Abstract
The brain requires steady delivery of oxygen and glucose, without which neurodegeneration occurs within minutes. Thus, the ability of the cerebral vasculature to maintain relatively steady blood flow in the face of changing systemic pressure, i.e., cerebral autoregulation, is critical to neurophysiologic health. Although the study of autoregulation dates to the early 20th century, only the recent availability of cerebral blood flow measures with high temporal resolution has allowed rapid, beat-by-beat measurements to explore the characteristics and mechanisms of autoregulation. These explorations have been further enhanced by the ability to apply sophisticated computational approaches that exploit the large amounts of data that can be acquired. These advances have led to unique insights. For example, recent studies have revealed characteristic time scales wherein cerebral autoregulation is most active, and specific regions wherein autonomic mechanisms are prepotent. However, given that effective cerebral autoregulation against pressure fluctuations results in relatively unchanging flow despite changing pressure, estimating the pressure-flow relationship can be limited by the error inherent in computational models of autoregulatory function. This review will focus on the autonomic neural control of the cerebral vasculature in health and disease from an integrative physiologic and perspective. It will also provide a critical overview of the current analytic approaches to understand cerebral autoregulation.
Keywords: sympathetic nervous system, autonomic nervous control, cerebrovasculature, human
As early as the mid 19th century, it was observed that asphyxia causes dilation of cerebral blood vessels (Donders, 1851), indicating that the cerebral circulation can adapt in the face of alterations in arterial gas concentrations (i.e., cerebrovascular reactivity). Near the turn of the century, Roy and Sherrington described local variations in cerebral blood flow in response to those in neural activity (Roy & Sherrington, 1890), showing that the cerebral vasculature is able to modulate regional blood flow in response to alterations in local metabolic demand (a phenomenon later termed as neurovascular coupling). They argued that vasoconstrictor nerves cause constriction in response to anoxia but that metabolic bi-products lead to the dilation of the cerebral blood vessels. Later, in the middle of the 20th century, it was observed that despite the increase in blood flow to areas of higher brain activity, and despite continuous fluctuations in arterial pressure, total blood supply to the brain remains remarkably constant (Cobb & Talbott, 1927;Landau et al., 1955;Lassen, 1959;Schmidt & Hendrix, 1938), suggesting that the cerebrovasculature is able to maintain constant global blood flow despite variations in regional flow and systemic arterial pressure (dubbed as cerebral autoregulation). This ability was recognized as being critical for protecting neural tissue from wide swings in blood flow (Lassen, 1959). Indeed, impaired cerebral autoregulation may underlie some of the secondary complications and outcomes in various pathological conditions that impact a disproportionate number of adults worldwide. For example, the degree of impairment in cerebral autoregulation is related to subsequent disability and death after traumatic brain injury (Lam et al., 1997), and predictive of delayed cerebral ischemia and secondary infarcts after subarachnoid hemorrhage (Budohoski et al., 2012;Budohoski et al., 2013). Thus, understanding cerebral autoregulation and its underlying mechanisms may be essential for devising effective prognostic and diagnostic options for numerous pathological conditions.
Earlier studies of cerebral autoregulation relied on inert gas and dilution methods, which were limited by both a poor time resolution and, in some cases, by very few observations (Panerai, 1998). Because of these limitations, and partly due to inaccessibility of computational power, most early studies relied on simple descriptive statistics at the population level to explore pressure – flow relationships. Nevertheless, despite these evident limitations, early studies did shed light on the basic concepts of cerebral autoregulation and laid the groundwork for more recent work. For example, in the 1950s, Lassen reviewed cerebral blood flow and arterial pressure across different groups of individuals with varying levels of mean pressure due to acute (e.g., drug-induced hypotension) or chronic (e.g., essential hypertension) conditions, and showed that cerebral blood flow is relatively constant across individuals with a wide range of arterial pressures (∼60 - 150 mmHg) (Lassen, 1959). This review was clearly limited by the inclusion of a diverse group of individuals with various pathological conditions and/or under varying pharmacological interventions. Nonetheless, this relation between steady-state levels of pressure and cerebral blood flow has obvious implications for pathological conditions (e.g., hypertension) and during various medical interventions (e.g., anesthesia). However, short-term variations in pressure and flow in healthy individuals are more pertinent to cerebrovascular control during daily activities (e.g., the transition from sitting to standing). But it was not until the recent availability of instrumentation with high temporal resolution that researchers became able to exploit rapid, beat-by-beat measurements of arterial pressure and cerebral blood flow, and to explore short-term cerebral autoregulation within individuals.
