Abstract
Recent clinical trials raised concerns regarding the cardiovascular toxicity of selective cyclooxygenase-2 (COX-2) inhibitors and COX-1 is now being reconsidered as a target for chemoprevention. Our aims were to determine whether selective COX-1 inhibition could delay or prevent cancer development and also clarify the underlying mechanisms. Data clearly showed that COX-1 was required for maintenance of malignant characteristics of colon cancer cells or tumor promoter-induced transformation of pre-neoplastic cells. We also successfully applied a ligand docking computational method to identify a novel selective COX-1 inhibitor, 6-C-(E-phenylethenyl)-naringenin (designated herein as 6CEPN). 6CEPN could bind to COX-1 and specifically inhibited its activity both in vitro and ex vivo. In colorectal cancer cells, it potently suppressed anchorage-independent growth by inhibiting COX-1 activity. 6CEPN also effectively suppressed tumor growth in a 28-day colon cancer xenograft model without any obvious systemic toxicity. Taken together, COX-1 plays a critical role in human colorectal carcinogenesis, and this specific COX-1 inhibitor merits further investigation as a potential preventive agent against colorectal cancer.
Keywords: colon cancer, natural product, cyclooxygenases, signal transduction
Introduction
Colorectal cancer is the third most common non-cutaneous malignancy and also the third leading cause of cancer-related death in the United States (1). Fortunately, during colorectal carcinogenesis, the transition from normal mucosa to adenoma and final carcinoma is a protracted event that offers opportunities for preventive interventions. Chemoprevention by targeting cyclooxygenase-2 (COX-2) has been used successfully and appears to be a promising strategy for prevention of colorectal cancer (2–4). However, recent clinical trial studies raised concerns regarding the cardiovascular toxicity of selective COX-2 inhibitors (5–6). Animal model studies further revealed that COX-2 plays a crucial role in cardio-protection (7–10), and thus persistent COX-2 inhibition might not be an ideal chemopreventive strategy.
Although evidence implicates a crucial role of COX-2 in colorectal cancer development, the idea that COX-2 is the only COX isoform involved in carcinogenesis has been challenged. For example, aspirin at low doses (81 mg per day) is widely accepted to be able to provide both cardioprotective and colon cancer chemopreventive effects (11–13). However, pharmacokinetic data analysis revealed that low doses of aspirin mainly target COX-1 rather than COX-2 (14–15). Genetic disruption of ptgs-1 (gene encoding for cyclooxygenase-1 (COX-1)) or ptgs-2 (gene encoding for COX-2) reduces intestinal polyposis to a similar extent (16–17). Importantly, targeting COX-1 was effective in preventing not only colon cancer but also other tumor types such as skin cancer and ovarian cancer (17–18). Deficiency of COX-1 reduced mouse skin tumorigenesis by 75%, whereas a COX-1 inhibitor (SC560) effectively attenuated epithelial ovarian tumor growth in multiple genetically engineered mouse models.
Thus, COX-1 is now being reconsidered as a target for chemoprevention (19–20). To gain a deeper insight into the role of COX-1 in human cancer development, we used a ligand docking computational method to identify a novel selective COX-1 inhibitor, 6-C-(E-phenylethenyl)- naringenin (i.e., 6CEPN). We then evaluated its chemopreventive activity against colon cancer both in vitro and in vivo.
Materials and Methods
Reagents and chemicals
6CEPN was chemically synthesized as described previously (21). CNBr-Sepharose 4B beads were purchased from Amersham Pharmacia Biotech (Peapack, NJ). All primary antibodies were purchased from Cell Signaling Technology (Beverley, MA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Cell culture
All cell lines used in this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained following ATTC instructions. Cells were cytogenetically tested and authenticated before being frozen. Each vial of frozen cells was thawed and maintained for a maximum of 20 passages.
Cell transfection
For either transient or lentiviral transfection in adherent cells, the jetPEI reagent (Qbiogen, Inc., Montreal, Quebec, Canada) was used following the manufacturer’s instructions. Full-length cDNAs for COX-1 and COX-2 (pCMV-SPORT6-COX-1 and pCMV-SPORT6-COX-2) and the 29-mer small hairpin RNA (shRNA) constructs against COX-1 and COX-2 were from Open Biosystems, Inc. (Huntsville, AL).
Cell growth assay
Cells were seeded (1×103 cells per well) in 96-well plates. After incubation for various times, 20 μL of CellTiter96 Aqueous One Solution (Invitrogen, Carlsbad, CA) were added. Cells were further incubated for 1 h at 37°C. Finally, the optical density was determined at 492 nm.
Anchorage-independent cell growth
In each well of a 6-well plate, cells (8×103) were suspended in Basal Medium Eagle (BME) medium (1 mL, with 10% FBS and 0.33% agar) and plated over a layer of solidified BME (3 mL, with 10% FBS and 0.5% agar). The cultures were incubated in a 37°C, 5% CO2 incubator for 7 d and colonies in soft agar were counted under a microscope equipped with the Image-Pro Plus software program (vs. 6, Media Cybernetics, Bethesda, MD).
In vitro pull-down assay
Recombinant COX-1 and COX-2 (0.5 μg) or endogenous cell lysates (500 μg) were incubated with 6CEPN-Sepharose 4B beads (100 μL, 50% slurry) in reaction buffer (50 mM Tris pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM DTT, 0.01% NP40, 2 μg/mL bovine serum albumin, 0.02 mM phenylmethysulfonyl fluoride (PMSF), 1× protease inhibitor mixture). Incubation with gentle rocking was performed overnight at 4°C. The beads were then washed a total of 5 times with washing buffer and proteins bound to the beads were analyzed by Western blotting.
