Abstract
The brain mechanisms by which sensory cues become transformed into expectations of impending events are a critical component of cognitive tuning of sensory processing. However, distinctions between the afferent processing of cue-related activity itself versus those mechanisms supporting the contextual meaning imparted to the cue remain limited. Do sensory cues with equal meaning engage similar patterns of brain activations even if they are delivered in separate modalities? To address this question, we used functional magnetic resonance imaging of an expectation paradigm in which cues were delivered with visual or innocuous thermal stimuli. Cues were designed to be highly meaningful since they predicted the delivery of high and low painful stimuli. As expected, the cues themselves activated unimodal sensory cortices. This cue modality-specific activation was transformed into a pattern of activity reflecting cue meaning. Cues signaling high pain produced greater activity in the left dorsolateral prefrontal cortex and anterior cingulate cortex. Such activity is consistent with the graded encoding of the magnitude of expected pain. In contrast, cues signaling low pain produced greater activity in the right intraparietal sulcus. This activation may reflect processes directing spatial attention to the stimulated body region in order to more accurately evaluate the relatively weak, low pain stimulus. Taken together, these findings indicate that cues arising from different sensory modalities ultimately engage common brain mechanisms that reflect the meaning of the cue. This meaning-related activity is presumably critical for preparing sensory systems to optimally process afferent information.
Introduction
Expectations of impending sensory events can be conceptualized as a sequence of multiple neural processes. First, expectations associated with an imminent sensory event are initiated by cues arising from one or more sensory modalities. Studies on expectations and placebo frequently utilize visual, auditory, or even somatosensory cues to inform the subject of impending painful stimuli [33, 24, 3]. Accordingly, modality-specific sensory areas are necessarily engaged in order to extract relevant sensory features during the initial processing of incoming cue information. Next, this cue-related sensory information needs to be invested with meaning based, in part, on prior knowledge and experience [17]. This process presumably involves episodic memory retrieval [6, 16, 36]. The investment of meaning subsequently leads to the formation of a mental representation of the impending event [17]. This mental representation is maintained in working memory and can be used to prepare the nervous system to optimally process expected sensory information [4, 7, 5, 17].
To date, the neural mechanisms supporting the sequence of events by which sensory input is transformed into an expectation of pain remain poorly characterized. In particular, distinctions between the afferent processing of cue-related activity itself versus those mechanisms reflecting the contextual meaning imparted to the cue remain limited. However, brain mechanisms reflecting the imparted meaning of the cue would be predicted to be identical regardless of the modality of the sensory input activated during cue presentation.
To better understand which expectation-related processes are cue modality-specific and which are cue meaning-related, we performed an fMRI study in which subjects were informed regarding the intensity of an impending noxious stimulus by prior presentation of either visual or innocuous thermal cues. In each modality, cues for low and high intensity stimuli were used to impart specific meaning to the cue. Functional MRI then was used to characterize brain activity both during (cue-presentation period) and after (cue-maintenance period) cue presentation. We hypothesized that activity related to cue meaning would be modality-independent and would engage common higher association areas.
Methods
Subjects
Both the psychophysical and MRI components of the study were completed by 19 right-handed, healthy volunteers (8 males and 11 females, age 23–35 years mean: 27.5 years). Twelve subjects were white, four Asian, two multi-racial, and one African-American. Two additional subjects completed the psychophysical training session, but their MRI data was not available for analysis due to equipment malfunctions. All subjects gave written, informed consent acknowledging that they would experience painful stimuli, that all procedures and manipulations had been clearly explained, and that they were free to withdraw at any time without prejudice. All procedures were approved by the Institutional Review Board of Wake Forest School of Medicine.
Stimulation procedures for noxious heat stimuli
A TSA II thermal stimulator (Medoc Ltd., Ramat Yishai, Israel) with a 16 × 16 mm contact surface was used for noxious heat stimulation. The baseline temperature was 35°C. The stimulus temperature was changed with rise and fall rates of 4.5°C/s for 47°C stimuli and 5°C/s for 50°C stimuli in order to ensure the optimal stimulus synchronization.
