Abstract
Objective
Capillary integrity continues to challenge critical care physicians worldwide when treating children with sepsis. Vascular growth factors, specifically angiopoietin (angpt)-1 and angpt-2, play opposing roles in capillary stabilization in septic patients, respectively. We aim to determine whether pediatric patients with severe sepsis/shock have persistently high angpt-2/1 ratios when compared to non-septic pediatric intensive care unit (PICU) patients over a 7-day period.
Design
Prospective, observational study. Patients were classified within 24h of admission into: non-systemic inflammatory response syndrome (non-SIRS), SIRS/sepsis, or severe sepsis/shock. Plasma levels of angpt-1 and angpt-2 were measured via ELISA. The angpt-2/1 ratio was graphically plotted and determined whether patients fell into ‘constant’ or ‘variable’ patterns.
Setting
Tertiary care center PICU.
Patients
Critically ill pediatric patients with varying sepsis severity.
Interventions
None
Measurements and Main Results
Forty five patients were enrolled (n=9 non-SIRS, n=19 SIRS/sepsis, and n=17 severe sepsis/shock). Gender, age, weight, comorbidities and PICU length of stay were not significantly different between the groups. Admission pediatric risk stratification scores and net fluid ins/outs were significantly elevated in the severe sepsis/shock group when compared (all p<0.05). Admission angpt-2 levels and angpt-2/1 ratios were significantly different in the severe sepsis/shock group when all groups were compared (both p<0.05). Additionally, the latter were significantly elevated in the severe sepsis/shock group at multiple time points (all p≤0.05) with the peak occurring on day 2 of illness. In a separate analysis, 32% of SIRS/sepsis and 82% of severe sepsis/shock had ‘variable’ angpt-2/1 ratio patterns compared to none in the control group (p<0.001).
Conclusions
Pediatric patients with severe sepsis and septic shock possess significantly elevated angpt-2/1 ratios during their first 3 days of illness which peak at day 2 of illness. A subset of these patients demonstrated ‘variable’ angpt-2/1 ratio patterns.
Keywords: Pediatric, capillary leak, shock, intensive care
INTRODUCTION
Nationally, sepsis remains one of the principal causes of pediatric mortality (1, 2) and despite advances in modern medicine, about 6 out of every 100,000 children will die from severe sepsis each year (3). Severe sepsis and septic shock, defined as sepsis plus organ failure, results from many different etiologies and is a significant health problem as illustrated by the increasing numbers of septic patients admitted to hospitals in the United States annually (4). Pediatric patients who develop organ dysfunction have increased morbidity and mortality when compared to those who do not (3, 5). Owing to a variety of different causes, there have been very few new therapies developed to combat severe sepsis.
In addition to upregulation of the host’s inflammatory response, another unifying characteristic between all septic etiologies and profound morbidity or death is endothelial activation (6). Capillary beds are highly complex structures that regulate blood flow through organs and allow for gas and nutrient exchange between blood and tissues. Endothelial cells are the principal cells of the microcirculation and are important components of our innate immunity. Upon activation, these cells can up-regulate leukocyte trafficking molecules and down-regulate cellular adhesion. Vascular growth factors, called angiopoietins (angpt), appear to play a prominent role in the signaling needed to accomplish this and possibly, the degree of endothelial activation seen in severe sepsis and septic shock.
Angiopoietin-1 and -2 are the best described growth factors and typically have opposing functions when bound to their tyrosine kinase receptor, Tie-2. Generally, angpt-1 is a Tie-2 receptor agonist, whereas angpt-2 is a Tie-2 receptor antagonist. When bound, angpt-1 possesses anti-inflammatory properties, stabilizes the capillary and promotes endothelial cell survival (7, 8). In most circumstances, angpt-2 blocks the Tie-2 receptor, destabilizes endothelial cells to allow for angiogenesis, and promotes inflammatory cell migration (8–10).
Many have shown increased circulating blood levels of angpt-2 in patients following a variety of inflammatory states, including cardiopulmonary bypass (11), trauma (12–14), acute lung injury (15–19) and sepsis (20–29) when compared to controls. Notably, most of these studies demonstrate worse outcomes when admission angpt-2 levels are elevated (15, 16, 18, 21, 23, 24, 28, 29). Among these studies, Mankhambo et. al. included the largest pediatric sample size but similarly, only included admission angiopoietin levels. Additionally, this study was performed on Malawian children, making generalizability to studies conducted in developed nations difficult (29). Furthermore, few of these studies describe angiopoietin levels or ratios past admission with none including children (12, 15, 21, 23, 24, 28). Therefore, we hypothesize that children with early-phase organ dysfunction as a result of severe sepsis and septic shock will have persistently elevated angpt-2/1 ratios when compared to critically ill children without organ dysfunction. Our study aims to characterize the temporal kinetics of angpt-1, angpt-2 and the angpt-2/1 ratio in children with varying degrees of sepsis during their first 7-days of illness.