Adoption of transcranial Doppler ultrasound imaging coupled with finger photoplethysmography in the 1980s allowed researchers to monitor beat-by-beat arterial blood pressure and cerebrovascular dynamics. While the pressure difference between the cerebral arteries and veins (that is, intracranial pressure) drives cerebral blood flow (assuming that cerebrospinal fluid pressure is roughly constant), the pressure in cerebral and peripheral veins is usually very close to the atmospheric pressure. Therefore, at least in healthy individuals, photoplethysmographic arterial pressure at the level of the head (e.g., at the finger in the supine position) adequately represents cerebral perfusion pressure. Though measurements via Doppler ultrasonography represent blood flow velocity and not flow, velocity can be used as a surrogate for flow as long as the diameter of the insonated artery remains constant. Several studies have shown that the diameter of the major cerebral arteries (e.g., middle cerebral artery) remains relatively constant despite transient changes in pressure induced by lower body negative pressure (Serrador et al., 2000) or thigh-cuff deflation (Newell et al., 1994). Moreover, under various stimuli, there are close correlations between relative changes in cerebral flow velocity and flow assessed via various techniques, such as xenon (133Xe), single-photon emission computed tomography (SPECT), MRI, and direct Fick calculations from the arterial to jugular venous oxygen difference (Jorgensen, 1995;Larsen et al., 1994;Larsen et al., 1995), Thus, blood flow velocity measured in the large cerebral arteries can be used as a surrogate for cerebral blood flow.
In the modern literature after the 1980s, a distinction has been made between ‘static’ and ‘dynamic’ cerebral autoregulation. Static autoregulation is described as operating over the timescale of several minutes to hours, and to represent the steady-state relationship between absolute arterial pressure and cerebral blood flow (similar to the pressure – flow relation demonstrated by Lassen (1959), but within an individual). Dynamic autoregulation, on the other hand, refers to the pressure – flow relation observed during transient changes in arterial pressure (e.g., with changes in posture), and takes place over several seconds or beats. However, some data suggest that there may be no physiologic basis for a distinction between mechanisms responsible for maintaining steady-state flow constant and those responsible for responding to short-term pressure changes. For example, there is a very close match (R2 = 0.87) between the percent change in cerebrovascular resistance in response to slow, drug-induced increases in pressure, and an autoregulatory index (described in the third section) derived from fast drops in arterial pressure induced by thigh-cuff release (Tiecks et al., 1995). This close match is despite the use of a pharmacologic agent, and despite the possibility that cerebral autoregulation may exhibit asymmetric behavior depending on whether pressure is increasing or decreasing (Aaslid et al., 2007;Tzeng et al., 2010). Thus, the close relation between indices of ‘static’ and ‘dynamic’ autoregulation does suggest that the two may simply represent the same phenomenon.
Most recent studies have focused on the characteristics of the autoregulatory responses to short-term, dynamic changes in pressure. These studies have shown consistently that cerebral autoregulation acts as a ‘high-pass filter’ (Hamner et al., 2004;Zhang et al., 1998). Fast, transient fluctuations in arterial pressure (e.g., due to respiration) are transmitted to the cerebral circulation almost linearly, whereas slower fluctuations that may result in greater sustained impact on neurophysiologic health (i.e., causing prolonged changes in cerebral perfusion) are effectively buffered against. More specifically, pressure – flow fluctuations slower than 10 – 12 seconds (i.e., < 0.1 Hz) demonstrate a markedly lower linear relation (e.g., coherence) with greater dampening (e.g., lower gain) and a pronounced time delay (e.g., a phase shift) (Figure 1).