Probes were placed on a specifically designed holder, after which the stimulated body region was positioned on the surface of the thermode. Noxious stimuli were delivered to the posterior aspect of the lower left leg. To minimize sensitization or adaptation, each experimental series was delivered to previously unstimulated skin regions.
Psychophysical training
Initially, all subjects were trained with thirty two, 5-s-duration stimuli (35–49°C) applied to the arm to give them experience in rating painful stimuli. Then subjects practiced the task by using two out of eight series of stimulations that were subsequently used in the scanner to ensure that they could feel the cues, adequately rate pain as well as to familiarize them with the task so as to minimize anxiety and expectation.
Experimental task
Each subject underwent 8 experimental series while in the MRI. Different types of trials within each series were pseudo-randomized, and task order was counterbalanced across subjects. Each 264-second experimental series consisted of four noxious stimuli of two intensities, low (47°C) and high (50°C). Both stimuli were 6 seconds in duration at the destination temperature (Fig. 1). Each noxious stimulus was preceded by a cue in either visual or thermal modality, informing subjects as to whether the upcoming stimulus would be of low or high intensity. Accordingly, we utilized a 2 × 2 factorial design to examine brain activation and psychophysical responses associated with the factors CUE MODALITY (visual versus somatosensory) and CUED PAIN INTENSITY (high versus low). For both modalities, cue delivery was completed 6 seconds before noxious stimuli onset.
Figure 1.
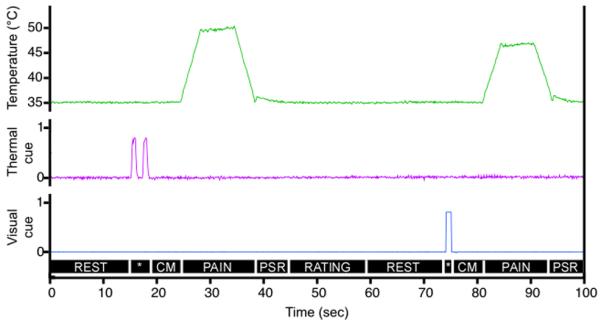
The temporal sequence of the task. Each noxious stimulus was preceded by a sensory cue (cue-presentation period, *) in either the visual (one or two white squares) or thermal (one or two 43°C pulses on the forearm) modality that informed subjects whether the upcoming stimulus would be of low or high intensity. The delivery of these cues was completed 6 s before noxious stimulation (cue-maintenance period, CM). Noxious stimuli were of two intensities – low (47°C) and high (50°C) and were 6 s in duration at the destination temperature (PAIN). There was a 6 s post-stimulus rest (PSR), and then subjects were allowed up to 14 s for stimulus intensity rating (RATING). Finally, there was a 15 s rest period (REST) before presentation of the next cue. Each functional imaging series involved the presentation of four such cue-stimulus-rating blocks.
Visual cues consisted of one (signaling low pain) or two (sequentially presented, signaling high pain) centrally-positioned white squares against the black background delivered via MRI-compatible goggles (Resonance Technology Inc, CA). Each square was presented for one second, and there was one-second interval between presentations when high intensity stimulus was cued.
Innocuous thermal cues completely paralleled visual cues in that each thermal “pulse” was one second in duration. These pulses were delivered to the posterior aspect of the left forearm via a second thermal stimulator with a 27 mm diameter contact surface (Medoc Pathway Model CHEPS). The baseline temperature was 35°C. The stimulus temperature was changed with rise and fall rates of 40°C/s to a target temperature of 43°C. One thermal pulse signaled low intensity stimuli and two thermal pulses signaled high intensity noxious stimuli. There was a one second interval between the sequential pulses.
Six seconds after the end of each noxious heat stimulus, subjects were asked to rate experienced pain intensity using a visual analogue scale (VAS) presented via MRI-compatible goggles. This scale was anchored with the descriptors `no pain intensity' and `most intense pain imaginable', and had a range from 0 to 10 [34]. This scale was visually identical to that described by Price et al [34]. Subjects were allowed 14 seconds to make the rating via MRI-compatible trackball (Resonance Technology Inc, CA). After the rating was performed, subjects had a 15-second rest period in addition to the remaining time from the allowed rating duration (14 seconds) before presentation of the next cue.
