Abstract
MetS is the manifestation of a cluster of cardiovascular (CV) risk factors and is associated with a three-fold increase risk of CV morbidity and mortality, which is suggested to be mediated, in part, by resting left ventricular (LV) systolic dysfunction. However, to what extent resting LV systolic function is impaired in MetS is controversial, and there are no data indicating whether LV systolic function is impaired during exercise. Accordingly, the objective of this study was to comprehensively examine LV and arterial responses to exercise in MetS individuals without diabetes and/or overt CVD compared to a healthy control population. CV function was characterized using Doppler echocardiography and gas exchange in MetS (n=27) vs. healthy controls (n=20) at rest and during peak exercise. At rest, MetS individuals displayed normal LV systolic function but reduced LV diastolic function vs. healthy controls. During peak exercise, individuals with MetS had impaired contractility; pump performance, and vasodilator reserve capacity vs. controls. A blunted contractile reserve response resulted in diminished arterial-ventricular coupling reserve and limited aerobic capacity in MetS vs. controls. These findings possess clinical importance as they provide insight to the pathophysiological changes in MetS that may predispose this population of individuals to an increased risk of CV morbidity and mortality.
Keywords: metabolic syndrome, systolic function, exercise reserve
Introduction
Individuals with the metabolic syndrome (MetS) are at a three-fold increased risk of cardiovascular disease (CVD) mortality than non-MetS individuals (Malik et al., 2004). Alarmingly, the prevalence of MetS in US adults is 34 percent and is on the rise due, in part, to rising rates of obesity (Mozumdar & Liguori, 2011), which will likely lead to a further increase in CVD incidence. This increased CVD mortality in MetS may be mediated, in part, by impaired left ventricular (LV) systolic function that has been noted at rest in some studies (Azevedo et al., 2007; Aijaz et al., 2008; Mahmud et al., 2009; Pagé et al., 2010) but not all (Chinali et al., 2004; de las Fuentes et al., 2007). However, many of the studies used load-dependent measures of systolic function (endocardial or midwall fractional shortening) or ejection fraction, which is more representative of the interaction between the LV and arterial system (Chantler et al., 2008a), as measures of LV systolic function (Azevedo et al., 2007; Aijaz et al., 2008; Mahmud et al., 2009; de las Fuentes et al., 2007). Further, the populations examined in these studies were confounded by including MetS patients with diabetes and/or overt CVD (Wong et al., 2005; de las Fuentes et al., 2007; Gong et al., 2009), and/or comparing MetS individuals to a control group that contained individuals with CVD (Wong et al., 2005; Azevedo et al., 2007; de las Fuentes et al., 2007; Aijaz et al., 2008; Mahmud et al., 2009; Pagé et al., 2010). Such confounding factors do not allow interpretations as to whether the severity of the changes in cardiovascular (CV) function is representative of MetS or other pathologies.
Importantly, existing evidence of LV dysfunction in MetS is limited to resting situations when the CV system is attempting to optimize chamber pumping efficiency (De Tombe et al., 1993). In contrast, during exercise the CV system is set to prioritize the output of the heart over energetic efficiency by coordinating changes in LV function, arterial tone, endothelial function, venous return, and autonomic signaling. Thus, it could be expected that LV abnormalities undetectable at rest, may emerge with exertion to limit exercise capacity in MetS and contribute to an impaired quality of life (Fletcher et al., 2001). However, to date, the pathophysiological alterations in LV function and the degree to which these changes influence the interaction of the heart with the arterial system during exercise in MetS is unknown. Thus, examining the LV functional changes during exercise may further provide pathophysiological insights into the CVD risk in MetS patients. Accordingly, the aim of this study was to determine the severity of LV and arterial dysfunction during exercise in MetS individuals without diabetes and/or overt CVD compared to a healthy control population. Further, we will examine resting LV systolic and diastolic function between MetS and healthy controls. We hypothesize that MetS individuals will have an impaired LV contractile response (peak end-systolic elastance and pre-load recruitable stroke work) to maximal exercise, leading to a blunted arterial-ventricular coupling response during exercise compared to healthy age-sex matched controls.
Methods
Study Population
Twenty-seven subjects with MetS were recruited into the study. MetS was defined according to the updated National Cholesterol Education Program: Adult Treatment Panel III (Huang, 2009) comprised of three out of the following five components: 1) obesity (waist men >102 cm, women >88 cm); 2) low HDL cholesterol (men<40 mg/dL; women<50 mg/dL); 3) hypertriglyceridemia (≥150 mg/dL); 4) elevated glucose (≥100 mg/dL), and; 5) elevated BP (130/85 mmHg or use of hypertensive medications). The MetS population was compared to 20 healthy controls free from CVD, as determined by a detailed history, physical examination, and a normal resting and exercise electrocardiogram. Exclusion criteria for MetS and healthy controls included abnormal resting and/or exercise electrocardiogram, diabetes mellitus (HbA1c ≥6.5 % or use of diabetic medications), pulmonary disease, angina, atrial fibrillation, aortic stenosis (or other types of valve disease), anemia, myocardial infarction, stroke, or coronary revascularization as assessed by a detailed medical history, and physical examination. No evidence of wall motion abnormalities were identified during the maximal exercise test. Subjects who participated in regular exercise, defined as greater than 30 minutes, three times/week were excluded to ensure similar physical activity levels between groups. All subjects provided written informed consent to participate that was approved by the WVU Institutional Review Board.
Study Design
Physiological assessments were performed between 7:00-10:00 AM, in a quiet, temperature-controlled room, after a 12-hour fast and abstinence from alcohol, caffeine, and vitamins. CV medications were withheld 24 hours prior to assessments. After a minimum 15 minutes of quiet rest subjects underwent supine, resting, non-invasive assessments of arterial and cardiac structure and function. Once supine assessments were completed, subjects moved to a modified monarch bike where upright rest and exercise measures of CV function were obtained.
Arterial Geometry and stiffness
In the supine position, B-mode ultrasound (GE Vivid i) 2-D images of the right common carotid artery were obtained 1–2 cm proximal to the carotid bifurcation to measure maximal lumen diameter, and intima-medial thickness (cIMT) following standard procedures (Roman et al., 2006). Cross-sectional area of the carotid artery was calculated as [(maximal lumen diameter/2)2 x π]-(maximal lumen diameter/2-cIMT)2 x π). Carotid circumferential stress was calculated as systolic BP x (carotid diameter in diastole/2). Carotid to femoral pulse wave velocity (cfPWV; central arterial stiffness) was measured by applanation tonometry (AtCor Medical, Sydney, Australia) (O'Rourke et al., 2001). ECG-gated waveforms were sequentially recorded. Aortic distance (D) was calculated as the difference in the distances from the carotid to the suprasternal notch and from the suprasternal notch to the femoral artery. Time delay was calculated using a foot-of-the-wave method.
Exercise Performance
Subjects underwent upright cycle maximal exercise testing. To optimize acquisition of the echo images, the back support of the cycle was set at approximately 130 degrees. Pedal speed was maintained at 50 rpm, and workloads increased by 25 W every 3 minutes until exhaustion. Oxygen consumed (VO2), carbon dioxide produced (VCO2), and the respiratory exchange ratio (RER=VCO2/VO2) were measured (ParvoMedics) throughout exercise. Subjective symptoms of fatigue (BORG score 6 to 20) (Borg, 1974), and BP’s (sphyg-momanometry) were recorded at the end of each workload.
Rest and Exercise Echocardiography
Echocardiograms were obtained using a GE Vivid i (GE Healthcare, Chalfont St. Giles, United Kingdom), portable ultrasound imaging system with a 5S-RS (2.0–5.0 MHz) Wideband Phased Array transducer. All echocardiograms were performed by experienced registered diagnostic cardiac sonographers. Adequate acoustic windows were available in 20 of 23 health controls and 27 of the 32 MetS participants. Thus, we only examined those individuals with adequate acoustic windows. At rest, standard 2-dimensional images were obtained in the following acoustic views; parasternal long axis, and apical 4 chamber view. Pulsed wave Doppler tracings of the mitral valve inflow velocity (recorded at the leaflet’s tips) were recorded in the apical 4 chamber view. Continuous/pulse wave Doppler tracings of the LV outflow track velocity were obtained in the apical 5 chamber view positioned 5 mm proximal to the aortic valve. Spectral tissue Doppler imaging was performed in the apical 4 chamber view with the gate sample positioned in the lateral corner and septal side of the mitral annulus. During exercise, the sonographer quickly acquired a 2-dimensional image of the parasternal long axis view to obtain the size of the LV outflow tract diameter (base of the aortic leaflets). The sonographer then focused on capturing: 4-chamber views to obtain cardiac volumes and mitral flow velocities; and 5-chamber views to obtain pulse-and continuous-wave Doppler-flow from the LV outflow track. During exercise the images were acquired approximately 1.5 minutes into each 3-minute exercise workload. If all images were not acquired within the time frame the duration of the exercise stage was extended to acquire those images. In 41 subjects all images were acquired within 90 seconds; however, in 6 individuals (4 MetS and 2 controls) the final exercise stage needed to be extended on average 40 seconds (range: 10–60 seconds) to acquire all images.
