Abstract
Rationale
A promising pharmacotherapy for alcohol use disorders has been positive allosteric modulators (PAMs) of the γ-aminobutyric acid receptor B (GABAB R) since GABAB R PAMs reduce ethanol drinking and self-administration in rodents.
Objective
The current studies investigated a novel, selective GABAB R PAM, ADX71441, in comparison to naltrexone in a protocol of ethanol binge-like drinking, drinking-in-the-dark (DID), and in a model of long-term, excessive drinking, intermittent access to ethanol (IA).
Methods
Male C57BL/6 J mice were given doses of ADX71441 (3, 10, 30 mg/kg, p.o.) before the fourth test day of repeated DID access to 20 % ethanol. Another group of mice had a history of 4 weeks of IA before ADX71441 (3, 10, 17 mg/ kg, p.o.) treatment. The opioid antagonist, naltrexone (0.1, 1, 10 mg/kg, i.p.), was administered to different groups of mice in both protocols as a positive control.
Results
In both DID and IA protocols, ADX71441 showed a selective and potent reduction of ethanol drinking, but not water drinking, while naltrexone had a more modest and transient effect on reducing ethanol drinking. The long-lasting effect of ADX71441 agrees with its plasma pharmacokinetics in showing peak concentrations at 2 h followed by a slow decay lasting well beyond 8 h.
Conclusions
These findings support previous studies demonstrating that GABAB R PAMs decrease voluntary ethanol intake without altering water intake. ADX71441 may be a worthwhile candidate for developing a treatment of alcoholism, yet its site of action in the brain and long-term pharmacological effects require further exploration.
Keywords: GABAB receptor, Positive allosteric modulator, Ethanol, Drinking-in-the-dark, intermittent access to alcohol, Naltrexone
Treatment of alcoholic-like drinking continues to be an urgent need in view of the large social and economic cost (Mohapatra et al. 2010). Current U.S. Food and Drug Administration-approved medications for alcoholism include acetaldehyde de-hydrogenase inhibitor disulfiram, glutamate antagonist acamprosate, and opioid antagonist naltrexone. However, these treatments are limited in their effectiveness to a substantial proportion of alcoholics (Carmen et al. 2004; Heilig et al. 2011).
A promising pharmacological target for alcohol-use-related disorders is the γ-aminobutyric acid receptor B (GABAB R) system, which includes the metabotropic receptors for the major inhibitory neurotransmitter. Activation of the GABAB receptor reduces the reinforcing properties of cocaine, heroin, and nicotine (Cousins et al. 2002; Fadda et al. 2003; Xi and Stein 1999; Vlachou et al. 2011). This receptor system also modulates a variety of ethanol-related behaviors. The first studies exploring the anti-ethanol effects of GABAB receptor activation involved the prototypic, direct agonist baclofen. Baclofen decreases two-bottle choice ethanol drinking as well as decreases the motivation for responding in fixed and progressive ratio schedules of ethanol reinforcement (Maccioni et al. 2005, 2007, 2008a; Daoust et al. 1987; Liang et al. 2006; Colombo et al. 2003; Anstrom et al. 2003; Walker and Koob 2007). These effects are dose-specific, as higher doses of baclofen increase ethanol self-administration and intake (Smith et al. 1992, 1999; Petry 1997; Czachowski et al. 2006). Baclofen has also shown promise in clinical trials, by reducing ethanol consumption, suppressing craving, and minimizing withdrawal symptoms in alcohol-dependent patients (for review, see Addolorato and Leggio 2010). This GABAB R agonist has been the only medication used in alcoholics with advanced alcoholic liver disease (Addolorato et al. 2007), including those with hepatitis C virus (Leggio et al. 2012). Though baclofen has found promise in recent human laboratory studies (Leggio et al. 2013), the clinical use of baclofen is limited by its short half-life and an unfavorable side effect profile that may include tolerance, sedation, and motor impairment (Addolorato et al. 2002; Bowery 2006; Evans and Bisaga 2009).
Similar to the anti-ethanol actions of baclofen, GABAB R-positive allosteric modulators (PAMs) like GS39783, CGP7930, BHF177, and rac-BHFF also effectively suppress ethanol intake in two-bottle choice drinking and self-administration protocols (Liang et al. 2006; Maccioni et al. 2007, 2008b, 2009b, 2010b, 2012; Orrù et al. 2005, 2012). These small molecules act at an independent binding pocket to modulate the GABAB receptor allosterically, instead of binding in the orthosteric site (Conn et al. 2009). In contrast to orthosteric agonists that bind to GABAB receptor everywhere, PAMs potentiate GABA only in those synapses where it is released. It has been proposed that this feature enables PAMs to produce less adverse effects and leads to less tolerance than direct agonists (May and Christopoulos 2003; Langmead and Christopolous 2006; Perdona et al. 2011; Urwyler 2011). Preclinical findings support this proposal, as ethanol-suppressing effects of GABAB R PAMs are associated with compensatory increases in water drinking (Orrù et al. 2005; Loi et al. 2013), while having no effect on self-administration of sucrose (Maccioni et al. 2007, 2008b, 2009, 2010). Microinfusion of GS39783 into the ventral tegmental area reduces ethanol-seeking behavior in rats without concurrent changes in spontaneous locomotor activity (Leite-Morris et al. 2009). Furthermore, GABAB R PAMs GS39783 and CGP7930 show anxiolytic-like profiles with improved side effect profiles compared to baclofen (Cryan et al. 2004; Mombereau et al. 2004a, b; Jacobson and Cryan 2008; but see Li et al. 2013).
Here we present a novel GABAB R PAM ADX71441 which received regulatory approval for phase I clinical studies (Addex Therapeutics Webpage). Previously, in a series of in vitro and in vivo studies, ADX71441 has been characterized as a potent, selective, reversible, and orally bioavailable GABAB R PAM active in models of pain, anxiety (Kalinichev et al. 2013a, under review), and overactive bladder (Kalinichev et al. 2013b, under review). Here we tested ADX71441 in mouse models of binge drinking and long-term high-dose drinking. Mice of the C57BL/6 J strain can engage in binge-like drinking in the initial hours of the dark phase (Rhodes et al. 2007) and can drink large amounts of ethanol in a 24-h period compared to other strains (Yoneyama et al. 2008). The current investigation uses a limited access model of binge-like drinking of 20 % ethanol, so-called drinking-in-the-dark (DID; Rhodes et al. 2005). In addition, we used a model of excessive long-term, voluntary, preferential, and dependence-producing drinking as a result of intermittent access to two-bottle choice (IA; Hwa et al. 2011). This model also provides an opportunity to evaluate specificity of the effect of the compound on ethanol vs. water and control for any nonspecific motor effects of the compound. In both experiments, opioid antagonist, naltrexone, was used as a positive control for medications already available in the clinic (Volpicelli et al. 1992; O’Malley et al. 1992; Kranzler and Kirk 2001; Anton et al. 2006). We hypothesized that ADX71441 would result in a robust and specific reduction of ethanol intake which is likely to be longer lasting than that of naltrexone, based on the differences in the duration of action between the two compounds (Kamdar et al. 2007; Kalinichev et al. 2013a, b, under review).