MATERIALS AND METHODS
Patient Recruitment and classification
With approval from the Pediatric Protocol Review Committee and the Human Investigation Committee at Yale University School of Medicine, we performed a prospective, observational study of critically ill pediatric patients with varying degrees of sepsis severity. Informed consent was obtained from parents and assent was obtained from subjects when appropriate. Patients who met inclusion criteria were enrolled within the first 24-hours of pediatric intensive care unit (PICU) admission during the study dates September 1, 2009 through December 31, 2011. All patients admitted to the PICU were evaluated for eligibility. Due to the maximum volume of blood potentially collected, patients weighing < 10 kg or with hematocrits < 25% were excluded. Since we were attempting to plot angpt levels over time, patients without anticipated blood draws or with an anticipated PICU length of stay < 48 hours were also excluded. Additionally, patients receiving, or having received steroids within the previous 7-days were excluded due to their potential affect on levels (30). Patients were classified within the first 24-hours of PICU admission into: non-systemic inflammatory response syndrome (non-SIRS), SIRS, sepsis, severe sepsis, or septic shock based on the 2005 pediatric sepsis and organ dysfunction definitions (31). Admission demographics were collected as well as admission Paediatric Index of Mortality (PIM2) and daily Paediatric Logistic Organ Dysfunction (PELOD) scores in an attempt to risk stratify patients (32–34). In addition, the total fluid administered within the first 24 hours, including fluid administered in the emergency room or operating room, was totaled. The total fluid output during this time period was subtracted from the total administered to give the net ins/outs.
Sample acquisition and analysis
Blood samples were obtained twice per day for the first 3-days and then once per day for the last 4-days, for a maximum of 7-days and 10 samples. Blood acquisition occurred in conjunction with lab draws ordered by the clinical team. Sample collection was discontinued when the patient was discharged from the PICU, after the 7-day study completion or when the clinical team deemed it unnecessary to draw further labs for patient care. Two milliliters of blood were collected in sodium citrate tubes and placed immediately in a refrigerator until centrifugation. The samples were centrifuged at 4,000 rpm for 10 minutes at 4° Celsius to separate the plasma from the cellular components. Plasma samples were stored in 1.5 mL Eppendorf© tubes at −80° Celsius until analysis.
Plasma levels of angpt-1 and angpt-2 were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Human Angiopoietin-1 and -2 Quantikine ELISA Kit, respectively, R&D Systems, Inc., Minneapolis, MN) using the provided manufacturer’s protocol.
Statistical analysis
Samples were batch analyzed with the investigators blinded to all demographic and clinical data. Due to the skewed nature of the data, angpt-1 and angpt-2 levels were expressed in medians with interquartile ranges (IQR). Categorical variables were analyzed using Fisher’s exact test, whereas continuous variables were analyzed using the Kruskal-Wallis test. The Mann-Whitney U test was used when comparing continuous variables between two groups. A mixed model repeated measurement analysis was performed to model for change over day 1, 2, 3 and 4 to determine both group effect and time effect. Group by time effect was also examined to assess the slope difference among groups. Data are presented as least square means with 95% confidence intervals. Additionally, a pair-wise comparison between groups after Bonferroni correction was performed. The angpt-2/1 ratio was graphically plotted over time and a researcher determined whether patients fell into ‘constant’ or ‘variable’ patterns for patients with 3 or more data points in order to determine trajectory. ‘Constant’ ratios were persistently below an angpt-2/1 ratio of 10 throughout the course, whereas ‘variable’ patterns consisted of ratios with some or all data points above this cutoff. Additionally, an area under the receiver operator curve (AUC) analysis was performed using logistic regression to assess the discriminatory ability of angpt-2 levels alone. A p-value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.2 (Cary, NC).
RESULTS
During the study period 1,995 critically ill patients were admitted to the PICU and were evaluated for study eligibility. Of these, 51 patients met eligibility criteria and 45 consented to participate (88% enrollment) (Figure 1). Plasma samples were obtained from critically ill children in each of the following categories: Non-SIRS PICU comparison group (n=9), SIRS (n=8), sepsis (n=11), severe sepsis (n=3) and septic shock (n=14). There was equal distribution of gender, age, weight and comorbid conditions across the three groups (Table 1). Admission PIM2 scores, PELOD scores and net ins/outs increased significantly as the severity of illness increased representing the early-phase organ dysfunction associated with sepsis, severe sepsis and septic shock (Table 1). Care was withdrawn at parental request on one patient in the control group. All other patients survived to discharge.
Figure 1. Patient Selection Flow Diagram.
LOS=length of stay, hrs=hours, kg=kilogram, IVIG=intravenous immunoglobulin, RRT=renal replacement therapy
Table 1.
Patient Admission Demographics
| Characteristic | Non-SIRS | SIRS/Sepsis | Severe Sepsis/Shock | P-value |
|---|---|---|---|---|
| Gender, N (%) | 0.667 | |||
| Female | 5 (55.6) | 7 (36.8) | 7 (41.2) | |
| Male | 4 (44.4) | 12 (63.2) | 10 (58.8) | |
| Age, years | 0.339 | |||
| Median (IQR) | 10.0 (2.8–15.0) | 9.5 (5.5–14.0) | 13.0 (8.5–15.0) | |
| Weight, kg | 0.301 | |||
| Median (IQR) | 30.0 (14.3–59.0) | 28.5 (16.3–39.3) | 38.0 (18.4–59.0) | |
| Comorbidity, N (%) | 1.000 | |||
| Present | 4 (44%) | 10 (53%) | 8 (47%) | |
| PIM2, % | 0.020 | |||
| Median (IQR) | 0.9 (0.8–1.0) | 3.2 (0.9–4.8) | 3.9 (1.2–6.0) | |
| PELOD | 0.008 | |||
| Median (IQR) | 0.0 (0.0–1.0) | 1.0 (0.0–2.0) | 10.0 (1.0–12.0) | |
| Net Ins/Outs, mL | 0.001 | |||
| Median (IQR) | 52 (−373.5–482.5) | 863 (492–1847) | 3970 (1578–6143) |
Patient demographic information upon admission to the PICU. N=number; IQR=interquartile range; kg=kilograms; PIM2=Paediatric Index of Mortality
One or more infectious organisms were isolated in 71% of the patients with sepsis, severe sepsis or septic shock (Supplemental Table 1). Due to the variety of etiologies for sepsis, correlations between angpt levels and causative organism could not be made. PICU length of stay in days was similar in the non-SIRS (median, 7 days; IQR, 3–12 days), SIRS/sepsis (median, 8 days; IQR, 5–13 days) and severe sepsis/septic shock (median, 5 days; IQR, 4–8 days; p=0.463) groups. However, the total hospital length of stay in days was significantly different when comparing the three groups (median, 11 days; IQR, 8–16 days, median, 18 days; IQR, 11–30 days, and median, 8 days; IQR, 5–12 days, respectively; p=0.05).