At the beginning of the imaging session but before the actual experimental series was initiated, all four cue types were delivered once each without subsequent noxious stimuli. Subjects were asked to rate the intensity of the noxious stimulus that would be expected to follow that particular cue. This was done in order to determine the magnitude of pain expected following each cue and to ensure that subjects retained information learned about the cues during the psychophysical session. However, there was no performance criterion participants had to meet in order to continue the experiment.
Psychophysical assessment and analysis
For both the training and fMRI acquisition series, subjects' responses were recorded using custom-written programs within the IDL software package (Research Systems, CO). They later were examined using a repeated-measures ANOVA to identify effects of cue type and stimulus intensity on rating magnitude. The timing of events recorded by the program additionally was used to construct regressors for the fMRI analysis. In addition, the timing of events was recorded using a digital chart recorder (Power-Lab: ADInstruments, Colorado Springs, CO).
Image acquisition and processing
Functional data were acquired on a 1.5 T General Electric echo-speed Horizon LX scanner with 1.5T 8-channel neurovascular coil. For functional imaging, blood oxygenation level-dependent images were acquired continuously in each contiguous plane by using echo-planar imaging [echo time (TE), 40ms; repetition time (TR), 2s; 28 × 5-mm-thick slices; 3.75 × 3.75 mm in-plane resolution; flip angle, 80°; no slice gap].
High-resolution structural scans were acquired using a BRAVO sequence (inversion time, 600 ms; TR, 11.49ms; flip angle, 12°; TE, 4.74ms; section thickness, 1 mm with no gap between sections; number of sections, 156; in-plane resolution, 0.9375 × 0.9375 mm).
The functional image analysis package FSL [Center for Functional Magnetic Resonance Imaging of the Brain (FMRIB), University of Oxford, Oxford, UK] was used for image processing and statistical analysis. The functional data were movement corrected, spatially smoothed by 5 mm with a 3-D isotropic Gaussian kernel, and temporally filtered by a nonlinear high-pass filter with a cutoff period of 100 s. Each subject's functional images were registered to their structural data using a six-parameter linear 3-D transformation and then were initially transformed into standard stereotaxic space [as defined by Montreal Neurologic Institute [39]] using a 12-parameter affine transformation. To further minimize spatial variation between subjects, these linearly transformed data were then nonlinearly transformed into standard space [1, 2].
Statistical analysis of regional signal changes within the brain
Statistical analysis of regional signal changes was performed on each acquisition series (first level analyses) using a general linear modeling approach with nonparametric local autocorrelation correction [11, 42]. In all analyses, the relationship of the predictive model function to MRI signal intensity was evaluated by calculating a t-statistic on a voxel-by-voxel basis. These t-values were then converted to Z-scores to allow p values to be calculated on the basis of Gaussian random field theory [43, 12, 10]. The predictive model functions for the general linear modeling analysis were derived as follows:
We created 14 regressors for analysis of cue presentation, cue maintenance, pain, post-stimulus rest, and rating periods. The cue presentation, cue maintenance, and pain periods each were described with four regressors based on the cue they were signaled with (thermal low, thermal high, visual low, visual high). All regressors were orthogonalized to each other to separate a true baseline. Each of the 14 regressors had a period of interest scaled as +1.
All regressors were convolved with a gamma-variate model of the hemodynamic response (delay 6s, SD 3 s) and its temporal derivative [8, 15, 42]. They then were filtered temporally using the same parameters that were applied to the functional images.
We performed inter-series fixed effects (second level) analyses within each subject separately for every regressor and proceeded to inter-subject group analysis (third level) using a random effects model in order to identify clusters significantly activated or deactivated compared to rest condition. Clusters of voxels exceeding a Z-score>2.3 and p<0.05 (mixed effect, corrected for multiple comparisons) were considered statistically significant [43].
