Abstract
Altered systemic hemodynamics following exercise can compromise cerebral perfusion and result in syncope. As the Wingate anaerobic test often induces pre-syncope, we hypothesized that a modified Wingate test could form the basis of a novel model for the study of post-exercise syncope and a test-bed for potential countermeasures. Along these lines, breathing through an impedance threshold device has been shown to increase tolerance to hypovolemia, and could prove beneficial in the setting of post-exercise syncope. Therefore, we hypothesized that a modified Wingate test followed by head-up tilt would produce post-exercise syncope, and that breathing through an impedance threshold device (countermeasure) would prevent post-exercise syncope in healthy individuals. Nineteen recreationally active men and women underwent a 60° head-up tilt during recovery from the Wingate test while arterial pressure, heart rate, end-tidal CO2, and cerebral tissue oxygenation were measured on a control and countermeasure day. The duration of tolerable tilt was increased by a median time of 3 min 48 sec with countermeasure compared to control (P < 0.05) and completion of the tilt test increased from 42% to 67% with countermeasure. During the tilt, mean arterial pressure was greater (108.0 ± 4.1 vs.100.4 ± 2.4 mmHg; P < 0.05) with countermeasure compared to control. These data suggest that the Wingate syncope test produces a high incidence of pre-syncope which is sensitive to countermeasures such as inspiratory impedance.
Keywords: Syncope, vasovagal, Post-exercise hypotension, Tilt-table test, Hypotension, Orthostatic, Anaerobic exercise, Orthostatic intolerance, Cerebrovascular circulation
INTRODUCTION
Post-exercise syncope (defined as loss of consciousness or development of pre-syncope signs and symptoms during recovery from a bout of physical activity or exercise) is an alarming response to exercise which can occur in apparently healthy individuals, including athletes, or in individuals with autonomic disorders.
It is plausible that the common reductions in blood pressure known as post-exercise hypotension can be large enough in magnitude to become symptomatic (Halliwill et al., 2013a); however, most reports of post-exercise syncope are likely to be incidents of neurally mediated syncope (formally called vasovagal syncope) (Freeman et al., 2011) that have occurred during recovery from exercise, with the underlying changes associated with post-exercise hypotension contributing to the onset of the event (Kosinski et al., 2000; Krediet et al., 2004; O’Connor et al., 2009). Several case studies of individuals who have reportedly fainted following physical activity have been examined, yet the physiology remains poorly understood (Tsutsumi & Hara, 1979; Tamura et al., 1990; Arad et al., 1993; Ziegelstein, 2004; Krediet et al., 2005, 2006). Important from these case studies is the recognition that cessation of exercise is a key step in the cascade leading to neurally mediated syncope (Krediet et al., 2005, 2006). Characteristically, these events occur when an individual is standing motionless during the first 5-10 min after exercise (Krediet et al., 2004).
At present, there are no widely accepted models for investigating the mechanisms of post-exercise syncope or to assess potential countermeasures. Along these lines, there is anecdotal evidence that the Wingate test of anaerobic power, a staple of undergraduate exercise physiology labs, results in pre-syncopal symptoms in many individuals. Furthermore, there is growing interest and utilization of high-intensity interval training (Gibala et al., 2012; Kessler et al., 2012) as an exercise intervention in a wide range of patients, providing further rationale for the study of recovery from intense efforts like the Wingate test. For this reason, we postulated that the Wingate test could be modified to serve as a robust model for the study of post-exercise syncope and development of countermeasures. The Wingate test is typically a 15- or 30-s maximal effort of cycling against a high resistance, which is then followed by a cool-down routine. Without a cool-down, individuals often experience lightheadedness and/or nausea, and in some cases syncope. Toward our goal, we tested the ability of an extended 60-s maximal effort followed by a 15-min head-up tilt, in the absence of a cool-down, to elicit pre-syncopal signs and symptoms. We asked the following three questions: What are the hemodynamics of upright recovery from this modified Wingate test? What is the underlying physiology in someone who becomes pre-syncopal in response to this test? What factors predict who becomes pre-syncopal in response to this test?
In addition to developing this potential model of post-exercise syncope, we sought to determine the effectiveness of a standardized countermeasure in the prevention of this response. An impedance threshold device designed to augment negative intrathoracic pressure during inspiration was adapted from research on hemorrhagic shock, in which it has been shown to increase venous return and ventricular pre-load (Lurie et al., 2002, 2004). Spontaneously breathing through such a device ameliorates the reduction in arterial pressure observed with central hypovolemia in healthy normotensive subjects (Convertino et al., 2005, 2006, 2007) and individuals with clinical orthostatic hypotension (Melby et al., 2007). Subjects in these studies also reported a reduction in symptoms of orthostatic stress (e.g., dizziness, nausea, and lightheadedness) with the device. Thus, we also asked the following two questions: Does an inspiratory threshold device work as a countermeasure to this model of post-exercise syncope? If so, by what mechanism does it provide protection?
In brief, our overarching aim of this study was to create a foundation for future work examining post-exercise syncope. We sought to study post-exercise syncope from a basic science and mechanistic perspective, as well as a translational perspective in order to better understand the physiology of this response and examine potential countermeasures for its treatment. We hypothesized the modified Wingate test would be effective at eliciting pre-syncope in healthy men and women. Additionally, we hypothesized that the use of an inspiratory threshold device would be an effective countermeasure and decrease the incidence of pre-syncopal signs and symptoms in this model.
METHODS
This study was approved by the Institutional Review Board of the University of Oregon. Each subject gave written informed consent before participating in the study.
Subjects
Nineteen healthy, nonsmoking, normotensive subjects (12 men and 7 women) between the ages of 18 and 30 yr participated in this study. Based on the subjects’ exercise habits over the previous 12 months and self-reported physical activity levels on two questionnaires (Baecke et al., 1982; Kohl et al., 1988), they were classified as recreationally active. Subjects were taking no medication other than oral contraceptives. Female subjects had a negative pregnancy test each study visit. Subjects completed a near-fainting experiences index (Schrezemaier et al., 2005), which indicated that they had normal orthostatic tolerance. Subject characteristics are summarized in Table 1.
TABLE 1. Subject characteristics.
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 22.2 ± 3.0 | (18 – 30) |
| Height (cm) | 179 ± 12 | (197 – 155) |
| Weight (kg) | 72.0 ± 15.6 | (43.8 – 107.6) |
| Body mass index (kg m−2) | 22.3 ± 2.5 | (18.2 – 28.1) |
| Baecke sport index (arbitrary units) | 3.1 ± 0.8 | (2.0 – 4.3) |
| Physical activity index (MET h week−1) | 97 ± 50 | (15 – 203) |
| Near-fainting experiences index (arbitrary units, from 0 to 20) |
1.9 ± 1.5 | (0 - 5) |
MET, metabolic equivalents; n = 19
Study Visits
Subjects reported for parallel experiments on two separate days. The order of experiments was randomized between a “control” day and a “countermeasure” day. For both study days, subjects reported for the study at least 3 h postprandial and abstained from caffeine and alcohol for 12 h and from exercise and all medications for 24 h before the study. Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of the oral contraceptive use.
Experimental Protocol
Studies took place in a thermoneutral environment (23°C). At the start of the protocol, subjects were laid in the supine position on an electronic tilt table (Colin Medical Instruments Corporation, Valley City, ND) for instrumentation. A baseline measurement period was completed at 45 min of rest, where arterial pressure, heart rate, end-tidal CO2, and cerebral tissue oxygenation were measured with subjects in the supine position. Subjects were de-instrumented for end-tidal CO2 and beat-by-beat arterial pressure and then moved to an upright seated position on a cycle ergometer (Excalibur Sport V2; Lode BV, Groningen, The Netherlands). Subjects performed a 5-min warm-up at a moderate resistance (100 W for males and 75 for females) and 60-80 RPM then completed a 1-min Wingate test of anaerobic power. The torque factor was set to 0.63 Nm for male subjects and 0.60 Nm for female subjects (Wingate for Windows software version 1; Lode BV, Groningen, The Netherlands). After exercise, subjects were immediately returned to the supine position on the tilt table, re-instrumented for beat-by-beat arterial pressure, and measurements of arterial pressure, heart rate, and cerebral tissue oxygenation were obtained. Two minutes after the completion of exercise, subjects were passively tilted to a 60° head-up position supported by a foot board (time to reach the head-up position was 7 s). At this time, subjects began breathing through either a mouthpiece (control) or a mouthpiece attached to an inspiratory threshold device (countermeasure) for the duration of the tilt and for measurement of end-tidal CO2. To ensure that subjects breathed entirely through their mouths, a nose clip was used. Continuous measurements of arterial pressure, heart rate, end-tidal CO2, and cerebral tissue oxygenation were taken in the head-up position until the subject was unable to continue with the tilt or 15 min had passed, whichever came first.