Cardiovascular Measurements
From the recorded echocardiograms LV structure and function were calculated as described below:
Left Ventricular Geometry and Remodeling
In the supine position, LV dimensions, wall thickness, and chamber volumes were determined in triplicate from 2-dimensional, M-mode, and Doppler spectra echocardiography using standard methods (Lang et al., 2006). Sex-specific LV hypertrophy (LVH) and geometry patterns, based on LV mass index and relative wall thickness (RWT) were defined as LV mass index >95 g/m2 for women or >115 g/m2 for men, and LV geometry was classified as normal, concentric remodeling, concentric LVH, or eccentric LVH (Lang et al., 2006).
Resting Diastolic Function
In the supine position, the medial mitral annular early diastolic velocity (e’) was determined by spectral tissue Doppler imaging (GE Vivid i) using standard methods. The e’ velocity is inversely related to the time constant of isovolumic relaxation (τ), derived from τ =(14.70–100e’)/0.15 (Ommen et al., 2000). Early (E) and late (A) transmitral flow velocities, the isovolumetric relaxation time (IVRT), and the deceleration time of early filling velocity (Dec T) were measured by pulsed-wave Doppler (GE Vivid i). End-diastolic pressure was estimated as EDP =11.96+0.596 x E/e’ (Ommen et al., 2000).
Left Ventricular Volumes and Contractility
In the upright seated rest position and during exercise, LV end diastolic (EDV), and end-systolic (ESV) volumes, along with ejection fraction (EF) were determined from Simpson’s biplane method; the recommended method for measurement of volumes (Lang et al., 2006). Cardiac index (Ci) was determined from the product of heart rate (HR) and stroke volume index. Load-independent measures of chamber contractility were examined as: 1) pre-load recruitable stroke work (PRSW [calculated from product of peak volumetric ejection rate from LV outflow Doppler and systolic BP, divided by EDV]), determined from the validated single-beat technique (Lee et al., 2003) and; 2) LV end-systolic elastance (Ees [calculated from BP, stroke volume, EF, and pre-ejection and systolic ejection time intervals from LV outflow Doppler]), determined by the validated single-beat technique (Chen et al., 2001) (see below).
Ees=[DBP−(ENd×SBP×0.9)]/[SV×ENd]
where SV is stroke volume (determined from the LV outflow dimension and pulse-wave Doppler), ENd is the normalized elastance value at the onset of ejection and DBP and SBP are diastolic and systolic pressures, respectively
ENd(est)=0.0275−0.165×EF+0.3656×(DBP/ESP)+0.515×ENd(avg)
where EF is Ejection fraction (determined from Simpson’s biplane method), ESP is end-systolic pressure (SBPx0.9), and ENd(avg) is given by a seven-term polynomial function:
ENd(avg)=Σi=0ai×tNdi
where ai are (0.35695, −7.2266, 74.249, −307.39, 684.54, −856.92, 571.95, −159.1) for i = 0 to 7, respectively. The value of tNd was determined by the ratio of pre-ejection period (R wave → flow-onset) to total systolic period (R-wave → end-flow), with the time at onset and termination of flow defined noninvasively from the aortic Doppler waveform.
The orientation of the LV outflow Doppler velocity was positioned 5 mm proximal to the aortic valve in the apical five chamber view. The change in each parameter from upright seated rest to peak exercise was used to characterize contractile reserve.
Arterial Function
Effective arterial elastance (Ea), a measure of the net arterial load, was calculated as end-systolic pressure (ESP)/stroke volume (SV), where ESP is approximated as 0.9 × systolic blood pressure (SBP) (Chantler et al., 2008a). Systemic vascular resistance index (SVRi) was calculated as mean arterial pressure (MAP) x 80/Ci. The change in each parameter from upright seated rest to peak exercise was used to characterize global arterial reserve.
Arterial-Ventricular Coupling
The LV and the central arteries have bidirectional interactions. These interactions were examined the depiction of the function of the LV and arterial system in terms of elastance i.e., LV chamber elastance (Ees) and arterial Elastance (Ea), and examining their ratio (arterial-ventricular coupling ratio: Ea/Ees) (Chantler et al., 2008a).
Scaling for Body Size
Adequate scaling of physiological measures for body size is essential for correct interpretation, and often the relationship between body size and physiological function may not be linear, a major assumption for the ratiometric scaling approach (Chantler et al., 2005). The allometric scaling approach accounts for this potential nonlinear relationship by normalizing physiological measures using exponential powers that linearize the relationship. In our data set, the relationship between body surface area (BSA) and cardiac volumes, cardiac output, and PRSW were adequately scaled for using the ratiometric scaling approach. No relationships were presented between BSA and Ees, or Ea. To account for differences in chamber size, Ees was normalized to end-diastolic volume (EDV). However, EDV was allometrically related to Ees and thus EDV to the power of 0.45 was used to scale Ees. The relationship between BSA and the CV parameters were examined using Pearson’s correlation. Initially, significant correlations were adjusted ratiometrically, y= a+bx+ ε, where b represents the slope of the line of best fit; a, the intercept on the y-axis; and ε, the additive residual error term. If significant correlations remained between the ratiometrically adjusted CV variable and BSA, then these data were adjusted allometrically, y=axbε, where y represents the CV variable, x represents BSA, a represents the proportionality coefficient or constant multiplier, b is the power function exponent, and ε represents the multiplicative residual error term as described in detail previously (Chantler et al., 2005).
Sample Size and Statistical analysis
Measurements of CV function were performed offline, by a single investigator who was blinded to group allocation. The intra-class correlation coefficient (ICC) for all echocardiographic variables was derived in a subset of subjects (n=8). At rest, the ICC for all variables, collected on two separate days, was >0.80. Similar results were obtained for echocardographic variables evaluated during peak exercise with all variables having an ICC>0.75 with the exception of the arterial-ventricular coupling ratio (ICC=0.63). Specifically, at peak exercise the ICCs for the following CV variables are: EDV = 0.83; SV = 0.80; ICT =0.75; LV ejection time = 0.84; EF = 0.83; SBP =0.94; DBP =0.86; Ees = 0.87; Ea = 0.90.
Based on previous data identifying differences in peak exercise Ees (≈4 mmHg/ml/m2) and the Ea/Ees ratio (≈0.10) between young and older healthy individuals (Najjar et al., 2004; Chantler et al., 2008b; Chantler et al., 2010) sample size was calculated as follows using G*Power 3.1: Assuming independence among subjects and a SD estimate of 6.5 mmHg/ml/m2 for peak Ees and 0.11 for the Ea/Ees ratio at peak exercise, eighteen subjects per group would provide us with an 80 percent power (2-sided α=0.05), to detect a difference of 4 mmHg/ml/m2 for peak Ees and 0.10 for peak Ea/Ees between any two groups.
All analyses were performed using SPSS version 20 (SPSS Inc, Chicago, Illinois). A two-tailed p≤0.05 was required for significance. Data are reported as mean ± SEM unless otherwise stated. Normality was evaluated by the Kolmogorov-Smirnov test. Categorical variables were compared by the chi-square test. Continuous variables were log transformed as necessary and compared between groups through ANCOVA adjusting for sex. The change from rest to peak exercise in CV parameters was calculated as a delta (max-rest) and this CV delta was then compared between groups using general linear models adjusted for sex as a covariate. The relationship between CV function and increasing exercise workloads was assessed by a two-way repeated-measures ANOVA with a time (exercise workloads) by group (MetS vs. healthy controls) interaction term. To account for structural differences (LV Mass, cfPWV, cIMT) between MetS and controls we performed a repeated-measures ANCOVA to examine the time by group interaction, adjusting for LV Mass, cfPWV, and cIMT as covariates. Lastly, in a subset of the population, individuals (MetS vs. controls) were matched by age, LV Mass, cfPWV, cIMT, peak HR, and respiratory exchange ratio, and analyzed using a repeated-measures ANOVA (see supplemental data).