Methods
Behavioral studies
Animals
Eight-week-old adult, male C57BL/6 J mice (Jackson Laboratories, Bar Harbor, ME) were group-housed for 1 week to acclimate to the vivarium of Tufts University (Medford, MA), which was maintained on a 12-h reversed light/dark cycle, 21± 2 °C temperature and 30 % humidity. Mice were then singly housed for the experiment in polycarbonate cages (28×17× 12 cm) with pine shaving as bedding. Rodent lab chow (Purina LabDiet 5001, PMI Nutrition International, Brentwood, MO) was continuously accessible through stainless steel wire mesh lids. In experiment 1, water was available except when ethanol was presented. In experiment 2, water was continuously available. All procedures were approved by the Tufts University Institutional Animal Care and Use Committee and were in accordance with the National Research Council’s “Guide for the Care and Use of Laboratory Animals” (2011).
Experiment 1: repeated drinking-in-the-dark
The first experiment used a repeated drinking-in-the-dark (DID; Cox et al. 2013) protocol, adapted from the original method by Rhodes et al. (2005). Three hours into the dark cycle, the standard water bottles were replaced with plastic 50 ml centrifuge tubes (Nalgene) containing 20 % ethanol solution (w/v) in tap water. Centrifuge tubes had a rubber stopper (No. 5, Fisher Scientific, Agawam, MA) with a sipper tube (Ancare Corp., Bellmore, NY) constructed with two ball bearings to prevent fluid loss. A cage with no animal present served as a daily control for ethanol spillage due to experimenter bottle handling. Fluid loss in this control condition was deducted from each individual’s intake value.
On test days 1–3, ethanol was accessible for 2 h. On test day 4, animals were weighed, administered the appropriate drug dose, and given ethanol access for 4 h. On day 4, hourly ethanol intake was measured in grams per kilogram of body weight (g/kg), milliliters (ml), and percent change from baseline to assess the time course of drug action. These four test days were followed by 3 days without ethanol, completing one DID cycle. These cycles of 7 days were repeated at least four times after 1 week of habituating mice to injections and the DID schedule. One group of mice (n =12) was given counterbalanced doses of ADX71441 (3, 10, 30 mg/kg) and 1 % carboxy methyl cellulose (CMC) vehicle, administered orally (p.o.) across 4 weeks of repeated DID cycles on each 4 h test day. A separate group of mice (n =12) received counterbalanced doses of naltrexone (0.1, 1, 10 mg/kg) and saline vehicle, administered intraperitoneally (i.p.) in 4 cycles of concurrent DID testing.
Experiment 2: intermittent access to ethanol
The second experiment aimed to test the experimental drugs in a procedure that generates excessive, voluntary ethanol drinking in mice, intermittent access (IA) to ethanol, as described by Hwa et al. (2011). Mice were given two bottles, one containing 20 % ethanol (w/v) and the other tap water for 24 h 3 days per week separated by 24–48 h (i.e., Monday, Wednesday, Friday). Mice were given two bottles of water during the remaining days of the week. The same 50-ml water bottles and sipper tubes were used as described in experiment 1. Mice were weighed before the ethanol and water bottles were given. The position of the ethanol bottle on the cage top was switched every ethanol and water session to avoid side preferences. A “drip cage” was also maintained in this experiment to control for experimenter spillage and evaporation. Mice were habituated to injections on non-ethanol drinking days.
After 4 weeks of IA, mice were tested for ethanol drinking after drug administration. Separate groups of mice (n =12/ group) were given repeated doses of ADX71441 (0, 3, 10, 17 mg/kg, p.o.) or naltrexone (0, 0.1, 1, 10 mg/kg, i.p.) before access to the two-bottle choice. After observing signs of mild reduction in motor activity in a few animals treated at 30 mg/ kg ADX71441, the dose range of ADX71441was limited to 17 mg/kg to avoid potential nonspecific effects in the IA procedure. Ethanol intake per body weight (g/kg), volume of ethanol intake (ml), volume of water intake (ml), and ethanol preference were evaluated 2, 4, and 24 h after drug injection. Ethanol preference was defined as the volume of ethanol intake divided by volume of total fluid intake, multiplied by 100.
After drug testing revealed that AD71441 most potently suppressed ethanol intake during 4 h access, blood from the submandibular vein of all animals was collected after 4 h of ethanol access to analyze for blood ethanol concentrations (BEC). Collection occurred over two additional test days for mice to obtain blood after a vehicle injection and after a 17-mg/kg ADX71441 injection. Blood samples were centrifuged at 1,000×g for 10 min, and plasma was extracted to use in an ethanol assay kit using a NAD-ADH reagent (Genzyme Diagnostics, Charlottetown, PE, Canada). Absorbance was measured with a SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA).
In vivo pharmacokinetic study
This pharmacokinetic study was performed in adult, male C57BL/6 J mice weighing 22–24 g (n =24) that were purchased from Charles River (L’Arbresle, France) and maintained in the animal facility of Addex Therapeutics under standard conditions. Animals were acclimated for at least 5 days before any experimentation. ADX71441 was given p.o. at 10 mg/kg (5 ml/kg), as suspension in 1 % CMC in water. Blood sampling was done by intracardiac puncture. Blood samples were collected up to 24 h post-dosing (three animals per time point) and plasma samples were stored frozen until analysis.
Plasma sample analysis
Sample analysis was performed using a tandem liquid chromatography (Waters UPLC system) coupled with mass spectrometry (API 3200, Applied Biosystems). Acquisition in mass spectrometry was done in MSMS electrospray-positive mode (MRM), monitoring the following MH transition: 437/ 143. The high performance liquid chromatography (HPLC) conditions used were a 1.5-min gradient with ammonium formate 10 mM pH 3.5/acetonitrile formic acid (15 mM) at 0.8 ml/min. To prepare plasma sample, 150 μl of acetonitrile (protein precipitation) was added to 50 μl of plasma spiked, respectively, with 10 μl DMSO for unknown samples or 10 μl of ADX71441 for calibration and QC samples. After vortexing and centrifugation (15 min, 4 °C, 13,200 rpm), a portion of the supernatant (100 μl) was transferred into the 384-well analytical plate. The limit of quantification was 1–5 ng/ml. The results generated with the analytical method were validated when 75 % of back-calculated calibration points remained within ±20 % of theoretical nominal concentrations and 66 % of the QC samples were within ±20 % of nominal theoretical concentrations.
Pharmacokinetic parameters
Calculation of pharmacokinetic parameters was performed using WinNonLin® Pro 5.2 software, Pharsight Corporation (Mountain View, CA). Noncompartmental analysis was performed using sparse sampling approach. The area under the concentration time curve (AUClast and AUC0–∞) was calculated by linear trapezoidal rule. Peak concentration (C max) and time for the peak concentration (T max) were the observed values at nominal sampling time. The elimination rate constant value (k) was obtained by linear regression of the log-linear terminal phase of the concentration–time profile using at least three declining concentrations (not including Cmax) in terminal phase. The terminal half-life value (T 1/2) was calculated using the equation ln2/k. For the calculation of the pharmacokinetic parameters, the nominal sampling times were used.
