Abstract
The E. coli stationary phase transcription factor RpoS is translated in response to small noncoding RNAs (sRNAs), which base pair with the rpoS mRNA leader. The bacterial Sm-like protein Hfq anneals sRNAs with their mRNA targets by simultaneously binding the mRNA and sRNA. Intriguingly, Hfq is recruited to the rpoS leader via AAN motifs far upstream of the sRNA. SHAPE chemical footprinting showed that the rpoS leader is divided into a far upstream domain, an Hfq binding domain, and a downstream inhibitory stem-loop containing the sRNA and ribosome binding sites. To investigate how Hfq promotes sRNA-mRNA base pairing from a distance, the natural AAN Hfq binding site was deleted, and artificial AAN binding sites were inserted at various positions in the rpoS leader. All the relocated AAN motifs restored tight Hfq binding in vitro, but only insertion at the natural position restored Hfq-dependent sRNA annealing in vitro and sRNA regulation of rpoS translation in vivo. Furthermore, U-rich motifs in the downstream inhibitory domain stabilized the rpoS mRNA-Hfq complex and contributed to regulation of rpoS expression. We propose that the natural Hfq binding domain is optimal for positive regulation because it recruits Hfq to the mRNA and allows it to act on incoming sRNAs without opening the inhibitory stem-loop when sRNA are absent.
Keywords: RNA-protein interactions, bacterial stress response, translational control, RNA chaperone, 5′ UTR
INTRODUCTION
Bacterial small RNAs regulate mRNA translation in response to environmental stress 1-3. A large class of sRNAs base pair with target mRNAs, inhibiting or activating translation by blocking or exposing the ribosome binding site 4. sRNA regulation requires Hfq, an RNA chaperone that facilitates sRNA and mRNA annealing and recruits additional proteins to the sRNA-mRNA complex 5, 6. Despite much progress in identifying sRNA and Hfq binding sites in mRNAs, the molecular “rules” for activation of translation by sRNAs are not fully understood 5.
A well-studied example of positive regulation by sRNAs, rpoS encodes an alternative stationary phase sigma factor that mediates the expression of many stress response genes 7. During exponential growth, translation of the rpoS mRNA is inhibited by a stable stem-loop that masks the Shine-Dalgarno sequence 8. Three E. coli sRNAs (DsrA, RprA, and ArcZ) up regulate rpoS translation by base pairing with the rpoS leader and opening the inhibitory stem, releasing the Shine-Dalgarno sequence for translation initiation 9.
An A-rich (AAN)4 motif upstream of the sRNA target site in rpoS is needed for sRNA regulation 10 and for Hfq to facilitate annealing of sRNAs to the rpoS mRNA 11, 12. These AAN motifs, which specifically bind the distal face of Hfq, are frequently found in mRNA targets 13, 14. By contrast, U-rich sequences present in the body and 3′ tail of many sRNAs interact with the lateral rim and proximal face of Hfq 15, 16. These distinct sRNA and mRNA binding surfaces on each face of Hfq position their complementary regions to interact with a conserved arginine patch on the rim that acts as the active site for RNA annealing 17. This arginine patch was proposed to orient sRNAs for base pairing 18 and is needed to catalyze the formation and release of RNA base pairs 17.
In vitro experiments on short, unstructured RNAs showed that Hfq anneals two RNAs most efficiently when bound less than 20 nucleotides away from the target, preferably on the 3′ side 19. This proximity requirement is partially overcome by secondary structure that brings Hfq site closer to the target.
Intriguingly, Hfq binding sites in bacterial mRNAs vary widely in their proximity to the sRNA binding site, and are found both upstream and downstream of the sRNA. Hfq binds shiA and sodB mRNAs <20 nucleotides away from the 3′ and 5′ of the sRNA annealing site, respectively 20, 21. Spot42 targets were also found to have AAN motifs within 14 nt of the target site 22. By contrast, flhA mRNA simultaneously binds Hfq distal and proximal surfaces at a distance of 50 nt and 30 nt to the OxyS sRNA binding site, respectively 23. The AAN motif in rpoS mRNA is ≥ 60 nt upstream of the sRNA binding site. These examples suggest that sequence elements within each mRNA may fine tune interactions between Hfq and the sRNA target region, potentially influencing the regulatory outcome.
Here we show that the location of Hfq binding is important for up-regulation of rpoS expression by sRNAs in E. coli. Using an improved model of the rpoS mRNA secondary structure as a guide, we deleted the natural (AAN)4 and A6 Hfq binding motifs and introduced artificial A18 binding sites at different positions upstream and downstream of the sRNA target site. Though in vitro assays showed that Hfq binds A18 sequences anywhere in the rpoS mRNA, Hfq only supports sRNA regulation in vivo when recruited to its original location or a site immediately upstream of the sRNA target site. We propose that the native Hfq binding site is optimal because it can communicate with the downstream inhibitory stem-loop through a flexible hinge between two structural domains of the rpoS leader.
RESULTS
The full-length rpoS leader forms three domains
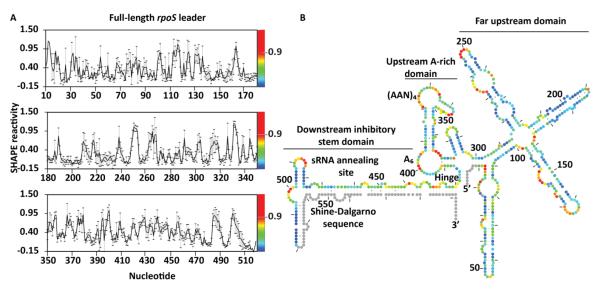
A previous model of a 323 nt fragment of the rpoS mRNA showed that the single-stranded (AAN)4 Hfq binding motif was imbedded in secondary structure upstream of the inhibitory stem-loop 12. To better predict how upstream sequences influence rpoS translation, we determined the secondary structure of the entire full-length rpoS leader using SHAPE to measure the backbone flexibility of individual nucleotide (Fig. 1A) 24, 25. SHAPE experiments were performed on transcripts starting from the rpoS promoter 567 bp upstream of the start codon and ending 30 nucleotides after the start codon. The scaled reactivity of each residue was converted to a pseudo-free energy term, ΔGSHAPE, and imported into the dynamic programming algorithm in the RNAstructure program to generate a secondary structure model (Fig 1B) 26-28.