In vitro COX enzyme assay
The effect of 6CEPN on COX activity was evaluated using a COX Inhibitor Screening Kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instructions.
Prostaglandin E2 (PGE2), thromboxane B2 (TXB2) and 6-keto prostaglandin F1α (6-keto PGF1α) determination
The measurement of PGE2 in the cell culture medium and TXB2 in mouse serum was performed using enzyme immunoassay kits from Cayman Chemical Company. In brief, cells (6×105) were plated in a 6-well plate with 10% serum. When cells reached 80% confluency, 1 mL fresh medium with 6CEPN or vehicle was added and cells were further incubated for 24 h. Supernatant fractions were collected for prostaglandin measurement according to the manufacturer’s instructions.
Western blot analysis
Protein samples (20 μg) were resolved by SDS-PAGE and transferred to Hybond C nitrocellulose membranes (Amersham Corporation, Arlington Heights, IL). After blocking, the membranes were probed with primary antibodies (1:1000) overnight at 4°C. The targeted protein bands were visualized using an enhanced chemiluminescence reagent (Amersham Corporation) after hybridization with a secondary antibody conjugated with horseradish peroxidase.
Xenograft mouse model
Athymic mice [Cr: NIH(S), NIH Swiss nude, 6–9 wk old] were obtained from Charles River and maintained under “specific pathogen-free” conditions based on the guidelines established by the University of Minnesota Institutional Animal Care and Use Committee. Mice were divided into 4 groups (n = 6 in each group). HT29 colon cancer cells (2×106 cells/100 μl) were suspended in serum free McCoy’s 5A medium and injected subcutaneously into the right flank of each mouse. 6CEPN dissolved in 5% (v/v) dimethyl sulfoxide (DMSO)/PEG400 was given to the mice by gavage every other day for total of 6 wk. Tumor volume and body weight were measured every other day. Venous blood was collected from mice post mortem by suctioning from the right ventricle using a syringe containing sodium citrate. Blood samples were then centrifuged at 2000 × g for 15 min, and the resulting supernatant fraction was designated as serum.
Molecular modeling
The 3-D structures of COX-1 and COX-2 were directly downloaded from the Protein Data Bank (PDB) for docking studies. COX-1 (PDB code 3KK6) is an X-ray diffraction structure with a resolution of 2.75 Å and COX-2 (PDB code 1PXX) is an X-ray diffraction structure with a resolution of 2.9 Å. The proteins were prepared for docking following the standard procedure outlined with the Protein Preparation Wizard in Schrödinger Suite 2012. All crystallographic waters were deleted and a 30-Å grid was generated on both COX-1 and COX-2 active sites to define the protein receptor for docking following the standard procedure outlined in Schrödinger’s GLIDE docking package. The Zinc natural database, FDA approved drug database, Traditional Chinese Medicine (TCMD), a flavonoid compound database, and our in-house library of compounds were each used in the virtual screening.
Statistical analysis
All cell line experiments were performed independently at least 3 times. Statistical analysis was performed using the Prism statistical package (Irvin, CA). Turkey’s t-test was used to compare data between 2 groups. One-way ANOVA and the Bonferroni correction were used to compare data between 3 or more groups. Values are expressed as means ± S. E. M. unless otherwise indicated. A p value of < 0.05 was considered statistically significant.
Results
COX-1 is required for maintaining colorectal cancer cell malignant characteristics
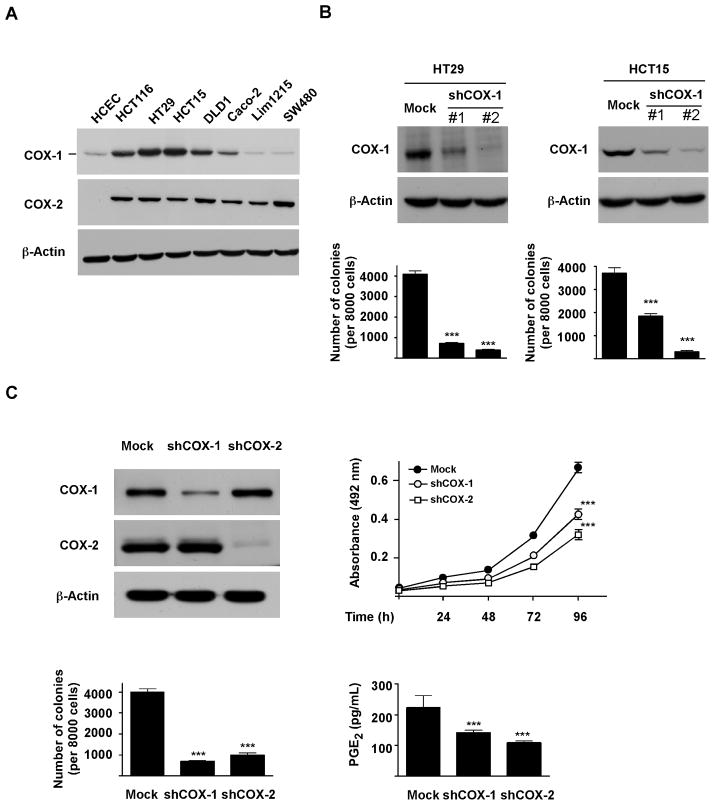
To determine whether COX-1 is directly associated with the tumorigenic properties of colon cancer cells, we first examined COX expression in normal and colon cancer cells (Fig. 1A). Consistent with previous reports (22–23), COX-1 is present both in normal and malignant colon cancer cells, whereas COX-2 is overexpressed only in colorectal cancer cells. Among the cell lines tested, HT29, HCT115 and DLD1 cells expressed relatively higher COX-1 levels, and were therefore chosen for subsequent studies.