Drugs
ADX71441 was synthesized at Addex Therapeutics (Tang et al. 2013, submitted). It was suspended in 1 % CMC and administered p.o. via gavage 30 min before animals were given access to ethanol. Naltrexone (NIDA) was dissolved in sterile saline administered i.p. 20 min before animals were given access to ethanol. All drugs were administered in a volume of 10 ml/kg. All doses are expressed as base.
Statistical analyses
The mean and standard error of the mean (SEM) were reported for all data. Statistical analyses were performed using SigmaStat version 3.1 (Systat Software, San Jose, CA). Test day intake data were analyzed using one-way analyses of variance (ANOVA) at individual time points, followed by Bonferroni post hoc analyses if significant main effects were revealed (p <0.05). Volume of ethanol intake (ml) and water intake (ml) were additionally analyzed as percent change from baseline transformations. Differences in BEC after drug treatment were also analyzed with a one-way ANOVA.
Results
Experiment 1: repeated drinking-in-the-dark
ADX71441
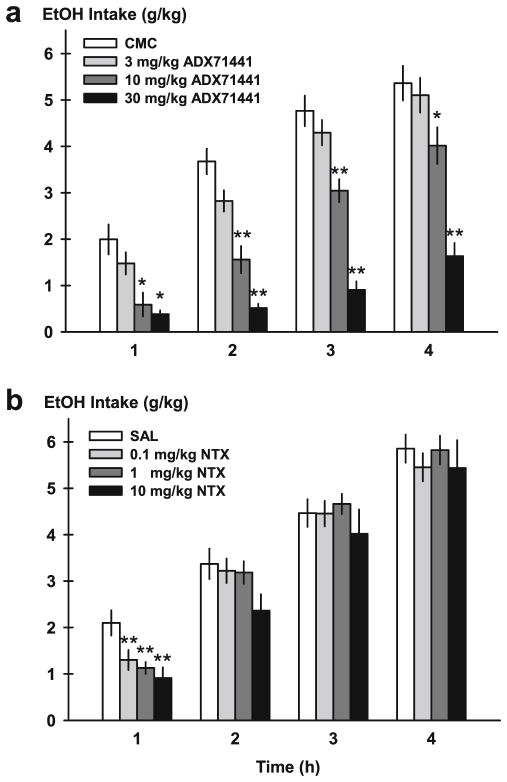
ADX71441 significantly and dose-dependently reduced DID ethanol intake (gram per kilogram) during the first, second, third, and fourth hours after ethanol access [F(3, 11)=8.41, p <0.001; F(3, 11)=30.00, p <0.001; F(3, 11)= 46.18, p <0.001; F(3, 11)=26.69, p <0.001] (Fig. 1a). Post hoc analysis revealed that only the higher doses of ADX71441 (10, 30 mg/kg) reduced ethanol intake [1 h—10 mg/kg, p <0.01 and 30 mg/kg, p <0.001; 2 h—10 mg/kg, p <0.001 and 30 mg/kg, p <0.001; 3 h—10 mg/kg, p <0.001 and 30 mg/kg, p <0.001; 4 h—10 mg/kg, p <0.05 and 30 mg/kg, p <0.001].
Fig. 1.
Ethanol intake (gram per kilogram) in mice on the fourth day of the repeated drinking-in-the-dark (DID) procedure. Animals were pretreated with either ADX71441 (3, 10, 30 mg/kg, p.o.) or its vehicle (1 % CMC, a) or naltrexone (NTX; 0.1, 1, 10 mg/kg, i.p.) and its vehicle (saline, SAL, b). Drinking from one bottle of 20 % ethanol was assessed every hour over the course of 4 h. Each point represents the observed mean (+SEM). *p <0.05, **p <0.01 vs. vehicle (n =12/group)
Similar to the effects on gram per kilogram intake, ADX71441 decreased volume of ethanol intake (ml) at every period of the 4-h DID procedure [F(3, 11)=7.95, p <0.001; F(3, 11)=28.99, p <0.001; F(3, 11)=44.08, p <0.001; F(3, 11)= 25.78, p <0.001; data not shown]. The two highest doses suppressed ethanol drinking at every hour [1 h—10 mg/kg, p <0.01 and 30 mg/kg, p <0.001; 2 h—10 mg/kg, p <0.001 and 30 mg/kg, p <0.001; 3 h—10 mg/kg, p <0.001 and 30 mg/ kg, p <0.001; 4 h—10 mg/kg, p <0.05 and 30 mg/kg, p <0.001]. When data were converted to percent change from 100 % baseline, results were again reflective of a long-lasting suppression of ethanol drinking at all four DID time points [F(3, 11)=8.41, p <0.001; F(3, 11)=30.81, p <0.001; F(3, 11)=47.83, p <0.001; F(3, 11)=27.72, p <0.001] (Table 1). Effects were driven by the two highest doses [1 h—10 mg/kg, p <0.01 and 30 mg/kg, p <0.001; 2 h—0 mg/kg, p <0.001 and 30 mg/kg, p <0.001; 3 h—10 mg/kg, p <0.001 and 30 mg/kg, p <0.001; 4 h—10 mg/kg, p <0.05 and 30 mg/kg, p <0.001]. Mean percent change from vehicle baseline by the highest dose of ADX71441 was approximately −70–80 % across the 4-h DID test.
Table 1.
Percent change from baseline drinking behavior of vehicle-treated controls following treatment with either ADX71441 (3, 10, 30 mg/kg, p.o.) or naltrexone (0.1, 1, 10 mg/kg, i.p.) on the test days of the drinking-in-the-dark (DID) procedure. Volumes of ethanol (ml) were measured at 1, 2, 3, and 4 h time periods during an access test session, and percent change was calculated from 100 % baseline. Each point represents the observed mean (+SEM)
| Drug dose | 1 h | 2 h | 3 h | 4 h | |
|---|---|---|---|---|---|
| ADX71441 | 3 mg/kg | −25.93±11.92 % | −23.12±7.29 % | −9.84±6.84 % | −4.83±8.82 % |
| 10 mg/kg | −70.44±13.55* | −57.53±8.28** | −36.11±5.39** | −25.11±7.68* | |
| 30 mg/kg | −80.73±4.11** | −86.04±4.05** | −80.99±4.39** | −69.57±3.89** | |
| Naltrexone | 0.1 mg/kg | −38.09±10.22* | −4.40±7.82 | −0.28±6.12 | −6.85±5.18 |
| 1 mg/kg | −46.29±6.09* | −5.49±7.14 | 4.39±4.88 | −0.53±5.30 | |
| 10 mg/kg | −56.67±11.04** | −29.84±10.43* | −10.03±11.79 | −7.16±10.27 |
p <0.05,
p <0.01 vs. vehicle injection
Naltrexone
Naltrexone had a significant drug effect on DID intake (gram per kilogram) during the first hour of ethanol access [F(3, 11)=8.90, p <0.001] (Fig. 1b). All doses of naltrexone significantly reduced ethanol drinking compared to vehicle [0.1 mg/kg, p <0.01; 1 mg/kg, p <0.001; 10 mg/kg, p <0.001]. Naltrexone had no effect on intake during any subsequent hour of ethanol access.