Figure 1. Structural model of the full-length rpoS leader.
(A) Normalized SHAPE reactivity of individual nucleotides, showing the average modification (black line) and standard deviation (error bars) for at least three independent trials. The relative reactivity is represented in the spectrum from red (more reactive) to blue (less reactive). (B) Secondary structure of the full-length rpoS leader predicted by RNAstructure using SHAPE modification as an energy constraint. Colors as in (A). Grey circles represent 5′ and 3′ sequences that were not mapped in our experiments. The far upstream, A-rich and inhibitory stem-loop domains are indicated. The hinge refers to the flexible helix that connects the A6 loop and the inhibitory stem.
As expected, our SHAPE data on the full-length leader was consistent with the regulatory elements identified previously. First, the 3′ end of the leader formed an inhibitory stem-loop between the sRNA annealing site and the Shine-Dalgarno sequence (Fig. 1B), identical to the structure based on genetic data 8 and structure probing of the truncated leader 12, 29. Second, the upstream (AAN)4 and A6 motifs that bind the distal face of Hfq were again predicted to reside in two single-stranded regions of the leader, separated by a short helix similar to our previous model 12.
On the other hand, our new model predicted three additional features likely to be important for translational control (Fig. 1B). First, the inhibitory stem was extended by 15 bp due to the base pairing between the upstream of the sRNA annealing site and the coding sequence. This extended base pairing is supported by reduced modification of the 5′ side of the stem (nt 435 – 450); the 3′ side (nt 570-590) is covered by the cDNA primer and was not assayed in our SHAPE experiments. The sRNA target site (nt 446-470) was less modified than the rest of the inhibitory stem, suggesting that base pairs between the sRNA target and the Shine-Dalgarno sequence are more stable than those involving the coding region. The longer inhibitory stem, however, may account for the lower basal expression of rpoS mRNAs containing the full-length leader 10.
Second, the hinge region between the inhibitory stem and the upstream AAN motifs was shortened and joined to the far upstream region as a four-way-junction. The moderate SHAPE reactivity of residues just downstream of the four-way-junction indicated that the hinge is dynamic and could allow for tertiary contacts between the upstream and downstream domains.
Third, the far upstream region was predicted to form four extended helices with very low SHAPE reactivity, indicating that the 5′ half of the leader forms a highly stable secondary structure. Thus, the full-length rpoS leader can be divided into three domains: the far upstream domain, the A-rich domain containing the strong Hfq binding site, and the downstream inhibitory stem domain. Based on the extent of modification, the A-rich domain and flexible hinge were more dynamic than other regions of the leader.
Ectopic Hfq binding sites rescue rpoS regulation in vivo
We next attempted to understand how the functional elements of the rpoS leader were organized to interact with Hfq, the regulatory sRNAs, and the translation machinery. Upstream (AAN)4 and A6 motifs specifically bind the distal face of Hfq and contribute to sRNA regulation 10, 12. We asked whether the position of the Hfq binding site is important for regulation by relocating A-rich motifs to other regions of the rpoS leader.
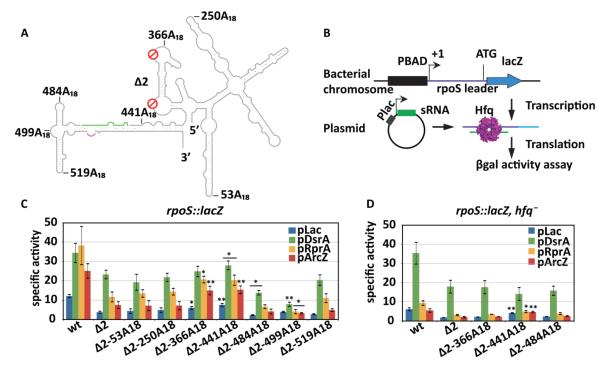
A double mutant (rpoSΔ2) lacking both the (AAN)4 and A6 motifs cannot bind Hfq tightly, and consequently Hfq no longer facilitates its annealing with the sRNAs 10. Using our secondary structure model as a guide, we inserted an A18 sequence cassette at various positions in the three domains of rpoSΔ2 (Fig. 2A). Insertion sites chosen for further study were in the far upstream region (positions 53 and 250), the original (AAN)4 loop (position 366), 5′ of the inhibitory stem (position 441), and the 3′ of the sRNA binding site (positions 484, 499, and 519) (Fig. 2A). All of these sites were designed to minimize perturbations to the mRNA structure, as predicted by MFOLD 30. Partial SHAPE modification data of Δ2-366A18 and Δ2-519A18 RNAs indicated these insertions do not grossly change the secondary structure of the inhibitory domain (data not shown).
Figure 2. Hfq location determines rpoS translational activation in vivo.
(A) Schematic representation of A18 insertions in the full-length rpoS leader lacking both A-rich motifs (Δ2). The positions are numbered from the rpoS transcription start site. The sRNA annealing site, green; Shine-Dalgarno, purple. (B) Experimental design. The rpoS leader sequence (606 nt) was fused with a chromosomal lacZ downstream of a PBAD promoter. DsrA, RprA, or ArcZ sRNA was overexpressed from the Plac promoter on plasmids. Hfq was expressed from its endogenous gene. (C) sRNA activation of rpoS-lacZ translation. Specific activity of β-galactosidase in strains carrying the rpoS-lacZ fusion listed below the x-axis, and transformed with the control plasmid pLac (blue), or plasmids overexpressing DsrA (green), RprA (yellow), and ArcZ (red). Error bars represent the standard deviation of three independent trials. Strains carrying Δ2-A18-lacZ fusions were compared to the one carrying Δ2-lacZ fusions for their specific activities by unpaired one-tail T-test. Significantly different expression level is marked as * (p < 0.05) or ** (p < 0.01). Expression levels of all the mutants were significantly lower than wt with p < 0.01. (D) sRNA activation of rpoS-lacZ fusions in an hfq− background. Same as in C. Mutant rpoS-lacZ fusions were compared to wt rpoS-lacZ fusion by unpaired one-tail T-test; * (p < 0.05) or ** (p < 0.01).