Results
Subject Characteristics
As expected, baseline CVD risk factors were significantly different between MetS and controls but not for age or sex as summarized in Table 1.
Table 1.
Clinical Characteristics
| Controls (n=20) |
MetS (n=27) |
p Value | |
|---|---|---|---|
| Age, year | 45 ± 2.5 | 49 ± 1.9 | 0.24 |
| Sex, female % | 65 | 63 | 0.57 |
| Height, cm | 169 ± 2 | 169 ± 2 | 0.92 |
| Weight, kg | 71 ± 2 | 103 ± 4 | <0.001 |
| Waist circumference, cm | 85 ± 5 | 119 ± 5 | <0.001 |
| Body Fat, % | 25 ± 2 | 39 ± 2 | <0.001 |
| Lean mass, kg | 53 ± 2 | 62 ± 2 | <0.01 |
| BSA, m2 | 1.80 ± 0.03 | 2.11 ± 0.04 | <0.001 |
| BMI, kg/m2 | 25.0 ± 0.8 | 36.0 ± 1.0 | <0.001 |
| SBP, mmHg* | 115 ± 2 | 134 ± 3 | <0.001 |
| DBP, mmHg* | 75 ± 1 | 83 ± 1 | <0.001 |
| Ejection Fraction, % | 61 ± 1 | 58 ± 1 | 0.06 |
| Triglycerides, mg/dL | 94 ± 10 | 170 ± 29 | 0.036 |
| HDL, mg/dL | 55 ± 3 | 43 ± 2 | <0.001 |
| Glucose, mg/dL | 93 ± 2 | 99 ± 1 | 0.018 |
| HbA1c, % | 5.35 ± 0.09 | 5.60 ± 0.07 | 0.03 |
| Insulin, µIU/mL | 6.1 ± 1 | 12.1 ± 1 | <0.001 |
| HOMA-IR | 1.32 ± 0.23 | 2.98 ± 0.37 | <0.001 |
| Hypertensive % | 0 | 56 % | <0.01 |
| Diabetes Mellitus % | 0 | 0 | |
| Medications | |||
| Hypertension | 0 | 56% | <0.05 |
| Cholesterol | 0 | 7% | <0.05 |
Values are mean ± 1 SEM; BSA: body surface area; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HDL: high density lipoprotein; HbA1c: hemoglobin A1c; HOMA-IR: homeostatic model assessment of insulin resistance.
SBP and DBP taken during screening visit
Cardiovascular Differences at Rest
Arterial Structure and Function
In MetS subjects, carotid diameter, carotid CSA and cIMT were larger (p<0.05) compared to controls. As such, carotid circumferential stress was larger in MetS (Table 2). Although net arterial load (Ea) and vascular resistance (SVRi) did not significantly differ between groups (Table 3), cfPWV was moderately increased (23%, p<0.01) in MetS vs. controls and remained significant after adjusting for MAP between groups (p=0.01), indicating an increase in arterial stiffness in MetS.
Table 2.
Measures of Cardiovascular Geometry at Supine Rest
| Controls (n=20) |
MetS (n=27) |
p Value |
|
|---|---|---|---|
| Arterial Geometry | |||
| cIMT, mm | 0.61 ± 0.02 | 0.72 ± 0.04 | 0.02 |
| Diastolic diameter, mm | 5.78 ± 0.12 | 6.17 ± 0.13 | 0.04 |
| Carotid CSA, mm2/m2 | 10.3 ± 0.32 | 12.7 ± 0.68 | <0.01 |
| Aortic CSA, cm2 | 2.52 ± 0.14 | 2.59 ± 0.11 | 0.68 |
| Cardiac Geometry | |||
| Septal wall thickness, cm | 0.79 ± 0.03 | 0.96 ± 0.03 | <0.01 |
| Posterior wall thickness, cm | 0.83 ± 0.04 | 0.94 ± 0.04 | 0.07 |
| LV internal dimension, cm | 4.34 ± 0.11 | 4.58 ± 0.08 | 0.09 |
| LV Mass, g | 136 ± 7 | 186 ± 11 | <0.01 |
| LV Mass Index, g/m2 | 75 ± 3 | 88 ± 5 | 0.046 |
| Relative Wall Thickness | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.41 |
| LV Hypertrophy % | 1 | 19 | 0.18 |
| Concentric Remodeling, % | 25 | 33 | 0.14 |
| Concentric Hypertrophy, % | 1 | 7 | 0.14 |
| Eccentric Hypertrophy, % | 0 | 7 | 0.14 |
Values are mean ± 1 SEM, p-values adjusted for sex; cIMT: carotid intima-medial thickness; CSA: cross-sectional area.
Table 3.
Measures of Left Ventricular Diastolic Function at Supine Rest
| Controls (n=20) |
MetS(n=27) | p Value | |
|---|---|---|---|
| LV Diastolic Function | |||
| E, m/s | 0.81 ± 0.04 | 0.82 ± 0.03 | 0.65 |
| A, m/s | 0.57 ± 0.03 | 0.73 ± 0.03 | <0.01 |
| E/A ratio | 1.47 ± 0.08 | 1.19 ± 0.07 | <0.01 |
| IVRT, m/s | 71 ± 3 | 74 ± 3 | 0.54 |
| Dec T, m/s | 220 ± 7 | 203 ± 7 | 0.10 |
| e’, m/s | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.03 |
| τ, ms | 28 ± 3 | 41 ± 3 | <0.01 |
| E/e’ | 6.46 ± 0.40 | 8.19 ± 0.59 | 0.02 |
| LV EDP, mmHg | 15.9 ± 0.24 | 16.9 ± 0.36 | 0.02 |
Values are mean ± 1 SEM, p-values adjusted for sex; E: peak velocity of the early diastolic mitral flow; A: peak velocity of the late diastolic mitral flow; E/A: E divided by A; IVRT: isovolumetric relaxation time; Dec T: mitral flow deceleration time of early filling velocity; e’: mitral annular early diastolic velocity; τ: time constant of isovolumetric relaxation; E/e’: E divided by e’; LV EDP: left ventricular end-diastolic pressure
Left Ventricular Structure and Function
LV Mass was 37 percent larger in MetS, but RWT was similar compared to controls. Thus, LVH was 18 percent greater in MetS than controls, with 33 percent of MetS presenting with concentric remodeling and 7 percent presenting with concentric and eccentric LVH (Table 2). In addition to these remodeling differences between groups; the MetS group tended to present with reduced supine LV diastolic function evident by a greater impaired LV relaxation (lower e’ and longer τ), a higher A-wave (thus the E/A ratio was reversed p<0.01), and a higher E/e’ (a predictor of elevated LV filling pressure), and EDP vs. controls (Table 3).
Comparisons of EDV, ESV, and SV were significantly elevated in MetS however, after adjusting to BSA, cardiac volume no longer differed between groups (Table 4). Cardiac index (Ci) and HR were also similar between groups. LV load-independent contractility as assessed by Ees, Ees·EDV0.45, and PRSW did not differ between MetS and controls at rest. The similarity between groups in Ees and Ea guaranteed that Ea/Ees (a measure of net cardiovascular performance) was also comparable between groups. Further, no differences in pre-ejection or total systolic ejection time were found between groups.
Table 4.
