Abstract
Objectives
We evaluated the effect of time intervals between the initiation of antiretroviral therapy (ART) and tuberculosis (TB) treatment on clinical outcomes in HIV-TB co-infected patients in an Asian regional cohort.
Methods
Adult HIV-TB co-infected patients in an observational HIV cohort database who had a known date of ART initiation and history of TB treatment were eligible for study inclusion. The time intervals between the initiation of ART and TB treatment were categorized as follows: TB diagnosed while on ART, early ART (<90 days after TB treatment), delayed ART (>90 days after TB treatment), and ART not started. Outcomes were assessed using survival analyses.
Results
A total of 768 HIV-TB co-infected patients were included in this study. Median CD4 T-cell count at TB diagnosis was 100 (IQR 40–208) cells/µL. The treatment outcomes between early ART and delayed ART initiation were not significantly different. Kaplan-Meier analysis indicated that mortality was highest for those diagnosed with TB while on ART (3.77 deaths per 100 person-years), and the prognoses of other groups were not different (in deaths per 100 person-years: 2.12 early ART, 1.46 delayed ART, and 2.94 ART not started). In a multivariate model, the interval between ART initiation and TB therapy did not significantly impact all-cause mortality.
Conclusions
The negative impact of delayed ART in patients co-infected with TB was not observed in this observational cohort of moderately to severely immunosuppressed patients. The broader impact of earlier ART in actual clinical practice should be monitored more closely.
Keywords: HIV, tuberculosis, antiretroviral therapy, antitubercular agents
Introduction
Tuberculosis (TB) is the most common opportunistic disease and cause of death in patients with HIV infection in developing countries (1). The two diseases are closely intertwined, and the number of co-infected patients continues to rapidly grow (2). The optimal time to begin antiretroviral therapy (ART) in patients with both TB and HIV has been carefully studied. Immune reconstitution inflammatory syndrome (IRIS), pharmacological interactions, and high pill burden have previously argued against a simultaneous therapy for both HIV and TB (3–6). In contrast, a delay in starting ART, specifically in severely immunosuppressed patients, is associated with disease progression and higher mortality (7, 8).
Previous World Health Organization (WHO) guidelines recommended that ART should be started between two and eight weeks after the start of TB therapy in persons with CD4 T-cell counts below 200 cells/µL, but the commencement of ART may be delayed for patients with CD4 T-cell counts above 200 cells/µL (9). Prospective studies have evaluated the optimal time for initiating ART in HIV/TB-coinfected persons (4), including randomized clinical trials in South Africa (SAPiT), Cambodia (CAMELIA), and global regions (STRIDE) (4, 10, 11). Both studies demonstrated a mortality reduction among those starting ART during the early part of TB therapy compared to those who started later or after completion of TB therapy. Current WHO guidelines now recommend that ART be initiated as soon as TB therapy is tolerated, ideally as early as two weeks and not later than eight weeks, regardless of CD4 T-cell counts (9). However, it is not clear how ART timing in TB co-infected patients impacts clinical outcomes in “real-life,” non-trial settings. Another issue is the risk of starting ART in a patient with underlying but quiescent TB, which may lead to unmasking of TB disease and IRIS in the absence of anti-TB treatment.
The objective of this analysis was to evaluate the effects of the time interval between the initiation of ART and TB treatment on clinical outcomes in a regional observational cohort of HIV-TB co-infected Asian patients.
Methods
Study population
We analysed data from the TREAT Asia HIV Observational Database (TAHOD), a prospective, observational cohort study of adults with HIV from 18 sites in the Asia-Pacific region (12). The structure of the database and standardized mechanisms for data collection and quality control have previously been described (12). Additional TB-related variables, such as diagnostic site of TB, diagnostic methods, TB treatment, mycobacterial resistance, and treatment outcomes were retrospectively collected for this analysis using a standardized form. All co-infected patients in TAHOD whose dates of TB diagnosis, TB therapy initiation, and ART initiation were known, and were aged 18 years or older at TB diagnosis were eligible for inclusion in this analysis.
