suggests an interaction with the Wnt pathway, which plays an important role in many human cancers including CRC38, 39. Is there a common underlying mode of CHD5 as tumor suppressor activity (TS)? We showed the fundamental concept that human CRC cell proliferation, migration and invasion are inhibited by CHD5 and its decrease expression consistent with TS activity in colon adenomas and tumors from patients, similar to other cancers11, 17–19, 22.
We have previously shown that CHD5, ICAM5 and GPNMB were predominantly methylated in AA colon cancer tumors when compared to Caucasians 10. CHD5 may be a sole potential gene candidate to be involved in early stages of oncogenic transformation and as such may be a useful biomarker for early colon cancer detection in this high risk population.
Acknowledgment
This work was supported in part by PHS grant from the National Institutes of Health, CTSA and by the RCMI.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Sharma S, O'Keefe SJ. Environmental influences on the high mortality from colorectal cancer in African Americans. Postgrad Med J. 2007;83:583–589. doi: 10.1136/pgmj.2007.058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller G, Babinsky VN, Ziegler B, et al. Genome-wide CpG island methylation analyses in non-small cell lung cancer patients. Carcinogenesis. 2013;34:513–521. doi: 10.1093/carcin/bgs363. [DOI] [PubMed] [Google Scholar]
- 3.Kondo Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med J. 2009;50:455–463. doi: 10.3349/ymj.2009.50.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formosa A, Lena AM, Markert EK, et al. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 5.Esteller M. Cancer epigenetics: DNA methylation and chromatin alterations in human cancer. Adv Exp Med Biol. 2003;532:39–49. doi: 10.1007/978-1-4615-0081-0_5. [DOI] [PubMed] [Google Scholar]
- 6.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 7.Hinoue T, Weisenberger DJ, Lange CP, et al. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Res. 2012;22:271–282. doi: 10.1101/gr.117523.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmer F, Brinkman AB, Assenov Y, et al. Comparative genome-wide DNA methylation analysis of colorectal tumor and matched normal tissues. Epigenetics. 2012;7:1355–1367. doi: 10.4161/epi.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson PM, Gotoh T, Kok M, White PS, Brodeur GM. CHD5, a new member of the chromodomain gene family, is preferentially expressed in the nervous system. Oncogene. 2003;22:1002–1011. doi: 10.1038/sj.onc.1206211. [DOI] [PubMed] [Google Scholar]
- 10.Mokarram P, Kumar K, Brim H, et al. Distinct high-profile methylated genes in colorectal cancer. PLoS One. 2009;4:e7012. doi: 10.1371/journal.pone.0007012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita T, Igarashi J, Okawa ER, et al. CHD5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J Natl Cancer Inst. 2008;100:940–949. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulero-Navarro S, Esteller M. Chromatin remodeling factor CHD5 is silenced by promoter CpG island hypermethylation in human cancer. Epigenetics. 2008;3:210–215. doi: 10.4161/epi.3.4.6610. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Lau KK, So LK, Lam YW. CHD5 is down-regulated through promoter hypermethylation in gastric cancer. J Biomed Sci. 2009;16:95. doi: 10.1186/1423-0127-16-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorringe KL, Choong DY, Williams LH, et al. Mutation and methylation analysis of the chromodomain-helicase-DNA binding 5 gene in ovarian cancer. Neoplasia. 2008;10:1253–1258. doi: 10.1593/neo.08718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai C, Ashktorab H, Pang X, et al. MicroRNA-211 expression promotes colorectal cancer cell growth in vitro and in vivo by targeting tumor suppressor CHD5. PLoS One. 2012;7:e29750. doi: 10.1371/journal.pone.0029750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashktorab H, Daremipouran M, Wilson M, et al. Transactivation of the EGFR by AP-1 Is Induced by Helicobacter pylori in Gastric Cancer. Am J Gastroenterol. 2007;102:2135–2146. doi: 10.1111/j.1572-0241.