Measures of Cardiovascular Function at Seated Rest
| Controls (n=20) |
MetS (n=27) |
p Value |
|
|---|---|---|---|
| LV Performance | |||
| EDV, ml | 87 ± 6 | 103 ± 5 | 0.02 |
| EDVi, ml/m2 | 48 ± 3 | 48 ± 2 | 0.92 |
| ESV, ml | 36 ± 3 | 49 ± 2 | <0.01 |
| ESVi, ml/m2 | 20 ± 1 | 21 ± 1 | 0.52 |
| SV, ml | 51 ± 3 | 59 ± 3 | 0.09 |
| SVi, ml/m2 | 28 ± 2 | 28 ± 1 | 0.76 |
| LV Contractility | |||
| Ees, mmHg/ml | 3.21 ± 0.33 | 2.91 ± 0.18 | 0.45 |
| Ees·EDV0.45 | 85 ± 6 | 91 ± 25 | 0.48 |
| PRSW, g/cm2 | 90 ± 4 | 94 ± 3 | 0.93 |
| PRSWi, g/cm2/m2 | 52 ± 2 | 51 ± 2 | 0.66 |
| Integrated indexes | |||
| Heart rate, bpm | 68 ± 2 | 66 ± 2 | 0.46 |
| Ea/Ees ratio | 0.91 ± 0.06 | 0.93 ± 0.07 | 0.93 |
| Cardiac Output | 3.43 ± 0.22 | 3.85 ± 0.20 | 0.18 |
| Cardiac Index, L/m2·min | 2.03 ± 0.12 | 2.16 ± 0.08 | 0.33 |
| Pre-ejection period, m/s | 70 ± 4 | 66 ± 3 | 0.36 |
| Systolic ejection time, m/s | 334 ± 5 | 337 ± 6 | 0.67 |
| Velocity-time integral, cm | 16.6 ± 0.5 | 18.8 ± 0.8 | 0.02 |
| Arterial Function | |||
| SBP, mmHg | 116 ± 3 | 127 ± 3 | <0.01 |
| MAP, mmHg | 90 ± 2 | 99 ± 2 | <0.01 |
| Ea, mmHg/ml | 2.71 ± 0.17 | 2.58 ± 0.15 | 0.58 |
| SVRi, dyne·m2/s·cm−5 | 1261 ± 78 | 1063 ± 75 | 0.08 |
| cf-PWV, m/s | 6.6 ± 0.2 | 8.0 ± 0.2 | <0.01≠ |
| Carotid Circum Stress, mmHg | 18.8 ± 0.8 | 23.4 ± 1.4 | 0.01 |
Values are mean ± 1 SEM, p-values adjusted for sex; ≠ adjusted for differences in mean arterial pressure; EDV: end-diastolic volume (i=index); SV: stroke volume; Ees: end-systolic elastance; PRSWi: preload recruitable stroke work index; Ea/Ees: arterial-ventricular coupling ratio; Ea: arterial elastance; SVRi: systemic vascular resistance index; cf-PWV: carotid to femoral pulse wave velocity.
Cardiovascular Responses to Upright Exercise
Exercise Performance and Cardiac Function
At peak exercise, in absolute terms, aerobic capacity (L.min) did not differ between MetS and controls; however, after adjusting for the known effect of body mass on VO2peak, peak aerobic capacity (in ml/min/kg of body mass and lean mass) was significantly reduced in MetS vs. controls. Further, MetS individuals had a lower ventilatory threshold than healthy controls, yet they achieved similar rates of perceived exercise exhaustion, exercise duration and peak workload as controls (Table 5).
Table 5.
Metabolic Performance During Exercise
| Controls (n=20) |
MetS (n=27) |
p Value | |
|---|---|---|---|
| Exercise duration, s | 869 ± 65 | 946 ± 49 | 0.34 |
| Peak respiratory exchange ratio | 1.15 ± 0.02 | 1.09 ± 0.02 | 0.01 |
| Peak work load, W | 113 ± 9 | 123 ± 5 | 0.29 |
| Peak Heart rate, bpm | 166 ± 4 | 154 ± 3 | 0.01 |
| Peak SBP, mmHg | 177 ± 8 | 191 ± 4 | 0.01 |
| Peak DBP, mmHg | 68 ± 4 | 77 ± 4 | 0.09 |
| Subjective effort score (6–20) | 19 ± 0.3 | 19 ± 0.3 | 0.56 |
| VO2peak, L/min | 1.75 ± 0.13 | 1.77 ± 0.12 | 0.98 |
| VO2peak indexed to BW, ml/kg/min | 24.6 ± 1.4 | 17.1 ± 0.9 | <0.01 |
| VO2peak indexed to LM, ml/kg/min | 32.7 ± 1.4 | 28.0 ± 1.0 | <0.01 |
| VO2 at ventilatory threshold, ml/kg/min | 16.8 ± 1.1 | 11.8 ± 0.8 | <0.01 |
| Time to ventilatory threshold, s | 638 ± 72 | 628 ± 57 | 0.91 |
| Ratio VO2 at ventilatory threshold to VO2peak, % | 65 ± 2 | 67 ± 2 | 0.58 |
| Ratio of observed to predicted VO2peak, % | 91 ± 5 | 69 ± 2 | <0.01 |
| VE/VO2 at VT, % | 29 ± 1 | 29 ± 1 | 0.70 |
| VE/VO2 at max | 41 ± 1 | 38 ± 1 | 0.10 |
| VE/VCO2 at max | 35 ± 1 | 35 ± 1 | 0.75 |
Values are mean ± 1 SEM, p-values adjusted for sex; BW, body weight; LM, lean mass.
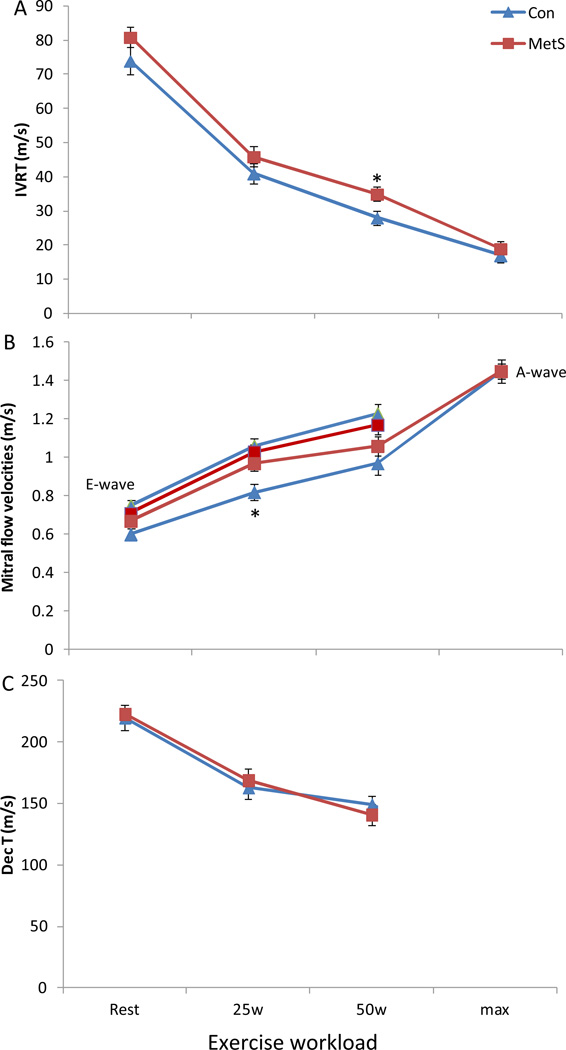
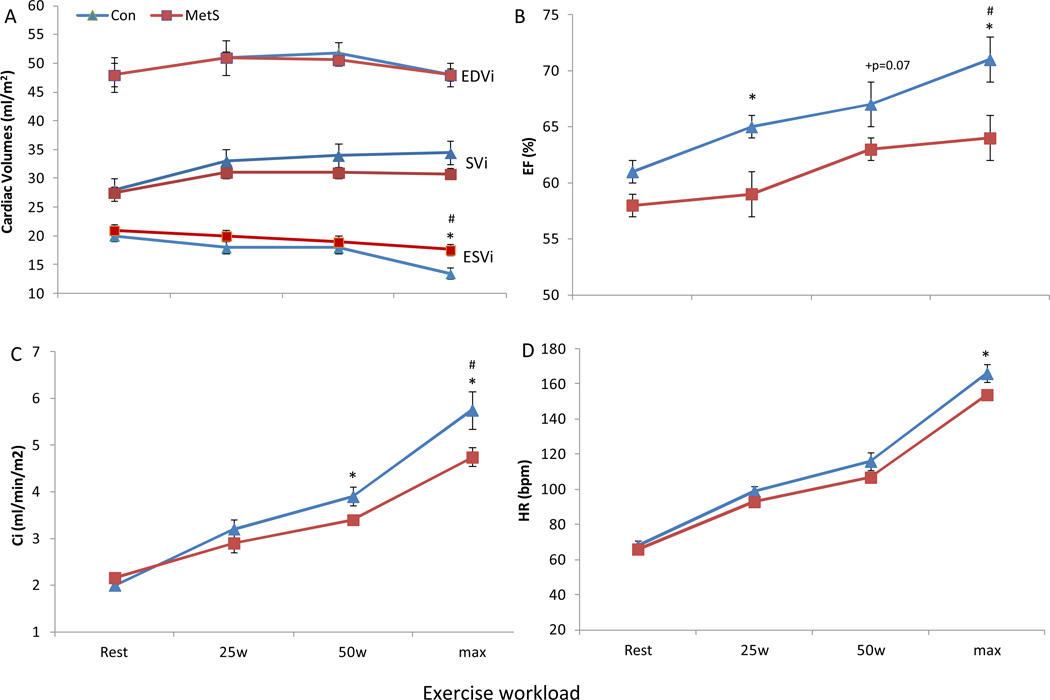
During exercise there were no significant time by group interactions for components of LV diastolic function, namely IVRT, E-wave, A-wave, and Dec T (Figure 1). No differences were found between controls and MetS in peak exercise pre-ejection rate (16±3 vs. 18±2 m/s, p=0.4) or total systolic ejection period (185±9 vs. 200±7m/s, p=0.2). Yet, MetS had a greater peak exercise velocity-time integral (2.60±0.13 vs. 2.42±0.14 cm, p=0.01) compared to controls. The change from rest to peak exercise in EDVi (p>0.9) and SVi (p=0.3) did not significantly differ between MetS and controls. However, there was a significant time by group interaction for the reduction in ESVi during exercise with MetS individuals having a blunted decrease vs. healthy controls (Figure 2). MetS individuals also demonstrated a blunted increase in EF (6%, p=0.02), and Ci (−24%, p<0.05) compared to controls, which were evident at submaximal workloads (Figure 2); whereas peak HR was lower at peak exercise in MetS. Importantly, LV contractility reserve (change from rest to peak exercise) was impaired in MetS compared to controls, evident by a blunted increase in Ees (−35%, p=0.01), and PRSWi (−26%, p=0.013) in MetS (Figure 3). Once again these differences were manifested at submaximal exercise workloads. Further, even after adjusting statistically the above parameters individually for either peak HR, LV Mass, cIMT, or cfPWV the results above did not differ significantly. Further, in a sub cohort of this population matched for resting LV Mass, cIMT, cfPWV, peak exercise HR and respiratory exchange ratio, the results above did not differ significantly (see supplemental data).