Tilt tests were terminated if arterial pressure fell markedly (≥10 mm Hg) or heart rate slowed suddenly (≥10 beats·min−1). In addition, subjects were asked to rate symptoms related to lightheadedness, nausea, and visual disturbances every 2 min, each on a scale of 0-3. Tilt tests were terminated if a subject reached either a 2 on any one symptom scale or a total of 3 on the three scales.
Countermeasure
The countermeasure was a small lightweight disposable plastic inspiratory threshold device (Advanced Circulatory Systems, Inc., Eden Prairie, MN). The device includes an expiratory valve that closes when the proximal airway pressure is less than atmospheric pressure and an inspiratory valve that opens at a preset negative pressure of approximately −7 cmH20. The device was designed to generate a negative inspiratory threshold pressure and to therefore generate a substantially more negative intrathoracic pressure during spontaneous inhalation (Convertino et al., 2005). There is little resistance (< 2 cmH20) during spontaneous exhalation. A threshold of −7 cmH20 was chosen for this study because this level has been previously shown to be tolerable and effective in increasing arterial blood pressures in healthy conscious humans (Convertino et al., 2004, 2005).
Measurements
Heart rate, arterial pressure, and systemic hemodynamics
Heart rate and arterial pressure were monitored throughout all experimental procedures. Heart rate was monitored using a 5-lead electrocardiogram (Cardiocap/5 Critical Care Monitor; Datex-Ohmeda, GE Healthcare). Arterial pressure was measured in the arm by automated oscillometry (Cardiocap/5 Critical Care Monitor; Datex-Ohmeda, GE Healthcare) and on a beat-by-beat basis by finger photoplethysmography (Finometer; Finapres Medical Systems BV, Arnhem, the Netherlands) of the middle finger of the right hand, which was supported at heart level by an arm rest. Using the Modelflow algorithm (adjusted for sex, age, height, and weight), relative changes in stroke volume were estimated from the arterial pressure pulse contour. This method has compared favorably during active and passive postural stress against thermodilution (Harms et al., 1999), Doppler ultrasound (van Lieshout et al., 2003; Sugawara et al., 2003), and inert gas re-breathing (van Dijk et al., 2005). While it is not considered a gold standard method, it provides additional insight into systemic hemodynamic changes during tilt. Cardiac output and total peripheral resistance were calculated from stroke volume, heart rate, and arterial pressure as appropriate.
Tissue oxygenation index
A near-infrared tissue oximeter (OxiplexTS model 99200 with cranial sensors; ISS Incorporated, Champaign, IL, USA) was used to indirectly and non-invasively measure cerebral tissue oxygenation. A light source and detectors were placed on each side of the subject’s forehead above the brow-line and as close to the hairline as possible. Photos were taken of the sensor placement on the first study day to ensure the same placement on the second study day.
End-tidal CO2
A capillary line connected to the mouthpiece or the inspiratory threshold device was used to sample end-tidal CO2 (CardioCap/5 Critical Care Monitor, Datex-Ohmeda, GE Healthcare) and corrected to partial pressure of CO2 using barometric pressure.
Data Analysis
Continuous signals from the electrocardiogram, capnogram, and arterial pressure derived signals were sampled at 250 Hz using a commercial analog-to-digital data acquisition system (WinDAQ, Dataq Instruments, Akron, OH). Tissue oxygenation index were sampled at 2 Hz using the commercial data acquisition system provided with the oximeter (OxiTS software; ISS Incorporated, Champaign, IL, USA).
Statistics
Due to the complex nature of the data, a variety of statistical tools were used to explore results. In order to maximize statistical power, male and female subjects were analyzed as a single group for most results, but we present information where appropriate on the influence of sex on responses. Throughout our statistical testing, differences were considered significant when P < 0.05, whereas P < 0.15 was considered indicative of a trend for a difference. For descriptive analysis, values are reported as means ± SD when it is important to convey subject heterogeneity (e.g., subject characteristics), as means ± 95% confidence limits when presenting group means in comparison to individual response and to document the “average” pattern of responses to the Wingate syncope test (e.g., in Figures 1-3), and as means ± SE where group means are directly compared (i.e., countermeasure vs. control). For hypothesis testing, we used three basic models. First, we used step-wise regression analyses to identify predictors of tilt duration using SAS Proc GLMSELECT (SAS v9.1; SAS Institute, Inc., Cary, NC) and also to identify responses that associated most strongly with the development of symptoms during the tilt. Criteria for a term remaining in the model was set at P < 0.15. Second, to determine whether the countermeasure had an effect on the duration of the head-up tilt, a fixed-effects partial likelihood survival analysis was completed (SAS v9.2; SAS Institute, Inc.). This statistical analysis is primarily reserved for larger subject populations (> 30 subjects). Therefore, in addition to this test, we compared tolerance times with a paired t-test. Third, hemodynamic and other outcome variables were analyzed with a two-way repeated measures ANOVA (condition vs. time) with a priori contrasts of specific condition-time combinations (SAS v9.2; SAS Institute, Inc., Cary, NC) for pre-exercise, post-exercise (supine), first 60-s head-up tilt, and final 30-s head-up tilt, as a means to explore the underlying physiology of recovery from the Wingate syncope test and the effect of the countermeasure on the initial response to tilt (first 60-s) and the point of tilt-test termination (final 30-s).
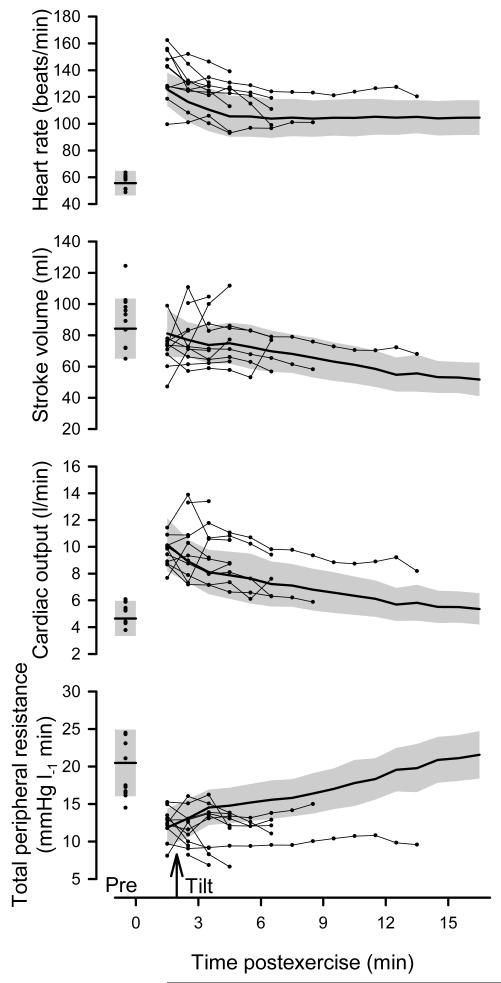
Figure 1.
Time course for heart rate, stroke volume, cardiac output, and total peripheral resistance on the control day. Heavy line and gray shading represent group means ± 95% confidence limits for a subset of subjects who completed the 15-min tilt (n = 8), measured in the supine position prior to exercise (Pre), supine immediately following exercise, and throughout 15-min head-up tilt on the control day. Scatter-plots prior to exercise (Pre) and after exercise (connected with light lines) indicate individual responses for those subjects who failed to complete the tilt (n=11).
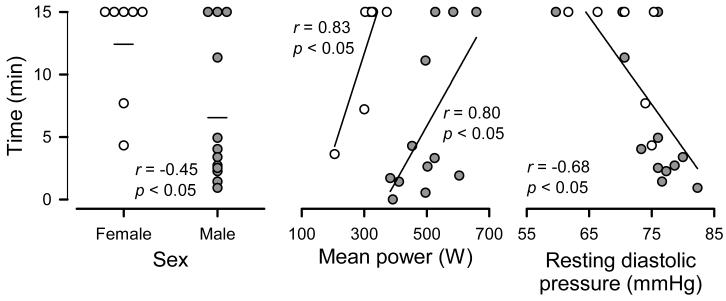
Figure 3.
Predictors of susceptibility to Wingate syncope test. The correlation between sex, mean power, and resting diastolic pressure vs. tilt duration across individual subjects on the control day is plotted, with open circles denoting female and closed circles denoting male subjects. In the left panel, mean times for men and women are displayed as horizontal lines. n = 19.
RESULTS
Pre-exercise hemodynamics and exercise performance
Pre-exercise hemodynamics were measured in the supine position after 45 min of rest. Heart rates were similar on the countermeasure and control days (58.7 ± 1.9 vs. 56.7 ± 1.8 beats·min−1; P = 0.37). Likewise, mean arterial pressures were similar on the countermeasure and control days (89.1 ± 1.4 vs. 89.5 ± 1.4 mmHg; P = 0.85). Table 2 shows the performance on the Wingate test for the countermeasure vs. the control day. Mean power, peak power, minimum power, time to peak power, and rate to fatigue were all similar between the two study days (all P > 0.05, paired t-test).