Finally, we performed a two-way ANOVA to identify the main effect of cue modality and cue meaning, as well as possible interactions between the two. This analysis was performed separately for both cue-presentation and cue-maintenance periods. Conjunction analysis was performed in order to identify regions commonly activated between visual and thermal cues [27]. These analyses were performed separately for both low and high cues for each period, as well as for all four conditions within the cue maintenance period (low thermal cue, low visual cue, high thermal cue, high visual cue).
Results
Psychophysics
Task validity was confirmed using psychophysical assessment. Analysis of expected pain intensity during 4 types of cues revealed that painful stimuli expected after high cues were believed to be significantly more painful than the ones expected after low cues (F=98.98, p < 0.0001; Fig. 2A). In addition, there was a significant effect of cue modality in that visual cues were expected to signal more intense pain than thermal cues (F=16.54, p=0.0007). Finally, there was a significant cue-modality vs cue-meaning interaction (F=22.13, p=0.0002) in which high cues behaved differently than low cues across cue modality. Post-hoc t-tests revealed that high cues delivered with visual stimuli had higher expected pain intensity than cues delivered with thermal stimuli (t=4.556; p<0.0002). In contrast, no significant differences in expected pain intensity were observed for the low cues (t=−1.329; p<0.2003).
Figure 2.
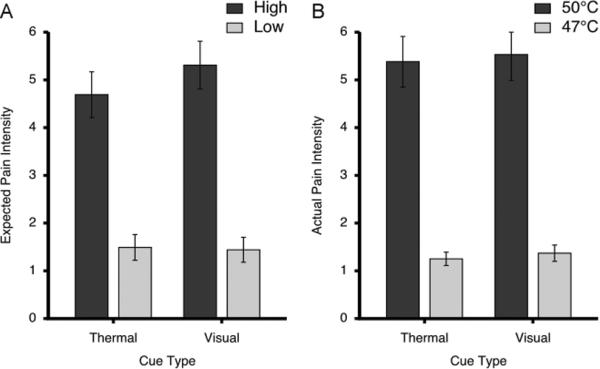
Expected and actual pain intensity ratings (mean±SEM) following different cue types. After training, ratings of expected pain (A) confirmed that subjects had learned that the high cues signaled intensely painful stimuli whereas low cues signaled mildly painful stimuli. Ratings of actual pain (B) evoked by 50°C and 47°C stimuli were largely consistent with the magnitude of expected pain and were strongly dependent upon stimulus temperature. The visual analogue scales used to provide pain intensity ratings ranged from 0 to 10.
The results from intensity ratings of delivered stimuli were similar to those of expected pain. 50°C stimuli were rated significantly higher compared to 47°C stimuli (F=99.99, p < 0.0001; Fig. 2B). In addition, noxious stimuli signaled with visual cues were rated significantly higher compared to those signaled with thermal cues (F=4.69, p=0.04). It is important to note that cue-related differences in pain intensity ratings were minute. High stimuli signaled with visual cues were only 0.15 VAS units greater than those signaled with thermal cues. Similarly, low stimuli signaled with visual cues were 0.11 VAS units greater than those signaled with thermal cues. In addition, there was no significant cue-modality vs cue-meaning interaction in actual pain ratings (F=0.13, p=0.72).
Cue presentation period activity
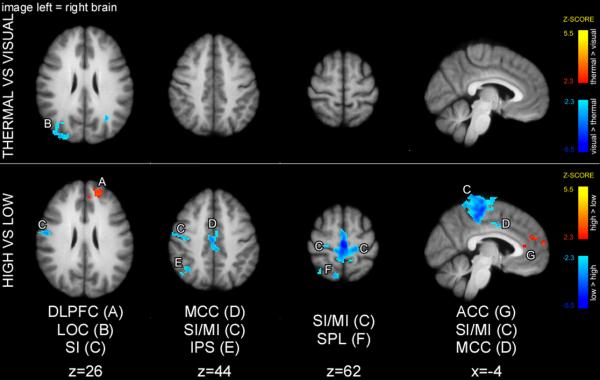
In a 2-way ANOVA (Fig. 3), thermal cues produced greater activity in SII consistent with early somatosensory processing, while visual cues produced greater activity of occipital cortex (V2) consistent with early visual processing. High cues produced greater activation in intraparietal sulcus (IPS), and low cues produced greater activation in left primary somatosensory area (SI). There was no cue-modality/cue meaning interaction.