Naltrexone also decreased the volume of ethanol drinking (ml) during the initial hour of DID [F(3, 11)=8.745, p <0.001; data not shown]. Post hoc tests confirmed that all doses of naltrexone suppressed DID drinking during the first hour [0.1 mg/kg, p <0.01; 1 mg/kg, p <0.01; 10 mg/kg, p <0.001]. Two, 3, and 4 h of ethanol intake was not altered by naltrexone. Percent decrease compared to baseline was also significant at the 1- and 2-h time periods [F(3, 11)=7.49, p <0.001; F(3, 11)= 3.20, p <0.05] (Table 1). All doses affected the initial 1-h percent decrease [0.1 mg/kg, p <0.05; 1 mg/kg, p <0.01; 10 mg/kg, p <0.001], while only the highest 10-mg/kg dose affected the 2-h percent decrease [p <0.05]. Mean percent decrease at the highest 10-mg/kg dose naltrexone was approximately 57 % during the initial hour.
Experiment 2: intermittent access to ethanol
ADX71441
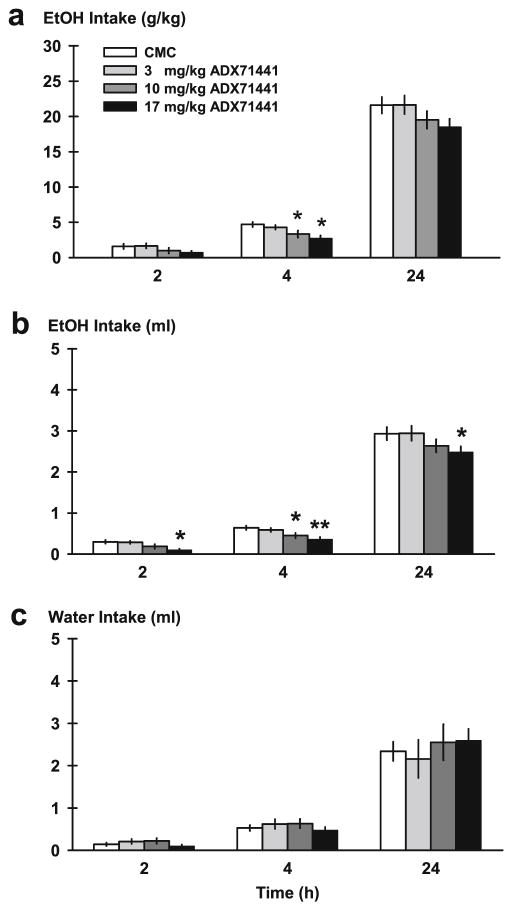
ADX71441 reduced ethanol drinking per unit of body weight (gram per kilogram) during the 4-h access period [F(3, 33)=6.66, p =0.001], but not during the initial 2-h access period (Fig. 2a). The highest two doses were most effective at decreasing ethanol drinking compared to vehicle [10 mg/kg, p <0.05; 17 mg/kg, p <0.001]; 24 h ethanol drinking (gram per kilogram) was also affected by ADX71441 [F(3, 33)=3.14, p <0.05]. Higher doses (10, 17 mg/kg) showed a trend of reducing ethanol drinking.
Fig. 2.
Ethanol intake in mice on the test day of the intermittent access to ethanol (IA) procedure. Animals were pretreated with either ADX71441 (3, 10, 17 mg/kg, p.o.) or its vehicle (1 % CMC) before being given two-bottle choice and their alcohol and water consumption was monitored at 2, 4, and 24 h time points (n =12/group). Ethanol intake per unit of body weight (gram per kilogram; a), volume of ethanol (b), and water intake (ml; c) are portrayed over the time course of a test day. Each point represents the observed mean (+SEM). *p <0.05, **p <0.01 vs. vehicle
Initial 2 h of ethanol intake (ml) was significantly attenuated after drug treatment [F(3, 33)=6.24, p <0.01] (Fig. 2b) specifically at the highest dose, 17 mg/kg, compared to vehicle [p <0.01]. This reduction in ethanol intake (ml) lasted across the 4-h time point [F(3, 33)=6.84, p =0.001] and the 24-h time point [F(3, 33)=3.60, p <0.05]. The two highest doses most effectively decreased drinking at the 4-h access period [10 mg/kg, p <0.05; 17 mg/kg, p <0.001], while only the highest dose was effective at the 24-h access period compared to vehicle [p <0.05]. When data were converted to percent change from vehicle, there was a significant reduction in 2 h of ethanol intake (ml) [F(3, 33]=6.24, p <0.01] (Table 2), seen at the 17-mg/kg dose [p <0.01]. Mean percent decrease was approximately 67 % during the initial 2-h binge. The calculated percent decrease lasted across the 4-h [F(3, 33)= 6.84, p =0.001] and 24-h time periods [F(3, 33)=3.60, p <0.05]. Similar to raw intake values, the 4-h percent change was most potently affected by the two highest ADX71441 doses [10 mg/kg, p <0.05; 17 mg/kg, p <0.001], and the 24-h percent change was affected by the highest 17-mg/kg dose [p <0.05].
Table 2.
Percent change from baseline drinking behavior of vehicle-treated controls following treatment with either ADX71441 (3, 10, 17 mg/kg, p.o.) or naltrexone (0.1, 1, 10 mg/kg, i.p.) on the test days of the intermittent access to ethanol (IA) procedure. Volumes of ethanol and water (milliliter) were measured at 2, 4, and 24 h time periods during an access test session, and percent change was calculated from 100 % baseline. Each point represents the observed mean (+SEM)
| Drug dose | 2 h | 4 h | 24 h | |
|---|---|---|---|---|
| Ethanol | ||||
| ADX71441 | 3 mg/kg | −3.92±12.96 % | −8.21±7.51 % | 0.31±6.16 % |
| 10 mg/kg | −37.54±18.17 | −29.60±10.55* | −10.12±5.36 | |
| 17 mg/kg | −66.39±10.30* | −44.07±9.06** | −15.30±4.81* | |
| Naltrexone | 0.1 mg/kg | −18.80±9.61 | 1.78±10.37 | 8.33±4.82 |
| 1 mg/kg | −7.28±7.98 | −13.05±8.42 | −2.34±2.31 | |
| 10 mg/kg | −35.59±7.14* | −26.55±6.66 | −7.74±2.73 | |
| Water | ||||
| ADX71441 | 3 mg/kg | 46.78±40.53 | 17.35±22.27 | −7.73±19.28 |
| 10 mg/kg | 55.56±45.39 | 19.72±20.18 | 9.05±18.21 | |
| 17 mg/kg | −26.90±22.63 | −9.62±13.96 | 10.83±11.73 | |
| Naltrexone | 0.1 mg/kg | −1.30±18.88 | −29.44±9.75 | 10.48±13.47 |
| 1 mg/kg | −28.31±9.98 | −17.50±12.68 | 2.65±10.05 | |
| 10 mg/kg | 5.59±21.71 | 30.81±22.77 | 18.27±12.04 | |
p <0.05,
p <0.01 vs. vehicle injection
IA water drinking (ml) was not affected by ADX71441 at any time period (Fig. 2c). Percent change from baseline water intake (ml) was not significantly altered by ADX71441 at any time point (Table 2). Ethanol preference was not significantly affected at any time point (data not shown).