Variables and definitions
The following variables were assessed: age at TB diagnosis; sex; reported route of HIV infection; prior AIDS diagnoses before TB; hepatitis B or C co-infection; HIV and TB treatment regimens, dates of starting and stopping, adverse events, and outcomes; CD4 T-cell count (cells/µL) and HIV viral load (copies/mL); development of IRIS. The most advanced US Centers for Disease Control and Prevention (CDC) clinical category recorded was used as the clinical status for the analysis (13). TB treatment outcomes were defined according to WHO TB reporting forms (14). A patient who was initially culture- or smear microscopy-positive at the beginning of TB treatment but who was smear-negative in the last month of treatment and on at least one previous occasion was considered as ‘cured.’ ‘Treatment failure’ was defined as i) a patient who is culture- or smear-positive at five months or later during initial TB treatment, or who is switched to a regimen including second-line TB drugs because of culture results showing multidrug-resistant (MDR)-TB, or ii) a previously-treated patient who is culture- or smear-positive at the end of a re-treatment regimen or who is switched to a regimen including second-line TB drugs because of culture results showing MDR-TB. A patient who completed treatment but did not meet criteria to be classified as ‘cured’ or a ‘treatment failure’ was considered as ‘treatment completed.’ ‘Died’ meant a patient who died from any cause during the course of TB treatment. A patient was considered as having ‘defauIted’ if TB treatment was interrupted for two or more consecutive months. A patient was considered as ‘transferred out’ if transferred to another health facility and the TB treatment outcome was not known. Immunological response (IR) at 12 months after ART initiation was defined as a rise in CD4 T-cell count of at least 100 cells/µL. The severity of adverse events of TB therapy was determined by a modified WHO toxicity grading scale (15). IRIS was diagnosed by the consensus case definitions of the International Network for the Study of HIV-associated IRIS (16). The exposures of interest were the time intervals between TB therapy and ART initiation, categorized into four groups according to the date of TB therapy and ART initiation, including: TB diagnosed while on ART, early ART (ART initiated within 90 days after TB therapy), delayed ART (ART initiated later than 90 days after TB therapy), and ART not started. Death was confirmed by local medical staff and reported using standardized Cause of Death (CoDe) forms. AIDS-defining illnesses were defined according to the modified 1993 CDC definitions (13).
Data analysis
Demographic and clinical characteristics were compared between patients in the four groups. All-cause mortality following TB diagnosis was examined using survival analysis methods. The survivor function for death of each group was compared using the Kaplan-Meier curve and the Cox proportional hazards model. For overall survival, time was calculated from the initiation of TB diagnosis and ended at the time of death, or the last follow-up visit. The final multivariate model included all covariates that remained significant at the 0.10 level (2-sided). All analyses were performed using SAS (version 9.1, SAS Institute Inc., Cary, North Carolina, USA) and STATA (version 10.1, StataCorp, College Station, Texas, USA).
Results
Demographic and clinical characteristics of subjects
A total of 768 HIV-TB co-infected patients were included in the analysis, including those diagnosed with TB while on ART (N=191), early ART (N=238), delayed ART (N=280), and not started on ART during the scope of the data collection (N=59). Durations between the initiation of ART and TB therapy varied (Table 1). Overall, 609 (79%) were male, with a median age of 34 years [interquartile range (IQR) 29–39], a median CD4 T-cell count at TB diagnosis of 100 (IQR 40–208) cells/µL, and a median HIV viral load of 81,650 (IQR 499–330,000) copies/mL (Table 2). The most common HIV exposure category was heterosexual contact (69%), followed by injection drug use (16%). The majority of patients had no prior AIDS-related illness reported (86%). NNRTI-based first-line regimens were prescribed to 615 (80%) patients and PI-based regimens to 39 (5%) patients. TB cases included pulmonary (42%), extrapulmonary (23%), and both (10%). In 25% of cases, the site of TB infection could not be identified. Age, sex, and reported route of infection were not different among the four groups (Table 2). The rate of prior AIDS diagnosis at TB diagnosis was highest in the group diagnosed while already on ART (29%), followed by the early ART group (13%). The CD4 T cell counts at TB diagnosis were highest in the group that had not yet started ART, followed by the group with TB diagnosed while on ART, the delayed ART group, and the early ART group.
Table 1.
Time intervals between the initiation of ART and TB treatment among HIV-TB co-infected patients (N=768)
| Duration between the initiation of ART and TB treatment (days) |
N (%) |
|---|---|
| ART after TB treatment | 518 (67%) |
| >365 | 79 (10%) |
| 181–365 | 90 (12%) |
| 91–180 | 110 (14%) |
| 61–90 | 71 (9%) |
| 31–60 | 76 (10%) |
| 15–30 | 41 (5%) |
| 0–14 | 51 (7%) |
| ART before TB treatment | 191 (25%) |
| >365 | 83 (11%) |
| 181–365 | 17 (2%) |
| 91–180 | 24 (3%) |
| 61–90 | 14 (2%) |
| 31–60 | 20 (3%) |
| 15–30 | 11 (1%) |
| 0–14 | 21 (3%) |
| ART was not started | 59 (8%) |
ART, antiretroviral therapy; TB, tuberculosis
Table 2.