TABLE 2. Performance during modified Wingate test.
| Control | Countermeasure | |
|---|---|---|
| Mean power (W) | 421 ± 28 | 415 ± 28 |
| Mean power, relative (W/kg) | 5.87 ± 0.17 | 5.76 ± 0.19 |
| Peak power (W) | 689 ± 62 | 687 ± 65 |
| Minimum power (W) | 263 ± 17 | 252 ± 17 |
| Time to peak (s) | 5.5 ± 0.5 | 6.0 ± 0.6 |
| Rate to fatigue (W/s) | 7.8 ± 1.0 | 8.0 ± 1.0 |
Values are Mean ± SE; n =19
Hemodynamics of upright recovery from the Wingate syncope test
Figures 1 and 2 show the time course for hemodynamic variables, as well as the cerebral tissue oxygenation index, end-tidal PCO2 and symptom scores on the control day (i.e., no countermeasure). To illustrate the “average” recovery during the Wingate syncope test independent of vasovagal reactions, and also explore what occurs in individuals who do not complete the 15-min tilt test, we chose a novel way of presenting the data in these figures. First, responses in eight subjects (42% of subjects) who were able to complete the 15-min tilt are documented as group means ± 95% confidence limits. Using this approach, differences over time (P < 0.05) are evident when confidence limits do not overlap. Superimposed on this “average” recovery are scatter-plots for each of the eleven individuals (58% of subjects) who failed to complete the 15-min tilt. No further statistical analyses were run on these time course data due to the issue of subject drop-out, rather they are interpreted descriptively as follows.
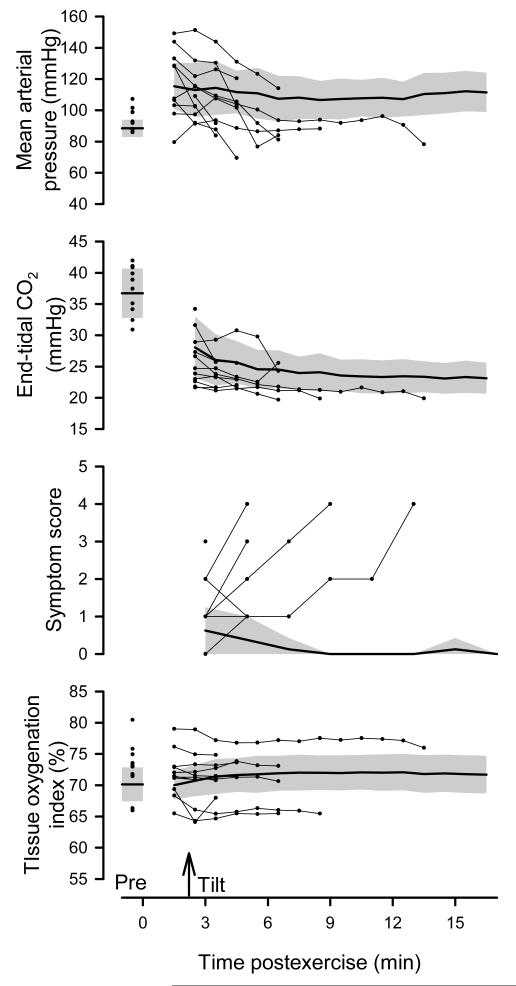
Figure 2.
Time course for mean arterial pressure, end-tidal CO2, symptom score, and tissue oxygenation index on the control day. Heavy line and gray shading represent group means ± 95% confidence limits for a subset of subjects who completed the 15-min tilt (n = 8), measured in the supine position prior to exercise (Pre), supine immediately following exercise, and throughout 15-min head-up tilt on the control day. Scatter-plots prior to exercise (Pre) and after exercise (connected with light lines) indicate individual responses for those subjects who failed to complete the tilt (n=11).
Figure 1 shows heart rate, stroke volume, cardiac output, and total peripheral resistance measured in the supine position prior to exercise, supine immediately following exercise, and throughout 15-min head-up tilt. Heart rate was elevated the entire post-exercise period (including the pre-tilt recovery and the 15-min tilt) in the group that completed the tilt vs. pre-exercise. Cardiac output was elevated in pre-tilt recovery and the first 3 min of tilt relative to pre-exercise, and was well maintained throughout the remainder of tilt. In contrast, stroke volume showed a progressive decline such that it was lower than pre-exercise during the last 3 min of tilt. Total peripheral resistance was reduced during pre-tilt recovery and the first minute of tilt, gradually increasing over time.
In the subjects who did not complete the tilt, some were above and some were below the average pattern for heart rate, stroke volume, and cardiac output such that no clear pattern was associated with the inability to complete the tilt except that, surprisingly, several fell above the upper 95% limit for heart rate and cardiac output. These subjects tended to fall below the average pattern for total peripheral resistance and many fell below the lower 95% limit for total peripheral resistance.
Figure 2 shows mean arterial pressure, end-tidal CO2, symptom score, and tissue oxygen index measured in the supine position prior to exercise, supine immediately following exercise, and throughout 15-min head-up tilt. Mean arterial pressure was well maintained at higher than pre-exercise levels throughout the entire post-exercise period (including the pre-tilt recovery and 15-min tilt) in the group that completed the tilt. End-tidal CO2 was reduced the entire post-exercise period (including the pre-tilt recovery and the 15-min tilt) in the group that completed the tilt vs. pre-exercise. After an elevation during the first minute of tilt, symptom scores remained low throughout the remainder of tilt. Tissue oxygen index was unchanged throughout recovery and head-up tilt.
In the subjects who did not complete the tilt, some were above and some were below the average pattern for mean arterial pressure such that no clear pattern was associated with the inability to complete the tilt, but several fell below the lower 95% limit for mean arterial pressure. These subjects tended to fall below the average pattern for end-tidal CO2 and some were below the 95% lower limit for end-tidal CO2. These subjects were consistently above the 95% upper limit for symptom scores. Some were above and some were below the average pattern for tissue oxygenation index such that no clear pattern was associated with the inability to complete the tilt.
Causes of tilt-test termination
Table 3 indicates the cause of tilt test termination in subjects who did not complete the 15-min tilt. In the 11 subjects in which the test was terminated before 15-min of tilt on the control day, the majority had developed hypotension of sudden onset in combination with pre-syncopal symptoms, including lightheadedness, nausea, and visual disturbances. While the general pattern was consistent with neurally mediated syncope, a relative bradycardia was only observed in one individual.
TABLE 3. Causes of tilt test termination.
| Subject | Control | Countermeasure |
|---|---|---|
| 2 | Hypotension, lightheadedness | |
| 3 | Lightheadedness, nausea | Dyspnea*, nausea, lightheadedness |
| 4 | Hypotension, nausea | Hypotension, nausea |
| 5 | Hypotension, lightheadedness, nausea | |
| 7 | Hypotension, lightheadedness, nausea | Hypotension, nausea |
| 9 | Hypotension, lightheadedness, vision loss |
Hypotension, lightheadedness, vision loss |
| 11 | Hypotension, lightheadedness, nausea | |
| 12 | Hypotension | Hypotension |
| 13 | Dyspnea*, hypotension | |
| 17 | Hypotension, lightheadedness, nausea | Dyspnea |
| 18 | Hypotension, lightheadedness | Hypotension, lightheadedness, nausea, vision loss |
| 19 | Hypotension, relative bradycardia, lightheadedness |
Reasons for early termination of head-up tilt testing. Empty cells signify the subject completed the entire 15 min head-up tilt test.
denotes individuals who did not tolerate the countermeasure due to dyspnea, and developed pre-syncopal signs or symptoms upon discontinuation of the countermeasure.
Predictors of susceptibility
Step-wise regression analyses identified several predictors of tilt duration, which was used as a marker of susceptibility to the Wingate syncope test. In one model we included as potential predictors sex, age, indices of physical activity, near-fainting experiences, and Wingate performance measures. Of these, female sex and higher Wingate mean power each associated with greater tilt duration (both P < 0.05); however, only sex independently correlated with tilt time as shown in Figure 3, as the relation with mean power interacted with sex. In an expanded model that also included resting hemodynamics, lower resting diastolic pressure was associated with greater tilt duration (P < 0.05), but sex and Wingate mean power dropped from the model (P > 0.15) when hemodynamics were added.