Figure 1.
COX-1 is required for maintenance of malignant characteristics of colorectal cancer cells. A, Western bolt analysis of COX-1 and COX-2 expression in human colorectal cancer and normal colon epithelial cells (HCEC). B, COX-1 is required for anchorage-independent growth of colorectal cancer cells. C, COX-1 and COX-2 are equally important for tumorigenic properties in human colorectal cancer cells. Knockdown of COX-1 or COX-2 in colon cancer cells was analyzed by Western blot. Mock and knockdown cells were then subjected to anchorage-dependent growth, anchorage-independent growth and PGE2 production assays as described in “Materials and Methods”. Cell growth was evaluated by MTS assay. Data are presented as means ± S.E.M. (n = 4). Anchorage-independent cell growth was evaluated by colony formation in soft-agar. Data are presented as means ± S.E.M. from 3 independent experiments. Production of PGE2 in supernatant fractions was measured by ELISA. Data are presented as means ± S.E.M. (n = 4). The asterisks (***) indicate a significant (p < 0.001) difference compared to Mock group.
Anchorage-independent growth ability is an ex vivo indicator and a key characteristic of the transformed cell phenotype (24). We thus questioned whether COX-1 inhibition would affect colon cancer cell growth under anchorage-independent conditions. Our results revealed that knocking down COX-1 expression in HT29 and HCT115 cells significantly decreased the number of colonies formed in soft agar compared with Mock-control cells (Fig. 1B).
We also compared the function of COX-1 with COX-2 in human colon cancer cells. Our results revealed that knocking down the expression of either COX-1 or COX-2 delayed cell growth, reduced the number of colonies formed in soft agar, and decreased PGE2 production in HT29 cells (Fig. 1C). Consistent with our findings, a previous animal study also indicated that both COX-1 and COX-2 contribute to PGE2 production in polyp formation (16).
Involvement of COX-1 in neoplastic transformation
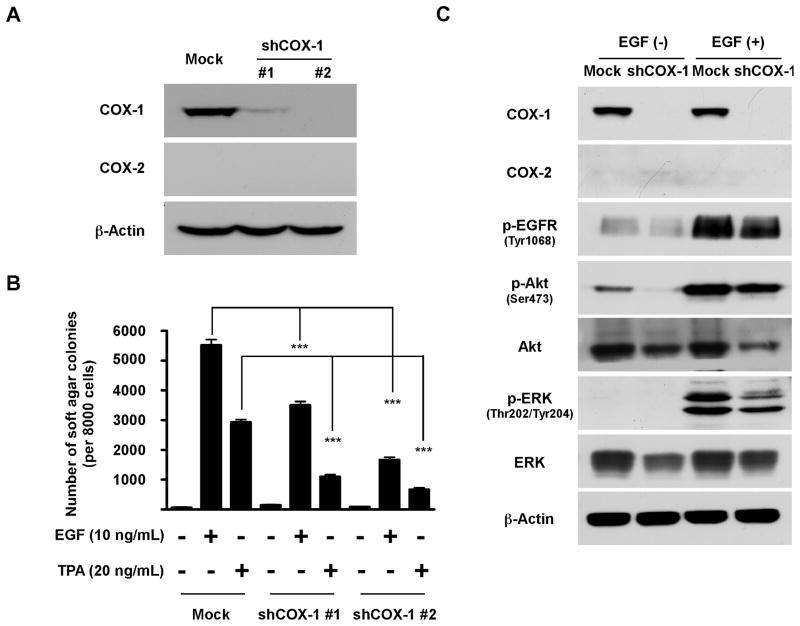
Based on the finding showing that knockdown of COX-1 greatly abrogated anchorage-independent cell growth, we hypothesized that COX-1 might also be involved in neoplastic transformation. The JB6 CI41 cell model is a promotion sensitive (P+) mouse epidermal skin cell line that provides a unique cell model to characterize the role of COX-1 in preneoplastic cells (25–26). We established two stable JB6 CI41 clones that express an shRNA targeting mouse COX-1 (Fig. 2A), and then tested the effects of COX-1 inhibition on tumor promoter (TPA or EGF)-induced cell transformation. Results indicated that either EGF- or TPA-induced cell transformation was markedly attenuated by knockdown of COX-1 (Fig. 2B). Additional results indicated that with EGF stimulation, EGFR downstream signaling cascades were substantially suppressed in the absence of COX-1 (Fig. 2C).
Figure 2.
COX-1 is involved in neoplastic transformation. A, knockdown of COX-1 in JB6 CI41 cells was analyzed by Western blot. B, EGF- or TPA-induced cell transformation is dramatically reduced by COX-1 knockdown. JB6 cells were grown in soft agar in the absence or presence of EGF (10 ng/mL) or TPA (20 ng/mL) and colonies were counted as described in “Materials and Methods”. Data are presented as means ± S.E.M. from 3 independent experiments. The asterisks (***) indicate a significant (p < 0.001) difference compared to Mock group. C, knockdown of COX-1 partially blocks EGFR signal transduction in EGF-induced cell transformation. After starvation for 24 h, JB6 cells were stimulated with EGF (10 ng/mL) for 15 min. Cell lysates were subjected to Western blot analysis.