Figure 3.
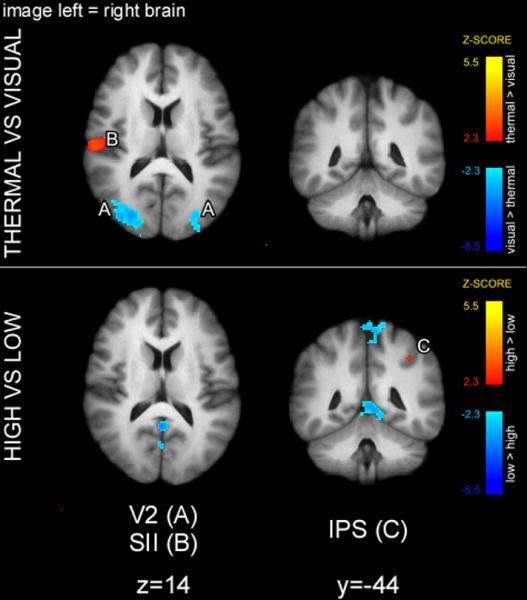
Early sensory areas exhibited cue modality-specific activation during the cue presentation period. The main effect of cue modality revealed that thermal cues produced greater activation of SII, whereas visual cues produced greater activation of V2. The main effect of cue meaning was limited to greater activation of IPS in high compared to low cue condition. Statistical maps were overlaid onto the mean high-resolution structural image derived from all subjects. Slice locations are relative to standard Montreal Neurologic Institute (MNI) stereotaxic space.
Cue maintenance period activity
A two-way ANOVA of the cue-maintenance period revealed a significant main effect of cue-modality in regions of occipital cortex (visual greater than thermal) (Fig. 4). This difference likely represents greater deactivation in low thermal compared to visual cue maintenance periods. In addition, a two-way ANOVA detected greater activations in the DLPFC and ACC in high compared to low trials, and greater activations of IPS in low compared to high trials. There was no cue-modality vs cue-meaning interaction.
Figure 4.
Main effect of sensory modality of the cue (thermal vs. visual) and main effect of cue meaning (high vs. low) during the cue maintenance period. The main effect of cue modality revealed greater activity in the dorsal occipital cortex/IPL following visual versus thermal cues. The main effect of cue meaning revealed that high cues produced greater activity in the DLPFC and ACC, whereas low cues produced greater activity in the IPS during maintenance periods. Statistical maps were overlaid onto the mean high-resolution structural image derived from all subjects. Slice locations are relative to standard MNI stereotaxic space.
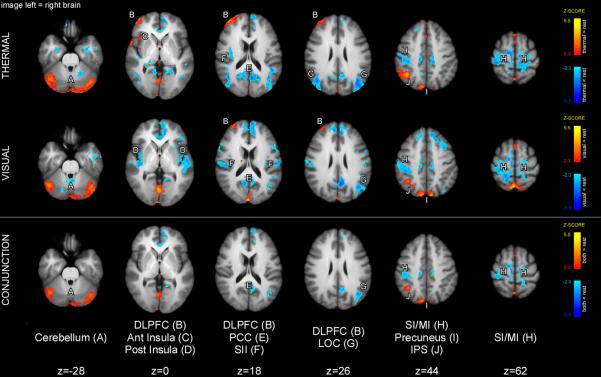
Conjunction analysis of low thermal and visual cue maintenance periods (Fig. 5) revealed significant activation overlap in parts of the cerebellum, precuneus, and the lower bank of the IPS, as well as significant deactivation overlap in the paracentral lobule. DLPFC activity (centered around Brodmann's area 10) was present in both low thermal and visual conditions; however, overlapping cluster sizes were not large enough to be determined significant by the conjunction analysis. Finally, low cue maintenance thermal period exhibited activation of the anterior insula.