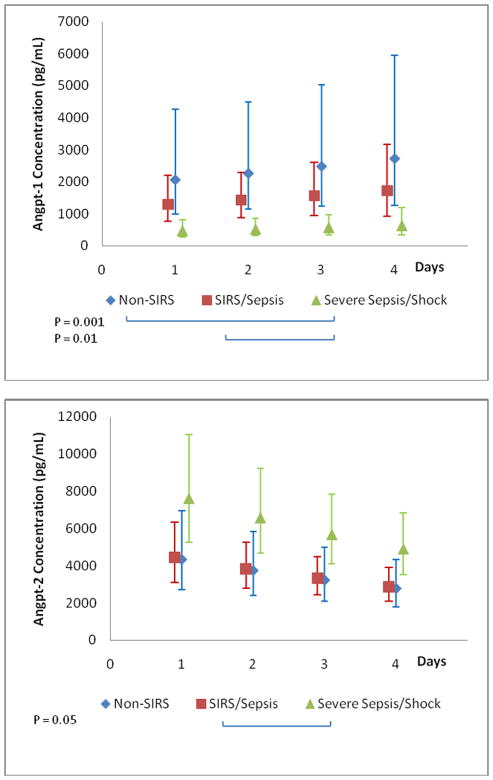
Due to discharges from the PICU, removal of access catheters, and the decreased need for clinical lab tests, sample data were not obtained on all patients for each study day (Supplemental Figure 1). The PICU admission plasma angpt-1 levels were not significantly different when comparing the non-SIRS, SIRS/sepsis, and severe sepsis/septic shock groups (p=0.137) (Figure 2A). Conversely, the PICU admission plasma angpt-2 levels were significantly different when comparing the three groups (p=0.004) (Figure 2B).
Figure 2. Admission Box and Whisker Plots.
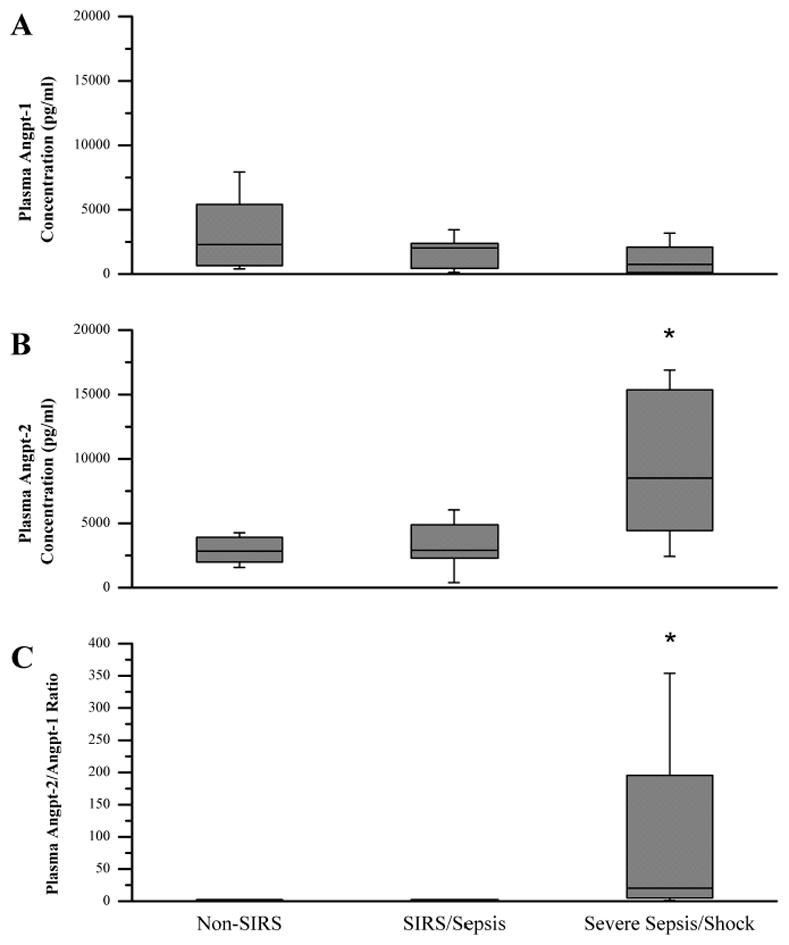
The non-SIRS (n=9), SIRS/sepsis (n=19) and severe sepsis/septic shock (n=17) were compared upon PICU admission. A. Plasma angpt-1 levels. B. Plasma angpt-2 levels. C. Angpt-2/1 ratio. *P < 0.05 compared with non-SIRS and SIRS/sepsis.