Effectiveness of countermeasure on tilt tolerance
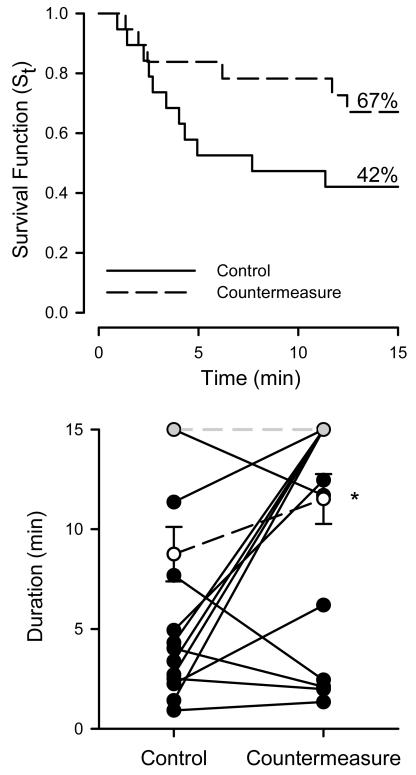
Figure 4 shows the proportion of subjects completing each minute of head-up tilt on the control vs. the countermeasure day. The median tolerance time (tilt time when the survival function is equal to 0.5) with countermeasure was 15 min compared to 7 min 41 s for control, and completion of the tilt test increased from 42% to 67% with countermeasure. Figure 4 also shows the individual tilt time durations with and without countermeasure. The duration of tolerable tilt was increased by a median time of 3 min 48 sec with countermeasure compared to control (P < 0.05), and was increased in 8 of the 11 subjects that did not complete the tilt test on the control day. Among fainters, the hazard ratio 0.32 with countermeasure relative to the control condition (P < 0.05). Table 3 indicates the cause of tilt test termination in subjects who did not complete the 15-min tilt. In the 8 subjects in which the test was terminated before 15-min of tilt on the countermeasure day, the majority had developed hypotension of sudden onset in combination with pre-syncopal symptoms, including lightheadedness, nausea, and visual disturbances. However, one terminated the test due to dyspnea that was attributed to the countermeasure, and two others rejected the countermeasure due to dyspnea and rapidly developed pre-syncopal signs and symptoms after coming off the countermeasure device.
Figure 4.
Survival function and tilt duration. Top panel: Proportion of subjects completing each stage of the head-up tilt shown as survival function. Solid line represents the control day; dotted line, countermeasure day. n = 19. Lower panel: Individual head-up tilt duration. Individual (closed circles, solid line) and group (open circles, dashed line; mean ± SE) head-up tilt durations on the control and countermeasure days for individuals who did not complete the tilt on one or more days (n = 12). * denotes P < 0.05 for countermeasure vs. control. Grey circles denote subjects who completed the tilt on both days (n = 7).
Hemodynamic effects of the countermeasure
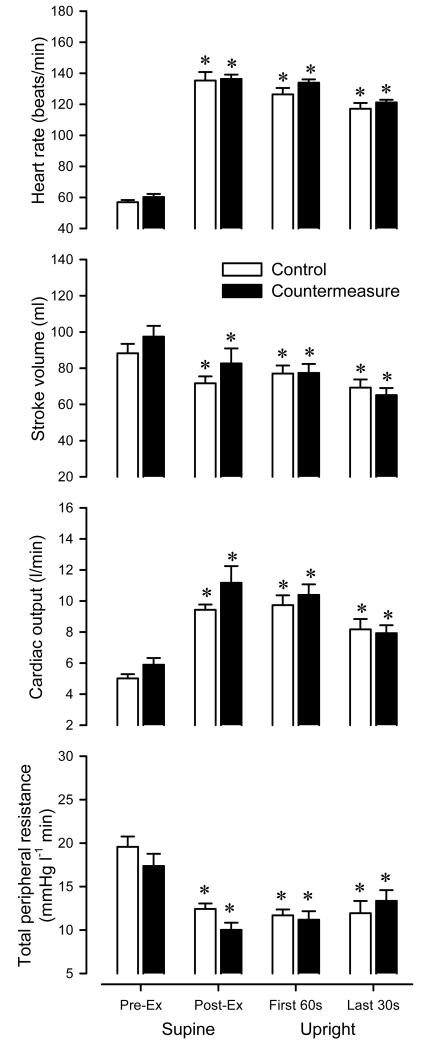
To explore the physiological basis for the countermeasure in the setting of this hemodynamic challenge, we analyzed the subset of subjects (n = 12) who failed to complete the 15-min tilt on at least one occasion (either on the control, countermeasure, or both days), eliminating the 7 subjects who completed the full 15-min tilt on both occasions. We specifically analyzed responses in the supine position prior to exercise, supine immediately following exercise, during the first 60-s of head-up tilt, and during the final 30-s of tolerated tilt (which in some cases was just prior to an early tilt termination, but in others was at the end of 15-min of tilt). These responses are presented in Figures 5 and 6.
Figure 5.
Control vs. countermeasure. Comparison of heart rate, stroke volume, cardiac output measured, and total peripheral resistance in the supine position prior to exercise, supine immediately following exercise, first 60-s of head-up tilt, and last 30-s of head-up tilt. Open bars denote control; Closed bars denote countermeasure. Values are means ± SE for individuals who did not complete the tilt on one or more days (n = 12). * P < 0.05 vs. pre-exercise.
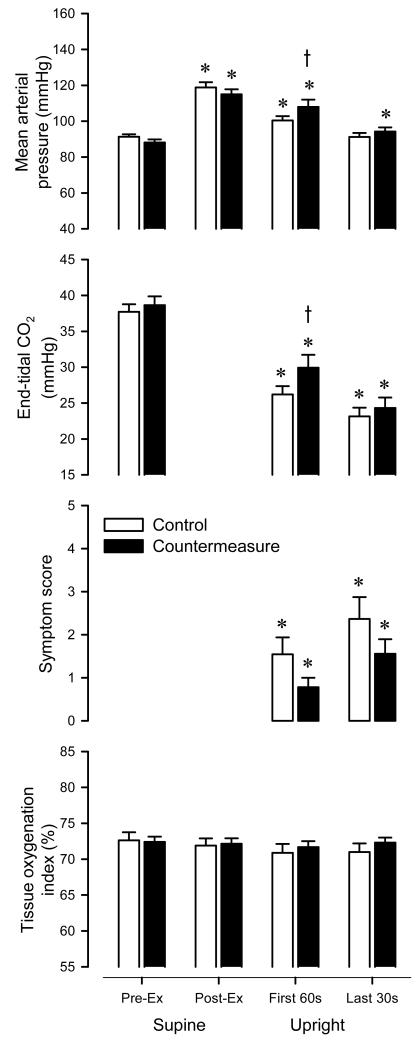
Figure 6.
Control vs. countermeasure. Comparison of mean arterial pressure, end-tidal CO2, symptom score, and tissue oxygenation index measured in the supine position prior to exercise, supine immediately following exercise, first 60-s of head-up tilt, and last 30-s of head-up tilt. Open bars denote control; Closed bars denote countermeasure. Values are means ± SE for individuals who did not complete the tilt on one or more days (n = 12). * P < 0.05 vs. pre-exercise. t P < 0.05 countermeasure vs. control.
Figure 5 shows the group averages for heart rate, stroke volume, cardiac output, and total peripheral resistance on the control and countermeasure days. Heart rate was elevated vs. pre-exercise at all time points (P < 0.05), with no clear differences in the pattern across conditions (P = 0.30). Stroke volume was reduced vs. pre-exercise at all time points (P < 0.05), but tended to be higher on the countermeasure vs. control day prior to tilt (P = 0.07). Cardiac output was elevated vs. pre-exercise at all time points (P < 0.05), with no clear differences in the pattern across conditions (P = 0.17). Total peripheral resistance was reduced vs. pre-exercise at all time points (P < 0.05), but tended to be lower on the countermeasure vs. control day prior to tilt (P = 0.05).
Figure 6 shows the group averages for and mean arterial pressure, end-tidal CO2, symptom score, and tissue oxygenation on the control and countermeasure days. Mean arterial pressure was elevated vs. pre-exercise at all time points (P < 0.05) except for the last 30-s on the control day, and mean arterial pressure was greater during tilt on the countermeasure vs. control day (P < 0.05). End-tidal CO2 was reduced vs. pre-exercise at all time points (P < 0.05), with less of a reduction during tilt on the countermeasure vs. control day (P < 0.05). Due to the marked effect on end-tidal CO2, we retrospectively estimated respiratory rate from the capnograph and found that breathing frequency was decreased during tilt on the countermeasure vs control day (14.5 ± 3.2 vs 18.4 ± 3.1 breaths/min; P < 0.05). Symptom scores were increased vs. pre-exercise during tilt (P < 0.05), with a trend for lower scores during tilt on the countermeasure vs. control day (P = 0.10). Tissue oxygenation index was unchanged vs. pre-exercise at all time points (P < 0.17), with a trend to be higher during tilt on the countermeasure vs. control day (P = 0.08).