Taken together, these findings indicated that COX-1 is required for both the maintenance of cancer cell malignant characteristics and neoplastic transformation.
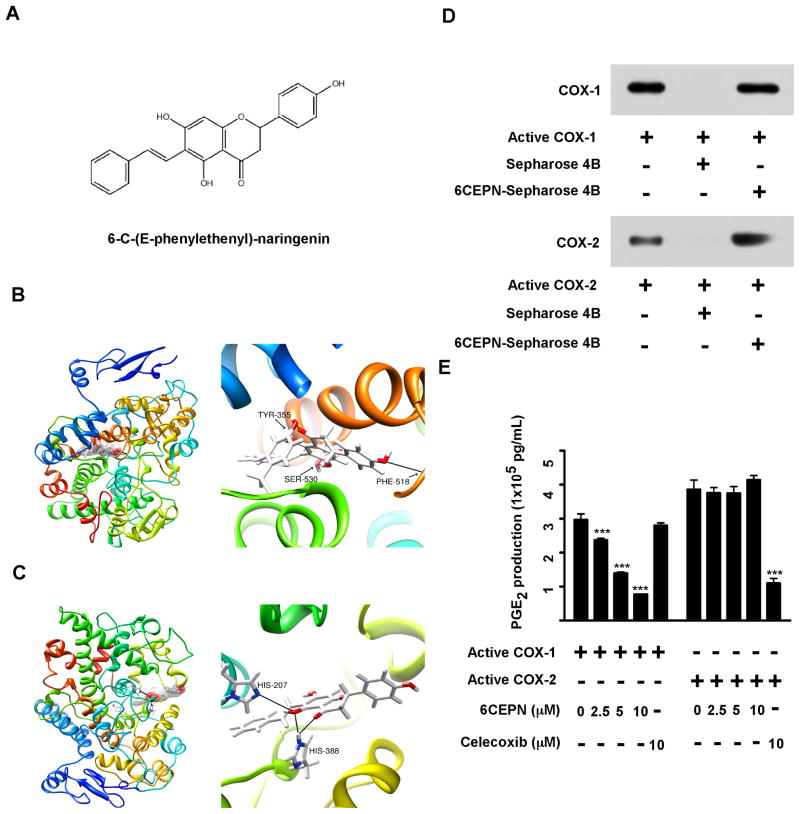
The predicted binding mode of 6-C-(E-phenylethenyl)-naringenin with COX-1
Although COX-1 is now being reconsidered as a target for colorectal cancer chemoprevention, only a few selective COX-1 inhibitors have been found (19). To identify a novel potent selective COX-1 inhibitor, we conducted an intensive molecular docking analysis using Glide v5.7 (16). We screened several libraries of compounds and 6-C-(E-phenylethenyl)-naringenin (6CEPN; Fig. 3A) was identified as a potential selective COX-1 inhibitor based on its docking scores against COX-1 and -2, respectively. Our computational modeling data clearly showed that 6CEPN could only bind to the COX-1 active site by forming 3 hydrogen bonds with Tyr355, Phe518 and Ser530 (Fig. 3B). This compound failed to bind to the COX-2 catalytic pocket due to a potential steric-hindrance effect–a phenomenon in which the enzyme is inaccessible to substrates with an improper molecular size as well as shape (Fig. 3C, left panel). However, even though 6CEPN failed to occupy the active pocket of COX-2, it still might bind to COX-2 in another region (i.e., His 207 and His388) (Fig. 3C, right panel).
Figure 3.
6CEPN is identified as a novel selective COX-1 inhibitor. A, chemical structure of 6CEPN. B, proposed molecular model of 6CEPN binding with COX-1. 6CEPN binds to the active site of COX-1 by forming 3 hydrogen bonds with Tyr355, Phe518 and Ser530. C, proposed molecular model of 6CEPN binding with COX-2. 6CEPN failed to occupy the active pocket of COX-2 (left panel) but might bind to COX-2 in another region (i.e., His207 and His388). D, 6CEPN binds with COX-1 and COX-2 in vitro. A pull-down assay was performed using recombinant COX-1 and COX-2 proteins. Proteins bound to the beads were analyzed by Western blotting. E, 6CEPN specifically inhibits COX-1 activity in vitro. The inhibitory activity of 6CEPN was evaluated using a COX Inhibitor Screening Kit (Cayman) according to the manufacturer’s instructions. Data are presented as means ± S.E.M. (n = 4). The asterisks (***) indicate a significant (p < 0.001) difference compared to each respective control group.
To validate the computational prediction, we performed an in vitro pull-down assay using 6CEPN-conjugated Sepharose 4B beads (Fig. 3D). Results revealed that both recombinant COX-1 and COX-2 bind with 6CEPN-Sepharose 4B beads, but not with Sepharose 4B beads alone in vitro. We then examined the potential inhibitory effect of 6CEPN against COX-1 and COX-2 enzyme activity using a COX inhibitor screening assay kit. Our data confirmed that 6CEPN selectively inhibited COX-1, but not COX-2, activity in vitro (Fig. 3E). These results clearly support our hypothesis that 6CEPN is a selective COX-1 inhibitor.