To determine whether these ectopic Hfq binding sites could support rpoS regulation in vivo (Fig. 2B), the rpoS leaders were fused to the lacZ reading frame in the E. coli chromosome as previously described 31. RpoS translation was induced by overexpression of DsrA, RprA, or ArcZ sRNA (pLac), and expression of the rpoS-lacZ fusion was obtained from the specific β-galactosidase activity of cell cultures in the late-log to early-stationary phase.
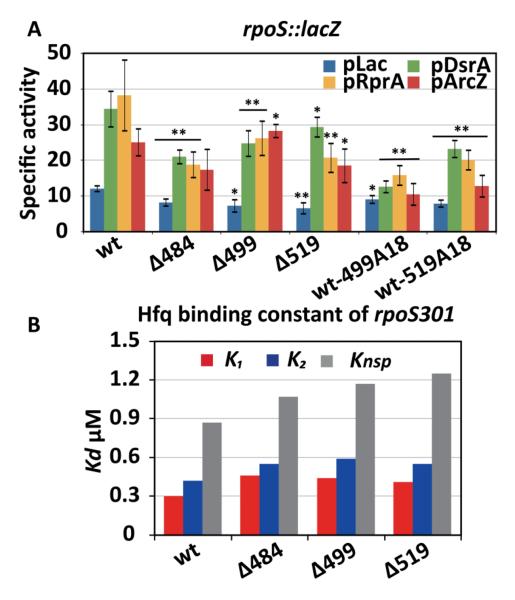
As expected, rpoSΔ2-lacZ fusions lacking the upstream AAN motifs were translated 30% - 50% less than the wild type rpoS-lacZ fusion, when DsrA, RprA or ArcZ sRNAs were over-expressed in the presence of IPTG (Fig. 2C, green, yellow and red bars). Basal expression of rpoSΔ2-lacZ in the presence of empty vector (pLac) was also 30% lower that for the wild type rpoS fusion (Fig. 2C, blue bars), indicating that the AAN motifs are important for activation by endogenous sRNAs. As cells over-expressing DsrA (Fig. 2C, green bars) can induce rpoS without Hfq 10, these A18 strains were least affected by deletion of the AAN motifs.
We next asked whether A18 insertions could rescue Hfq-dependent translation of rpoSΔ2. When the A18 Hfq binding site was located far upstream in the rpoS leader (positions 53 and 250), the expression levels remained the same, suggesting these sites are too far from the sRNA target site. When A18 was inserted at the natural location of the (AAN)4 motif (position 366) or 5′ of the sRNA target site (position 441), basal and activated expression improved about 2-fold compared to rpoSΔ2 (Fig. 2C, Δ2-366A18 and Δ2-441A18), suggesting that activation by endogenous and over-produced sRNAs is restored when Hfq binds near the sRNA target site.
By contrast, A18 insertions 3′ of the sRNA target site did not rescue rpoS expression (positions 484, 499, and 519). In fact, insertions at positions 484 and 499 decreased the level of expression by half under some conditions (Fig. 2C, Δ2-484A18 and Δ2-499A18). Therefore, the level of expression depended on the position of the A18 insertion and was optimal when this sequence was placed just upstream of the sRNA (Fig. 2C).
rpoS regulation requires 5′ Hfq binding in vivo
If the rpoSΔ2-A18 fusions rescue expression by recruiting Hfq to the mRNA, then deleting hfq from the cells should erase any change of expression induced by A18 insertions. We deleted hfq from strains containing the rpoS::lacZ fusions, and re-measured the ability of sRNAs to activate rpoS translation. Deleting hfq lowered expression of all the rpoS fusions tested (Fig. 2D), apart from strains over-expressing DsrA that do not require Hfq to induce rpoS expression 10. Moreover, the Δ2-366A18 and Δ2-484A18 fusions were expressed at a level similar to the rpoSΔ2 mutant in the hfq− strains. This indicates that the different activities of the A18-containing leaders arise from their ability to bind Hfq, and not from a difference in the mRNA stability or translation levels.
When A18 was inserted at position 441 immediately 5′ of the sRNA binding site, expression was slightly higher even without Hfq. We speculate that this insertion weakens the inhibitory stem-loop, causing leaky repression of translation initiation. None of the rpoSΔ2-A18 reporters were as active as the wt rpoS::lacZ fusion, possibly because the (AAN)4 deletion alters the structure of the Hfq binding domain 10. Nonetheless, the ability of A18 insertions to partially restore rpoS translation in the presence of Hfq suggested that sRNA regulation requires recruitment of Hfq to an AAN binding site within 80 nt upstream of the sRNA.
A18 insertion restores tight Hfq binding in vitro
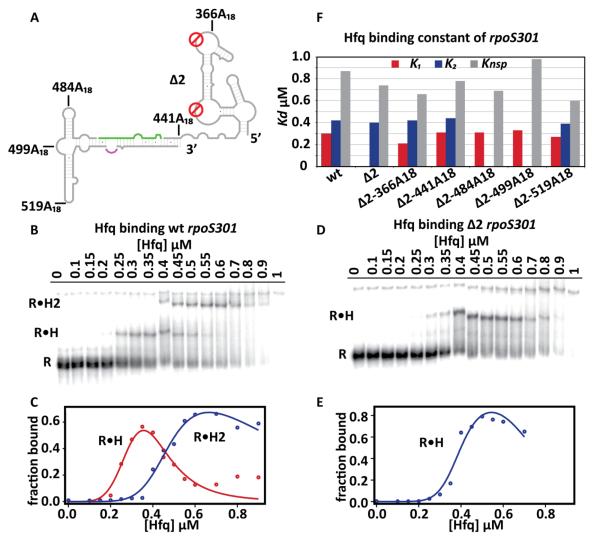
We next analyzed how the A18 insertions at different positions affect Hfq binding to the rpoS mRNA leader. Based on our structural model of the full-length leader (Fig. 1B), we designed a new 301 nt rpoS leader that lacks the far upstream helices (nt 9–300) that are not required for Hfq binding 12, but retains the 5′ fragment (nt 1–8) that forms part of the hinge region. The resulting rpoS301 leader contained the upstream (AAN)4 and A6 motifs, the flexible hinge, and the downstream inhibitory stem (Fig. 3A). It migrated as single species in native polyacrylamide gel, indicating more uniform folding than the previous rpoS323 truncation 10, 12.