Factors associated with symptoms and effects of the countermeasure
Step-wise regression analyses identified several factors associated with symptoms during tilt. For these models we included as potential factors the change from resting for arterial pressure, end-tidal CO2, and tissue oxygenation index, as well as experimental condition (countermeasure vs. control), and time post-exercise. Table 4 highlights the strongest associations which were retained in the model for lightheadedness symptom scale, nausea symptom scale, visual disturbances symptom scale, and a combined symptom scale. It is worth noting that all of the symptom scores decreased with time post-exercise, both the lightheadedness and nausea scores were inversely related to tissue oxygen index (symptoms increased with decreasing tissue oxygenation index), and the nausea score was inversely related to diastolic pressure and end-tidal CO2. Of note, the nausea score decreased with countermeasure independent of these other factors, suggesting the countermeasure improves some as yet unaccounted for factor related to nausea. The combined symptom score, as sum of the three scores, was inversely related to tissue oxygenation index, diastolic pressure, end-tidal CO2, and decreased with increased time post-exercise.
TABLE 4. Factors associated with symptoms.
| Estimate ± SE | r | P | |
|---|---|---|---|
| Lightheadedness scale | |||
| Time post-exercise (min) | −0.025 ± 0.009 | −0.234 | < 0.05 |
| Δ Tissue oxygenation index (%) | −0.046 ± 0.017 | −0.224 | < 0.05 |
| Nausea scale | |||
| Δ Tissue oxygenation index (%) | −0.039 ± 0.012 | −0.236 | < 0.05 |
| Δ Diastolic pressure (mmHg) | −0.009 ± 0.002 | −0.152 | < 0.05 |
| Countermeasure | −0.143 ± 0.057 | −0.167 | < 0.05 |
| Δ End-tidal PCO2 (mmHg) | −0.017 ± 0.006 | −0.133 | < 0.05 |
| Time post-exercise (min) | −0.014 ± 0.006 | −0.203 | < 0.05 |
| Visual disturbances scale | |||
| Time post-exercise (min) | −0.005 ± 0.002 | −0.147 | < 0.05 |
| Combined symptom scale | |||
| Time post-exercise (min) | −0.051 ± 0.014 | −0.276 | < 0.05 |
| Δ Tissue oxygenation index (%) | −0.076 ± 0.026 | −0.268 | < 0.05 |
| Δ End-tidal PCO2 (mmHg) | −0.032 ± 0.010 | −0.078 | < 0.05 |
| Δ Diastolic pressure (mmHg) | −0.010 ± 0.005 | −0.077 | < 0.05 |
DISCUSSION
Little is known about the prevalence of isolated syncope during recovery from exercise in the normal population, or any clinical population. While many case reports exist, most of these assessed individuals who have presented to the clinic after suffering from a syncopal episode post-exercise (Halliwill et al., 2013b) and other seldom provide physiological insight beyond documentation of reduced orthostatic tolerance following exercise. Therefore, a primary focus of this investigation was to develop an exercise model for inducing post-exercise syncope in healthy men and women in order to examine the physiology underlying this phenomenon. Subsequent to this, we wanted to test the effectiveness of a standardized countermeasure (an impedance threshold device) at alleviating pre-syncopal signs and symptoms. Thus, in addition to developing this potential model of post-exercise syncope, we sought to determine whether the model was “sensitive” to intervention.
We hypothesized the modified Wingate test would be effective at eliciting pre-syncope in healthy men and women. One major finding of this study is that our modified Wingate test in combination with orthostatic stress is an effective model for examining post-exercise syncope, inducing pre-syncope in the majority (58%) of individuals. This is markedly higher than the incidence of ~ 8% pre-syncope during similar head-up tilt in the general population (Kosinski & Grubb, 1994; Natale et al., 1995). Additionally, we hypothesized that the use of an inspiratory threshold device would be an effective countermeasure and decrease the incidence of pre-syncopal signs and symptoms in this model. Thus, another major finding was that spontaneous breathing through an inspiratory threshold device proved beneficial at increasing tolerance time (~ 4 min, similar to what it does during lower body negative pressure (Convertino et al., 2007)) and decreased the number of individuals with pre-syncope (down to 33%). Taken together, this set of observations demonstrates that the Wingate syncope test is a valid model for post-exercise fainting associated with high-intensity exercise, and that the responses to the test are modifiable, i.e., responsive to interventions. Beyond these important observations, we have asked a series of descriptive and mechanistic questions regarding this model of post-exercise syncope as well as one potential countermeasure.
What are the hemodynamics of upright recovery from this modified Wingate test?
In humans, upright posture challenges the cardiovascular system as it is associated with a translocation of ~ 70% of total blood volume below the heart into the compliant veins of dependent limbs and pelvic organs (Rowell, 1986), resulting in reduced central venous pressure and a lessening of cardiac output. Thus, we were surprised to observe that in individuals who completed the Wingate syncope test, the hemodynamic profile included elevations in cardiac output during upright recovery from exercise relative to supine levels prior to exercise. Therefore, the primary hemodynamic challenge faced during the Wingate syncope test is the marked peripheral vasodilation, not a lessening of cardiac output. In the balance between elevated cardiac output and peripheral vasodilation, a high cardiac output is insufficient to meet the demands of elevated conductance. This hemodynamic pattern is consistent with one prior report on the Wingate test, in which recovery was assessed in the seated and supine position (Kilgour et al., 1995). It is also consistent with what is observed following longer duration but more moderate exercise, when recovery is assessed in the supine position (Halliwill et al., 2013a). However, it stands in contrast to what is seen following 45-min moderate intensity running in the heat, when recovery was assessed during a 15-min head-up tilt (McCord et al., 2008). In this alternative model for post-exercise syncope, cardiac output was decreased relative to pre-exercise supine levels, indicating that different models of post-exercise syncope (intense but short anaerobic exercise vs. longer moderate aerobic exercise in the heat) result in different forms of cardiovascular strain on the individual.
In the face of a marked post-exercise hyperemia following the Wingate test, arterial pressure is well-maintained at or above pre-exercise supine levels when measured at the heart, although the modest elevations in heart-level pressure shown in Figure 1 (~ 20 mmHg) would be the equivalent of a ~ 7 mmHg reduction in arterial pressure at brain level (see Appendix for details of the this estimation). That said, it is controversial whether height-adjusted arterial pressure is the best indicator of perfusion pressure for the cerebral circulation. The root of the controversy is a lack of certainty regarding the degree to which cerebral circulation acts like a siphon, or more like a starling resistor (Gisolf et al., 2005; Hicks & Munis, 2005). It is accepted that the anterior veins (i.e., internal and external jugular veins) collapse during upright posture (Dawson et al., 2004), but there is a shift in venous drainage to posterior veins (vertebral and spinal epidural veins) which appear to be protected from collapse (Valdueza et al., 2000). Therefore, if one or more of these posterior pathways remain patent, a siphon effect would counterbalance the hydrostatic effects on the arterial side, and cerebral perfusion pressure would equal the arterial to central venous pressure difference, with both pressures taken at heart level. We must note that this assumes that the cerebral circulation is operating above the microcirculatory critical closing pressure, as the microcirculation may behave as starling resistor and collapse if the arterial pressure at brain level falls below the critical closing pressure (in which case, cerebral perfusion pressure would equal the brain-level arterial pressure to closing pressure difference). Estimates of the cerebral critical closing pressure are on the order of 20 to 40 mmHg in humans, and so we presume that there is a patent microcirculatory pathway throughout the cardiac cycle in the context of our study, and hence, cerebral perfusion pressure was slightly reduced if one doesn’t accept the presence of a siphon effect, and slight increased if one accepts it.
Regardless of whether or not there are modest changes in cerebral perfusion pressure, there are likely to be substantial reductions in cerebral perfusion and cerebral oxygenation under these circumstances, as the sustained post-exercise hyperventilation decreased end-tidal CO2 (~ 14 mmHg) to levels that would be associated with a ~ 40% reduction in cerebral perfusion (Low et al., 2008). Additionally, Rasmussen et al. (Rasmussen et al., 2006) looked at cerebral CO2 vascular reactivity following strenuous exercise and found it to be increased following exercise, concluding that arterial CO2 appears to be the primary factor influencing changes in cerebral blood flow during and after prolonged exercise. Despite an expectation of diminished cerebral perfusion secondary to severe hypocapnia and mild pressure reductions, our indirect measure of cerebral tissue oxygenation remained unchanged on average, but many subjects felt modest symptoms of lightheadedness, nausea, or visual disturbances and many of these symptoms were associated with reductions in cerebral tissue oxygenation, perfusion pressure, and end-tidal CO2.
The potential role of autoregulation in this model also merits discussion. While cerebral autoregulation is well-maintained following moderate intensity exercise (Ogoh et al., 2007; Murrell et al., 2007; Willie et al., 2013), two studies suggest that more intense exercise may produce a lasting cerebral autoregulatory deficit. Specifically, Ogoh et al. (Ogoh et al., 2005) found reduced dynamic cerebral autoregulation (as assessed by transfer function) during heavy cycle exercise and Bailey et al. (Bailey et al., 2011) found reduced dynamic cerebral autoregulation (as assessed by thigh-cuff release) during recovery from a recumbent cycle peak test. Thus, it seems likely that cerebral perfusion is less protected from sudden onset hypotension during the Wingate syncope test, but we have not directly assessed this in the current study.