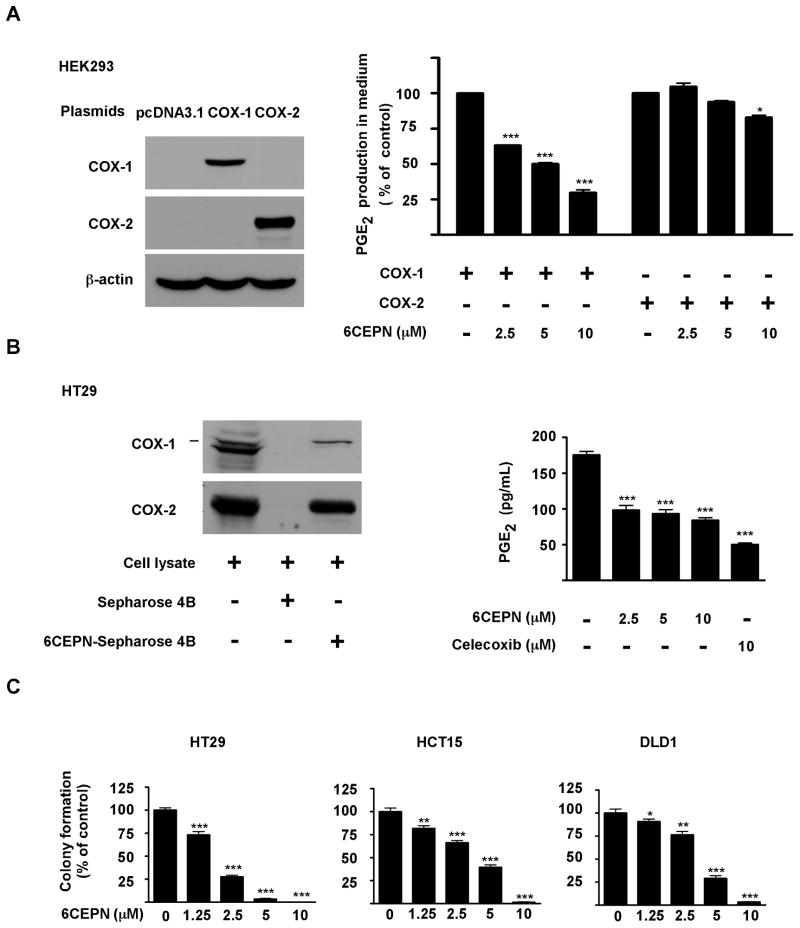
6CEPN suppresses human colorectal cancer cell growth
Next, we determined whether 6CEPN could selectively inhibit COX-1 activity in cells. Human embryonic kidney HEK293T cells were transiently transfected with a COX-1 or COX-2 plasmid (Fig. 4A, left panels) and then treated with 6CEPN. Data regarding PGE2 release in supernatant fractions clearly indicated that 6CEPN selectively inhibited COX-1 activity rather than COX-2, especially at low doses (Fig. 4A, right panel). We also confirmed that 6CEPN binds to endogenous COX-1 (Fig. 4B, left panels) and lowers PGE2 production in colon cancer cells (Fig. 4B, right panel). Moreover, 6CEPN potently inhibited anchorage-independent growth in a dose-dependent manner (Fig. 4C) in 3 colon cancer cell lines. 6CEPN at 2.5 or 5μM caused a decrease of more than 70–90% compared with untreated control HT29 cells, which highly express COX-1.
Figure 4.
6CEPN targets COX-1 ex vivo. A, 6CEPN specifically inhibits COX-1 activity in HEK293T cells. The effector plasmids (COX-1 and COX-2) and control plasmid (pcDNA3.1) were transiently transfected into HEK293 cells using jetPEI reagent (Qbiogen) following the manufacturer’s instructions. After 24 h, transfection reagents were removed. Cells were then incubated with 6CEPN for 24 h, and supernatant fractions were collected for PGE2 measurement using an enzyme immunoassay kit (Cayman). Data are presented as means ± S.E.M. (n = 4). The asterisks indicate a significant (*, p < 0.05; ***, p < 0.001) difference compared to each respective control group. B, 6CEPN targets COX-1 in HT29 cells. COX-1 proteins in HT29 cell lysates were pulled down and analyzed by Western blotting (left panels). 6CEPN inhibits PGE2 production in HT29 cells (right panels). Production of PGE2 was measured by ELISA. Data are presented as means ± S.E.M. (n = 4). The asterisks (***) indicate a significant (p < 0.001) difference compared to control group. C, 6CEPN inhibits anchorage-independent growth of human colorectal cancer cells. HT29, HCT15 or DLD1 cells were grown in soft agar for 7 d and colonies counted as described in “Materials and Methods”. Data are presented as means ± S.E.M. from 3 independent experiments. The asterisks indicate a significant (**, p < 0.01; **, p < 0.01) difference compared to each respective control group.