Figure 3. Hfq binds rpoS mRNA with A18 insertions.
(A) Schematic representation of the rpoS301 leader that lacks the far upstream domain. (B) Hfq titrations of uniformly labeled wt rpoS301 at 25°C. Free rpoS (R) binds one or two Hfq multimers (R•H and R•H2). A smear of complexes at the top of the gel in high Hfq are not shown. Around 1% of the rpoS301 formed a slow migrating band that did not bind Hfq. (C) Fraction of bound wt rpoS301 as a function of [Hfq] was fit to eq. 2 (Methods). Red, R•H (k1); blue, R•H2 (k2). The fraction of R•H and R•H2 decreased at high [Hfq] due to formation of high molecular weight (non-specific) complexes. (D,E) Hfq titrations of uniformly labeled rpoS301 Δ2 at 25°C as in B and C, except data were fit to eq. 1. (F) Comparison of the Hfq binding constants among rpoS301 wt, Δ2, and Δ2-A18 fusions. Data for Δ2-A18 fusions are shown in Fig. S1; Kd values are listed in Table S1. For rpoS301 wt, S.D. < 0.02 μM (7%) among 3+ trials.
We previously found that a 323 nt rpoS RNA bound multiple Hfq hexamers in native (TBE) polyacrylamide gels, described by one tight and several nonspecific binding sites with dissociation constants KT = 0.28 μM and Knsp = 1 μM Hfq monomer 12. To better discriminate between specific and non-specific Hfq binding, we supplemented the running buffer with 2 mM Mg2+ to stabilize the folded RNA. Under these conditions, we observed two specific rpoS•Hfq complexes (R•H and R•H2) for WT rpoS323 (data not shown) and rpoS301 (Fig. 3B) with dissociation constants K1 = 0.30 μM and K2 = 0.42 μM Hfq monomer corresponding to distinct transitions in the Hfq titrations (Fig. 3C). Our model also included a nonspecific binding term (Knsp = 0.87 μM) to account for high molecular weight complexes that did not enter the gel.
The rpoS301Δ2 RNA, which lacks the upstream AAN motifs, formed only one specific Hfq complex (Fig. 3D), with K2 = 0.40 μM Hfq monomer (Fig. 3E). Therefore, the Δ2 mutation abolished the strongest Hfq binding site, corresponding to interactions with the (AAN)4 and A6 motifs (K1 ~ 0.3 μM Hfq monomer) 12. The second binding site, K2 ~ 0.4 μM, also behaved like a specific binding interaction, because it generated a second stable RNP and produced very similar Kd values for wild type and Δ2 rpoS301. By contrast, non-specific binding produced high molecular weight complexes that were poorly resolved in these gels. Because low affinity non-specific complexes (0.7–1 μM Hfq) were treated collectively in our partition function, the resulting Knsp values varied considerably among different rpoS RNAs.
With our optimized rpoS301 truncation and improved Hfq titration, we measured Hfq binding constants for all the rpoSΔ2-A18 fusions. All the A18 insertions restored tight Hfq binding with K1 values comparable to the wild type rpoS301 (0.26 to 0.33 μM; Fig. 3F, red bars). Like WT rpoS, rpoSΔ2-366A18, Δ2-441A18, and Δ2-519A18 formed a second specific complex with K2 ~ 0.4 μM (Fig. 3F, blue bars, and Fig. S1A, B and E). Surprisingly, the downstream insertions Δ2-484A18, and Δ2-499A18 (Fig. S1C and D) formed the first Hfq complex but not the second complex (Fig. 3F, blue bars). These insertions may disrupt the second binding site, or indirectly disrupt the second RNP by changing the rpoS mRNA conformation. In summary, all the A18 insertions tested were capable of binding Hfq, showing that recognition is independent of context. However, insertions at positions 484 and 499 in the inhibitory stem-loop domain disrupted a second specific interaction with Hfq.
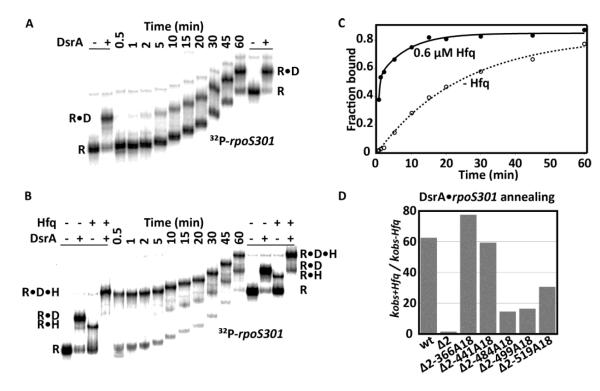
5′ Hfq binding facilitates DsrA•rpoS301 annealing in vitro
While Hfq was able to bind A18 sequences at any position in the rpoS leader, only A18 insertions 5′ of the sRNA target sequence rescued sRNA regulation in vivo. We next determined whether Hfq must be recruited to a specific location in the rpoS leader to facilitate annealing of DsrA sRNA with the rpoS301 RNA using native gel mobility shift assays. As expected, while WT rpoS301 annealed with DsrA slowly in the absence of Hfq (Fig. 4A), it rapidly formed a ternary complex with DsrA and 0.6 μM Hfq (Fig. 4B). Consistent with previous studies 10, the initial annealing rate with Hfq was 2.5 min−1, 62 times faster than with no Hfq (0.04 min−1; Fig. 4C,D). By contrast, rpoS301Δ2 was unable to form the ternary complex, and the annealing kinetics was about the same with and without Hfq (0.018 min−1 and 0.012 min−1, respectively; Fig. S2A).
Figure 4. Upstream A18 insertions rescue sRNA annealing by Hfq.