Characteristics of patients with HIV-TB co-infection, by ART initiation category
| Characteristic | Total N=768 |
TB diagnosed while on ART N=191 |
Early ART N=238 |
Delayed ART N=280 |
ART not started N=59 |
|---|---|---|---|---|---|
| Age at TB diagnosis, median years (IQR) |
34 (29, 39) | 35 (30, 41) | 33.5 (29, 39) | 33 (29, 38.5) | 32 (30, 37) |
| Sex, male | 609 (79%) | 149 (78%) | 206 (86%) | 207 (74%) | 47 (80%) |
| Reported exposure route of HIV infection |
|||||
| Heterosexual contact | 530 (69%) | 129 (68%) | 163 (68%) | 197 (70%) | 41 (69%) |
| Homosexual contact | 67 (9%) | 27 (14%) | 18 (8%) | 18 (7%) | 4 (7%) |
| Injecting drug use | 128 (16%) | 24 (12%) | 43 (18%) | 51 (18%) | 10 (17%) |
| Other/unknown | 43 (6%) | 11 (6%) | 14 (6%) | 14 (5%) | 4 (7%) |
| Prior AIDS-defining diagnosis at TB diagnosis |
|||||
| No | 662 (86%) | 136 (71%) | 208 (87%) | 263 (94%) | 55 (93%) |
| Yes | 106 (14%) | 55 (29%) | 30 (13%) | 17 (6%) | 4 (7%) |
| Hepatitis B co-infection | |||||
| No | 465 (61%) | 106 (56%) | 136 (57%) | 188 (67%) | 35 (59%) |
| Yes | 46 (6%) | 10 (5%) | 15 (6%) | 20 (7%) | 1 (2%) |
| Not tested | 257 (33%) | 75 (39%) | 87 (37%) | 72 (26%) | 23 (39%) |
| Hepatitis C co-infection | |||||
| No | 303 (39%) | 72 (38%) | 92 (39%) | 123 (44%) | 16 (27%) |
| Yes | 116 (15%) | 27 (14%) | 32 (13%) | 51 (18%) | 6 (10%) |
| Not tested | 349 (46%) | 92 (48%) | 114 (48%) | 106 (38%) | 37 (63%) |
| CD4 T-cell count at TB diagnosis, median cells/µL (IQR) |
100 (40, 208) | 151 (50, 243) | 66 (28, 142) | 119 (48, 238) | 357 (125, 505) |
| HIV viral load at TB diagnosis, median copies/mL (IQR) |
81650 (499, 330000) |
707.5 (<400, 80027) |
206902 (42400, 4880000) |
170000 (11104, 552216) |
132518 (41135, 466718) |
| Initial ART | |||||
| NRTI+NNRTI | 615 (80%) | 145 (76%) | 213 (90%) | 257 (92%) | --- |
| NRTI+PI | 39 (5%) | 19 (10%) | 5 (2%) | 15 (5%) | --- |
| Other combination | 114 (15%) | 27 (14%) | 20 (8%) | 8 (3%) | --- |
| Type of TB | |||||
| Pulmonary | 321 (42%) | 84 (44%) | 91 (38%) | 125 (45%) | 21 (36%) |
| Extrapulmonary | 178 (23%) | 57 (30%) | 50 (21%) | 58 (21%) | 13 (22%) |
| Both | 74 (10%) | 15 (8%) | 25 (11%) | 30 (11%) | 4 (6%) |
| Unknown | 195 (25%) | 35 (18%) | 72 (30%) | 67 (24%) | 21 (36%) |
| Median days of total TB therapy (IQR) |
275 (186,427) | 256 (183, 379.5) |
275.5 (185, 411) |
303.5 (214, 534) |
190 (111, 281) |
| Median days between TB therapy and ART initiation (IQR) |
--- | Before TB therapy: 223 (51, 790) |
After TB therapy: 42 (17, 64) |
After TB therapy: 212 (137.5, 407.5) |
--- |
Data are frequencies and percentages in parentheses, unless otherwise indicated. TB, tuberculosis; ART, antiretroviral therapy; IQR, interquartile range; NRTIs, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
TB treatment outcomes
Within the TB treatment outcomes categories, 240 (31%) individuals were considered cured, 435 (57%) completed treatment, 3 (0.4%) were treatment failures, 21 (3%) died, 29 (4%) defaulted, and 9 (1%) transferred out (Table 3). Overall survival was 91% during the follow-up period, with the highest death rate per 100 person-years in the group diagnosed with TB while on ART (3.77/100 person-years), followed by the group not started on ART (2.94/100 person-years), the early ART group (2.12/100 person-years), and then the delayed ART group (1.46/100 person-years). The incidence of new AIDS-defining illnesses excluding TB was highest in the group not started on ART (13.66/100 person-years), followed by the group diagnosed with TB while on ART (5.06/100 person-years), the early ART group (4.24/100 person-years), and then the delayed ART group (2.91/100 person-years). The rate of favorable outcomes (i.e., cured or treatment completed) was lowest in the group not started on ART. IRIS was most common in the early ART group.