Figure 5.
Brain activations (orange-yellow) and deactivations (blue) during the low cue maintenance period. Conjunction analysis revealed that cues of both modalities produced activations in the cerebellum, IPS, and regions of SPL/precuneus during the cue-maintenance period. Slightly different yet partially overlapping portions of the right DLPFC were activated during both thermal and visual cue maintenance periods although the area of overlapping activity was not sufficiently large to be a statistically reliable conjunction. Activation of common/similar areas is consistent with the processing of cue meaning. Statistical maps were overlaid onto the mean high-resolution structural image derived from all subjects. Slice locations are relative to standard MNI stereotaxic space.
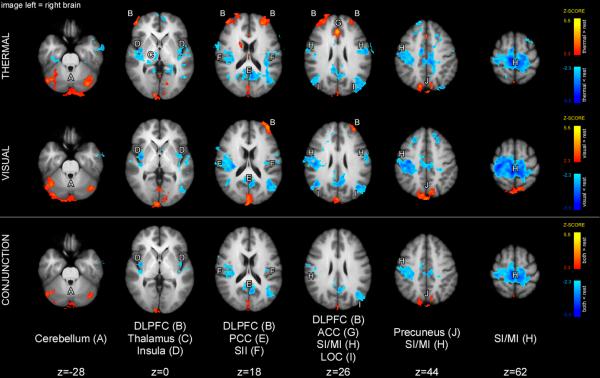
Conjunction analysis of high thermal and visual cue maintenance periods revealed a significant overlap of activations in the cerebellum and precuneus and deactivations in the paracentral lobule and SII (Fig. 6). Both conditions exhibited activation of the DLPFC predominately in Brodmann's area 10. However, the overlap was not large enough to be considered significant by conjunction analysis. Finally, thermal high cue maintenance period showed activation in the ACC and caudate.
Figure 6.
Brain activations (orange-yellow) and deactivations (blue) during the high cue-maintenance period. Similar to the low cue-maintenance period, conjunction analysis revealed that cues of both modalities produced activations in the cerebellum and regions of SPL/precuneus during the high cue-maintenance period. In contrast to the low cue maintenance period, partially overlapping portions of the left DLPFC were activated during both thermal and visual cue-maintenance periods although the area of overlapping activity was not sufficiently large to be a statistically reliable conjunction. Activation of common/similar areas is consistent with processing of cue meaning. Statistical maps were overlaid onto the mean high-resolution structural image derived from all subjects. Slice locations are relative to standard MNI stereotaxic space.
In order to obtain further insight into mechanisms reflecting cue meaning we performed an additional conjunction analysis incorporating all four conditions within the cue maintenance period (low thermal cue, low visual cue, high thermal cue, high visual cue). This analysis indicated that common portions of the right cerebellar hemisphere were activated during all four conditions (results not shown).
Pain period activity
A two-way ANOVA (results not shown) performed over the whole brain revealed a significant main effect of noxious stimulus intensity. Specifically, areas that have been shown previously to exhibit graded activations at different intensities of noxious stimuli (i.e., SI, SII, ACC, insula, putamen and thalamus) had greater activity during 50°C (high) compared to 47°C (low) stimulus conditions. However, there was no main effect of cue-modality in areas known to be involved in nociceptive processing. This absence of a detectable effect of cue-modality on pain-related brain activation is consistent with the minute effect of cue-modality on pain intensity ratings. However, we did detect greater activity in the occipital cortex during thermal versus visual cue conditions. There was no noxious stimulus-intensity vs cue-modality interaction.
Discussion
Expectation of an impending sensory event is a complex process that encompasses multiple components ranging from cue processing to the formation of a mental representation of the impending experience. The current study was designed to determine if sensory cues with equal meaning engage similar patterns of brain activations even if they are delivered in separate modalities. As hypothesized, early sensory processing of the cues occurred in modality-specific cortices, whereas later processing related to expectations about the intensity of the impending stimulus was localized to common brain areas that are positioned to alter the processing of noxious information.