Due to the agonist/antagonist interaction with the Tie-2 receptor, we also determined the angpt-2/1 ratio for the three groups. Similarly to the admission angpt-2 levels, the PICU admission angpt-2/1 ratio was significantly different when comparing the non-SIRS, SIRS/sepsis, and severe sepsis/septic shock groups (median, 1.6; IQR, 0.5–2.4, median, 1.5; IQR, 1.3–2.6, and median, 20.5; IQR, 4.8–195.3, respectively; p=0.002) (Figure 2C). The angpt-2/1 ratio continued to be significantly elevated in the severe sepsis/septic shock group when compared to both the non-SIRS and SIRS/sepsis groups for the first 3-days of illness before returning to baseline by day 5 of illness (Figure 3). Additionally, the severe sepsis/septic shock angpt-2/1 ratio peaked on day 2 of illness (median, 29.0; IQR, 7.7–107.8) when compared to the non-SIRS and SIRS/sepsis groups on that day (median, 1.1; IQR, 0.6–1.8 and median, 1.8; IQR, 1.0–10.3, respectively; p=0.001) (Figure 3). The angpt-2 level alone on day 2 showed a significant AUC to distinguish between patients with severe sepsis/septic shock versus all others (AUC=0.77, 95% confidence interval 0.61–0.93) (Figure 4). At the optimal cutoff for angpt-2 (3,955 pg/mL), there was a sensitivity of 76%, specificity of 74%, positive predictive value of 68% and negative predictive value of 81% for predicting more severe illness.
Figure 3. Angpt-2/1 Ratio versus Time.
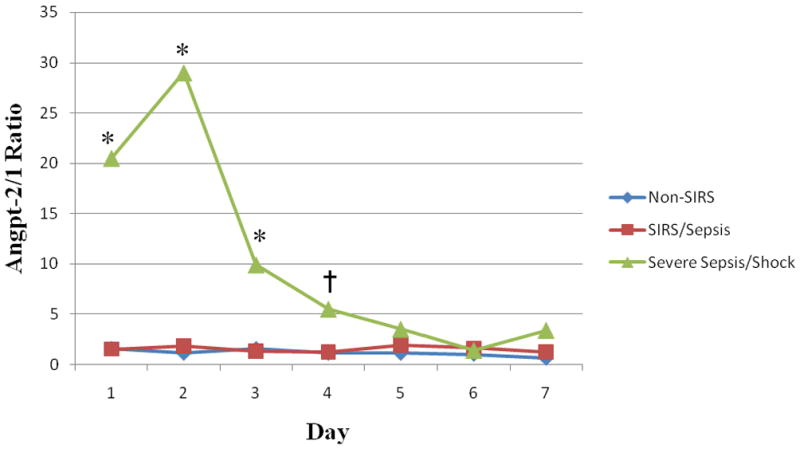
The non-SIRS (n=9), SIRS/sepsis (n=19) and severe sepsis/septic shock (n=17) plasma angpt-2/1 ratios were compared during the first 7 days of illness. Medians were plotted for the day they were collected. For days where multiple samples were obtained (i.e. days 1–3), the first sample of the day was plotted only. *P ≤ 0.01 and †P ≤ 0.05 comparing severe sepsis/septic shock with non-SIRS and SIRS/sepsis.
Figure 4. Receiver Operator Curve for Predicting Severe Sepsis/Septic Shock.
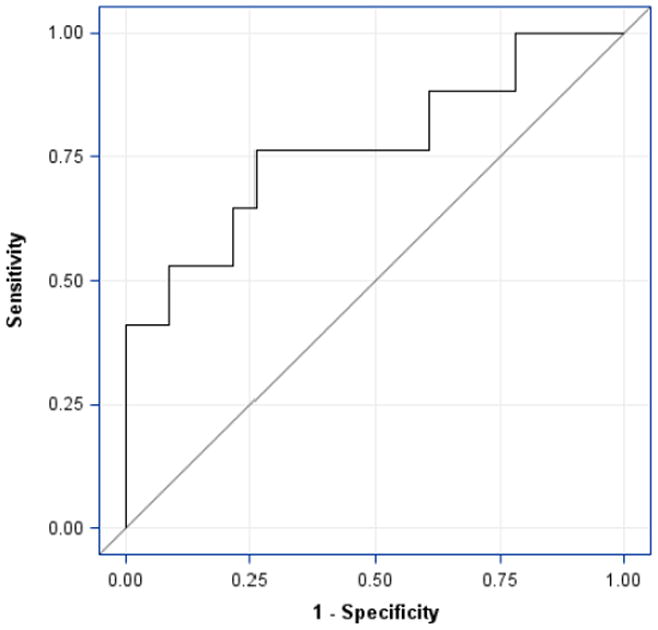
Only the first sample of day 2 was plotted. The severe sepsis/septic shock plasma angpt-2 levels (n=17) were compared to all others (n=23) on day 2 of illness. The area under the receiver operator curve was 0.77 (95% confidence interval, 0.61–0.93). The optimal cutoff for angpt-2 resulted in a 76% sensitivity, 74% specificity, 68% positive predictive value, and 81% negative predictive value for predicting severe sepsis or septic shock.