Table 3.
Clinical outcomes of patients with HIV-TB co-infection, by ART initiation category
| Outcome | Total N=768 |
TB diagnosed while on ART N=191 |
Early ART N=238 |
Delayed ART N=280 |
ART not started N=59 |
|---|---|---|---|---|---|
| TB treatment outcomes, N (%) | |||||
| Cured | 240 (31%) | 61 (32%) | 79 (33%) | 92 (33%) | 8 (14%) |
| Treatment completed | 435 (57%) | 99 (52%) | 137 (58%) | 171 (61%) | 28 (47%) |
| Treatment failure | 3 (0%) | 0 (0%) | 0 (0%) | 1 (0%) | 2 (3%) |
| Died during TB treatment | 21 (3%) | 10 (5%) | 3 (1%) | 1 (0%) | 7 (12%) |
| Defaulted | 29 (4%) | 8 (4%) | 7 (3%) | 6 (2%) | 8 (14%) |
| Transferred out | 9 (1%) | 4 (2%) | 2 (1%) | 2 (1%) | 1 (2%) |
| Current treatment without failure |
20 (3%) | 6 (3%) | 7 (3%) | 5 (2%) | 2 (3%) |
| Missing | 11 (1%) | 3 (2%) | 3 (1%) | 2 (1%) | 3 (5%) |
| Mortality | |||||
| Follow-up, person-years | 2973 | 611 | 896 | 1025 | 441 |
| Deaths | 70 | 23 | 19 | 15 | 13 |
| Rate per 100 person-years, 95% CI |
2.35 (1.86, 2.98) | 3.77 (2.50, 5.67) | 2.12 (1.35, 3.32) | 1.46 (0.88, 2.43) | 2.94 (1.71, 5.07) |
| New AIDS-defining illness, excluding TB |
|||||
| Follow-up, person years | 2531 | 533 | 777 | 824 | 395 |
| New AIDS cases | 138 | 27 | 33 | 24 | 54 |
| Rate per 100 person-years, 95% CI |
5.45 (4.62, 6.44) | 5.06 (3.47, 7.38) | 4.24 (3.02, 5.97) | 2.91 (1.95, 4.34) | 13.66 (10.46, 17.83) |
| AIDS-defining illness (excluding TB) or death |
|||||
| Follow-up, person-years | 2531 | 533 | 777 | 824 | 395 |
| New AIDS cases or death | 189 | 46 | 48 | 32 | 63 |
| Rate per 100 person-years, 95% CI |
7.47 (6.48, 8.61) | 8.62 (6.46, 11.51) |
6.17 (4.65, 8.19) | 3.88 (2.75, 5.49) | 15.93 (12.45, 20.40) |
| IRIS, N (%) | 34 (4%) | 8 (4%) | 14 (6%) | 11 (4%) | 1 (2%) |
| Immunological response at 12 months after ART initiation* |
|||||
| No | 167 (33%) | 52 (40%) | 49 (30%) | 66 (31%) | --- |
| Yes | 338 (67%) | 77 (60%) | 115 (70%) | 146 (69%) | --- |
| Incomplete information | 204 | 62 | 74 | 68 | --- |
| Virological response at 12 months after ART initiation** |
|||||
| Yes | 38 (18%) | 13 (25%) | 7 (12%) | 18 (17%) | --- |
| No | 178 (82%) | 40 (75%) | 50 (88%) | 88 (83%) | --- |
| Test not done | 493 | 138 | 181 | 174 | --- |
| Toxicities of TB therapy greater than grade 3 |
22 (3%) | 4 (2%) | 3 (1%) | 13 (5%) | 2 (3%) |
Data are frequencies and percentages in parentheses, unless otherwise indicated.