Brain areas engaged during cue presentation period
As expected, visual cues produced reliable activation in the occipital cortex (V2). In contrast, thermal cues produced activation of SII. Activation in these areas is consistent with early processing of visual and somatosensory information. Activation of these unimodal sensory cortices validates the experimental design by confirming that cue modality-related activation can be distinguished from cue meaning-related activity.
Common processes engaged during the cue-maintenance period
During the cue-maintenance period, separate conjunction analyses for high and low cues detected common activations between thermal and visual conditions in the precuneus and cerebellum. Many cognitive processes during the cue maintenance period could engage brain mechanisms that would be largely common across both cue modality and cue meaning. For example, after the initial sensory processing of the cue, it must be invested with meaning based, in part, on recollection of previous experiences following the same cue type. The precuneus, as well as other parts of the PPC, has been implicated in memory recall [16, 36]. However, overlap in activation of the precuneus between high and low meaning conditions was not sufficiently large to be significant in conjunction of all four conditions.
In contrast, the cerebellum was the only brain region to exhibit significant overlapping activity during conjunction of all four conditions. Given the role of the cerebellum in motor control of withdrawal in nociception, it is possible that cerebellar activation represents preparation for such behaviors [30]. However, such motor control-related activity would be predicted to be dependent on the expected magnitude of the impending stimulus [17]. There are also a number of non-motor functions that the cerebellum might perform during the cue-maintenance period. First, the cerebellum has been implicated in generalized aversive processing with overlapping clusters of activity in response to noxious heat stimuli and passive viewing of unpleasant images [25]. Second, the cerebellum is important for the timing of events [38]. In the present experiment, painful stimuli always started at a predictable time after the delivery of the cue. Thus, cerebellar activity may have contributed to the processing of temporal predictions of the onset of the noxious stimulus. Third, the cerebellum has been hypothesized to be involved in encoding of internal models of mental representations in the cortex [14]. Taken together, the cerebellum is positioned to perform a number of non-motor, cognitive functions that are present in our experimental design.
Meaning-dependent processes during the cue-maintenance period
Analysis of variance revealed that three brain regions exhibited responses consistent with cue meaning and independent of cue modality. These regions include the DLPFC, ACC, and IPS.
The prefrontal cortex is implicated in active maintenance of goals, rules, and expectations in both animal and human studies [22]. The magnitude of activation of the prefrontal cortex has been shown to be related to the magnitude of expected pain [17]. In the present study, DLPFC had greater activation in high compared to low cue-maintenance conditions, consistent with its role in encoding the magnitude of expected pain. In addition, prefrontal cortex is positioned to provide signals that might influence activity throughout much of the brain, including areas responsible for perception. Activity in DLPFC has been linked to placebo analgesia and decreased activity in pain-sensitive brain regions [41]. It also has been linked to decreases in perceived pain intensity and affect, possibly through modulation of cortico-subcortical and cortico-cortical pathways [20]. TMS disruption of DLPFC has been shown to block the effects of placebo analgesia, also consistent with its role in the generation and/or maintenance of sensory set [18].
The ACC was another brain region that exhibited greater activation during the high compared to low cue maintenance period. Several studies have demonstrated that the ACC plays a role in anticipation of painful sensory stimuli [32, 17]. Moreover, Koyama et al. have proposed that the ACC and DLPFC have unique role in maintaining mental representations of impending events since these regions exhibit activation which is significantly related to the magnitude of expected pain intensity [17]. Similar to the DLPFC, the ACC is activated during placebo and other cognitive manipulations that may modulate pain [29, 41, 44]. This region has anatomic connections with the anterior insula which may be engaged to modulate nociceptive processing [21, 26, 40, 37].
IPS was commonly activated by both visual and thermal cues during low but not high cue-maintenance periods. IPS has been implicated in top-down attentional control, especially before stimulus presentation [9, 13, 23]. Because low pain was expected to be significantly less intense than high pain, IPS activation may be correlated with the direction of attention to the stimulated body region to improve accuracy of ratings of low pain stimuli [28, 19]. As such, this pattern of activation is consistent with the cue preparing the system to optimally process information from the impending stimulus.