Evaluation of cardiovascular toxicity of 6CEPN
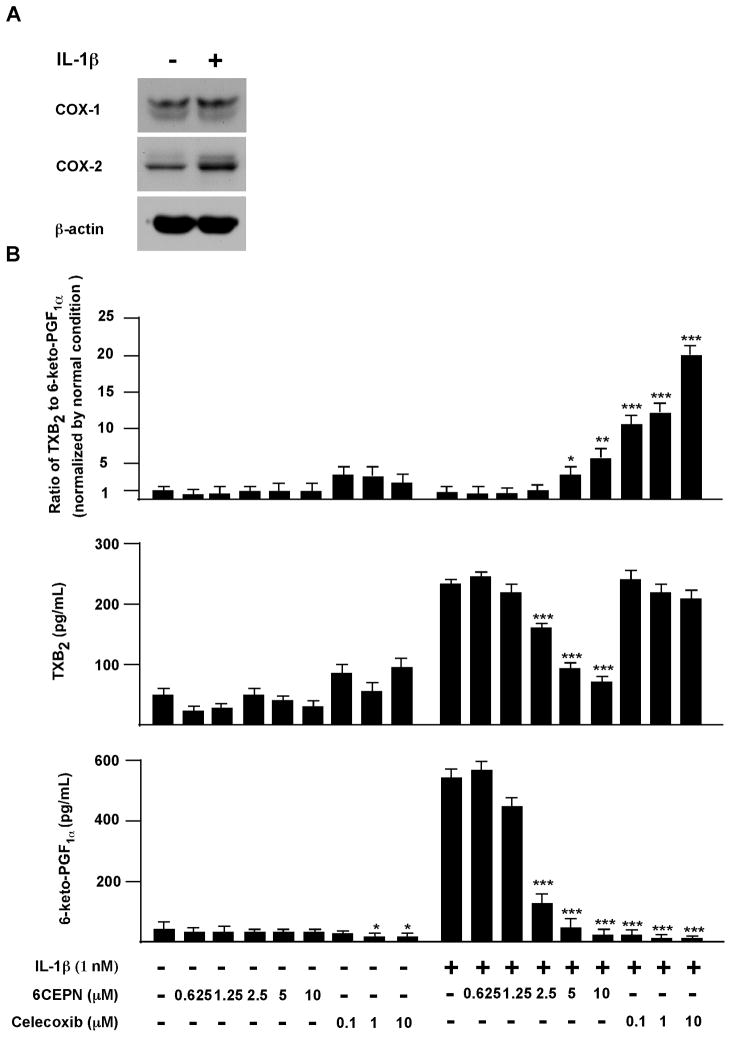
Next, we evaluated the potential cardiovascular toxicity of 6CEPN in an in vitro model using human umbilical vein endothelial cells (HUVECs). Although undetectable under normal physiological conditions, COX-2 is markedly induced and exerts a cardiac protective role under pathophysiological conditions such as during cardiac ischemia or reperfusion injury. The imbalance between COX-1-derived pro-thrombotic thromboxane A2 (TXA2) and COX-2-relateded antithrombotic prostacyclin (PGI2) production is suspected to contribute to the cardiovascular side effects of selective COX-2 inhibitors. Additionally, the ratio of TXB2 (i.e., the stable breakdown product of TXA2) to 6-keto-PGF1α (i.e., the hydrolysis product of PGI2) has been used as one of the biomarkers for COX-2 inhibition-related cardiovascular toxicity (7, 9–10, 19–20, 27–28). To mimic pro-inflammatory conditions, HUVECs were treated with IL-1β, an inflammatory cytokine implicated in vascular diseases. IL-1β stimulation resulted in a remarkable increase in COX-2 expression as well as 6-keto-PGF1α synthesis (Fig. 5A). 6CPEN, but not celecoxib, had a modest but significant inhibitory effect on TXB2 synthesis (Fig. 5B, middle panels). In contrast, celecoxib, but not 6CPEN, could potently suppress 6-keto-PGF1α synthesis (Fig. 5B, lower panels). More importantly, the ratio of TXB2/6-keto-PGF1α was significantly (p < 0.001) increased by the selective COX-2 inhibitor celecoxib even at very low doses (e.g., 0.1 μM), but only weakly disturbed by 6CEPN (Fig. 5B, upper panels), suggesting that cardiovascular toxicity is caused by celecoxib but not by 6CEPN.
Figure 5.
Effect of 6CEPN on human umbilical vein endothelial cells. A, Western blot analysis of COX-1 and COX-2 protein expression in human umbilical vein endothelial cells. To simulate pro-inflammatory conditions observed in many vascular diseases, cells were incubated with 1 nM IL-1β for 8 h. B, effects of 6CEPN treatment on the ratio of TXB2 to 6-keto-PGF1α. HUVECs were seeded in a six-well-plate (6×105 cells per well). At 70–80% confluence, cells were pretreated with 1 mL fresh medium containing DMSO or compounds for 2 h and then IL-1β (1 nM) was added together with compounds for another 8 h incubation. Supernatant fractions were collected for prostaglandin measurement. Data are presented as means ± S.E.M. (n = 4). The asterisks indicate a significant (*, p < 0.05; **, p < 0.01; ***, p < 0.001) difference compared to each respective control group.
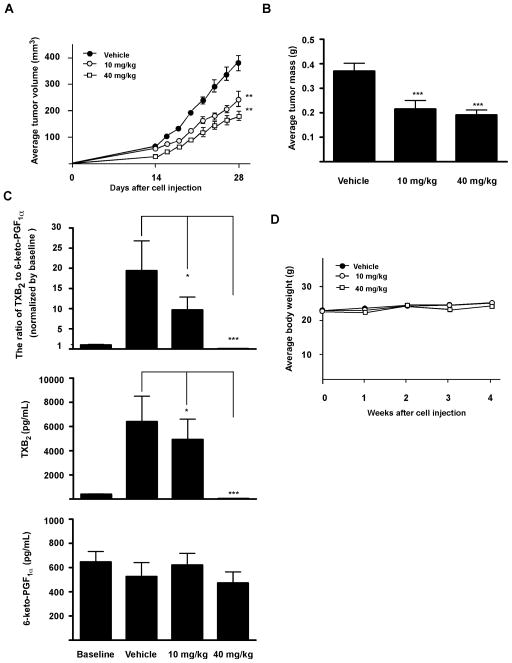
6CEPN suppresses tumor growth by inhibiting COX-1 activity in vivo
Based on both in vitro and ex vivo data, we determined whether 6CEPN could suppress tumor growth in vivo. Results (Fig. 6A) indicated that 28 days of continuous 6CEPN treatment (10 or 40 mg/kg body weight) by gavage significantly reduced tumor volume by 31% or 59%, respectively. Similar inhibitory effects were observed on final tumor mass (Fig. 6B). Classic COX-1 inhibitors such as aspirin and ibuprofen are known exert their cardio-protective activity by inhibiting platelet COX-1 activity, resulting in decreased synthesis of TXA2, but not PGI2 (13, 19, 27–28). Similar results were obtained with 6CEPN treatment in this study (Fig. 6C). Importantly, the ratio of TXB2 to 6-keto-PGF1α was greatly attenuated by continuous 6CEPN treatment, suggesting that 6CEPN might provide cardio-protective effects. Moreover, based on body weight data (Fig. 6D), general appearance and organ histology, 6CEPN was well-tolerated in mice and no obvious systemic toxicity (e.g., diarrhea or bleeding in the digestive tract) was observed during the entire period of drug treatment.