Naltrexone
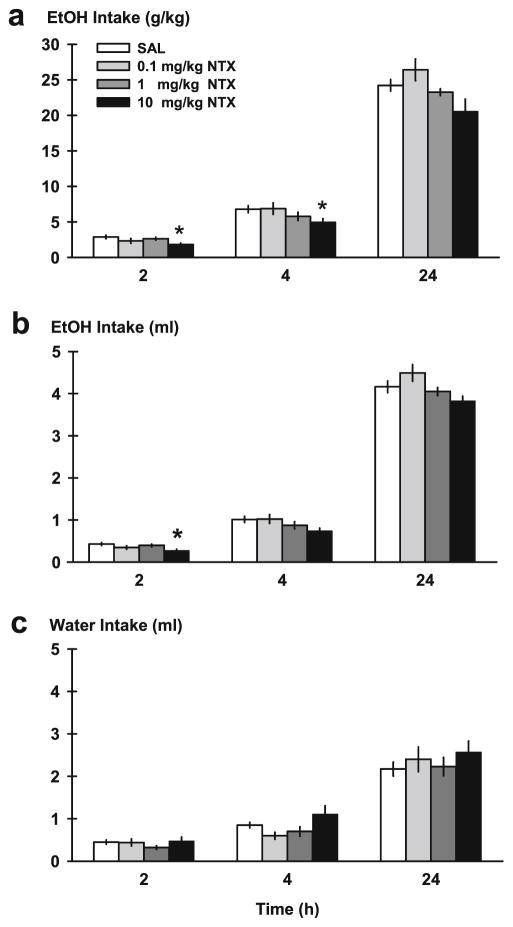
Naltrexone had a significant effect on ethanol drinking (gram per kilogram) after the 2-h access to two-bottle choice [F(3, 33)=3.63, p <0.05] (Fig. 3a). Post hoc tests revealed that only the highest dose of naltrexone, 10 mg/kg, decreased ethanol drinking (gram per kilogram) compared to saline vehicle [p <0.05]. Naltrexone also attenuated ethanol drinking (gram per kilogram) at the 4-h time point [F(3, 33)=3.19, p <0.05], which was driven by the highest dose, as well [p <0.05]. Although there was a main effect for drug treatment after 24 h access [F(3, 33)=5.02, p <0.01], daily ethanol drinking (gram kilogram) was not significantly altered by any naltrexone dose compared to saline vehicle, albeit there was a trend of reduction at the highest dose.
Fig. 3.
Ethanol intake in mice on the test day of the IA procedure. Animals were pretreated with naltrexone (NTX; 0.1, 1, 10 mg/kg, i.p.) or saline (SAL) before being exposed to two-bottle choice and their ethanol and water consumption was monitored at 2, 4, and 24 h time points (n =12/group). Ethanol intake per unit of body weight (gram per kilogram; a), volume of ethanol (b), and water intake (milliliter; c) are portrayed over the time course of a test day. Each point represents the observed mean (+SEM). *p <0.05 vs. SAL
Naltrexone affected volume of ethanol intake (ml) in the initial 2-h access period [F(3, 33)=3.67, p <0.05] (Fig. 3b), specifically at the highest 10-mg/kg dose [p <0.05]. A treatment effect on volume of ethanol intake also lasted until the 4-[F(3, 33)=3.00, p <0.05] and 24-h time points [F(3, 33)= 5.06, p <0.01], but no naltrexone doses were different from vehicle. When fluid intake data were presented as percent change from saline vehicle, there was a significant reduction of ethanol (ml) by naltrexone treatment at 2 h [F(3, 33)=3.67, p <0.05] (Table 2), evident at the highest dose [p <0.05]. Mean percent decrease caused by 10 mg/kg naltrexone was approximately 36 %.
Water intake (ml) was not changed at any time point by naltrexone (Fig. 3c). Percent change for water intake (ml) was also not altered, as raw water intake (ml) remained unchanged (Table 2). Since the volume of ethanol intake was transiently attenuated by naltrexone, ethanol preference as a function of total fluid was also affected [F(3, 33)=5.02, p <0.01], but ethanol preference at the 4- or 24-h time points was not (data not shown).
After 4 h access, BEC were 47.73±9.18 mg/dl after vehicle treatment ranging from 16.19 to 122.32 mg/dl. Average BEC after 17 mg/kg ADX71441 were 35.74±5.34 with values ranging from 11.37 to 67.58 mg/dl. BEC after 4 h IA were not statistically different between treatments.
In vivo pharmacokinetic study
Following acute oral administration at 10 mg/kg, ADX71441 plasma concentrations increased rapidly (average of 538±51 ng/ ml at 0.25 h) and reached the maximum 2 h following drug administration (mean Cmax of 673±50 ng/ml) (Fig. 4; Table 3). Peak plasma concentrations were followed by a slow decay such that mean plasma concentration at 4 and 24 h represented 72 and 5 % of the mean Cmax, respectively (Table 3). Terminal half-life was estimated to be 5 h after oral administration.
Fig. 4.
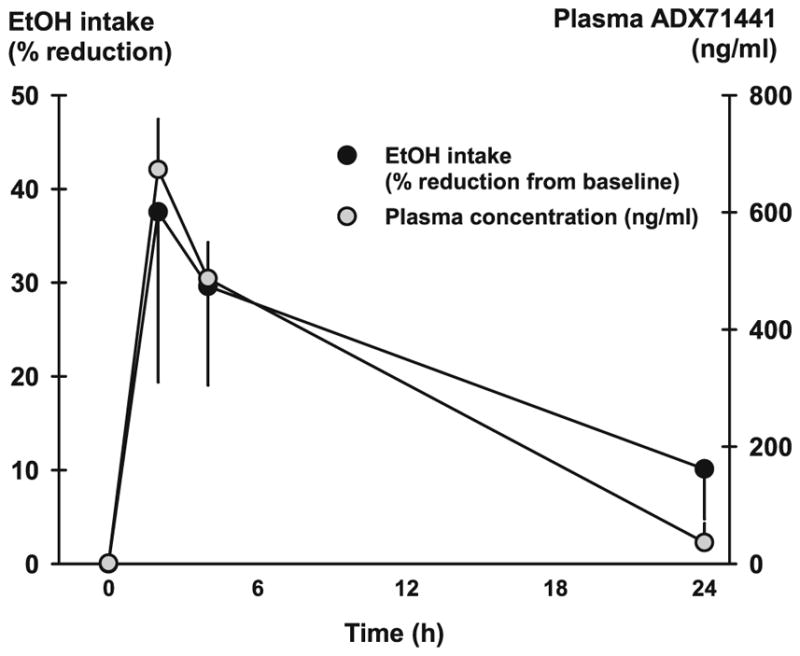
Mean plasma concentration of ADX71441 (nanogram per milliliter) after p.o. administration of 10 mg/kg to male C57BL/6 mice (n =24). ADX71441-induced reduction in 20 % ethanol intake (ml) across 24 h in C57BL/6 J mice (n =12) in the intermittent access protocol. Portrayed is the percent change in ethanol intake from the vehicle baseline across 2, 4, and 24 h
Table 3.