(A) 200 nM DsrA with uniformly labeled wt rpoS301 without Hfq at 25°C over 60 min. Markers of R and R•D complexes were generated by incubating the same annealing reaction at 25°C for 2 hrs and were loaded before the first time point or after the last time point. (B) 200 nM DsrA binding to uniformly labeled wt rpoS301 with 0.6 μM Hfq as in B, with four markers showing R, R•D, R•H, and R•D•H complexes. Free rpoS301 migrated as multiple bands 0.5 min after the reaction started, which was not observed in B and may reflect a transient conformational change upon addition of Hfq. (C) Binding kinetics of rpoS and DsrA in the absence (open circles) and presence of Hfq (closed circles). No Hfq, kobs-Hfq = 0.04 min−1 (82.6%); 0.6 μM Hfq, combined R•D and R•D•H complexes formed with a fast phase kobs+Hfq = 2.50 min−1 (52 %) and slow phase kobs+Hfq = 0.12 min−1 (33%). (D) DsrA annealing rates of wt, Δ2, and Δ2-A18 rpoS301 RNAs reported as the ratio of the fast phase kobs+Hfq and kobs-Hfq. Data for Δ2 and Δ2-A18 insertions are shown in Fig. S2; rate constants are listed in Table S1.
We then compared the DsrA annealing kinetics with or without Hfq for all the rpoS301 RNAs containing A18 insertions. All the insertions except Δ2-441A18 had little effect on the annealing rate in the absence of Hfq (ranging from 0.011 to 0.016 min−1), suggesting that the DsrA•rpoS mRNA interaction alone was not altered by an A18 insertion at most positions (Fig. S2, left column). The Δ2-441A18 RNA was almost unable to base pair with DsrA without Hfq (Fig. S2, C left column), possibly because the insertion at nt 441 changes the rpoS mRNA structure (Fig. 3A).
All the A18 insertions allowed Hfq to facilitate sRNA annealing to some degree, depending on the location. First, unlike rpoSΔ2, they all formed stable ternary complexes with Hfq and DsrA (Fig. S2, middle column), indicating that any A-rich Hfq binding site in the rpoS leader was necessary and sufficient for stable ternary complexes. Second, Hfq accelerated DsrA annealing with all the Δ2-A18 RNAs (Fig. S2, right column), but insertions 5′ of the sRNA restored the annealing kinetics to the same level as WT rpoS301, while insertions 3′ of the sRNA did not (Fig. 4D). These data agreed with our in vivo results, that Hfq promotes sRNA-rpoS mRNA interactions most effectively when the AAN motif is placed 5′ of the sRNA annealing site.
Downstream A-/U-rich sequences stabilize rpoS mRNA conformation
Interestingly, positions 484, 499, and 519 are each adjacent to short A-/U-rich patches that might interact directly with Hfq or might form tertiary interactions in the rpoS leader. We first confirmed whether those A-/U-rich sequences are functionally important by converting them to G-/C-rich sequences (Fig. 2A; Δ484, Δ499, and Δ519), and measuring up regulation of those mutants by Hfq and sRNAs using β-galactosidase assay described above. The Δ484, Δ499, and Δ519 mutations all reduced expression of rpoS-lacZ significantly compared to the wild type rpoS leader (Fig. 5A). Mutation of the U-rich loop at position Δ484 was the most detrimental, decreasing expression levels by average 40%, followed by Δ519 with expression levels decreased by ~30%, and Δ499 with a moderate 20% decrease of expression levels.
Figure 5. Downstream A-/U-rich motifs contribute to regulation of rpoS translation.
(A) sRNA activation of rpoS::lacZ fusions in vivo, as in Fig. 2C. Hairpin loops at the positions indicated were changed to C-/G-rich sequences, in the wt rpoS background. Significantly different expression of mutants from the wt rpoS-lacZ fusion is marked as * (p < 0.05) or ** (p < 0.01). (B) Hfq binding to wt and mutated rpoS301 RNAs was measured as in Figure 3 and fit to eq. 2 as shown in Fig. S3.
We next tested whether these A-/U-rich sequences were Hfq binding sites. The Δ484, Δ499, and Δ519 mutants all formed two specific Hfq complexes in vitro (Fig. S3, left column), suggesting that both tight and weak Hfq binding sites were retained even though insertions at these positions disrupted the weak complex. All three single mutations weakened Hfq binding overall, however, increasing K1 from 0.3 μM to 0.45 μM, and K2 from 0.4 μM to 0.55 μM (Fig. 5B). While we cannot exclude the possibility that the A-/U-rich sequences contact Hfq directly, these data suggested that these loops fold the rpoS leader in a manner that favors Hfq recognition.
If our original A18 insertions at positions 499 and 519 (Δ2-499A18 and Δ2-519A18) lowered rpoS expression by disrupting tertiary interactions formed by the A-/U-rich sequences at those positions, inserting A18 into the wild type rpoS leader at positions 499 and 519 should cause a similar loss of function. In fact, both wt-499A18 and wt-519A18 showed reduced expression compared to WT rpoS::lacZ (Fig. 5A). We found that wt-499A18 was more deleterious than wt-519A18 (60 - 64% and 33% - 57% reduction, respectively), consistent with our results with Δ2-499A18 and Δ2-519A18 (Fig. 2C). Together, these data suggest that the downstream A-/U-rich sequences are important for rpoS function because they stabilize the tertiary conformation of the mRNA and Hfq recognition, and that this accounts for why Hfq binding sites downstream of the sRNA target site reduce rpoS up-regulation.
DISCUSSION
Position of the AAN motif in the rpoS leader affects Hfq regulation
Most bacterial mRNAs regulated by sRNAs contain AAN motifs that bind Hfq 5. The upstream (AAN) motifs in the rpoS mRNA leader are required for Hfq-dependent regulation of rpoS translation by sRNAs in E. coli 10 and accelerated sRNA-mRNA base pairing in vitro 11, 12. By relocating the AAN motif within the rpoS leader, we found that Hfq recognized this motif at all the positions tested, but Hfq-dependent regulation was only rescued when its binding site was placed close to the 5′ side of the sRNA annealing site. Therefore, the location of Hfq within the rpoS leader determines its chaperone function and its ability to act in translational control.