What is the underlying physiology in someone who becomes pre-syncopal in response to this test?
Given the nature of the changes we observed during the Wingate syncope test, it is clear that while few subjects were symptom free, some were hemodynamically stable, and others went from hemodynamic stability to pre-syncope abruptly. One can say that during orthostatic stress following the Wingate test, arterial pressure is well-maintained, until it isn’t. The sudden onset of hypotension in combination with pre-syncopal symptoms, including lightheadedness, nausea, and visual disturbances were consistent with neurally mediated syncope, but in general the bradycardia component of this response was absent in this model. As stated above, symptoms tracked loosely with factors one would expect to impact on cerebral perfusion and cerebral tissue oxygenation, yet it remains possible that non-traditional signals underlie the development of some of the symptoms (i.e., they may develop independent of measured changes in cerebral perfusion or cerebral tissue oxygenation). The hemodynamics preceding the onset of pre-syncope were variable, but some patterns emerge such as high heart rates, low total peripheral resistance, and low end-tidal CO2. One could argue that these hemodynamic patterns are not consistent with the “empty heart syndrome” often described as a putative trigger for neurally mediated syncope (Joyner, 2009), and are more consistent with a centrally generated signal for sudden onset vasodilation and hypotension (Hainsworth, 2003).
What factors predict who becomes pre-syncopal in response to this test?
In trying to predict what type of subject was more susceptible to the Wingate syncope test, it is surprising that men were more susceptible than women. In general, women have decreased orthostatic tolerance compared to men, however, men more commonly suffer from post-exercise syncope than women (Halliwill et al., 2013b). Likewise, it is intriguing that subjects with lower resting diastolic pressures fared better in this test than those with higher resting pressures. We can only speculate that these are 1) spurious correlations in a small subject pool or 2) associated with longer-term adaptations in cerebral autoregulation, cerebral sensitivity to CO2, or other factors which were not assessed in the current study. It is also unclear what underlies the relationship between mean power and susceptibility, in which greater mean power, indicative of a greater metabolic cost, would be associated with greater vasodilation of the previously exercised skeletal muscle, yet correlated with greater tolerance to head-up tilt.
By what mechanism does inspiratory resistance provide protection against post-exercise syncope?
The inspiratory threshold device was developed with the idea of exploiting the respiratory pump. During inspiration, intrathoracic pressure is lowered through the depression of the diaphragm and this increases the pressure gradient between the abdomen and the thorax, causing the inferior vena cava to discharge blood centrally. For this reason, increased negative intrathoracic pressure during spontaneous inspiration represents a natural mechanism for enhancing venous return and preload to the heart. By decreasing intrathoracic pressure during inspiration, the inspiratory threshold device had been shown to increase venous return and ventricular pre-load (Lurie et al., 2002, 2004), effectively elevating cardiac output and arterial blood pressure in patients during cardiac arrest (Plaisance et al., 2000) or severe central hypovolemia and lower body negative pressure (Convertino et al., 2007; Ryan et al., 2008). Along these lines, Rickards et al. (Rickards et al., 2008) found the device could reduce the severity of orthostatic symptoms during a squat-stand test as well as during central hypovolemia induced by lower body negative pressure (Rickards et al., 2007). Thus, it was reasonable to expect that increasing inspiratory resistance would have similar effects on the Wingate syncope test and that the device would function by increasing venous return to the heart. Our results suggest that the effects are more complicated than an exploitation of the respiratory pump in this model of post-exercise syncope.
First, we provide clear evidence that in many individuals, use of the countermeasure was beneficial. However, the inspiratory threshold device was not well tolerated by all subjects. Indeed, several subjects reported severe dyspnea, to the point where the countermeasure had to be discontinued in some subjects (15%). This has not been reported in other experiments using the inspiratory threshold device and is likely to be specific to the considerable respiratory drive experienced by subjects following the Wingate test, believed to arise from the metabolic acidosis of severe exercise.
Second, the inspiratory threshold device did not produce a clear effect on stroke volume or cardiac output during head-up tilt following exercise. Likewise, there was no clear effect on total peripheral resistance; yet mean arterial pressure during head-up tilt was increased. This suggests that the effect size on hemodynamics was small relative to our statistical power, and also that factors in addition to central hemodynamics may be important to this model of syncope and how the use of the inspiratory threshold device improved head-up tilt tolerance and reduced symptoms.
Third, the inspiratory threshold device altered ventilation and this resulted in higher end-tidal CO2, suggesting that the degree of hypocapnic cerebral vasoconstriction would be lessened by the device. The magnitude of the effect could benefit cerebral perfusion by ~ 10% (Low et al., 2008). Along these lines, we noted a trend for better cerebral oxygenation and this was associated with fewer symptoms. Of note, symptom ratings for nausea were lessened by the countermeasure independent of measured hemodynamics. In contrast, symptom ratings such as lightheadedness and visual disturbances were lessened by improvements in arterial pressure and in end-tidal CO2, both associated with the countermeasure.
Lastly, an unappreciated aspect of the greater intrathoracic negative pressure excursions is that it has the potential to augment cerebral perfusion via an increase in the cerebral siphon effect. As discussed above, there is controversy over the existence of a cerebral siphon effect in upright humans (Gisolf et al., 2005; Hicks & Munis, 2005). However, venous drainage to posterior veins (vertebral and spinal epidural veins) increases in the upright position, and these veins appear to be protected from collapse (Valdueza et al., 2000). Therefore, if one or more of these posterior pathways remain patent, a siphon effect would not only counterbalance the hydrostatic effects of orthostasis, but could function to augment the cerebral perfusion pressure when intrathoracic pressures become more negative during breathing against an inspiratory resistance.
Thus, it appears that, unlike other conditions of compromised cardiac function, in the context of upright recovery from intense anaerobic exercise, manipulation of inspiratory resistance provides benefits by more than just an enhanced venous return to the heart. Some combination of effects may be at play, including but not limited to small improvements in central hemodynamics, amelioration of hypocapnic cerebral vasoconstriction, and an augmented perfusion gradient across the cerebral circulation by a siphon effect.
Methodological considerations
Studies on syncope are full of technical challenges, including how to deal with subject dropout. We have attempted some novel approaches and argue that they faithfully capture the physiology of this particular model of post-exercise syncope. That said, several experimental design issues warrant specific discussion. First, due to the repeated stresses of this protocol, concern for subject welfare, and consideration of how best to encourage subjects to push hard during the exercise period, we did not take subjects to loss of consciousness, but rather used strict pre-syncopal symptom and sign criteria as our endpoint for head-up tilt. We argue this is a valid methodology for the questions we are addressing in this experiment, as the development of pre-syncope is a relevant clinical outcome. Second, we did not anticipate to uncover a potential disconnect between well-maintained arterial pressure and the development of pre-syncopal symptoms. Further, at the time of this investigation, we did not have access to a transcranial Doppler system. Thus, we do not have measurements of cerebral perfusion. Recent investigations suggest some heterogeneity in cerebral hemodynamics across the major conduit vessels providing blood flow to the central nervous system (Sato et al., 2012). So, it is not entirely clear whether use of transcranial Doppler of the middle cerebral artery (commonly measured in studies such as this) would have been of benefit in interpreting our results. However, in lieu of cerebral vascular measurements, we used transcranial near-infrared spectroscopy to monitor cerebral tissue oxygenation. This complicated measurement is generally interpreted as representing a balance between local metabolism and local perfusion such that a relative under-perfusion or over-perfusion can be documented. This method has been used previously to demonstrate a decrease in cerebral oxygenation at the onset of neurally mediated syncope (Rao et al., 2010). Lastly, to gain greater temporal resolution for central hemodynamics such as stroke volume and cardiac output, we relied on an indirect index of stroke volume provided by the arterial pulse contour analysis. In this context, this method does not provide accurate absolute measures of hemodynamics, but has been shown to track corresponding changes in these derived variables during acute interventions such as in the current study (Matsukawa et al., 2004; Krediet et al., 2005). It has some shortcomings in heat-stress (Shibasaki et al., 2011), but these are unlikely to affect our results.
Perspectives
Vast numbers of undergraduate kinesiology students attest to the discomfort associated with the Wingate test of anaerobic power. The current study provides some insight into the physiology that underlies this anecdotal representation, and provides a novel model for the study of one form of post-exercise syncope: syncope that follows short but severe whole body exercise. While we explored a specific countermeasure, use of an inspiratory threshold device, we did not anticipate that this would become the first line of defense in undergraduate physiology labs, nor do we anticipate medical personnel handing out these devices at athletic events. On the other hand, this experiment demonstrates that post-exercise syncope can be averted in many individuals by physical manipulation of the cardiorespiratory system. Syncope is not a fixed outcome, but a risk that can be mitigated.