CD4 T-cell counts increase of 100 cells/µL from ART initiation up to 12 month visit (testing range: 6–18 months)
HIV viral load <500 copies/mL (6–18 months)
ART, antiretroviral therapy; TB, tuberculosis; IRIS, immune reconstitution inflammatory syndrome.
Effects of time intervals between the initiation of ART and TB treatment on overall survival
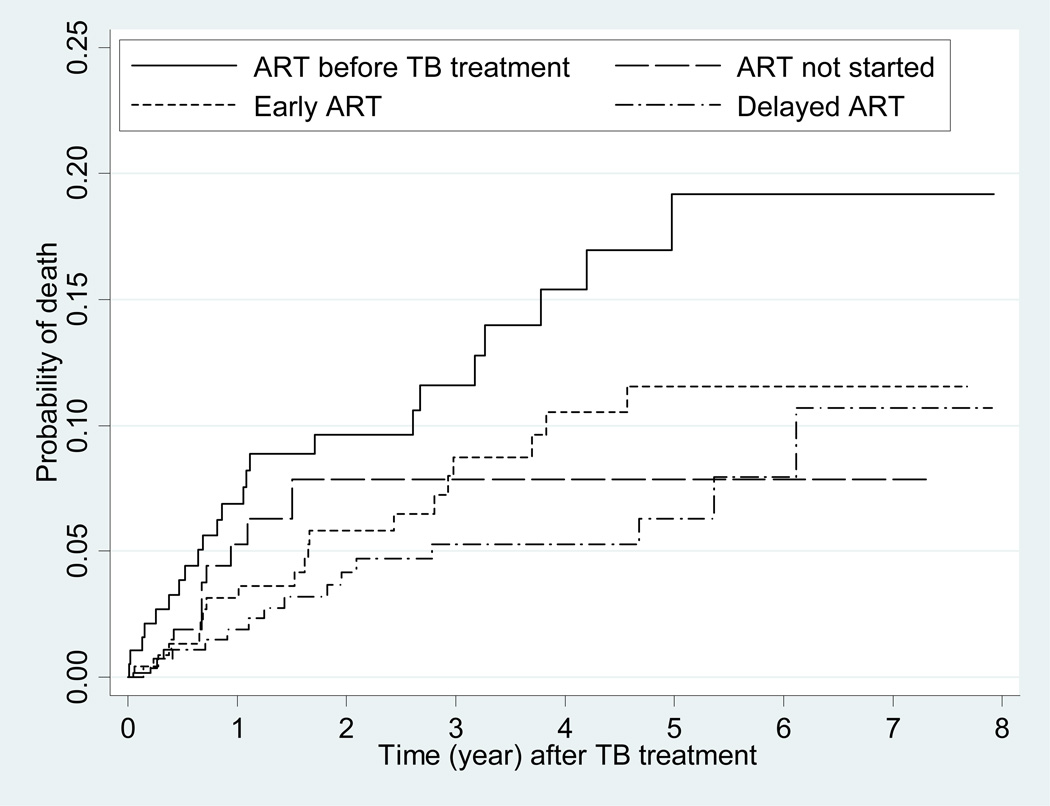
We evaluated the prognostic significance of the time intervals between the initiation of ART and TB therapy in Cox proportional hazards analysis of all-cause mortality (Table 4). There were 70 deaths reported during 2973 person-years of follow-up [an incidence of 2.35 per 100 person-years, 95% confidence interval (CI) 1.86–2.98]. Patients with early ART or delayed ART had a lower risk of mortality than those in the group with TB diagnosed while on ART (p<0.01). However, the difference between early ART and delayed ART was not significantly different (p=0.46). In the multivariate model, injection drug users were at significantly higher risk of death compared with other HIV exposure risks (adjusted hazard ratio=1.96, 95% CI 1.05–3.66, p=0.035). The interval between the initiation of ART and TB therapy did not have a significant impact on all-cause mortality in the multivariate model. The Kaplan-Meier curve summarizing time to death indicated that mortality was highest for the group diagnosed with TB while on ART; the prognoses of other groups were not significantly different (Fig. 1).
Table 4.