Figure 6.
Chemopreventive activity of 6CEPN in a HT29 colon cancer xenograft model. A, effect of 6CEPN on tumor growth. B, effect of 6CEPN on tumor mass. C, effect of continuous 6CEPN treatment on the ratio of TXB2 to 6-keto-PGF1α. D, effect of 6CEPN on body weight of mice. Chemopreventive activity of 6CEPN was evaluated in a 28-day colon cancer xenograft model. Before cell injection, mice were pretreated with 6CEPN for 14 days. 6CEPN dissolved in 5% (v/v) dimethyl sulfoxide (DMSO)/PEG400 was given to the mice by gavage every other day for a total of 42 days. Data are presented as means ± S.E.M. (n = 12 mice). The asterisks indicate a significant (*, p < 0.05; ***, p < 0.001) difference compared to vehicle control group.
Discussion
In this study, we confirmed a critical role for COX-1 in colorectal cancer. Phenotypically, COX-1 knockdown or catalytic inactivation in colon cancer cells resulted in an obvious reduction of malignant characteristics, including anchorage-dependent and -independent cell growth as well as in the production of endogenous PGE2. Importantly, COX-1 was also required for genotoxic carcinogen-induced malignant transformation in pre-neoplastic cells. All of these findings provided an explanation as to why genetic disruption of ptgs-1 reduces cancer incidence both in skin and colon.
Although more attention has been given to COX-2 as a key player in the development of various cancers, accumulating evidence indicates that COX-1 is equally as important as COX-2 for carcinogenesis, especially in skin and colon (16–17). The immediate-early phase of prostaglandin production is reportedly mediated by constitutive expression of COX-1, whereas the later phase of prostaglandin production is dependent on the induction of COX-2 (29). On the other hand, PGE2 can transactivate the EGFR kinase cascade in colon cancer cells, which is dependent on the extracellular release of an EGF-like ligand (30), while activation of EGFR might conversely stimulate COX-2 biosynthesis (31). Overall, our data suggest the possibility that both COX-1 and COX-2 contribute to colon cancer development by cooperating with the EGFR signaling pathway, which modulates tumorigenesis through multiple biological effects including anchorage independent cell growth (32). Therefore, although COX-1 is generally described as constitutively expressed both in malignant and normal colon tissues, this might be an oversimplification because the COX enzymes are known to function in the production of prostaglandins, which is either due to increased protein expression, catalytic activity, or both (27).
In the present study, we successfully applied a ligand docking computational method and discovered a novel selective COX-1 inhibitor (6CEPN). Notably, 6CEPN, chemically appears to be a natural product-based compound, a hybrid molecule of stilbene with a flavonoid structure. Compared with other known COX-1 inhibitors, it has a unique carbon skeleton that might present a new leading COX-1 inhibitor (19, 27, 33). To identify compounds that show higher selective COX-1 inhibition, more structure-activity studies are needed.
Another question to be addressed is whether selective COX-1 inhibition causes gastrointestinal toxicity. Conventional NSAIDs are known to normally produce much stronger gastrointestinal toxicity than selective COX-2 inhibitors. Considering its high expression in the gastrointestinal tract, COX-1 is commonly believed to protect the gastrointestinal tract, and thus selective COX-1 inhibition is still a controversial issue (19–20, 27). However, no direct evidence exists to support selective inhibition of COX-1 as the cause of gastrointestinal side effects. Notably, homozygous ptgs-1 (genes coding for COX-1) mutant mice do not exhibit gastric lesions even though their PGE2 production in the gastrointestinal tract is just 1% that of wildtype mice (16). Pharmacological inhibition of COX further suggested that inhibition of both COX-1 and COX-2 was required for NSAIDs-induced gastrointestinal toxicity. In the Wistar rat, neither a selective COX-1 inhibitor (SC560) nor a COX-2 inhibitor (celecoxib) could cause obvious gastric damage. However, their combination results in severe gastrointestinal side effects (34). In the present study, our COX-1 inhibitor, 6CEPN, was also well tolerated in mice, and no obvious GI toxicity (e.g., diarrhea and bleeding in digestive tract) was observed during the entire period of drug treatment.