Our study identifies three factors that determine how the position of the AAN motif affects Hfq regulation. First, experiments using short unstructured RNAs showed that Hfq must interact with both the sRNA and mRNA to catalyze the formation of base pairs 17, and is most effective when bound within 20 nt of the sRNA target site 19. Although the rpoS (AAN)4 motif is about 60 nt upstream of the sRNA binding site, our results suggest the flexible hinge allows the Hfq-binding domain to fold back on the inhibitory stem-loop, placing the lateral rim of Hfq where it can engage an incoming sRNA and its mRNA complement. In agreement with this hypothesis, A18 insertions far upstream (positions 53 and 250) or far downstream (position 519) of the sRNA binding site did not restore Hfq regulation, suggesting that the necessary tertiary interaction with the inhibitory stem was abolished.
Second, downstream A-/U-rich sequences may help position Hfq to interact with the sRNA binding site. Our results show that A-/U-rich sequences at positions 484 and 499 in the rpoS leader contribute to Hfq regulation, as replacing them with A18 or mutating them reduced Hfq regulation in vivo and weakened Hfq binding in vitro. The loop at position 484 was protected by Hfq in ribonuclease footprinting experiments on a minimal rpoS mRNA and may interact with Hfq directly 29. These nucleotides may also make RNA interactions that stabilize the overall conformation of the rpoS leader.
Third, A18 insertions in the inhibitory stem-loop of rpoS disturb the balance between translation repression and activation and are thus disadvantageous for sRNA regulation. This was suggested by the observation that the A18 insertion at the natural (AAN)4 site (position 366) restored Hfq function better than the A18 insertion immediately 5′ of the sRNA binding site (position 441), though in vitro studies predicted the latter should be more effective.
The A18 insertion next to the sRNA target site may hinder DsrA base pairing. In agreement with that, Δ2-441A18 barely annealed to DsrA in the absence of Hfq, although Hfq accelerated this reaction almost 60-fold. Additionally, the A18 insertion in the inhibitory stem-loop likely destabilizes its secondary structure, as basal expression of rpoS Δ2-441A18 was slightly elevated when DsrA was over-expressed in the hfq− strain (Figure 2D) or in the pLac control in the hfq+ strain (Figure 2C). Higher basal translation initiation compensated for the reduced sRNA annealing rate, so that expression of Δ2-441A18 in the wt background was equal to expression of Δ2-366A18.
General implications for Hfq regulation of other mRNAs
In mRNAs regulated by Hfq and sRNAs, the AAN binding motif occurs at various positions with respect to the start of the open reading frame and the binding sites for sRNAs 20-23. The three factors discussed above suggest how the structural context of Hfq binding can modulate the efficiency of sRNA regulation in other mRNAs.
First, the optimal location for Hfq likely depends on whether the sRNA increases or decreases expression. In many negatively regulated mRNAs in which the sRNA represses translation by base pairing with the Shine-Dalgarno sequence, Hfq binds near the sRNA target site 32. Our results predict this proximity between Hfq and the sRNA target maximizes the ability of Hfq to facilitate sRNA annealing 19. In some cases, Hfq binds so close to the Shine-Dalgarno sequence that Hfq directly competes with ribosome entry 21. In studies using artificial mRNA and sRNA mimics, a stable Hfq-sRNA-mRNA ternary complex recruits RNase E to the message via interactions with a 5′ monophosphate on the sRNA 33. Moreover, Hfq was shown to directly recruit RNase E to ptsG mRNA for SgrS sRNA mediated degradation 34. Thus, strong Hfq binding sites near the site of regulation may make translation repression or mRNA turnover more efficient.
On the other hand, positively regulated mRNAs tend to adopt more complicated secondary structures that inhibit translation initiation in the absence of sRNA but open to permit ribosome binding when the sRNA is present 5. In these examples, Hfq may bind far from the sRNA, in which case it is presumably delivered to the site of the action by the mRNA structure. We found that the rpoS leader is organized into distinct domains dedicated to inhibition and Hfq binding. Our results suggest this organization will be generally advantageous for positive control, because Hfq can be recruited to the mRNA without disrupting its inhibitory self-structure. This organization may also allow Hfq to release the sRNA-mRNA double helix once annealing is complete.
Finally, other sequence elements in the mRNA may fine-tune or stabilize Hfq interactions. Downstream A-/U-rich patches in rpoS stabilize Hfq binding to the upstream (AAN)4 motif and improve Hfq-dependent regulation, consistent with previous findings that Hfq restructures certain mRNAs 35, 36. In flhA mRNA, a second Hfq binding site was identified that interacted with the proximal face of Hfq and enhanced binding of the distal face to AAN motifs 23. Alternatively, A-/U-rich patches in the mRNA may help displace the sRNA from the lateral rim of Hfq, where the body of the sRNA has been shown to bind 18. Though further work is needed to fully understand how Hfq interacts with its mRNA targets, the results presented here demonstrate that the ability of Hfq to act in sRNA regulation depends on its structural context.
MATERIALS AND METHODS
SHAPE footprinting
Full-length rpoS leader RNA was renatured by heating to 75 °C for 1 min and cooling to room temperature for 5 min before use. For SHAPE modification, rpoS mRNA (0.5 pmol) was combined with 2 μL 5X TNK buffer (50 mM Tris-HCl pH 8.0, 250 mM NaCl, 250 nM KCl), 2 μL 5X Hfq buffer (50 mM Tris–HCl pH 7.5, 1 mM EDTA, 250 mM NH4Cl) and 2 μL TE in 10 μL total volume and incubated up to 2 hr at 25°C. 1-methyl-7-nitroisatoic anhydride (1M7; 1 μL 5 mM in anhydrous DMSO) was added and allowed to react completely. Reactions were diluted to 200 μL with 0.1 mg/mL carrier tRNA, extracted with phenol and chloroform, the RNA precipitated with ethanol and resuspended in 2 μL water. Modified RNA was analyzed by primer extension with dye-labeled primers as previously described 12, 24 using a Beckman CEQ8000.
Raw CE peaks were assigned and integrated using ShapeFinder 37. After background subtraction, peak volumes were scaled to the average of the top 10% of values after exclusion of outliers using model free statistics 27. Data from 7-8 independent experiments were averaged and the one-sided Grubb’s test used to pick single outliers between different experiments. Average SHAPE reactivities (negative numbers were set to zero) were used to constrain secondary structures predicted by RNAstructure 27.