One can imagine the development of a physical countermeasure specific to this post-exercise syncope model that is not dependent on a device, but rather is based on coaching a breathing strategy (Lucas et al., 2013) or postural adjustment. While physical maneuvers such as squatting and muscle tensing have been evaluated in individuals with autonomic dysfunction (Krediet, 2002; Krediet et al., 2004, 2005; Groothuis et al., 2007), these have not been systematically assessed in healthy individuals post-exercise (although Eichna et al. established the principle in 1947 by having subjects move their legs in place on a tilt table to avert severe post-exercise hypotension) (Eichna et al., 1947). We propose that different models of post-exercise syncope (intense but short anaerobic exercise vs. longer moderate aerobic exercise in the heat) will each be amenable to countermeasures that act specifically on the underlying physiology of each model, seeing post-exercise syncope as a spectrum of challenges that needs a quiver of fixes, rather than a single physiology fixed by a single “one-size fits all” solution. For this particular model, the recent interest in high intensity interval training (Gibala et al., 2012; Kessler et al., 2012) provides a rationale for prioritizing its study and development of interventions.
Conclusion
Our overarching aim of this study was to create a foundation for future work examining post-exercise syncope. We found that our modified Wingate test in combination with orthostatic stress is an effective model for examining post-exercise syncope, inducing pre-syncope in the majority of individuals. Further, spontaneous breathing through an inspiratory threshold device proved beneficial at increasing tolerance time (~ 4 min) and decreasing the number of individuals developing pre-syncope. In combination, this demonstrates that the Wingate syncope test is a valid model for at least one form of post-exercise syncope, and that the responses to the test are receptive to physical interventions.
NEW FINDINGS.
What is the central question of this study?
Does a modified version of the Wingate anaerobic power test produce pre-syncopal signs and symptoms in healthy individuals?
Does an inspiratory threshold device work as a countermeasure against post-exercise syncope?
What is the main finding and its importance?
A modified Wingate test is a good model to induce post-exercise syncope and syncopal symptoms can be ameliorated by an inspiratory threshold device.
ACKNOWLEDGEMENTS
This research was supported in part by grants from the American Heart Association, Grant-in-Aid 11GRNT5490000, and National Institutes of Health Grant HL115027. The authors have no conflict of interest to report. This study was conducted by Alisha N. Lacewell in partial fulfillment of the requirements for the degree of Masters of Science at the University of Oregon.
Source of Funding: This research was supported in part by grants from the American Heart Association, Grant-in-Aid 11GRNT5490000, and National Institutes of Health Grant HL115027.
APPENDIX
Arterial pressure at brain level was estimated using the following equation: Pbrain = Pheart - Sin [60°]·d·g·ρ·k, where Pbrain is arterial pressure at the level of the brain, Pheart is arterial pressure at the level of the heart, d is the distance from heart to the brain (estimated to be 0.40 m), g is the acceleration due to gravity (9.81 m/s2), ρ is the density of blood (1050 kg/m3), and k is a conversion factor (0.0075 mmHg per 1.0 pascals). Thus, when the subjects are tilted to 60°, arterial pressure at brain level is estimated to be 26.8 mmHg lower than arterial pressure measured at the heart.
Footnotes
AUTHOR CONTRIBUTIONS
ANL, SAR, and JRH were responsible for conception and design of the study. ANL, TMB, and JRH performed the experiments, analysis, and interpretation. ANL and JRH prepared the figures and drafted the manuscript. All authors edited a draft of the article and approved the final manuscript.
Conflicts of Interest The authors have no conflict of interest to report.
REFERENCES
- Arad M, Solomon A, Roth A, Atsmon J, Rabinowitz B. Postexercise syncope: evidence for increased activity of the sympathetic nervous system. Cardiology. 1993;83:121–123. doi: 10.1159/000175957. [DOI] [PubMed] [Google Scholar]
- Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Evans KA, McEneny J, Young IS, Hullin DA, James PE, Ogoh S, Ainslie PN, Lucchesi C, Rockenbauer A, Culcasi M, Pietri S. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp Physiol. 2011;96:1196–1207. doi: 10.1113/expphysiol.2011.060178. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Ratliff DA, Crissey J, Doerr DF, Idris AH, Lurie KG. Effects of inspiratory impedance on hemodynamic responses to a squat-stand test in human volunteers: implications for treatment of orthostatic hypotension. Eur J Appl Physiol. 2005;94:92–399. doi: 10.1007/s00421-005-1344-1. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Ratliff DA, Eisenhower KC, Warren C, Doerr DF, Idris AH, Lurie KG. Inspiratory impedance effects on hemodynamic responses to orthostasis in normal subjects. Aviat Space Environ Med. 2006;77:486–493. [PubMed] [Google Scholar]
- Convertino VA, Ratliff DA, Ryan KL, Cooke WH, Doerr DF, Ludwig DA, Muniz GW, Britton DL, Clah SD, Fernald KB, Ruiz AF, Idris A, Lurie KG. Effects of inspiratory impedance on the carotid-cardiac baroreflex response in humans. Clin Auton Res. 2004;14:240–248. doi: 10.1007/s10286-004-0180-4. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Ryan KL, Rickards CA, Cooke WH, Idris AH, Metzger A, Holcomb JB, Adams BD, Lurie KG. Inspiratory resistance maintains arterial pressure during central hypovolemia: implications for treatment of patients with severe hemorrhage. Crit Care Med. 2007;35:1145–1152. doi: 10.1097/01.CCM.0000259464.83188.2C. [DOI] [PubMed] [Google Scholar]
- Dawson EA, Secher NH, Dalsgaard MK, Ogoh S, Yoshiga CC, González-Alonso J, Steensberg A, Raven PB. Standing up to the challenge of standing: a siphon does not support cerebral blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2004;287:R911–R914. doi: 10.1152/ajpregu.00196.2004. [DOI] [PubMed] [Google Scholar]
- Van Dijk N, de Bruin I, Gisolf J, Rianne de Bruin-Bon HACM, Linzer M, Van Lieshout JJ, Wieling W. Hemodynamic effects of leg crossing and skeletal muscle tensing during free standing in patients with vasovagal syncope. J Appl Physiol. 2005;98:584–590. doi: 10.1152/japplphysiol.00738.2004. [DOI] [PubMed] [Google Scholar]
- Eichna LW, Horvath SM, Bean WB. Post-exertional orthostatic hypotension. Am J Med Sci. 1947;213:641–654. doi: 10.1097/00000441-194706000-00001. [DOI] [PubMed] [Google Scholar]
- Freeman R, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisolf J, Gisolf A, van Lieshout JJ, Karemaker JM. The siphon controversy: an integration of concepts and the brain as baffle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R627–R629. doi: 10.1152/ajpregu.00709.2004. [DOI] [PubMed] [Google Scholar]
- Groothuis JT, van Dijk N, Ter Woerds W, Wieling W, Hopman MTE. Leg crossing with muscle tensing, a physical counter-manoeuvre to prevent syncope, enhances leg blood flow. Clin Sci. 2007;112:193–201. doi: 10.1042/CS20060241. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Syncope: what is the trigger? Heart. 2003;89:123–124. doi: 10.1136/heart.89.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilation: what happens after we exercise? Exp Physiol. 2013a;98:7–18. doi: 10.1113/expphysiol.2011.058065. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Sieck DC, Romero SA, Buck TM, Ely MR. Blood pressure regulation X: What happens when the muscle pump is lost? Post-exercise hypotension and syncope. Eur J Appl Physiol (In Review) 2013b doi: 10.1007/s00421-013-2761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci. 1999;97:291–301. [PubMed] [Google Scholar]
- Hicks JW, Munis JR. The siphon controversy counterpoint: the brain need not be “baffling”. Am J Physiol Regul Integr Comp Physiol. 2005;289:R629–R632. doi: 10.1152/ajpregu.00810.2004. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Orthostatic stress, haemorrhage and a bankrupt cardiovascular system. J Physiol. 2009;587:5015–5016. doi: 10.1113/jphysiol.2009.181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med. 2012;42:489–509. doi: 10.2165/11630910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kilgour RD, Mansi JA, Williams PA. Cardiodynamic responses during seated and supine recovery from supramaximal exercise. Can J Appl Physiol. 1995;20:52–64. doi: 10.1139/h95-004. [DOI] [PubMed] [Google Scholar]