Although our findings in this study are promising, several significant questions remain unanswered. For example, whether the inhibitory potential of 6CEPN in vivo was due to a direct suppression of COX-1 activity (not COX-2) is still unclear. Addressing this question is challenging with current technologies because COX-1 and COX-2 normally share the same substrate (arachidonic acid) and yield the same product (PGH2) in vivo. Thus, differentiating between their respective activities is not possible at this time. Another issue is the relevance of the JB6 cell model to colon carcinogenesis. JB6 Cl41 cells are a promotion sensitive (P+) mouse epidermal cell line. This cell line enables the study of genetic susceptibility to promotion of transformation, and thus might provide a unique cell model to characterize activated COX-1 in pre-neoplastic cells. TXA2 production in vivo is known to be associated with COX-1. 6CEPN could markedly lower the serum levels of TXA2 in mice. However, we did not collect pharmacokinetic data in this study, and therefore whether 6CEPN reached tumors directly or only inhibited COX-1 activity pharmacologically is still unknown.
In summary, this study was conducted to characterize the role of COX-1 in colorectal cancer and evaluate the clinical potential of a selective COX-1 inhibitor in chemoprevention. The present work together with previous studies by others should provide insight into the potential application of selective COX-1 inhibitors in colorectal cancer chemoprevention (16–17, 19, 34–35).
Acknowledgments
Grant Support:
This work was supported by The Hormel Foundation and National Institutes of Health grants R37 CA081064 and ES016548 and NCI Contract Numbers HHSN-261200533001C-NO1-CN-53301 and N01-CN-43309-18018-01WA 13B.
Footnotes
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed.
Authors’ Contributions
Zigang Dong, Feng Zhu and Ronald A. Lubet designed and supervised the experiments; Hanyong Chen performed computational studies; Ka Wing Cheng and Mingfu Wang prepared candidate chemicals; Haitao Li, Tatyana Zykova and Naomi Oi performed experiments and analyzed data; Haitao Li and Ann M. Bode prepared the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. The New England journal of medicine. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 3.Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, et al. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer research. 2001;61:1733–40. [PubMed] [Google Scholar]
- 4.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. The New England journal of medicine. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 5.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England journal of medicine. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. The New England journal of medicine. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 7.Camitta MG, Gabel SA, Chulada P, Bradbury JA, Langenbach R, Zeldin DC, et al. Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia/reperfusion injury but are protected by acute preconditioning. Circulation. 2001;104:2453–8. doi: 10.1161/hc4401.098429. [DOI] [PubMed] [Google Scholar]
- 8.Inserte J, Molla B, Aguilar R, Traves PG, Barba I, Martin-Sanz P, et al. Constitutive COX-2 activity in cardiomyocytes confers permanent cardioprotection Constitutive COX-2 expression and cardioprotection. Journal of molecular and cellular cardiology. 2009;46:160–8. doi: 10.1016/j.yjmcc.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10197–202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, et al. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7548–52. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998;114:873–7. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. The New England journal of medicine. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. The New England journal of medicine. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 14.Gray PA, Warner TD, Vojnovic I, Del Soldato P, Parikh A, Scadding GK, et al. Effects of non-steroidal anti-inflammatory drugs on cyclo-oxygenase and lipoxygenase activity in whole blood from aspirin-sensitive asthmatics vs healthy donors. British journal of pharmacology. 2002;137:1031–8. doi: 10.1038/sj.bjp.0704927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. The New England journal of medicine. 1984;311:1206–11. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 16.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer research. 2000;60:4705–8. [PubMed] [Google Scholar]
- 17.Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer research. 2002;62:3395–401. [PubMed] [Google Scholar]
- 18.Daikoku T, Wang D, Tranguch S, Morrow JD, Orsulic S, DuBois RN, et al. Cyclooxygenase-1 is a potential target for prevention and treatment of ovarian epithelial cancer. Cancer research. 2005;65:3735–44. doi: 10.1158/0008-5472.CAN-04-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrone MG, Scilimati A, Simone L, Vitale P. Selective COX-1 inhibition: A therapeutic target to be reconsidered. Current medicinal chemistry. 2010;17:3769–805. doi: 10.2174/092986710793205408. [DOI] [PubMed] [Google Scholar]
- 20.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728–35. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng KW, Wong CC, Cho CK, Chu IK, Sze KH, Lo C, et al. Trapping of phenylacetaldehyde as a key mechanism responsible for naringenin’s inhibitory activity in mutagenic 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine formation. Chemical research in toxicology. 2008;21:2026–34. doi: 10.1021/tx800220h. [DOI] [PubMed] [Google Scholar]
- 22.Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer research. 2004;64:3694–700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 23.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer research. 1995;55:3785–9. [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:609–13. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Huang C, Brown RE, Ma WY. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997;272:9962–70. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. Journal of medicinal chemistry. 2007;50:1425–41. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- 28.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:272–7. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy ST, Tiano HF, Langenbach R, Morham SG, Herschman HR. Genetic evidence for distinct roles of COX-1 and COX-2 in the immediate and delayed phases of prostaglandin synthesis in mast cells. Biochemical and biophysical research communications. 1999;265:205–10. doi: 10.1006/bbrc.1999.1658. [DOI] [PubMed] [Google Scholar]
- 30.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nature medicine. 2002;8:289–93. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 31.Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:657–62. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humtsoe JO, Kramer RH. Differential epidermal growth factor receptor signaling regulates anchorage-independent growth by modulation of the PI3K/AKT pathway. Oncogene. 2010;29:1214–26. doi: 10.1038/onc.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jachak SM. Cyclooxygenase inhibitory natural products: current status. Current medicinal chemistry. 2006;13:659–78. doi: 10.2174/092986706776055698. [DOI] [PubMed] [Google Scholar]
- 34.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–14. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 35.Takeda H, Sonoshita M, Oshima H, Sugihara K, Chulada PC, Langenbach R, et al. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer research. 2003;63:4872–7. [PubMed] [Google Scholar]