Figure 1. LV Diastolic function at rest and during exercise.
The change from rest to max exercise in (A) isovolumetric relaxation time (IVRT), (B) early (E) and late (A) mitral inflow velocities, and (C), the deceleration time of early filling velocity (Dec T) in MetS and controls. No significant time by group interactions for IVRT or Dec T were evident between MetS and controls during exercise. Further at peak exercise no differences were observed between groups for the early (E) and late (A) mitral inflow filling velocities, however, at moderate workloads the E- and A-waves become superimposed at extreme workloads the A-wave dominates over the E-wave. Further, once the E- and A-waves become superimposed we are not able to calculate the Dec T and thus we have only reported the Dec T for rest, 25 and 50w time points. *p<0.05 MetS vs. controls; # p<0.05 time by group interaction. Data presented as means ± SEM.
Figure 2. Cardiac pump performance at rest and during upright exercise.
The change from rest to max exercise in (A) cardiac volumes, (B) ejection fraction (EF), (C) cardiac index (Ci), and (D) heart rate (HR) in MetS and controls. During exercise the change from rest to max exercise in EDVi and SVi was similar between MetS and healthy controls. A significant time by group interaction was identified for the reduction of ESVi during exercise with MetS displaying a blunted decrease vs. controls. Compared to controls MetS also demonstrated a blunted increase in EF and Ci at max exercise, which was evident at submaximal workloads. Peak HR was significantly lower in MetS vs. controls. *p<0.05 MetS vs. controls; #p<0.05 time by group interaction. Data presented as means ± SEM.
Figure 3. Cardiac contractility and arterial-ventricular coupling response to exercise.
The change from rest to max exercise in (A) pre-load recruitable stroke work index (PRSWi), (B) end-systolic elastance (Ees) and effective arterial-elastance (Ea), (C) and the arterial-ventricular coupling ratio (Ea/Ees) in MetS and controls. Compared to controls, MetS displayed a blunted increase in Ees and PRSWi at max exercise. During exercise the combination of a blunted increase in Ees and a comparable change in Ea between groups resulted in a blunted Ea/Ees ratio in MetS compared to controls. *p<0.05 MetS vs. controls; #p<0.05 time by group interaction. Data presented as means ± SEM.
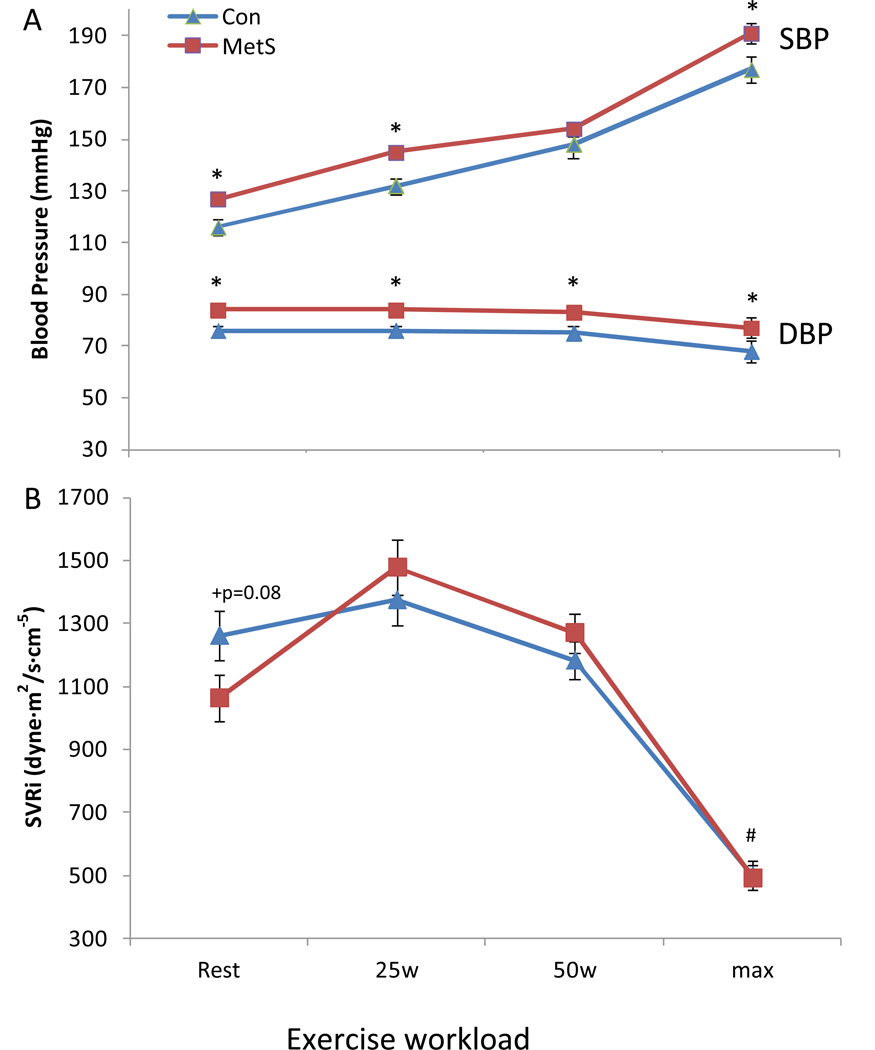
Arterial Function and AVC Reserve
During exercise the change in Ea did not differ between groups (p>0.2) (Figure 3B). The combination of an impaired contractile reserve in MetS, but a similar change in Ea between groups during exercise considerably limited the Ea/Ees response in MetS (25% impaired decrease, p<0.05) compared to controls (Figure 3A). Submaximal and peak SBP and DBP were higher in MetS than controls (Figure 4A), and a significant time by group interaction was noted for the change in SVRi during exercise. We further examined these data by statistically adjusting the above CV variables for LV Mass, cIMT, or cfPWV, and with the exception of SVRi (p=0.1 time by group interaction), the results above did not differ significantly. Similarly, the results above did not differ significantly from the sub cohort analysis (see supplemental data).
Figure 4. Pressure and vascular resistance response during exercise.