Limitations
In the present study, we sought to deliver highly parallel cues in two modalities: thermal and visual. Because the thermal cue stimuli had to be innocuous, brief, and readily detectable, we were limited in the range of temperatures that could be employed. Moreover, because the thermal cues were delivered via a single contact stimulator, we were similarly limited in the spatial configuration of the stimuli. Accordingly, we used sequential delivery of heat pulses and, consequently, sequential delivery of visual stimuli to signal the intensity of impending stimuli. Therefore, the low and high cue presentation periods were unequal in duration, which may have affected the magnitude of activity detected.
Second, the present study was not directed at the evaluation of the effect of cue meaning on the magnitude of the perceived pain. This would have necessitated the use of non-cued or miscued conditions. The use of non-cued unpredictable noxious stimuli would likely elevate general anxiety during the “rest” period and thereby possibly engage additional attentional mechanisms during processing of the noxious stimulus itself. Finally, we did not want to introduce uncertainty about cue meaning by using occasional miscued trials because this also can differentially engage endogenous pain inhibitory mechanisms [31]. The effect of incorrect cues on processing of non-painful and painful stimuli has been examined in detail elsewhere [35, 41, 17].
Summary and significance
In conclusion, cues of different sensory modalities produce common patterns of activation during the expectation of painful stimuli. Modality-independent areas that exhibit this pattern likely are involved in the extraction of cue meaning, retrieval of past memories and experiences, and the formation of a mental set for an impending sensory event.
Acknowledgements
We thank the staff of The Center for Biomolecular Imaging for their technical support.
Funding: This work was supported by the National Institutes of Health (R01 NS39426).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors of this manuscript report no conflict of interest.
References
- [1].Andersson JLR, Jenkinson M, Smith SM. FMRIB technical report. 2007. Non-linear optimisation. TR07JA1. [Google Scholar]
- [2].Andersson JLR, Jenkinson M, Smith SM. FMRIB technical report. 2007. Non-linear registration, aka Spatial normalisation. TR07JA2. [Google Scholar]
- [3].Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- [5].Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- [6].Cabeza R, McIntosh AR, Tulving E, Nyberg L, Grady CL. Age-related differences in effective neural connectivity during encoding and recall. Neuroreport. 1997;8(16):3479–3483. doi: 10.1097/00001756-199711100-00013. [DOI] [PubMed] [Google Scholar]
- [7].Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- [8].Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- [9].Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- [10].Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- [11].Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- [12].Friston KJ, Worsley KJ, Frackowiak RJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- [13].Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- [14].Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- [15].Josephs O, Turner R, Friston K. Event-related f MRI. Hum Brain Mapp. 1997;5(4):243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- [16].Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- [17].Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krummenacher P. Prefrontal cortex mediated control of expectations in placebo analgesia. Schmerz. 2011;25(4):440–443. doi: 10.1007/s00482-011-1049-9. [DOI] [PubMed] [Google Scholar]
- [19].Lobanov OV, Quevedo AS, Hadsel MS, Kraft RA, Coghill RC. Frontoparietal mechanisms supporting attention to location and intensity of painful stimuli. Pain. 2013;154(9):1758–1768. doi: 10.1016/j.pain.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- [21].Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- [22].Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- [23].Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cereb Cortex. 2007;17(11):2703–2712. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- [24].Moseley GL, Arntz A. The context of a noxious stimulus affects the pain it evokes. Pain. 2007;133(1–3):64–71. doi: 10.1016/j.pain.2007.03.002. [DOI] [PubMed] [Google Scholar]
- [25].Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31(10):3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mufson EJ, Mesulam MM. Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212(1):23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- [27].Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- [28].Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting spatial discrimination of pain. J Neurosci. 2007;27(13):3388–3394. doi: 10.1523/JNEUROSCI.5128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia--imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- [30].Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- [31].Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci. 2003;7(5):197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- [32].Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- [33].Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- [35].Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20(19):7438–7445. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31(12):4407–4420. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29(9):2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- [39].Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]
- [40].Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262(2):271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- [41].Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- [42].Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- [43].Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- [44].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]