A mixed model analysis was performed which confirmed that all three outcomes differed significantly across groups during the first 4-days of illness (group effect: angpt-1 p=0.002; angpt-2 p=0.006; angpt-2/1 ratio p<0.001). Among the groups, only the angpt-2 level decreased significantly over time (time effect: angpt-2 p=0.01). Pair-wise comparisons between the groups after Bonferroni multiple correction determined that the significant difference was between the severe sepsis/septic shock group and both non-SIRS and SIRS/sepsis for angpt-1 and the angpt-2/1 ratio (Figure 5). The trajectories of angpt-2 levels were significantly different between patients infected with Gram positive and Gram negative organisms (p-0.001). Angpt-2 levels were initially more elevated and significantly decreased daily by 18% over the first 4-days in patients infected with Gram positive organisms (n=10; p<0.001). Patients infected with Gram negative organisms displayed a 10% increase in angpt-2 levels (n=5; p=0.1) (Figure 6). Similarly, the trajectories were significantly different between patients with sepsis requiring inotropes and those off inotropes (p=0.04). Angpt-2 levels were also initially higher and significantly decreased daily by 20% over the first 4-days in patients with sepsis requiring inotropes (septic shock n=14; p=0.007). Whereas, levels did not vary in patients with sepsis off inotropes (sepsis and severe sepsis n=14; p=0.94) (Figure 7).
Figure 5. Group Trajectories with Pair-wise Group Comparison.
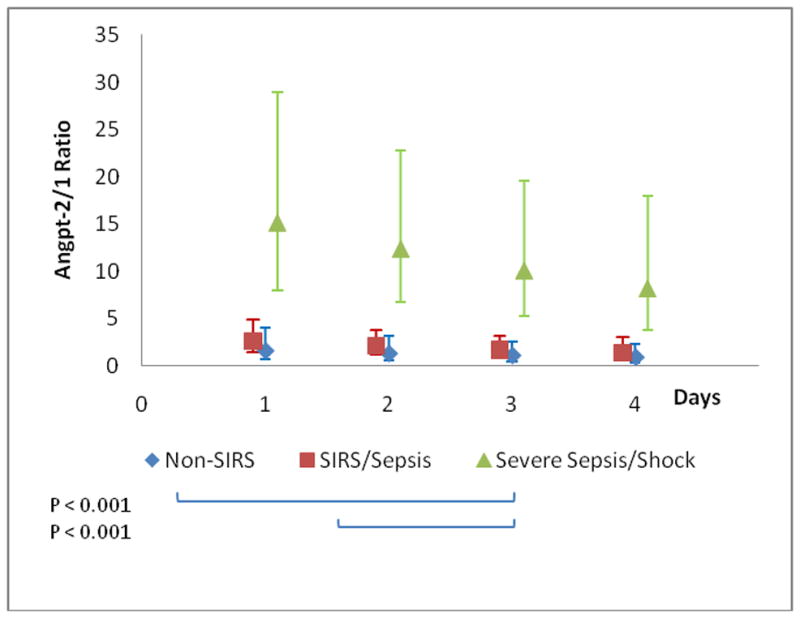
Geometric least square means and 95% confidence intervals are plotted. A. Plasma angpt-1 levels. B. Plasma angpt-2 levels. C. Angpt-2/1 ratio.
Figure 6. Angpt-2 Level Trajectory: Gram Negative versus Positive Infection.
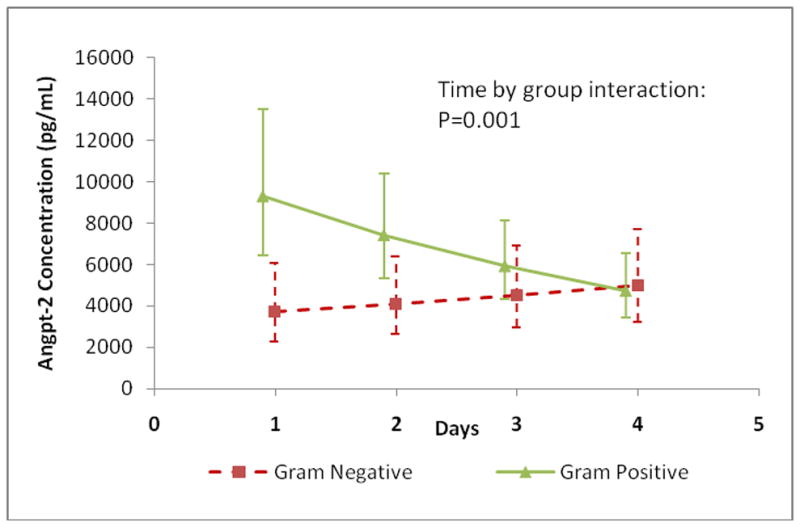
Angpt-2 level trajectories were plotted for the first 4-days of illness. Angpt-2 levels decreased by 18% per day in patients infected with Gram positive organisms (n=10; p<0.001) and increased by 10% in patients infected with Gram negative organisms (n=5; p=0.1). The Gram positive group decreased 34% more daily compared to the Gram negative group (p=0.001).
Figure 7. Angpt-2 Level Trajectory: Septic Patients With or Without Inotropes.