The change from rest to max exercise in (A) systolic (SBP) and diastolic (DBP) blood pressure, and (B) systemic vascular resistance index (SVRi) in MetS and controls. Systolic BP and diastolic BP were higher in MetS vs. controls at submaximal and maximal workloads. +p=0.08 vs. controls; *p<0.05 MetS vs. controls; #p<0.05 time by group interaction. Data presented as means ± SEM.
Discussion
The present study provides the first comparison of LV and arterial structure and function responses to exercise in non-diabetic MetS individuals without overt CVD. This finding is of significant clinical interest, and a growing public health concern. Our study provides evidence of LV systolic dysfunction during exercise including limitations in peripheral vasodilation accumulating in a blunted arterial-ventricular coupling reserve and impaired peak aerobic capacity in MetS. These data demonstrate that pathophysiological CV alterations occur in the earliest stages of MetS development, prior to any evidence of chronic disease such as diabetes and/or overt CVD, and that impaired LV systolic function during exercise occurs prior to evidence of LV systolic dysfunction at rest.
Resting LV and Arterial Structure and Function
Previous studies examining CV alterations in MetS patients (with or without diabetes) have been limited to characterizing differences in LV and arterial structure and function at rest. MetS patients are traditionally characterized as having increased cIMT (range 9–16%), and PWV (range 13–32%) (Scuteri et al., 2004; Lin et al., 2010). The results of the present study are similar to previous findings with MetS individuals presenting with a 21 percent higher PWV, and an 18 percent higher cIMT. They also confirm the presence of increased LV Mass in MetS (in the absence of CVD and/or diabetes) reported by existing studies (Scuteri et al., 2004; de las Fuentes et al., 2007).
Although the LV structural differences in MetS are fairly well agreed upon, consensus is lacking with regard to changes in LV function. Some studies (Chinali et al., 2004; Azevedo et al., 2007; de las Fuentes et al., 2007; Aijaz et al., 2008; Mahmud et al., 2009; Pagé et al., 2010), but not all (Chinali et al., 2004; de las Fuentes et al., 2007), have suggested that MetS individuals have LV systolic dysfunction at rest (Azevedo et al., 2007; Aijaz et al., 2008; Mahmud et al., 2009; Pagé et al., 2010). However, many of these studies used EF as a measure of systolic function, which despite its conventional clinical application, is a rather poor prognostic measure of systolic function as it is potentially influenced by loading conditions and chamber remodeling (Kass et al., 1987). Further, the populations examined in these studies were confounded by the inclusion of MetS patients with diabetes (Wong et al., 2005; de las Fuentes et al., 2007; Aijaz et al., 2008) and moderate stenosis (Pagé et al., 2010), conditions that would exacerbate symptoms of LV dysfunction. In addition, control subjects included people with hypertension (Aijaz et al., 2008; Mahmud et al., 2009; Pagé et al., 2010), obesity (de las Fuentes et al., 2007), diabetes (Wong et al., 2005; Azevedo et al., 2007; Aijaz et al., 2008), and many were taking CV medications (Wong et al., 2005; Pagé et al., 2010). Such confounding factors do not allow interpretation as to whether the changes in LV function are representative of MetS or of other existing pathologies. Using load-independent measures of contractility (Ees, or Ees•EDV, and PRSWi) we found no evidence of LV dysfunction in MetS. The present findings cannot exclude the possibility of cellular abnormalities in the cardiomyocytes of MetS individuals. However, we did identify differences in resting diastolic function in MetS versus healthy controls (Table 4), which has been previously reported in some (Chinali et al., 2004; Mahmud et al., 2009) but not all (Cuspidi et al., 2004; Schillaci et al., 2006) studies. It has been postulated that LV diastolic dysfunction is a pre-cursor of LV systolic dysfunction and heart failure with a preserved EF (HFpEF) (Kitzman et al., 2001; Bella et al., 2002; Schannwell et al., 2002). In addition, abnormalities of LV relaxation, i.e. grade 1 diastolic dysfunction, confer a two-fold increase in all-cause and cardiac mortality (Bella et al., 2002). This statistic highlights the clinical importance of recognizing the early, and/or subclinical, changes in diastolic function at rest in individuals with MetS.
Aerobic Capacity and Cardiovascular Reserve Function
Exercise provides a powerful tool to examine the response of the LV and arterial systems to stress and to assess functional reserve. If LV function is impaired in MetS individuals it would likely be revealed during exercise, and to promote intolerance to exercise.
Widely regarded as a load-independent measure of LV chamber performance, the change from rest to peak exercise in Ees is blunted with advancing age and may be limited further in the presence of disease (Chantler & Lakatta, 2012). Although at rest Ees is determined by geometric and biochemical properties that regulate LV end-systolic stiffness (i.e. structural changes from LV hypertrophy or fibrosis) (Borlaug & Kass, 2011), acute changes in Ees, such as those observed during exercise reflect inherent changes in LV contractile function (Chantler et al., 2008a). Using load-independent measures of LV contractility, we identified a blunted Ees, and PRSWi reserve capacity in MetS that manifested at submaximal workloads (Figure 3). Ha et al. (Ha et al., 2011) noted a blunted increase in s’ in MetS during exercise; however the extent of the LV function impairment was limited by the load dependence of s’ and the lack of a healthy control for comparison. In the present study, impaired LV contractility was accompanied by altered cardiac pump performance, and an impaired vasodilator reserve capacity in MetS. Importantly, impaired contractile function and vasodilator capacity resulted in a blunted arterialventricular coupling response to exercise. Ea/Ees is a key determinant of CV performance, cardiac energetics and exercise capacity (Chantler et al., 2008a). At rest in healthy individuals, Ea/Ees varies from 0.5-1.0 to ensure maximal cardiac power and chamber efficiency (De Tombe et al., 1993). During exercise, Ea/Ees decreases due to an acute mismatch between Ees and Ea to optimize cardiac performance (Chantler et al., 2008a). While resting Ea/Ees did not differ between our groups, the Ea/Ees response to exercise was considerably blunted, which in part contributed to reduced peak aerobic capacity in MetS. Similar impairments in Ea/Ees have been reported during acute maximal exercise with advancing age and have been suggested to explain, in part, diminished CV functional capacity in the elderly (Chantler & Lakatta, 2012).
In addition to the blunted arterial-ventricular coupling response to exercise we, like others (Tjonna et al., 2008; Jae et al., 2010), have shown that peak aerobic capacity is reduced in MetS compared to healthy controls. However, this finding only became evidenced once peak VO2 was indexed to body weight or lean mass. It is well known that peak aerobic capacity is positively associated with body size, in particular lean body mass (de Simone et al., 1997; Batterham et al., 1999; Collis et al., 2001), with a larger body mass requiring a greater demand for oxygen. This would suggest that the similar absolute peak VO2 (L.min) between MetS and healthy controls is due to MetS having a greater body/lean mass requiring a greater oxygen demand and less likely due to having similar physical fitness levels as controls. Thus, in order to assess CV function independently of the effect of body size, we need to adjust for body size in individuals. Adjusting for differences in body size has been shown to be important for revealing pathophysiological effects of obesity on arterial health (Chirinos et al., 2009), and for a more accurate detection of adverse CV events (de Simone et al., 2005; Chirinos et al., 2010).
Mechanisms limiting CV reserve in MetS individuals remain speculative. But, it is likely that multiple factors are involved, including sympathetic nervous system activity, activation of the renin-angiotensin-aldosterone system, and cardiac metabolism during exercise (Engeli et al., 2000; Peterson et al., 2004; Turhan et al., 2004). Importantly, the blunted CV response is unlikely to be attributed to differences in exercise effort exercise duration, maximal workload, and peak BORG were similar between groups (Table 5). Further, the blunted CV responses in MetS remained evident even after: a) adjusting statistically for peak HR and the respiratory exchange ratio (which were higher in MetS vs. controls); and b) in the subgroup analyses in which subjects were matched for peak HR and the respiratory exchange ratio. Impairments in contractile reserve observed in the present study may be mediated in part by altered calcium handling, as the influx of calcium is a primary determinant of cardiac performance. Specifically, contractile dysfunction as a result of alterations in sarcoplasmic reticulum calcium handling has been implicated in aging and type 2 diabetes (Poirier et al., 2001; Lakatta & Sollott, 2002). Although there was no sign of wall motion abnormalities during exercise in MetS, the use of upright echocardiography may have missed subtle wall motion abnormalities (Ryan et al., 1993). Thus this blunted systolic function in MetS could, in part, be a manifestation of early myocardial ischemia even in the presence of normal wall motion during exercise.