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 17.Bagchi A, Papazoglu C, Wu Y, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128:459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Bagchi A, Mills AA. The quest for the 1p36 tumor suppressor. Cancer Res. 2008;68:2551–2556. doi: 10.1158/0008-5472.CAN-07-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Chen H, Fu S, Xu ZM, Sun KL, Fu WN. The involvement of CHD5 hypermethylation in laryngeal squamous cell carcinoma. Oral Oncol. 2011;47:601–608. doi: 10.1016/j.oraloncology.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 20.White PS, Thompson PM, Gotoh T, et al. Definition and characterization of a region of 1p36.3 consistently deleted in neuroblastoma. Oncogene. 2005;24:2684–2694. doi: 10.1038/sj.onc.1208306. [DOI] [PubMed] [Google Scholar]
- 21.Aarts M, Dannenberg H, deLeeuw RJ, et al. Microarray-based CGH of sporadic and syndrome-related pheochromocytomas using a 0.1-0.2 Mb bacterial artificial chromosome array spanning chromosome arm 1p. Genes Chromosomes Cancer. 2006;45:83–93. doi: 10.1002/gcc.20268. [DOI] [PubMed] [Google Scholar]
- 22.Zhao R, Yan Q, Lv J, et al. CHD5, a tumor suppressor that is epigenetically silenced in lung cancer. Lung Cancer. 2012;76:324–331. doi: 10.1016/j.lungcan.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Brim H, Kumar K, Nazarian J, et al. SLC5A8 Gene, A Transporter of Butyrate: A Gut Flora Metabolite, Is Frequently Methylated in African American Colon Adenomas. PLoS One. 2011;6:e20216. doi: 10.1371/journal.pone.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver SS, Musselman CA, Srinivasan R, Svaren JP, Kutateladze TG, Denu JM. Multivalent recognition of histone tails by the PHD-fingers of CHD5. Biochemistry. 2012 doi: 10.1021/bi3006972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagarman JA, Motley MP, Kristjansdottir K, Soloway PD. Coordinate regulation of DNA methylation and H3K27me3 in mouse embryonic stem cells. PLoS One. 2013;8:e53880. doi: 10.1371/journal.pone.0053880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illingworth RS, Bird AP. CpG islands--'a rough guide'. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Ohm JE, McGarvey KM, Yu X, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widschwendter M, Fiegl H, Egle D, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 29.Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potts RC, Zhang P, Wurster AL, et al. CHD5, a brain-specific paralog of Mi2 chromatin remodeling enzymes, regulates expression of neuronal genes. PLoS One. 2011;6:e24515. doi: 10.1371/journal.pone.0024515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong RR, Chan LK, Tsang TP, et al. CHD5 Downregulation Associated with Poor Prognosis in Epithelial Ovarian Cancer. Gynecol Obstet Invest. 2011;72:203–207. doi: 10.1159/000323883. [DOI] [PubMed] [Google Scholar]
- 32.Lang J, Tobias ES, Mackie R. Preliminary evidence for involvement of the tumour suppressor gene CHD5 in a family with cutaneous melanoma. Br J Dermatol. 2011;164:1010–1016. doi: 10.1111/j.1365-2133.2011.10223.x. [DOI] [PubMed] [Google Scholar]
- 33.Garcia I, Mayol G, Rodriguez E, et al. Expression of the neuron-specific protein CHD5 is an independent marker of outcome in neuroblastoma. Mol Cancer. 2010;9:277. doi: 10.1186/1476-4598-9-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashktorab H, Schaffer AA, Daremipouran M, Smoot DT, Lee E, Brim H. Distinct genetic alterations in colorectal cancer. PLoS One. 2010;5:e8879. doi: 10.1371/journal.pone.0008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brim H, Hasszadeh H, Schaffer AA, et al. Genomic Aberrations in an African American Colorectal Cancer Cohort Reveals a MSI-Specific Profile and Chromosome X Amplification in Male Patients. PLoS One. 2012 doi: 10.1371/journal.pone.0040392. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavallo RA, Cox RT, Moline MM, et al. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 37.Roose J, Molenaar M, Peterson J, et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 38.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 39.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]