Pharmacokinetic parameters following oral administration of 10 mg/kg ADX71441 to male C57BL/6 mice (n =24). Pharmacokinetic data shown are peak concentration (Cmax), area under the concentration time curves (AUClast and AUC0-∞), the terminal half-life value (T1/2), and time for the peak concentration (Tmax)
| Dose (mg/kg) | Plasma concentration ± SD at 1 h (ng/ml) | Plasma concentration ± SD at 4 h (ng/ml) | Plasma concentration ± SD at 24 h (ng/ml) | C max ± SD (ng/ml) | AUClasta ± SD (ng h/ml) | AUC0–8 (ng h/ml) | T 1/2 (h) | T max (h) |
|---|---|---|---|---|---|---|---|---|
| 10 (p.o.) | 629±61 | 487±62 | 36.4±33 | 673±50 | 6,700±309 | 6,970 | 5.3 | 2 |
Tlast=24 h
Discussion
In this set of studies, ADX71441 represents a novel, potent, and highly selective GABAB PAM that shows promise for the treatment of excessive ethanol drinking. Specifically, in mice, acute, oral administration of ADX71441 suppressed ethanol drinking for the entire 4-h “binge” in DID and reduced ethanol intake for up to 24 h in the IA protocol. The ethanol-suppressing effects of ADX71441 appeared remarkably specific, as the compound had no effect on water drinking. Naltrexone, used in this study as a clinically available positive control, acted transiently to also selectively reduce ethanol drinking in both the DID and IA protocols.
ADX71441 decreased ethanol intake in the DID and IA protocols, which extends the reductions in DID intake by GABAB agonist baclofen (Moore et al. 2007; 2009). Others have also found decreases in ethanol drinking in a scheduled high ethanol consumption procedure and operant self-administration for ethanol reinforcement by baclofen (Tanchuck et al. 2011). The highest dose of baclofen tended to decrease water intake and decrease operant self-administration of sucrose, indicative of nonspecific motor effects, confirming the need for GABAB R activators that do not exert undesired side effects. Some have also found that baclofen reduced ethanol intake only in outbred mice that drank low levels of ethanol but did not alter drinking in mice that drank high levels (Villas Boas et al. 2012). In the current studies, we found that mice that drink in excess (ca. 20 g/kg/24 h) on the IA protocol have significant reductions in ethanol drinking caused by ADX71441. This report is among the first studies of decreased drinking with a GABAB R PAM seen in C57BL/6 J mice (Orrù et al. 2012).
The current findings are in accordance with past research with GABAB R PAMs and ethanol drinking collected in rats that were selectively bred for increased ethanol intake and preference (Bell et al. 2006; Colombo et al. 2006). GABAB R PAMs CGP7930, GS39783, BHF177, and rac-BHFF have been found to reduce ethanol intake and operant self-administration of ethanol in Indiana ethanol-preferring (P) and Sardinian ethanol-preferring (sP) rats (Liang et al. 2006; Maccioni et al. 2007; 2008b; 2009; Orrù et al. 2005; Loi et al. 2013). In the current study, novel GABAB R PAM ADX71441 reduced ethanol intake in C57BL/6 J mice, an inbred strain with a high ethanol preference. With the two-bottle choice method, we observed no specific decreases in water intake or any nonspecific decreases due to motor impairment at any time point in animals treated with ADX71441. Importantly, we confirm other findings with GABAB R PAMs showing that ethanol-reducing effects are selective to ethanol and do not affect self-administration of a nondrug fluid such as water drinking or sucrose drinking.
Mice showed a long-lasting reduction in ethanol intake caused by ADX71441 treatment that persisted as long as 24 h. These data are in accord with the long half-life of the compound following oral administration in rodents. This is a valuable feature because short-acting drugs can cause animals to compensate for the initial void in ethanol drinking by later overdrinking. Orrù et al. (2005) found that repeated daily treatment of GS39783 and CGP7930 lasted for over 24 h when 10 % ethanol and water were continuously available. The efficacy of GS39783 and CPG7930 lasted during the first 2–3 days of treatment, but daily injections of rac-BHFF dose-dependently reduced daily ethanol drinking for up to 7 days (Loi et al. 2013). The positive control treatment, naltrexone, was effective transiently, for 1 h, in the DID protocol while it suppressed ethanol intake (ml) for 2 h in the IA protocol. This short-acting nature of naltrexone is in agreement with past findings showing the drug suppressing DID intake for only 2 h (Kamdar et al. 2007). In contrast, ADX71441 reduced DID ethanol drinking for the entire 4-h binge episode as well as IA ethanol intake (milliliter and gram per kilogram) for up to 24 h. Future studies are needed to investigate the effects of repeated, chronic ADX71441 treatment in order to determine the long-term effectiveness of this compound. Previously ADX71441 showed efficacy in the MIA-induced chronic os-teoarthritic pain model in the rat after acute as well as subchronic (once daily for 7 days) administration (Kalinichev et al. 2013a, b, under review). Also, the anxiolytic efficacy of GS39783 was also shown after both acute and chronic dosing (Mombereau et al. 2004b). Based on this, we can hypothesize that the alcohol intake-suppressant effect of ADX71441 will persist after repeated, chronic treatment regime. This is clinically important, as medication adherence can be problematic in alcoholics (Weiss 2004), which may also contribute to considerable variation in efficacy of naltrexone (Swift et al. 2011).