An additional potential mechanism for the impaired CV response to exercise in MetS may be depressed systemic peripheral vasodilation in the resistance vessels. An impaired SVRi response to exercise is evident in HFpEF and is known to contribute to limited exercise capacity in systolic heart failure, and is thought to be a result of a reduction in NO generation (Borlaug et al., 2006; Abudiab et al., 2013). In accordance with these findings, we observed a blunted reduction in SVRi during exercise in MetS vs. controls. Indeed, endothelial dysfunction is a common denominator linking MetS, type 2 diabetes, and CVD (Diamant & Tushuizen, 2006) suggesting that therapy targeted to promoting the bioavailability and/or stimulating the generation of NO may effectively improve systemic vasodilatation during exercise in MetS.
The MetS is characterized by structural changes to the heart (increase LV Mass and remodeling) and conduit arteries (increased cIMT and cfPWV) (Scuteri et al., 2004; Scuteri et al., 2010). These structural changes are not only predictors of CV events (myocardial or cerebral infarction) (Laurent et al., 2003; Protopsaltis et al., 2009) and mortality (Vlachopoulos et al., 2010; Stevens et al., 2013) but they are also predictors of poor aerobic capacity (Hundley et al., 2001; Kokkinos et al., 2007; Jae et al., 2010; Jae et al., 2012). It is plausible that the LV and arterial structural differences noted between MetS and healthy controls could contribute to the blunted CV response during exercise. However, after adjusting for LV mass, cIMT, or cfPWV in the statistical models, the blunted Ees, PRSWi, Ci and EF response from rest to peak exercise in MetS remained. We further examined these relationships by matching healthy controls and MetS by LV mass, cIMT, and cfPWV. Once again, MetS individuals demonstrated a blunted CV response to exercise compared to healthy controls in this sub cohort. These results suggest that the structural CV differences between MetS and healthy controls do not fully account for the impaired exercise CV response in MetS.
Previous literature has suggested a link between LV diastolic dysfunction and exercise intolerance (Poirier et al., 2000; de las Fuentes et al., 2007). Although the focus of this study was to examine the extent of LV systolic dysfunction during exercise (and thus echocardiographic views were optimized to examine systolic function), we were able to compare some components of LV diastolic function during exercise between MetS and controls. No differences in IVRT, and Dec T were evident between groups. Further, the early (E) and late (A) mitral inflow filling velocities, during submaximal workloads, did not differ between groups. At moderate exercise workloads there is a high incidence of fusion of the E and A-wave, thus from 50 W onwards the A-wave dominated and no differences were noted between groups at peak exercise (Figure 2). Unfortunately the acquisition of LV diastolic parameters during exercise is cumbersome, limiting our ability to fully characterize the extent of exercise diastolic function. Therefore we cannot eliminate the possibility that diastolic abnormalities during exercise may have contributed to the CV reserve deficits.
Limitations
Due to the small sample size and cross-sectional nature of our study, we cannot infer causality between MetS and CV abnormalities. Although pressure and flow were not directly measured, but rather estimated from non-invasive surrogates, these have been previously validated against invasive hemodynamic measurements performed at rest (Chen et al., 2001; Lee et al., 2003). However, the non-invasive measurements of end-systolic elastance and pre-load recruitable stroke work have not been validated during exercise. Further, our cardiac data may be underestimated due to a systematic underestimation of LV volumes from 2-D echocardiography (Tischler & Plehn, 1995; Gottdiener et al., 2004) and to the challenge of acquiring echocardiograhic images during exercise, but this technique has been successfully used by others (Borlaug et al., 2010; Tartière-Kesri et al., 2012), to which we observe similar values and responses in our subjects, suggesting fidelity in our data. Although, it is possible that the lower peak HR and respiratory exchange ratio in MetS may have contributed to their blunted CV response to exercise, this impaired CV response remained evident in a sub cohort of individuals matched for peak HR and the respiratory exchange. The overall significance and strength of our study is that our MetS patient population did not have the disease-related confounders and/or medications seen in most of the existing studies, which allows to report a comprehensive examination of resting and exercise arterial and LV measures in MetS individuals prior to the development of chronic disease.
Conclusion
This study demonstrates that individuals with MetS display evidence of impaired systolic contractile function, vasodilator, and cardiac pump function reserve capacity during exercise. These deficits contribute to abnormal arterial-ventricular coupling and exercise intolerance in individuals with MetS without diabetes and/or overt CVD. Whether the development of MetS and associated CV changes, and the progression to MetS together with diabetes is along the same pathophysiological pathway to heart failure warrants further investigation.
Supplementary Material
New Findings.
-
What is the central question of this study?
Metabolic syndrome is associated with a three-fold increased risk of cardiovascular disease mortality, which may be mediated in part, by impaired LV systolic function. The severity of LV and arterial dysfunction during dynamic exercise in MetS individuals without diabetes and/or overt cardiovascular disease has not previously been explored.
-
What is the main finding and its importance?
Cardiovascular function was characterized at rest and during peak exercise using echocardiography and gas exchange. During exercise MetS individuals displayed impaired LV contractility, a blunted arterial-ventricular coupling reserve, and limited aerobic capacity. These findings provide insight to the pathophysiological changes that may occur to predispose MetS to an increased risk of cardiovascular disease.
Acknowledgements
The authors thank Charles Murray and Diana Stofcheck for their help with the echocardiography.
Sources of Funding
This study was supported in part by the American Heart Association 11CRP7370056 (Dr Chantler), National Heart, Lung, Blood Institute T32- HL090610 (Sara Fournier), and the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
Sara Fournier, Brian Reger, Paul Chantler: conception, design, data analysis, drafting and revising of the manuscript; David Donley, Daniel Bonner, Jefferson Frisbee, Melissa Olfert, Mark Olfert: data collection, interpretation of results, writing and reviewing of the manuscript; Bradford Warden, Wissam Gharib, Conard Failinger: medical supervision, assisted interpretation of electrocardiograms, drafting and reviewing of the manuscript
Disclosures
All authors report that there are no conflicts of interest, financial or otherwise in connection with the submitted article to disclose.