Cox proportional hazards analysis of all-cause mortality for HIV-TB co-infected patients (N=768)
| Variable | Follow up in person- years |
Deaths | Unadjusted HR |
P-value | Adjusted HR |
95 % CI |
P-value |
|---|---|---|---|---|---|---|---|
| Age at ART initiation, years | |||||||
| ≤30 | 854 | 19 | 1 | 1 | |||
| 30–41 | 1463 | 31 | 0.96 | 0.886 | 1.14 | (0.63, 2.04) |
0.667 |
| >41 | 657 | 20 | 1.38 | 0.311 | 1.62 | (0.84, 3.15) |
0.151 |
| Sex | |||||||
| Male | 2332 | 61 | 1 | 1 | |||
| Female | 641 | 9 | 0.53 | 0.075 | 0.52 | (0.25, 1.06) |
0.072 |
| Reported exposure route of HIV infection |
|||||||
| Heterosexual contact | 2235 | 44 | 1 | 1 | |||
| Homosexual contact | 290 | 4 | 0.70 | 0.503 | 0.55 | (0.19, 1.53) |
0.252 |
| Injecting drug use | 311 | 17 | 2.27 | 0.005 | 1.96 |
(1.05, 3.66) |
0.035 |
| Other/unknown | 136 | 5 | 1.72 | 0.252 | 1.42 | (0.56, 3.61) |
0.464 |
| Prior AIDS diagnosis at ART initiation | |||||||
| No | 2547 | 57 | 1 | 1 | |||
| Yes | 426 | 13 | 1.39 | 0.284 | 1.68 | (0.90, 3.12) |
0.102 |
| Hepatitis B co-infection | |||||||
| No | 1804 | 30 | 1 | 1 | |||
| Yes | 187 | 5 | 1.64 | 0.306 | 1.47 | (0.57, 3.83) |
0.425 |
| Not tested | 982 | 35 | 2.17 | 0.002 | 2.60 |
(1.58, 4.28) |
<0.001 |
| Hepatitis C co-infection | |||||||
| No | 1304 | 23 | 1 | 1 | |||
| Yes | 320 | 10 | 1.47 | 0.310 | 0.70 | (0.28, 1.71) |
0.431 |
| Not tested | 1349 | 37 | 1.51 | 0.118 | 1.05 | (0.47, 2.38) |
0.902 |
| CD4 T-cell count (cells/µL) at TB diagnosis |
|||||||
| ≤100 | 716 | 22 | 1 | 1 | |||
| >101 | 893 | 15 | 0.55 | 0.076 | 0.69 | (0.35, 1.36) |
0.282 |
| Missing | 1364 | 33 | 0.89 | 0.679 | 0.96 | (0.55, 1.67) |
0.890 |
| HIV viral load (copies/ml) at TB diagnosis |
|||||||
| <500 | 113 | 4 | 1 | 1 | |||
| ≥500 | 26 | 1 | 1.17 | 0.886 | 1.52 | (0.17, 13.74) |
0.707 |
| Missing | 2833 | 65 | 0.74 | 0.552 | 0.84 | (0.30, 2.32) |
0.733 |
| Type of TB | |||||||
| Pulmonary | 1116 | 36 | 1 | 1 | |||
| Extrapulmonary | 730 | 21 | 0.94 | 0.825 | 1.02 | (0.57, 1.84) |
0.937 |
| Both | 255 | 5 | 0.59 | 0.269 | 0.65 | (0.25, 1.65) |
0.360 |
| Not tested or reported | 871 | 8 | 0.32 | 0.003 | 0.31 |
(0.14, 0.70) |
0.005 |
| Time intervals between initiation of TB therapy and ART initiation |
|||||||
| TB diagnosed while on ART | 611 | 23 | 1 | 1.00 | |||
| ART not yet started | 441 | 13 | 0.63 | 0.197 | 0.80 | (0.39, 1.66) |
0.551 |
| Early ART after TB therapy | 896 | 19 | 0.61 | 0.114 | 0.69 | (0.37, 1.28) |
0.239 |
| Delayed ART after TB therapy | 1025 | 15 | 0.51 | 0.050 | 0.57 | (0.29, 1.12) |
0.101 |
TB, tuberculosis; ART, antiretroviral therapy.
Bold text denotes adjusted hazard ratios with significant p values; TB, tuberculosis; ART, antiretroviral therapy.
Fig. 1.
Time to death based on intervals between the initiation of antiretroviral therapy (ART) and tuberculosis (TB) treatment.
Discussion
TB is highly prevalent in Asia, accounting for 55% of all global cases in 2008 (16). Our group previously reported TB to be the most common AIDS-defining diagnosis (45%) in TAHOD patients, and that survival after TB was 50% lower than that of patients without an AIDS-defining illness (17). Clinical trial data have shown that the optimal time to begin ART in HIV-TB co-infected patients is earlier than has previously been recommended (10, 18). The CAMELIA trial in Cambodia showed that early ART (within two weeks) improved survival in patients with CD4 T-cell counts lower than 50 cells/µL (18). These results were confirmed by two additional trials: the global STRIDE study and the South African SAPiT trial (11, 19). In these trials, early or immediate ART was also associated with an increased risk of IRIS, but did not result in poorer overall survival. Although the risk-benefit balance of earlier initiation of ART after initiation of TB treatment is not clear for patients with higher CD4 T-cell counts, WHO changed TB-HIV management guidelines on the basis of these robust results.