The IA procedure is informative because it generates excessive levels of voluntary ethanol drinking in C57BL/6 J mice (Melendez 2011; Hwa et al. 2011, 2013; Rosenwasser et al. 2012; but see Crabbe et al. 2012). The repeated cycles of ethanol access and deprivation are thought to generate periods of bingeing and withdrawal over time, mimicking a transition to ethanol dependence. Compared with ethanol naïve mice, mice that have undergone repeated cycles of DID do not show preliminary indicators of a dependent-like state such as anxiety-like behavior, ataxia, and handling-induced convulsions (Cox et al. 2013). We have previously shown that months of chronic IA produce tonic–clonic convulsions 8 h after access to ethanol (Hwa et al. 2011). More research needs to be done using GABAB R PAMs to relieve ethanol with-drawal symptoms. ADX71441 may have effects upon other indices of withdrawal such as anxiety-like behavior, as GABAB R PAMs have also been investigated in tests of anxiety (Cryan et al. 2004; Mombereau et al. 2004a, b; Jacobson and Cryan 2008, but see Li et al. 2013). Previously, ADX71441 showed anxiolytic-like effects in the elevated plus maze and marble burying tests in mice (Kalinichev et al. 2013a, b, under review). An anxiolytic profile of a GABAB R PAM may alleviate the anxiogenic withdrawal state in alcoholics and thus offer an additional benefit in the treatment of alcoholism.
Studies have started to examine the role of GABAB receptors in the brain and ethanol drinking. In addition to dopamine (DA) neurons, the ventral tegmental area (VTA) is populated by GABAergic cells that influence the activity of DA neurons, which can also send projections to a variety of brain regions (Kalivas et al. 1990; Omelchenko and Sesack 2009; Ciccarelli et al. 2012). GABAB(1) R transcription levels are elevated in the striatum of outbred mice showing high ethanol preference (Ribeiro et al. 2012), and microinjection of GS39783 into the ventral tegmental area (VTA) inhibits ethanol-seeking behavior (Leite-Morris et al. 2009). Future studies using microin-jections of ADX71441 in the VTA or striatum would further the knowledge put forward by the current study. GABAB R PAM-induced inhibitory activation of GABAB R in ethanol-withdrawn animals might counterbalance the enhanced function of glutamate excitatory neurotransmission.
In conclusion, we showed robust, long-lasting, and specific reductions in alcohol consumption in mice following acute, oral administration of a novel GABAB R PAM ADX71441. This may offer strong evidence that the compound can be considered for treatment of alcoholism. Further studies involving efficacy after repeated administration and effects on withdrawal can provide additional valuable insights.
Acknowledgments
This study was supported by funding both from Addex Therapeutics and from NIH RO1 AA013983 awarded to K.A.M. The authors would like to thank Rachel Doyle and Kevin Norman for their technical assistance as well as Emily Newman and Professor Joseph F. DeBold for their guidance.
Footnotes
Conflict of interest: In addition to NIH funding, this work was, in part, sponsored by Addex Therapeutics. M.K., H.H., and S.P. are employees of Addex Therapeutics, Geneva, Switzerland. Addex supplied the experimental compound and pharmacokinetic data. L.S.H. and K.A.M. have received contract funding from Addex Therapeutics and designed and performed the experiments. All authors contributed to the writing and editing of the manuscript.
Contributor Information
Lara S. Hwa, Department of Psychology, Tufts University, 530 Boston Avenue, Medford, MA 02155, USA
Mikhail Kalinichev, Addex Therapeutics, Geneva, Switzerland.
Hasnaà Haddouk, Addex Therapeutics, Geneva, Switzerland.
Sonia Poli, Addex Therapeutics, Geneva, Switzerland.
Klaus A. Miczek, Email: klaus.miczek@tufts.edu, Department of Psychology, Tufts University, 530 Boston Avenue, Medford, MA 02155, USA. Department of Neuroscience, Tufts University, Boston, MA 02111, USA
References
- Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Current pharmaceutical design. 2010;16(19):2113–2117. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Demonicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol and Alcoholism. 2002;37(5):504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D’Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen and alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27(6):900–908. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donavan DM, Gastfriend DR, Hosking JD, et al. Combined pharmaco-therapies and behavioral interventions for alcohol dependence. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. Review: the alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11(3):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor: a site of therapeutic benefit. Current opinion in pharmacology. 2006;6(1):37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Carmen B, Angeles M, Ana M, Maria AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99(7):811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Ciccarelli A, Calza A, Panzanelli P, Concas A, Giustetto M, Sassoè-Pognetto M. Organization of GABAergic synaptic circuits in the rat ventral tegmental area. PloS One. 2012;7(10):e46250. doi: 10.1371/journal.pone.0046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Review: phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and-non-preferring (sNP) rats. Addict Biol. 2006;11(3):324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology. 2003;167(3):221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nature reviews Drug discovery. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Roberts D, de Wit HD. GABAB receptor agonists for the treatment of drug addiction: a review of recent findings. Drug and alcohol dependence. 2002;65(3):209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, Kash TL, Thiele TE. Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary etOH intake but not other dependence-like phenotypes. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harkness JH, Spence SE, Huang LC, Metten P. Intermittent availability of ethanol does not always lead to elevated drinking in mice. Alcohol and alcoholism. 2012;47(5):509–517. doi: 10.1093/alcalc/ags067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N, N′-dicyclopentyl-2-methyulsulfanyl-5natro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABAB agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30(5):812–818. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F. GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol. 1987;4(6):469–472. doi: 10.1016/0741-8329(87)90087-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Bisaga A. Acute interaction of baclofen in combination with alcohol in heavy social drinkers. Alcohol Clin Exp Res. 2009;33(1):19–30. doi: 10.1111/j.1530-0277.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50(1):1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12(11):670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35(11):1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, DeBold JF, Miczek KA. Alcohol in excess: CRF1 receptors in the rat and mouse VTA and DRN. Psychopharmacology. 2013;225(2):313–327. doi: 10.1007/s00213-012-2820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Evaluation of the anxiolytic-like profile of the GABAB receptor positive allosteric modulator CGP7930 in rodents. Neuropharmacology. 2008;54(5):854–862. doi: 10.1016/j.neuropharm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Girard F, Haddouk H, Heroux M, Royer-Urios I, Bessif A, Mahious N, Rizzo O, Derouet F, Jouin D, Schneider M, Bonnet B, Campo B, Poli S. The drug candidate ADX71441, a novel, potent and selective positive allosteric modulator of GABAB receptor, with potential for treatment of anxiety, pain and spasticity. J Pharmacol Exp Ther. 2013a under review. [Google Scholar]
- Kalinichev M, Palea S, Haddouk H, Royer-Urios I, Guilloteau V, Lluel P, Saporito M, Schneider M, Poli S. ADX71441, a novel, potent and selective positive allosteric modulator of GABAB receptor, shows efficacy in rodent models of overactive bladder. The British Journal of Pharmacology. 2013b doi: 10.1111/bph.12517. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric agonists. J Pharmacol Exp Ther. 1990;253(2):858–866. [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192(2):207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25(9):1335–1341. [PubMed] [Google Scholar]