Construction of rpoS mutants and A18 insertions
The pUC18 plasmids of full-length wt and Δ2 rpoS were published previously and were used as templates for generating rpoS mutations and A18 insertions in this study 10, 12. The plasmid carrying wt rpoS was used to derive Δ484, Δ499, Δ519, wt-499A18, and wt-519A18; the plasmid carrying Δ2 rpoS was used to derive Δ2-366A18, Δ2-441A18, Δ2-484A18, Δ2-499A18, and Δ2-519A18. All the constructs were made by inverse PCR as described previously 38. The primers for generating mutations or insertions are listed in Table S2.
β-Galactosidase assays
Bacterial strains (Table S3) containing chromosomal rpoS::lacZ fusions were constructed from strain PM1205 as previously described 10. DNA fragments for making the recombinant bacterial strains and in vitro transcription were generated by PCR amplification of the corresponding pUC18 plasmids with primers Pbad-rpoS-F and lacZ-rpoS10aa-R (Table S2). The hfq− strains were generated by P1 phage transduction 39. The strains were transformed with pLac, pDsrA, pRprA, or pArcZ plasmids that were previously published 10.
Overnight cultures of transformed cells were diluted 1:500 into fresh LB medium containing ampicillin (100 μg/mL), arabinose (0.2%), and IPTG (100 μM). They were grown at 37°C with 250 rpm agitation for about 4-6 hrs or until their OD600 reached 0.6-0.8. The OD600 was measured, and the cultures were lysed assayed for β-galactosidase activity as described previously 8. The reaction velocities were determined using a microplate reader (Molecular Device Thermomax), and the specific activity was calculated as Vmax/OD600. The Vmax of each culture was the average of 3 aliquots from the same culture, and the reported specific activity of the strain was the average of at least 3 independent experiments.
RNA Preparation
In vitro transcription templates of rpoS301 were generated by PCR amplification of the corresponding pUC18 plasmids with primers rpoS301F and rpoS576R (Table S2). The unlabeled RNAs were transcribed with T7 RNA polymerase from the PCR templates described above. The RNAs were purified by denaturing PAGE and recovered by phenol-chloroform extraction and ethanol precipitation, as described previously 40. The 32P labeled rpoS301 was transcribed in vitro in the presence of α32P-ATP 12, 29 and purified through Chroma spin+ TE-100 columns (Clontech Laboratories).
Hfq Preparation
Wild-type E.coli Hfq was overexpressed and harvested as previously described 41. The cells were homogenized (Avestin EmulsiFlex-C3) in 50 mL lysis buffer (50 mM HEPES pH 7.5, 0.5 M NaCl, 20 mM imidazole, 5% glycerol), followed by 1 hr DNase I treatment on ice. The cell lysate was centrifuged and filtered prior to loading on a prepared 5 mL Hi-Trap Co2+ column. The column was washed with 50 mL lysis buffer and 150 mL wash buffer (50 mM HEPES pH 7.5, 1 M NaCl, 20 mM imidazole, 5% glycerol), the eluted with 25 mL wash buffer containing 250 mM imidazole. The eluant was concentrated to 5 mL and dialyzed overnight against 1 L 20 mM HEPES pH 7.5, 100 mM NaCl, 0.5 mM EDTA. The protein sample was loaded on a UNO S6 ion exchange column (Bio-Rad), washed with dialysis buffer at 2 mL/min flow rate for 30 min and eluted with a linear gradient of 0.1 M to 1 M NaCl. The desired fractions were pooled and dialyzed against 1 L of Hfq storage buffer (50 mM Tris-HCl pH 7.5, 1 mM EDTA, 250 mM NH4Cl, 10% glycerol).
Hfq Binding
The equilibrium binding reactions with Hfq and rpoS301 RNA were assembled as previously described 12. After 10 min at room temperature, 2 μL aliquots were loaded on a native 6% polyacrylamide gel in 1X THEM2 (66 mM HEPES, 34 mM Tris, 0.1 mM EDTA, 2 mM MgCl2). The fractions of bound rpoS, fRH, were quantified as previously described 29 and were fit with a partition function (IGOR Pro, WaveMetrics). For rpoS constructs that formed only one specific complex with Hfq, we assumed two unequal independent binding sites
| (1a) |
| (1b) |
in which [Hfq] is the concentration of Hfq monomers, K1 is the dissociation constant of the specific complex, K2 is the apparent dissociation constant of nonspecific binding, and n is the Hill coefficient. Similarly, for rpoS constructs that formed two specific Hfq complexes, R•H and R•H2 fractions were fit to the second and third terms of a partition function assuming three unequal independent binding sites:
| (2a) |
| (2b) |
| (2c) |
DsrA annealing
Reactions to measure the annealing kinetics of DsrA and rpoS301 were assembled as previously described 12, except that the native gel was done in 1X THEM2 buffer as described above. The fractions of bound rpoS were quantified as previously described 29 and were fit with either a single or double exponential rate equation (IGOR Pro, WaveMetrics)
| (3) |
| (4) |
in which f , f1, and f2 are the fractions of the reaction that followed the corresponding annealing rate, t is the annealing time, kobs1 is the apparent annealing rate for the slow phase reaction, kobs2 is the apparent annealing rate for the fast phase reaction.
Supplementary Material
Highlights.