Our observational study showed that the treatment outcomes and overall mortality of patients with TB initiated on early ART did not significantly differ from those with delayed ART. The lower overall mortality rate in our cohort of 2.35 per 100 person-years and different end points may be possible reasons why we could not see a difference between the groups. Specifically, the rates of death in the CAMELIA trial were 8.28 per 100 person-years in the early ART group, and 13.77 per 100 person-years in the delayed ART group (18). The primary composite end point of the global STRIDE study was new AIDS-defining illness or death, and they proved the benefit of early ART group among patients with CD4 T-cell counts less than 50 cells/µL (11). Due to the small numbers of cases in our cohort within the multiple treatment categories and CD4 strata, we did not perform subgroup analyses breaking down the CD4 T-cell count categories.
Patient data for our study were collected before regional HIV-TB treatment recommendations were changed and reflect how TB management was practically implemented for patients with moderate to severely suppressed immune systems. To our knowledge, this study is the first in our region to compare clinical outcomes of non-trial patients who were diagnosed with TB after ART initiation to those who had TB prior to ART initiation. Interestingly, our data showed that those diagnosed with TB while on ART may have a poorer prognosis than those who had TB prior to ART initiation. High incidence rates of TB have been reported shortly after ART initiation both in developed countries and in resource-limited settings (20). Among 191 individuals who developed TB while on ART in our cohort, 108 (56%) had TB treatment within one year after initiating ART. Incident TB during ART can arise because of residual immunodeficiency, and some cases of previously subclinical disease may manifest because of restoration of TB antigen-specific immune responses. A subset of these cases may have inflammatory symptoms consistent with IRIS (21). While the use of ART decreases the risk of developing TB by 70–90% (22–24), our data reinforce the importance of latent TB screening and treatment and the recommendation to carefully exclude TB prior to ART initiation to avoid unmasking TB and its associated morbidity and mortality (25–27).
It is unclear why patients diagnosed with TB while on ART had a poorer prognosis compared to the other groups with ART after TB treatment. The patients who started ART before TB diagnosis were more likely to have prior AIDS diagnoses, suggesting that they could have had a history of greater immunodeficiency compared to those who started ART after TB diagnosis. Some of those who had previously initiated ART also had first-line ART failure at the time of TB diagnosis, which could result in poorer outcomes than ART-naïve patients starting on a suppressive antiretroviral regimen. TB as unmasking IRIS may have worse prognosis than TB without IRIS or TB with paradoxical IRIS.
The study’s limitations include the observational nature of the data, and the retrospective collection of some of the TB-related variables. Another issue is generalizability to other clinical centers in the region. TAHOD participating sites are generally urban referral centers, and patients are enrolled who are considered likely to remain in long-term follow-up. The site and patient selection criteria consequently prevent broad generalizations, but also offer the opportunity to gather reliable longitudinal data in a non-trial setting. Furthermore, only 40% of TB cases were microbiologically diagnosed, and testing for TB drug susceptibility was not a consistent practice. We arbitrarily defined early ART with a cut-off of 90 days, although previous reports have used different cut-offs such as 14 or 60 days. However, when we compared the outcomes between 60 days and 90 days, the results were similar with regards to the hazard ratios for death (data not shown).
In summary, our data demonstrate that treatment outcomes and overall mortality of HIV-TB co-infected patients who started ART within 90 days of TB treatment did not differ from those of started ART later in this observational cohort of moderately to severely immunosuppressed patients. In addition, overall mortality was highest among patients who were diagnosed with TB while on ART. TB screening efforts prior to ART initiation should be reinforced in HIV care settings in the region. As revised guidelines to initiate ART earlier for co-infected patients are more widely implemented, further studies will be needed to evaluate the broader clinical impact of early ART initiation for patients with TB in Asia.
Acknowledgments
The authors would like to thank participating TAHOD sites, steering committee members (Appendix 1), and the patients who participated in this study. This work was supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS [IeDEA; U01AI069907]. The TREAT Asia HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above. JYC involvement was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2005412)
Appendix 1.