- Langmead CJ, Christopolous A. Allosteric agonists of 7TM receptors: expanding the pharmacological toolbox. Trends Pharmacol Sci. 2006;27(9):475–481. doi: 10.1016/j.tips.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Zambon Z, Caputo F, Kenna GA, Swift RM, Addolorato G. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561–564. doi: 10.1016/j.addbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchhione SR, Shoaff JF, Addolorato G, Swift RM, Kenna GA. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Biochem Behav. 2013;103(4):784–791. doi: 10.1016/j.pbb.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Morris KA, Kerestes HB, Colombo G. Intra-ventral tegmen-tal area injection of the GABA B receptor positive allosteric modulator GS39783 inhibits ethanol seeking behavior in rats. Alcohol: Clinical and Experimental Research. 2009;33:226A. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- Li X, Risbrough VB, Cates-Gatto C, Kaczanowska K, Finn MG, Roberts AJ, Markou A. Comparison of the effects of the GABAB receptor positive modulator BHF177 and the GABAB receptor agonist baclofen on anxiety-like behavior, learning, and memory in mice. Neuropharmacology. 2013;70:156–167. doi: 10.1016/j.neuropharm.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart P, Lawrence AJ. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50(5):632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Loi B, Maccioni P, Lobina C, Carai MA, Gessa GL, Thomas AW, Malherbe P, Colombo G. Reduction of alcohol intake by the positive allosteric modulator of the GABAB receptor, rac-BHFF, in alcohol-preferring rats. Alcohol. 2013;47(1):69–73. doi: 10.1016/j.alcohol.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug and Alcohol Dependence. 2008a;95(3):284–287. doi: 10.1016/j.drugalcdep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Carai MA, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. Reduction of alcohol’s reinforcing and motivational properties by the positive allosteric modulator of the GABAB receptor, BHF177, in alcohol-preferring rats. Alcohol Clin Exp Res. 2009;33(10):1749–1756. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MA, Gessa GL, Colombo G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABAB receptor, GS39783—comparison with the effect of the GABAB receptor direct agonist, baclofen. Alcohol Clin Exp Res. 2008b;32(9):1558–1564. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Pes D, Orrù A, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulator of the GABAB receptor, GS39783, on alcohol self-administration in alcohol-preferring rats. Psychopharmacology. 2007;193(2):171–178. doi: 10.1007/s00213-007-0776-1. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Serra S, Vacca G, Orrù A, Pes D, Agabio R, Addolorato G, Carai MAM, Gessa GL, Colombo G. Baclofen-induced reduction of alcohol reinforcement in alcohol-preferring rats. Alcohol. 2005;36(3):161–168. doi: 10.1016/j.alcohol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Thomas AW, Carai MA, Gessa GL, Malherbe P, Colombo G. The positive allosteric modulator of the GABAB receptor, rac-BHFF, suppresses alcohol self-administration. Drug and alcohol dependence. 2010;109(1):96–103. doi: 10.1016/j.drugalcdep.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Zaru A, Loi B, Lobina C, Carai MA, Gessa GL, Capra A, Mugnaini C, Pasquini S, Corelli F, Hyytiä P, Lumeng L, Colombo G. Comparison of the effect of the GABAB receptor agonist, baclofen, and the positive allosteric modulator of the GABAB receptor, GS39783, on alcohol self-administration in 3 different lines of alcohol-preferring rats. Alcohol Clin Exp Res. 2012;36(10):1748–1766. doi: 10.1111/j.1530-0277.2012.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3(5):551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35(4):652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S, Patra J, Popova S, Duhig A, Rehm J. Social cost of heavy drinking and alcohol dependence in high-income countries. International Journal of Public Health. 2010;55(3):149–157. doi: 10.1007/s00038-009-0108-9. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, van der Putten H, Cryan JF. Altered response to benzodiazepine anxiolytics in mice lacking GABA B(1) receptors. Eur J Pharmacol. 2004a;497:119–120. doi: 10.1016/j.ejphar.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABAB receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004b;29:1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Moore EM, Boehm SL. Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behavioral neuroscience. 2009;123(3):555. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., II GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use and laboratory animals. 8. National Academies Press; Washington: 2011. [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence—a controlled study. Arch Gen Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63(10):895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrù A, Fujani D, Cassina C, Conti M, Di Clemente A, Cervo L. Operant, oral alcoholic beer self-administration by C57BL/6J mice: effect of BHF177, a positive allosteric modulator of GABAB receptors. Psychopharmacology. 2012;222(4):685–700. doi: 10.1007/s00213-012-2672-6. [DOI] [PubMed] [Google Scholar]
- Orrù A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulators of the GABAB receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol. 2005;525(1):105–111. doi: 10.1016/j.ejphar.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Perdona E, Constantini VJ, Tessari M, Martinelli P, Carignani C, Valerio E, Mok MH, Zonzini L, Visentini F, Gianotti M, Gordon L, Rocheville M, Corsi M, Capelli AM. In vitro and in vivo characterization of the novel GABAB receptor positive allosteric modulator, 2-{1-[2-(4-chlorophenyl)-5-methylpyrazolo[1,5-a]pyrimidin-7-yl]-2-piperidinyl}ethanol (CMPPE) Neuropharmacology. 2011;61(5):957–966. doi: 10.1016/j.neuropharm.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Experimental and clinical psychopharmacology. 1997;5(3):183. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain and Behavior. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro AF, Correia D, Torres AA, Villas Boas GR, Rueda AVL, Camarini R, Chiavegatto S, Boerngen-Lacerda R, Brunialti-Godard AL. A transcriptional study in mice with different ethanol-drinking profiles: possible involvement of the GABAB receptor. Pharmacol Biochem Behav. 2012;102(2):224–232. doi: 10.1016/j.pbb.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S. Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol. 2012;18(3):496–507. doi: 10.1111/j.1369-1600.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Boyle AE, Amit Z. The effects of the GABAB agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol. 1999;17(3):231–240. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol and Alcoholism. 1992;27(3):227–231. [PubMed] [Google Scholar]
- Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. Journal of Studies on Alcohol and Drugs. 2011;72(6):1012. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA. Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol. 2011;45(1):33–44. doi: 10.1016/j.alcohol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Riguet E, Bonnet B, Campo B, Kalinichev M, Poli S, Girard F, Gibelin A, Mallah K, Le Poul E, Bessis A-S, Donovan-Rodriguez T, Mordant C, Mutel V, Rocher J-P. Discovery of triazinediones as novel GABAB receptor positive allosteric modulators for the treatment of pain. ACS Medicinal Chemistry Letters. 2013 submitted. [Google Scholar]
- Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011;63(1):59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- Villas Boas GR, Zamboni CG, Peretti MC, Correia D, Rueda AVL, Camarini R, Brunialti-Godard AL, Boerngen-Lacerda R. GABAB receptor agonist only reduces ethanol drinking in light-drinking mice. Pharmacol Biochem Behav. 2012;102(2):233–240. doi: 10.1016/j.pbb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Vlachou S, Guery S, Froestl W, Bannerjee D, Benedict J, Finn MG, Markou A. Repeated administration of the GABAB receptor positive modulator BHF177 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine seeking in rats. Psychopharmacology. 2011;215(1):117–128. doi: 10.1007/s00213-010-2119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutryric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31(1):11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacology in patients with alcohol and opioid dependence. Addiction. 2004;99(11):1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290(3):1369–1374. [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]