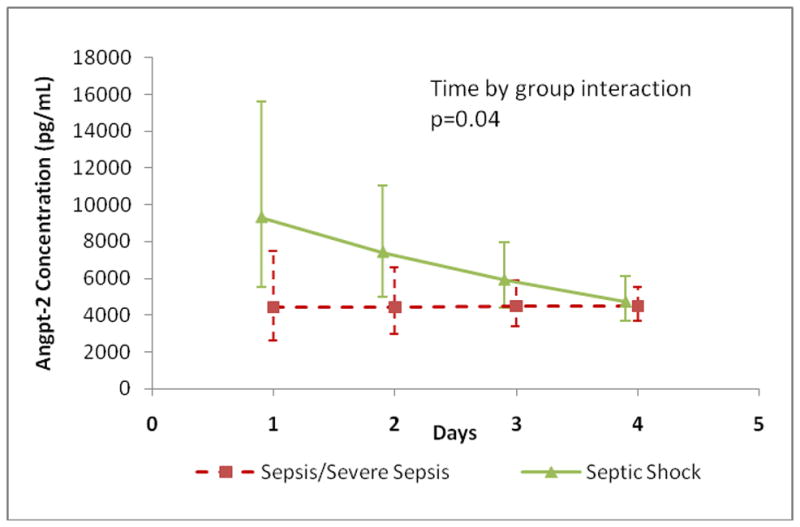
Angpt-2 level trajectories were plotted for the first 4-days of illness. Angpt-2 levels decreased by 20% per day in patients with sepsis requiring inotropic support (n=14; p=0.007) and did not vary in those without an inotropic requirement (n=14; p=0.94). The group with sepsis on inotropes decreased 26% more daily compared to the non-inotrope group (p=0.04).
Two independent investigators examined individual patient angpt-2/1 ratio versus time plots in order to classify them into ‘constant’ or ‘variable’ patterns (Supplemental Figure 2). Following classification, no patients with a ‘variable’ angpt-2/1 ratio pattern were identified in the non-SIRS group (0%), 31.6% (n=6) were identified in the SIRS/sepsis group and 82.4% (n=14) were identified in the severe sepsis/septic shock group (p<0.001) (Figure 8). Angpt-2 levels were higher in the ‘variable’ group and decreased by 22% per day over the first 4-days (p<0.001) but had minimal change in the ‘constant’ group (p=0.94) (Figure 9).
Figure 8. Percent ‘Variable’ Angpt-2/1 Ratio versus Time Pattern.
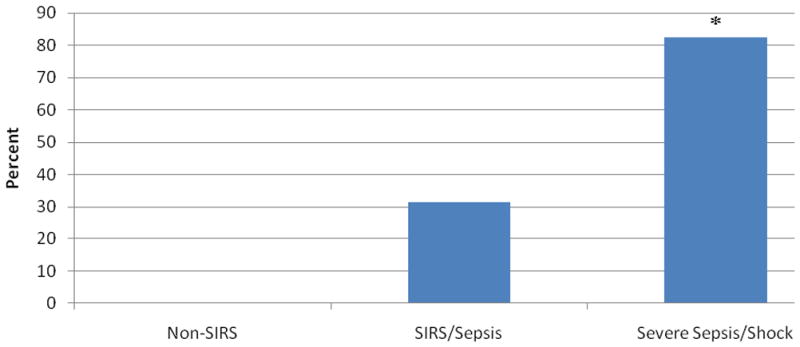
Individual angpt-2/1 versus time patient plots were examined to determine whether a ‘variable’ or ‘constant’ ratio pattern existed. The non-SIRS (0%), SIRS/sepsis (31.6%) and severe sepsis/septic shock (82.4%) variable patterns were compared. *P < 0.001 compared with non-SIRS and SIRS/sepsis.
Figure 9. Angpt-2 Level Trajectory: Constant versus Variable Patterns.
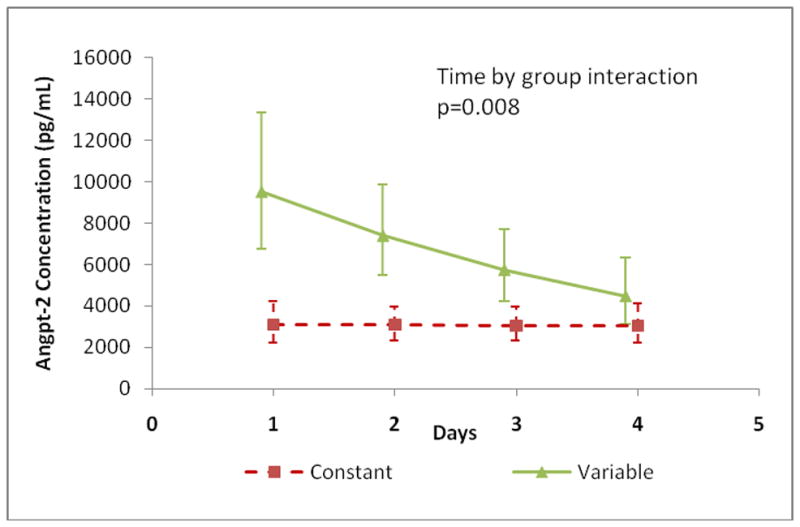
Angpt-2 level trajectories were plotted for the first 4-days of illness. Angpt-2 levels decreased by 22% per day in patients with ‘variable’ patterns (n=20; p<0.001) and did not change in the ‘constant’ group (n=25; p=0.94). The ‘variable’ group decreased 28% more daily compared to the ‘constant’ group (p=0.008).
DISCUSSION
Similar to other groups, we demonstrate here that patients admitted to the PICU with severe sepsis and septic shock possess elevated plasma levels of angpt-2 and the angpt-2/1 ratio, when comparing them to PICU patients with or without SIRS and with sepsis. Angpt-2 is preformed and stored in endothelial Weibel-Palade bodies and is released upon endothelial cell activation during times of stress (35). Other investigators have established that using human serum with high levels of angpt-2 reduced cultured endothelial cell barrier integrity through the phosphorylation of myosin light chain kinase. This disruption was rescued with the addition of angpt-1 (19, 36). Therefore, due to the agonist/antagonist relationship with the Tie-2 receptor, absolute angpt levels may be less clinically relevant than the angpt-2/1 ratio when predicting morbidity or directing potential therapies.