Figure 1.
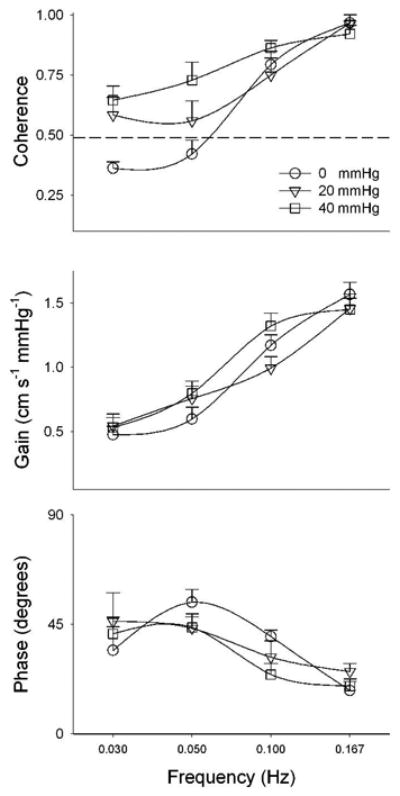
Cross-spectral coherence, gain and phase relations between arterial pressure and cerebral flow fluctuations at rest (i.e., spontaneous fluctuations, 0 mmHg) and during two levels of oscillatory lower body negative pressure. The dashed line in the first panel shows 0.49 coherence, which is statistically significant for how cross-spectral coherence was calculated for this data. Data from Hamner et al. (2004)
Another major advance that stands out in the modern literature is adoption of more sophisticated approaches to data analysis, exploiting the ability to collect numerous measurements and perform high-speed computer calculations. These advances have provided significant insights to the nature and physiologic effectors of cerebral autoregulation. This review will delineate the current state of these insights, with a specific focus on the autonomic control of the cerebral autoregulation. In addition, there is some evidence that there may be some interplay between autoregulation and other effectors of cerebral blood flow (vasoreactivity and neurovascular coupling), and understanding these interactions can facilitate a more integrative view of cerebrovascular regulation. Therefore, the second section of this review provides an overview of these interactions. It should also be noted that while the development of analytic methods for understanding autoregulation has a relatively short history, these methods span a wide range from simple linear models in the time- and frequency-domain to complicated nonlinear models. All methods have inherent mathematical limitations, and understanding the assumptions and premises that underlie analytic paradigms is critical to draw correct physiologic inferences from the data. Therefore, the third part of this review provides an overview of contemporary analytic approaches to cerebral autoregulation and their underlying assumptions. Our purpose is not to provide an exhaustive treatment of all analytic approaches to cerebral autoregulation, but rather to provide an overview of the strengths and limitations of methods that were used in the studies we review in the first two parts.