References
- Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013 doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aijaz B, Ammar KA, Lopez-Jimenez F, Redfield MM, Jacobsen SJ, Rodeheffer RJ. Abnormal Cardiac Structure and Function in the Metabolic Syndrome: A Population-Based Study. Mayo Clinic Proceedings. 2008;83:1350–1357. doi: 10.4065/83.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo A, Bettencourt P, Almeida P, Santos A, Abreu-Lima C, Hense H-W, Barros H. Increasing number of components of the metabolic syndrome and cardiac structural and functional abnormalities - cross-sectional study of the general population. BMC Cardiovascular Disorders. 2007;7:17. doi: 10.1186/1471-2261-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham AM, Vanderburgh PM, Mahar MT, Jackson AS. Modeling the influence of body size onV˙o 2 peak: effects of model choice and body composition. Journal of Applied Physiology. 1999;87:1317–1325. doi: 10.1152/jappl.1999.87.4.1317. [DOI] [PubMed] [Google Scholar]
- Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- Borg GA. Perceived exertion. Exerc Sport Sci Rev. 1974;2:131–153. [PubMed] [Google Scholar]
- Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin. 2011;29:447–459. doi: 10.1016/j.ccl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired Chronotropic and Vasodilator Reserves Limit Exercise Capacity in Patients With Heart Failure and a Preserved Ejection Fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global Cardiovascular Reserve Dysfunction in Heart Failure With Preserved Ejection Fraction. Journal of the American College of Cardiology. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P, Nussbacher A, Gerstenblith G, Schulman SP, Becker LC, Fleg JL, Ferrucci L, Lakatta EG, Najjar SS. Attenuating The Age-associated Deficit In Peak Arterial-ventricular Coupling With Sodium Nitroprusside: 2450: Board #58 June 4 9:00 AM - 10:30 AM. Medicine & Science in Sports & Exercise. 2010;42:630. 610.1249/1201.MSS.0000385756.0000372940.ee. [Google Scholar]
- Chantler PD, Clements RE, Sharp L, George KP, Tan LB, Goldspink DF. The influence of body size on measurements of overall cardiac function. Am J Physiol Heart Circ Physiol. 2005;289:H2059–H2065. doi: 10.1152/ajpheart.00022.2005. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Lakatta E. Arterial-Ventricular Uncoupling with Age and Disease. Frontiers in Physiology. 2012;3 doi: 10.3389/fphys.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of Applied Physiology. 2008a;105:1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Najjar SS. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. American Journal of Physiology-Heart and Circulatory Physiology. 2008b;295:H145–H153. doi: 10.1152/ajpheart.01179.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, Resnick HE, Lee ET, Best LG, de Simone G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study) Am J Cardiol. 2004;93:40–44. doi: 10.1016/j.amjcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P investigators obotA. Arterial Load and Ventricular-Arterial Coupling. Hypertension. 2009;54:558–566. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St. John-Sutton M, Rietzschel ER. Left Ventricular Mass: Allometric Scaling, Normative Values, Effect of Obesity, and Prognostic Performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis T, Devereux RB, Roman MJ, de Simone G, Yeh J, Howard BV, Fabsitz RR, Welty TK. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation. 2001;103:820–825. doi: 10.1161/01.cir.103.6.820. [DOI] [PubMed] [Google Scholar]
- Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, Leonetti G, Magrini F, Zanchetti A. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:1991–1998. doi: 10.1097/00004872-200410000-00023. [DOI] [PubMed] [Google Scholar]
- de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, Davila-Roman VG. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- de Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR, Greco R, Witt S, Contaldo F. Stroke Volume and Cardiac Output in Normotensive Children and Adults: Assessment of Relations With Body Size and Impact of Overweight. Circulation. 1997;95:1837–1843. doi: 10.1161/01.cir.95.7.1837. [DOI] [PubMed] [Google Scholar]
- de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB. Normalization for body size and population-attributable risk of left ventricular hypertrophy: The Strong Heart Study. American Journal of Hypertension. 2005;18:191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- De Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264:H1817–H1824. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- Diamant M, Tushuizen ME. The metabolic syndrome and endothelial dysfunction: common highway to type 2 diabetes and CVD. Curr Diab Rep. 2006;6:279–286. doi: 10.1007/s11892-006-0061-4. [DOI] [PubMed] [Google Scholar]
- Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000;35:1270–1277. doi: 10.1161/01.hyp.35.6.1270. [DOI] [PubMed] [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Pina IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- Gong HP, Tan HW, Fang NN, Song T, Li SH, Zhong M, Zhang W, Zhang Y. Impaired left ventricular systolic and diastolic function in patients with metabolic syndrome as assessed by strain and strain rate imaging. Diabetes Res Clin Pract. 2009;83:300–307. doi: 10.1016/j.diabres.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ha TH, Seo HS, Choo WJ, Choi J, Suh J, Cho YH, Lee NH. The Effect of Metabolic Syndrome on Myocardial Contractile Reserve during Exercise in Non-Diabetic Hypertensive Subjects. J Cardiovasc Ultrasound. 2011;19:176–182. doi: 10.4250/jcu.2011.19.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. A Comprehensive Definition for Metabolic Syndrome. Disease Models & Mechanisms. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- Jae SY, Heffernan K, Fernhall B, Choi YH. Cardiorespiratory fitness and carotid artery intima media thickness in men with type 2 diabetes. J Phys Act Health. 2012;9:549–553. doi: 10.1123/jpah.9.4.549. [DOI] [PubMed] [Google Scholar]
- Jae SY, Heffernan KS, Fernhall B, Oh YS, Park WH, Lee MK, Choi YH. Association between cardiorespiratory fitness and arterial stiffness in men with the metabolic syndrome. Diabetes Res Clin Pract. 2010;90:326–332. doi: 10.1016/j.diabres.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76:1422–1436. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- Kokkinos P, Pittaras A, Narayan P, Faselis C, Singh S, Manolis A. Exercise Capacity and Blood Pressure Associations With Left Ventricular Mass in Prehypertensive Individuals. Hypertension. 2007;49:55–61. doi: 10.1161/01.HYP.0000250759.71323.8b. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Laurent S, Katsahian S, Fassot C, Tropeano A-I, Gautier I, Laloux B, Boutouyrie P. Aortic Stiffness Is an Independent Predictor of Fatal Stroke in Essential Hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284:H744–H750. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
- Lin H-F, Liu C-K, Liao Y-C, Lin R-T, Chen C-S, Juo S-HH. The risk of the metabolic syndrome on carotid thickness and stiffness: Sex and age specific effects. Atherosclerosis. 2010;210:155–159. doi: 10.1016/j.atherosclerosis.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Mahmud A, Almuntaser I, Brown A, King G, Crean P, Feely J. Left ventricular structural and functional changes in the metabolic syndrome. J Cardiometab Syndr. 2009;4:81–88. doi: 10.1111/j.1559-4572.2008.00043.x. [DOI] [PubMed] [Google Scholar]
- Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the Metabolic Syndrome on Mortality From Coronary Heart Disease, Cardiovascular Disease, and All Causes in United States Adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- Mozumdar A, Liguori G. Persistent Increase of Prevalence of Metabolic Syndrome Among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34:216–219. doi: 10.2337/dc10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, Becker LC, Lakatta EG. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–522. doi: 10.1046/j.0306-5251.2001.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- Pagé A, Dumesnil JG, Clavel M-A, Chan KL, Teo KK, Tam JW, Mathieu P, Després J-P, Pibarot P. Metabolic Syndrome Is Associated With More Pronounced Impairment of Left Ventricle Geometry and Function in Patients With Calcific Aortic Stenosis: A Substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) Journal of the American College of Cardiology. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil J-G. Diastolic Dysfunction in Normotensive Men with Well-Controlled Type 2 Diabetes: Importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- Poirier P, Garneau C, Bogaty P, Nadeau A, Marois L, Brochu C, Gingras C, Fortin C, Jobin J, Dumesnil JG. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2000;85:473–477. doi: 10.1016/s0002-9149(99)00774-2. [DOI] [PubMed] [Google Scholar]
- Protopsaltis J, Kokkoris S, Korantzopoulos P, Milionis HJ, Karzi E, Anastasopoulou A, Filioti K, Antonopoulos S, Melidonis A, Giannoulis G. Prediction of long-term functional outcome in patients with acute ischemic non-embolic stroke. Atherosclerosis. 2009;203:228–235. doi: 10.1016/j.atherosclerosis.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. American Society of Echocardiography Report: Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society for Vascular Medicine and Biology. Vascular Medicine. 2006;11:201–211. doi: 10.1177/1358863x06070511. [DOI] [PubMed] [Google Scholar]
- Ryan T, Segar DS, Sawada SG, Berkovitz KE, Whang D, Dohan AM, Duchak J, White TE, Foltz J, O'Donnell JA, et al. Detection of coronary artery disease with upright bicycle exercise echocardiography. J Am Soc Echocardiogr. 1993;6:186–197. doi: 10.1016/s0894-7317(14)80489-6. [DOI] [PubMed] [Google Scholar]
- Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–39. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vaudo G, Mannarino E. Different Impact of the Metabolic Syndrome on Left Ventricular Structure and Function in Hypertensive Men and Women. Hypertension. 2006;47:881–886. doi: 10.1161/01.HYP.0000216778.83626.39. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Najjar SS, Orru' M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. European Heart Journal. 2010;31:602–613. doi: 10.1093/eurheartj/ehp491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SM, Reinier K, Chugh SS. Increased Left Ventricular Mass as a Predictor of Sudden Cardiac Death: Is it Time to Put it to The Test? Circulation: Arrhythmia and Electrophysiology. 2013;6:212–217. doi: 10.1161/CIRCEP.112.974931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartière-Kesri L, Tartière J-M, Logeart D, Beauvais F, Cohen Solal A. Increased Proximal Arterial Stiffness and Cardiac Response With Moderate Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- Tischler MD, Plehn JF. Applications of stress echocardiography: beyond coronary disease. J Am Soc Echocardiogr. 1995;8:185–197. doi: 10.1016/s0894-7317(05)80407-9. [DOI] [PubMed] [Google Scholar]
- Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome: A Pilot Study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhan H, Erbay AR, Yasar AS, Bicer A, Sasmaz H, Yetkin E. Impaired coronary blood flow in patients with metabolic syndrome: documented by Thrombolysis in Myocardial Infarction (TIMI) frame count method. Am Heart J. 2004;148:789–794. doi: 10.1016/j.ahj.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Wong CY, O'Moore-Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol. 2005;96:1686–1691. doi: 10.1016/j.amjcard.2005.07.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.