Additionally, we show that the angpt-2/1 ratio continues to be significantly elevated in pediatric patients with severe sepsis and septic shock for the first 3-days of critical illness, peaking at day 2 of illness. Angpt-2 levels on day 2 alone also discriminate between patients with severe sepsis and septic shock compared to the rest of the cohort with a sensitivity of 76%, negative predictive value of 81% and AUC of 0.77. In a recent study, angpt-2 was shown to enhance thrombin-induced cell permeability in cultured endothelial cells via the disruption of vascular endothelial (VE)-cadherins. Further supporting the concept that the angpt-2/1 ratio is likely more important than absolute levels, the addition of angpt-1 attenuated this response (37).
To further explore the different trajectories for the non-SIRS, SIRS/sepsis and severe sepsis/septic shock groups we performed mixed model repeated measurements analysis with post hoc pair-wise group comparisons. These analyses were used for correlated measurements over time. Group by time interaction was examined to determine if there were significant differences in slopes of time trend between groups. The significant group effect confirmed that the group means were truly different. Elevated angpt-2 levels were also seen in patients infected with Gram positive organisms and in those with sepsis requiring inotropes. However, interpretation of these results should be made with caution due to the very small patient cohort.
Lastly, we noticed that there were distinct differences between the patients’ angpt-2/1 ratios versus time plots. Patients either possessed a stable, unvarying pattern or a fluctuating, saw-tooth pattern. We labeled those with a ratio consistently below 10 throughout their illness as ‘constant’ and the others as ‘variable.’ After re-classifying each patient plot with their disease category, we found that more than 80% of the severe sepsis/septic shock group possessed a ‘variable’ pattern with just over 30% possessing this pattern in the SIRS/sepsis group. When angpt-2 levels were plotted for both the ‘constant’ and ‘variable’ patterns, distinct trajectories were identified. A common explanation why new sepsis therapies have not performed well clinically is that they are being tested on too heterogeneous of a population. Most studies use inadequate clinical definitions to define at-risk patients which have produced disappointing results (38). To our knowledge, this is the first time that anyone has graphically shown that all patients in a specific sepsis category are not the same. It has been previously reported that other pro-inflammatory cytokines, specifically tumor necrosis factor alpha and interleukin-6, and infectious agents, such as lipopolysaccharide, can decrease angpt-2 release from human lung microvascular endothelial cells (27). The ‘variable’ pattern seen in this subset of patients could be secondary to the modulatory effects of these circulating inflammatory mediators on angpt-2 release at that specific time-point in their illness. Assuming that the “sicker” patients possess the most ‘variable’ pattern with the highest angpt-2 and angpt-2/1 ratios, then this initial pattern could potentially be used as an early prognostic indicator or as a new way to classify patients for future sepsis research.
There are a few limitations to our study that are worth discussing. First, PICU length of stay did not change across the three groups. This was likely a result of a variety of factors, some of which are unrelated to our study. However, the similar PICU length of stay illustrates that the non-SIRS PICU comparison group were also critically ill patients and not healthy controls. This group possessed a similar percent of patients with underlying comorbid conditions, as well; making the angpt-2/1 ratio elevations described truly the result of sepsis associated organ failure and not from being critically ill. Additionally, since this study was an attempt to plot angiopoietin level trajectory over time, we excluded patients who were not anticipated to receive blood draws or to remain in the PICU for > 48 hours. These patients also would likely predominantly reside in the non-SIRS and SIRS groups. By excluding these types of patients, we selected patients with longer predicted lengths of stay.
Next, per our protocol, samples were only obtained in conjunction with blood tests ordered by the clinical care team. This allowed us to have a high enrollment rate but may have resulted in some missed sample acquisition. Since we limited the total amount of blood removal to be ≤ 2.5% total blood volume, our median patient ages were between 9.5 and 13 years. Conclusions about younger age groups cannot be made at this time. Finally, we selected consecutive patients based on the admitting team’s impression that a particular patient would remain in the PICU for at least 48 hours. This may represent a degree of selection bias. However, we felt that this was necessary to be able to adequately follow and compare angiopoietin levels between groups over a 7-day period. Despite these limitations, we were able to enroll a high percentage of eligible patients (88%) and collect over 250 sequential samples in 45 patients for analysis.
CONCLUSIONS
Pediatric patients who develop organ dysfunction as a result of sepsis have significantly elevated angpt-2 levels upon admission and angpt-2/1 ratios during their first 3 days of illness. Subsets of these patients demonstrate ‘variable’ angpt-2/1 ratio versus time patterns. We speculate that this may be useful as an early prognostic marker or a new way to classify septic patients for future study.
Supplementary Material
Sample Size by Study Day
Maximum samples by study day: Non-SIRS (n=9), SIRS/sepsis (n=19) and severe sepsis/septic shock (n=17). Samples were collected in conjunction with clinical tests. If consent was obtained after the last clinical test of the day, no sample was obtained.
Individual Patient Angpt-2/1 Ratio versus Time Plot
‘Constant’ ratios were persistently below an angpt-2/1 ratio of 10 throughout the course, whereas ‘variable’ patterns consisted of ratios with some or all data points above this cutoff. A. Constant Pattern. B. Variable Pattern.