- Kohl HW, Blair SN, Paffenbarger RS, Macera CA, Kronenfeld JJ, Paffenbarger RS., Jr. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- Kosinski D, Grubb BP, Karas BJ, Frederick S. Exercise-induced neurocardiogenic syncope: clinical data, pathophysiological aspects, and potential role of tilt table testing. Europace. 2000;2:77–82. doi: 10.1053/eupc.1999.0065. [DOI] [PubMed] [Google Scholar]
- Kosinski DJ, Grubb BP. Neurally mediated syncope with an update on indications and usefulness of head-upright tilt table testing and pharmacologic therapy. Curr Opin Cardiol. 1994;9:53–64. doi: 10.1097/00001573-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Krediet CTP. Management of vasovagal syncope: Controlling or aborting faints by leg crossing and muscle tensing. Circulation. 2002;106:1684–1689. doi: 10.1161/01.cir.0000030939.12646.8f. [DOI] [PubMed] [Google Scholar]
- Krediet CTP, Wilde AAM, Halliwill JR, Wieling W. Syncope during exercise, documented with continuous blood pressure monitoring during ergometer testing. Clin Auton Res. 2005;15:59–62. doi: 10.1007/s10286-005-0241-3. [DOI] [PubMed] [Google Scholar]
- Krediet CTP, Wilde AAM, Halliwill JR, Wieling W. Post-exercise vasovagal syncope. In: Civera R, Esquivias G, Bland J, Brignole M, Mitjans A, Granell R, Wieling W, editors. Syncope Cases. Blackwell; Malden, MA: 2006. pp. 46–48. [Google Scholar]
- Krediet CTP, Wilde AAM, Wieling W, Halliwill JR. Exercise related syncope, when it’s not the heart. Clin Auton Res. 2004;14(Suppl):25–36. doi: 10.1007/s10286-004-1005-1. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Toska K, van Lieshout EJ, Eriksen M, Walløe L, Wesseling KH. Beat-to-beat noninvasive stroke volume from arterial pressure and Doppler ultrasound. Eur J Appl Physiol. 2003;90:131–137. doi: 10.1007/s00421-003-0901-8. [DOI] [PubMed] [Google Scholar]
- Low DA, Wingo JE, Keller DM, Davis SL, Zhang R, Crandall CG. Cerebrovascular responsiveness to steady-state changes in end-tidal CO2 during passive heat stress. J Appl Physiol. 2008;104:976–981. doi: 10.1152/japplphysiol.01040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJE, Lewis NCS, Sikken ELG, Thomas KN, Ainslie PN. Slow breathing as a means to improve orthostatic tolerance: a randomized sham-controlled trial. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00128.2013. DOI: 10.1152/japplphysiol.00128.2013. [DOI] [PubMed] [Google Scholar]
- Lurie K, Zielinski T, McKnite S, Idris AH, Yannopoulos D, Raedler C, Sigurdsson G, Benditt DG, Voelckel W. Treatment of hypotension in pigs with an inspiratory impedence threshold device: A feasibility study. Crit Care Med. 2004;32:1555–1562. doi: 10.1097/01.ccm.0000131207.29081.a2. [DOI] [PubMed] [Google Scholar]
- Lurie K, Zielinski T, Voelckel W, McKnite S, Plaisance P. Augmentation of ventricular preload during treatment of cardiovascular collapse and cardiac arrest. Crit Care Med. 2002;30:162–165. doi: 10.1097/00003246-200204001-00009. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Kobayashi T, Nakamoto T, Murata J, Komine H, Noso M. Noninvasive evaluation of cardiac output during postural change and exercise in humans: comparison between the modelflow and pulse dye-densitometry. Japan J Physiol. 2004;54:153–160. doi: 10.2170/jjphysiol.54.153. [DOI] [PubMed] [Google Scholar]
- McCord JL, Pellinger TK, Lynn BM, Halliwill JR. Potential benefit from an H1-receptor antagonist on postexercise syncope in the heat. Med Sci Sports Exerc. 2008;40:1953–1961. doi: 10.1249/MSS.0b013e31817f1970. [DOI] [PubMed] [Google Scholar]
- Melby D, Lu F, Sakaguchi S, Zook M, Benditt DG. Increased impedence to inspiration ameliorates hemodynamic changes associated with movement to upright posture in orthostatic hypotension: A randomized blinded pilot study. Heart Rhythm. 2007;4:128–135. doi: 10.1016/j.hrthm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Murrell C, Wilson L, Cotter JD, Lucas S, Ogoh S, George K, Ainslie PN. Alterations in autonomic function and cerebral hemodynamics to orthostatic challenge following a mountain marathon. J Appl Physiol. 2007;103:88–96. doi: 10.1152/japplphysiol.01396.2006. [DOI] [PubMed] [Google Scholar]
- Natale A, Akhtar M, Jazayeri M, Dhala A, Blanck Z, Deshpande S, Krebs A, Sra JS. Provocation of hypotension during head-up tilt testing in subjects with no history of syncope or presyncope. Circulation. 1995;92:54–58. doi: 10.1161/01.cir.92.1.54. [DOI] [PubMed] [Google Scholar]
- O’Connor FG, Levine BD, Childress MA, Asplundh CA, Oriscello RG. Practical management: a systematic approach to the evaluation of exercise-related syncope in athletes. Clin J Sport Med. 2009;19:429–434. doi: 10.1097/JSM.0b013e3181b732c3. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–H1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fisher JP, Purkayastha S, Dawson EA, Fadel PJ, White MJ, Zhang R, Secher NH, Raven PB. Regulation of middle cerebral artery blood velocity during recovery from dynamic exercise in humans. J Appl Physiol. 2007;102:713–721. doi: 10.1152/japplphysiol.00801.2006. [DOI] [PubMed] [Google Scholar]
- Plaisance P, Lurie KG, Payen D. Inspiratory Impedance During Active Compression-Decompression Cardiopulmonary Resuscitation : A Randomized Evaluation in Patients in Cardiac Arrest. Circulation. 2000;101:989–994. doi: 10.1161/01.cir.101.9.989. [DOI] [PubMed] [Google Scholar]
- Rao RP, Danduran MJ, Dixon JE, Frommelt PC, Berger S, Zangwill SD. Near infrared spectroscopy: guided tilt table testing for syncope. Pediatric cardiology. 2010;31:674–679. doi: 10.1007/s00246-010-9683-z. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol. 2006;96:299–304. doi: 10.1007/s00421-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Rickards CA, Cohen KD, Bergeron LL, Burton L, Khatri PJ, Lee CT, Ryan KL, Cooke WH, Doerr DF, Lurie KG, Convertino VA. Inspiratory resistance, cerebral blood flow velocity, and symptoms of acute hypotension. Aviat Space Environ Med. 2008;79:557–564. doi: 10.3357/asem.2149.2008. [DOI] [PubMed] [Google Scholar]
- Rickards CA, Ryan KL, Cooke WH, Lurie KG, Convertino VA. Inspiratory resistance delays the reporting of symptoms with central hypovolemia: association with cerebral blood flow. Am J Physiol Regul Integr Comp Physiol. 2007;293:R243–50. doi: 10.1152/ajpregu.00087.2007. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human circulation: Regulation during physical stress. Oxford University Press; New York, NY: 1986. [Google Scholar]
- Ryan KL, Cooke WH, Rickards CA, Lurie KG, Convertino VA. Breathing through an inspiratory threshold device improves stroke volume during central hypovolemia in humans. J Appl Physiol. 2008;104:1402–1409. doi: 10.1152/japplphysiol.00439.2007. [DOI] [PubMed] [Google Scholar]
- Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol. 2012;97:1272–1280. doi: 10.1113/expphysiol.2012.064774. [DOI] [PubMed] [Google Scholar]
- Schrezemaier C, Gehrking JA, Hines SM, Low PA, Benrud-Larson LM, Sandroni P. Evaulation of orthostatic hypotension: Relationshp of a new self-report instrument to laboratory-based measures. Mayo Clin Proc. 2005;80:330–334. doi: 10.4065/80.3.330. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol. 2011;300:R486–R491. doi: 10.1152/ajpregu.00505.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand. 2003;179:361–366. doi: 10.1046/j.0001-6772.2003.01211.x. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Onodera O, Kodera K, Igarashi Y, Miida T, Aizawa Y, Izumi T, Shibata A, Takano S. Atrial standstill after treadmill exercise test and unique response to isoproterenol infusion in recurrent postexercise syncope. Am J Cardiol. 1990;65:533–535. doi: 10.1016/0002-9149(90)90829-p. [DOI] [PubMed] [Google Scholar]
- Tsutsumi E, Hara H. Syncope after running. Br Med J. 1979;280:1480. doi: 10.1136/bmj.2.6203.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdueza JM, von Münster T, Hoffman O, Schreiber S, Einhäupl KM. Postural dependency of the cerebral venous outflow. Lancet. 2000;355:200–201. doi: 10.1016/s0140-6736(99)04804-7. [DOI] [PubMed] [Google Scholar]
- Willie CK, Ainslie PN, Taylor CE, Eves ND, Tzeng Y-C. Maintained cerebrovascular function during post-exercise hypotension. Eur J Appl Physiol. 2013;113:1597–1604. doi: 10.1007/s00421-012-2578-3. [DOI] [PubMed] [Google Scholar]
- Ziegelstein RC. Near-syncope after exercise. JAMA. 2004;292:1221–1226. doi: 10.1001/jama.292.10.1221. [DOI] [PubMed] [Google Scholar]