Regulation of rpoS translation by sRNAs requires an AAN binding site for Hfq protein
The Hfq binding domain is structurally distinct from the translation control domain
The AAN binding site was relocated to 7 new positions in the rpoS mRNA
Hfq enables sRNA regulation in E. coli and in vitro only when bound 5′ of the sRNA
Structural context of Hfq binding determines the efficiency of sRNA regulation
ACKNOWLEDGEMENTS
The authors thank S. Panja for Hfq purification, and N. Majdalani and S. Gottesman for assistance and bacterial strains. This work was supported by a grant from the National Institute of General Medicine (R01 GM46686) to S.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benjamin JA, Desnoyers G, Morissette A, Salvail H, Masse E. Dealing with oxidative stress and iron starvation in microorganisms: an overview. Can. J. Physiol. Pharmacol. 2010;88:264–272. doi: 10.1139/Y10-014. [DOI] [PubMed] [Google Scholar]
- 2.Mandin P, Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 2010;29:3094–3107. doi: 10.1038/emboj.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstle K, Klatschke K, Hahn U, Piganeau N. The small RNA RybA regulates key-genes in the biosynthesis of aromatic amino acids under peroxide stress in E. coli. RNA Biol. 2012;9:458–468. doi: 10.4161/rna.19065. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a003798. 10.1101/cshperspect.a003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobrero P, Valverde C. The bacterial protein Hfq: much more than a mere RNA-binding factor. Crit. Rev. Microbiol. 2012;38:276–299. doi: 10.3109/1040841X.2012.664540. 10.3109/1040841X.2012.664540; 10.3109/1040841X.2012.664540. [DOI] [PubMed] [Google Scholar]
- 7.Hengge-Aronis R. Stationary phase gene regulation: what makes an Escherichia coli promoter sigmaS-selective? Curr. Opin. Microbiol. 2002;5:591–595. doi: 10.1016/s1369-5274(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 8.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Updegrove T, Wilf N, Sun X, Wartell RM. Effect of Hfq on RprA-rpoS mRNA pairing: Hfq-RNA binding and the influence of the 5′ rpoS mRNA leader region. Biochemistry. 2008;47:11184–11195. doi: 10.1021/bi800479p. [DOI] [PubMed] [Google Scholar]
- 12.Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008;14:1907–1917. doi: 10.1261/rna.1110608. 10.1261/rna.1110608; 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikulecky PJ, Kaw MK, Brescia CC, Takach JC, Sledjeski DD, Feig AL. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat. Struct. Mol. Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Link TM, Valentin-Hansen P, Brennan RG. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19292–19297. doi: 10.1073/pnas.0908744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauer E. Structure and RNA-binding properties of the bacterial Lsm protein Hfq. RNA Biol. 2013;10:610–618. doi: 10.4161/rna.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panja S, Schu DJ, Woodson SA. Conserved arginines on the rim of Hfq catalyze base pair formation and exchange. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt521. 10.1093/nar/gkt521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer E, Schmidt S, Weichenrieder O. Small RNA binding to the lateral surface of Hfq hexamers and structural rearrangements upon mRNA target recognition. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9396–9401. doi: 10.1073/pnas.1202521109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panja S, Woodson SA. Hfq proximity and orientation controls RNA annealing. Nucleic Acids Res. 2012;40:8690–8697. doi: 10.1093/nar/gks618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevost K, Salvail H, Desnoyers G, Jacques JF, Phaneuf E, Masse E. The small RNA RyhB activates the translation of shiA mRNA encoding a permease of shikimate, a compound involved in siderophore synthesis. Mol. Microbiol. 2007;64:1260–1273. doi: 10.1111/j.1365-2958.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 22.Beisel CL, Updegrove TB, Janson BJ, Storz G. Multiple factors dictate target selection by Hfq-binding small RNAs. EMBO J. 2012;31:1961–1974. doi: 10.1038/emboj.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salim NN, Feig AL. An upstream Hfq binding site in the fhlA mRNA leader region facilitates the OxyS-fhlA interaction. PLoS One. 2010;5:e13028. doi: 10.1371/journal.pone.0013028. doi: 10.1371/journal.pone.0013028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson KA, Merino EJ, Weeks KM. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- 25.Mitra S, Shcherbakova IV, Altman RB, Brenowitz M, Laederach A. High-throughput single-nucleotide structural mapping by capillary automated footprinting analysis. Nucleic Acids Res. 2008;36:e63. doi: 10.1093/nar/gkn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE) J. Am. Chem. Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- 27.Deigan KE, Li TW, Mathews DH, Weeks KM. Accurate SHAPE-directed RNA structure determination. Proc. Natl. Acad. Sci. U. S. A. 2009;106:97–102. doi: 10.1073/pnas.0806929106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Low JT, Weeks KM. SHAPE-directed RNA secondary structure prediction. Methods. 2010;52:150–158. doi: 10.1016/j.ymeth.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lease RA, Woodson SA. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J. Mol. Biol. 2004;344:1211–1223. doi: 10.1016/j.jmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandin P, Gottesman S. A genetic approach for finding small RNAs regulators of genes of interest identifies RybC as regulating the DpiA/DpiB two-component system. Mol. Microbiol. 2009;72:551–565. doi: 10.1111/j.1365-2958.2009.06665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Lay N, Schu DJ, Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J. Biol. Chem. 2013;288:7996–8003. doi: 10.1074/jbc.R112.441386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandyra KJ, Said N, Pfeiffer V, Gorna MW, Vogel J, Luisi BF. The seed region of a small RNA drives the controlled destruction of the target mRNA by the endoribonuclease RNase E. Mol. Cell. 2012;47:943–953. doi: 10.1016/j.molcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda Y, Yagi M, Morita T, Aiba H. Hfq binding at RhlB-recognition region of RNase E is crucial for the rapid degradation of target mRNAs mediated by sRNAs in Escherichia coli. Mol. Microbiol. 2011;79:419–432. doi: 10.1111/j.1365-2958.2010.07454.x. [DOI] [PubMed] [Google Scholar]
- 35.Geissmann TA, Touati D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soper TJ, Doxzen K, Woodson SA. Major role for mRNA binding and restructuring in sRNA recruitment by Hfq. RNA. 2011;17:1544–1550. doi: 10.1261/rna.2767211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasa SM, Guex N, Wilkinson KA, Weeks KM, Giddings MC. ShapeFinder: a software system for high-throughput quantitative analysis of nucleic acid reactivity information resolved by capillary electrophoresis. RNA. 2008;14:1979–1990. doi: 10.1261/rna.1166808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Russell DW. Inverse PCR. CSH Protoc. 2006;2006:10–1101. doi: 10.1101/pdb.prot3487. [DOI] [PubMed] [Google Scholar]
- 39.Moore SD. Assembling new Escherichia coli strains by transduction using phage P1. Methods Mol. Biol. 2011;765:155–169. doi: 10.1007/978-1-61779-197-0_10. [DOI] [PubMed] [Google Scholar]
- 40.Zaug AJ, Grosshans CA, Cech TR. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry. 1988;27:8924–8931. doi: 10.1021/bi00425a008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang A, Wassarman KM, Ortega J, Steven AC, Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.