The TREAT Asia HIV Observational Database (TAHOD)
CV Mean, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; PCK Li* and MP Lee, Queen Elizabeth Hospital, Hong Kong, China; N Kumarasamy*, S Saghayam and C Ezhilarasi, YRG Centre for AIDS Research and Education, Chennai, India; S Pujari*, K Joshi and A Makane, Institute of Infectious Diseases, Pune, India; TP Merati*, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; E Yunihastuti*, D Imran and A Widhani, Working Group on AIDS Faculty of Medicine, University of Indonesia/ Cipto Mangunkusumo Hospital, Jakarta, Indonesia; S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; JY Choi*, SH Han and JM Kim, Division of Infectious Diseases, Dept. of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; CKC Lee*, BLH Sim and R David, Hospital Sungai Buloh, Kuala Lumpur, Malaysia; A Kamarulzaman*† and A Kajindran, University of Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines; YMA Chen*, WW Wong and LH Kuo, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; OT Ng*, A Chua, LS Lee and A Loh, Tan Tock Seng Hospital, Singapore; P Phanuphak*, K Ruxrungtham and M Khongphattanayothin, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Kiertiburanakul*‡, S Sungkanuparph and N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; T Sirisanthana*, R Chaiwarith and W Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand; VK Nguyen*, VH Bui and TT Cao, National Hospital for Tropical Diseases, Hanoi, Vietnam; TT Pham*, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam; AH Sohn*, N Durier* and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, J Zhou* and A Jiamsakul, The Kirby Institute, The University of New South Wales, Sydney, Australia.
* TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair.
References
- 1.Reid A, Scano F, Getahun H, et al. Towards universal access to HIV prevention, treatment, care, and support: the role of tuberculosis/HIV collaboration. Lancet Infect Dis. 2006;6:483–495. doi: 10.1016/S1473-3099(06)70549-7. [DOI] [PubMed] [Google Scholar]
- 2.Karim SS. Durban 2000 to Toronto 2006: the evolving challenges in implementing AIDS treatment in Africa. AIDS. 2006;20:N7–N9. doi: 10.1097/01.aids.0000247110.51338.73. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 4.Blanc FX, Havlir DV, Onyebujoh PC, Thim S. Goldfeld AE and Delfraissy JF. Treatment strategies for HIV-infected patients with tuberculosis: ongoing and planned clinical trials. J Infect Dis. 2007;196(Suppl 1):S46–S51. doi: 10.1086/518658. [DOI] [PubMed] [Google Scholar]
- 5.Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984–996. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- 6.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Kwara A, Carter EJ, Rich JD, Flanigan TP. Development of opportunistic infections after diagnosis of active tuberculosis in HIV-infected patients. AIDS Patient Care STDS. 2004;18:341–347. doi: 10.1089/1087291041444069. [DOI] [PubMed] [Google Scholar]
- 8.Breen RA, Smith CJ, Cropley I, Johnson MA, Lipman MC. Does immune reconstitution syndrome promote active tuberculosis in patients receiving highly active antiretroviral therapy? AIDS. 2005;19:1201–1206. doi: 10.1097/01.aids.0000176221.33237.67. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach 2010 version. WHO. 2010 Available at: http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 10.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 14.WHO. Implementing the WHO Stop TB Strategy : a handbook for national TB control programmes. WHO. 2008 Available at: http://www.who.int/tb/publications/2008/en/ [PubMed]
- 15.WHO. WHO handbook for reporting results of cancer treatment. WHO. 1979 Available at: http://whqlibdoc.who.int/offset/WHO_OFFSET_48.pdf.
- 16.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J, Elliott J, Li PC, et al. Risk and prognostic significance of tuberculosis in patients from The TREAT Asia HIV Observational Database. BMC Infect Dis. 2009;9:46. doi: 10.1186/1471-2334-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdool Karim Q, Abdool Karim SS, Baxter C, et al. The SAPIT trial provides essential evidence on risks and benefits of integrated and sequential treatment of HIV and tuberculosis. S Afr Med J. 2010;100:808–809. doi: 10.7196/samj.4621. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Wood R. Incidence of tuberculosis during highly active antiretroviral therapy in high-income and low-income countries. Clin Infect Dis. 2005;41:1783–1786. doi: 10.1086/498308. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 22.Girardi E, Sabin CA, d'Arminio Monforte A, et al. Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 23.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 24.Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One. 2007;2:e826. doi: 10.1371/journal.pone.0000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Improving the diagnosis and treatment smear-negative pulmonary and extrapulmonar tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings. WHO. 2007 Available at: http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf.
- 26.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD000171.pub2. CD000171. [DOI] [PubMed] [Google Scholar]
- 27.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]