Acknowledgments
Funding: Research funding (JG) and biostatistical collaboration (KT, VN, FL) was provided by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
We would like to recognize and thank Kellie Martino for her work identifying and recruiting patients in the early stages of this project. We would also like to thank Rayman Choo-Wing and Mansoor Syed for their ELISA expertise. Finally, we would like to thank all of the Yale-New Haven Children’s Hospital PICU nursing staff for acquiring blood samples in these patients.
References
- 1.Kochanek KD, Kirmeyer SE, Martin JA, et al. Annual summary of vital statistics: 2009. Pediatrics. 2012;129(2):338–348. doi: 10.1542/peds.2011-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 3.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 4.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2011;40(3):754–761. doi: 10.1097/CCM.0b013e318232db65. [DOI] [PubMed] [Google Scholar]
- 5.Proulx F, Joyal JS, Mariscalco MM, et al. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10(1):12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 6.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101(10):3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 7.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98(8):1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–558. doi: 10.1016/j.it.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 10.van Meurs M, Kumpers P, Ligtenberg JJ, et al. Bench-to-bedside review: Angiopoietin signalling in critical illness - a future target? Crit Care. 2009;13(2):207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano JS, Jr, Lahni PM, Bigham MT, et al. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 2008;34(10):1851–1857. doi: 10.1007/s00134-008-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng MT, Stammers AT, Kwon BK. Vascular Disruption and the Role of Angiogenic Proteins After Spinal Cord Injury. Transl Stroke Res. 2011;2(4):474–491. doi: 10.1007/s12975-011-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giamarellos-Bourboulis EJ, Kanellakopoulou K, Pelekanou A, et al. Kinetics of angiopoietin-2 in serum of multi-trauma patients: correlation with patient severity. Cytokine. 2008;44(2):310–313. doi: 10.1016/j.cyto.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247(2):320–326. doi: 10.1097/SLA.0b013e318162d616. [DOI] [PubMed] [Google Scholar]
- 15.Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: Prognostic and pathogenetic significance*. Crit Care Med. 2012;40(6):1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong T, McClintock DE, Kallet RH, et al. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med. 2010;38(9):1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63(10):903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 19.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conroy AL, Glover SJ, Hawkes M, et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med. 2012;40(3):952–959. doi: 10.1097/CCM.0b013e3182373157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricciuto DR, dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39(4):702–710. doi: 10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 22.Kumpers P, Hafer C, David S, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med. 2010;36(3):462–470. doi: 10.1007/s00134-009-1726-7. [DOI] [PubMed] [Google Scholar]
- 23.Siner JM, Bhandari V, Engle KM, et al. Elevated serum angiopoietin 2 levels are associated with increased mortality in sepsis. Shock. 2009;31(4):348–353. doi: 10.1097/SHK.0b013e318188bd06. [DOI] [PubMed] [Google Scholar]
- 24.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med. 2009;35(9):1567–1574. doi: 10.1007/s00134-009-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12(6):R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliano JS, Jr, Lahni PM, Harmon K, et al. Admission angiopoietin levels in children with septic shock. Shock. 2007;28(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 27.Orfanos SE, Kotanidou A, Glynos C, et al. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit Care Med. 2007;35(1):199–206. doi: 10.1097/01.CCM.0000251640.77679.D7. [DOI] [PubMed] [Google Scholar]
- 28.David S, Mukherjee A, Ghosh CC, et al. Angiopoietin-2 may contribute to multiorgan dysfunction and death in sepsis. Crit Care Med. 2012;40(11):3034–3041. doi: 10.1097/CCM.0b013e31825fdc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mankhambo LA, Banda DL, Jeffers G, et al. The role of angiogenic factors in predicting clinical outcome in severe bacterial infection in Malawian children. Crit Care. 2010;14(3):R91. doi: 10.1186/cc9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aghai ZH, Faqiri S, Saslow JG, et al. Angiopoietin 2 concentrations in infants developing bronchopulmonary dysplasia: attenuation by dexamethasone. J Perinatol. 2008;28(2):149–155. doi: 10.1038/sj.jp.7211886. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 32.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 33.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 34.Leteurtre S, Duhamel A, Grandbastien B, et al. Paediatric logistic organ dysfunction (PELOD) score. Lancet. 2006;367(9514):897. doi: 10.1016/S0140-6736(06)68371-2. author reply 900–892. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 36.Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87(7):603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 37.van der Heijden M, van Nieuw Amerongen GP, van Bezu J, et al. Opposing effects of the angiopoietins on the thrombin-induced permeability of human pulmonary microvascular endothelial cells. PLoS One. 2011;6(8):e23448. doi: 10.1371/journal.pone.0023448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall JC. Sepsis: rethinking the approach to clinical research. J Leukoc Biol. 2008;83(3):471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample Size by Study Day
Maximum samples by study day: Non-SIRS (n=9), SIRS/sepsis (n=19) and severe sepsis/septic shock (n=17). Samples were collected in conjunction with clinical tests. If consent was obtained after the last clinical test of the day, no sample was obtained.
Individual Patient Angpt-2/1 Ratio versus Time Plot
‘Constant’ ratios were persistently below an angpt-2/1 ratio of 10 throughout the course, whereas ‘variable’ patterns consisted of ratios with some or all data points above this cutoff. A. Constant Pattern. B. Variable Pattern.