1. Autonomic Control of Cerebral Autoregulation
Sympathetic Control of Cerebral Autoregulation
It is known that there are nerve fibers along the arteries of the brain that have connections to the cervical sympathetic chain (Edvinsson, 1975), and it would seem logical that these neural elements subserve some function. In the sixties, Guyton and associates (Guyton & Sagawa, 1961;Sagawa & Guyton, 1961;Sagawa et al., 1962) isolated the cerebral circulation of one dog from his peripheral circulation, by supplying it from another, donor dog. The carotid sinus nerves of the recipient dog were cut, eliminating baroreceptor and chemoreceptor responses to pressure changes. Under these circumstances, the recipient dog showed no signs of cerebral autoregulation, but instead the cerebral blood flow simply tracked pressure over a range of 20 to 140 mmHg. From this preparation, it appears that sympathetic control may be required to maintain constant flow, and other possible mechanisms (i.e., vascular myogenic responses or endothelial nitric oxide release) may have insignificant effects in counteracting pressure changes. It is also possible that anesthesia, non-pulsatile pressure, and/or neural damage during the long surgery could conceivably explain these results. Nonetheless, they do at least suggest that, in dogs, sympathetic pathways may play an important role in cerebral autoregulation. In humans, several studies have provided inferential data in support of sympathetic control. For example, those with more severe carotid stenosis, which can markedly impair autonomic control (Nasr et al., 2005), show greater fluctuations in flow in response to changes in pressure (Hu et al., 1999). This infers that impaired autonomic control consequent to carotid stenosis results in impaired counter-regulatory capacity of the cerebral vasculature. Other data also support this inference. For example, in response to acute sympathoexcitatory stimuli, such as isometric handgrip (Ainslie et al., 2005), simulated orthostatic stress (Guo et al., 2006), and the cold pressor test (Wilson et al., 2005) cerebrovascular resistance increases, suggesting reflex sympathetic activation plays a role in cerebral blood flow control.
However, despite the inferential data, there has been a long-standing skepticism that the sympathetic system, indeed any neural system has an important role in the regulation of cerebral blood flow (Heistad & Marcus, 1978;Strandgaard & Sigurdsson, 2008). As a result, examinations of sympathetic neural control of cerebral autoregulation in humans have been especially scarce. Only two studies have directly examined this possibility via pharmacologic blockade. Zhang et. al. (2002) explored the cross-spectral relationship between arterial pressure and cerebral blood flow fluctuations elicited via oscillatory lower body negative pressure (OLBNP) before and after ganglionic autonomic blockade via trimethaphan. After ganglionic blockade, both arterial pressure and cerebral blood flow decreased, but the gain relation between pressure and flow fluctuations was almost doubled, indicating that the degree of cerebral counter-regulation against pressure fluctuations was reduced. This result clearly suggests that intact autonomic neural control is important for cerebral autoregulation. However, ganglionic blockade abolished both sympathetic and cholinergic nervous control, and so the observed effect could have been due to impairment of either or both. In a more recent study, we explored the cross-spectral pressure – flow relationship during OLBNP before and after alpha-adrenergic blockade via phentolamine (Hamner et al., 2010). We found that when the vascular effect of sympathetic nervous outflow was blocked, the linear relation between pressure and flow fluctuations (i.e., coherence) increased significantly, and almost doubled at lower frequencies, affirming a role for sympathetic mechanisms in autoregulation.
Cholinergic Control of Cerebral Autoregulation
While it has long been known that the cerebrovascular bed is also innervated by cholinergic nerve fibers (Edvinsson, 1975), earlier studies on whether the cholinergic system also plays a role in cerebral autoregulation yielded equivocal results. On one hand, in anaesthetized dogs, stimulation of superficial petrosal nerve (which supplies cholinergic fibers to cerebral vessels) and intra-arterial acetylcholine infusions were reported to produce vasodilation in cerebral arteries in a graded manner, proportional to stimulation frequency and drug dose (D'Alecy & Rose, 1977). On the other hand, in cats, stimulation of the same nerve did not alter cerebral blood flow (Busija & Heistad, 1981). Thus, the animal work makes the presence of cholinergic vasodilator control in the cerebral circulation far from clear.
In humans, acetylcholinesterase inhibitors (which increase acetylcholine release from the nerve terminals) are used clinically for pathological conditions that compromise cerebrovascular function, such as Alzheimer's disease (Birks & Harvey, 2006;Howard et al., 2012) and vascular dementia (Roman et al., 2010). Though it is yet unclear whether the positive effect of acetylcholinesterase inhibitors in these conditions are related to alterations in cerebral blood flow, this effect is suggestive of at least some role for cholinergic nervous control in cerebral flow regulation. In addition, the finding that ganglionic autonomic blockade impairs cerebral autoregulation (Zhang et al., 2002; see above) also suggests some role for cholinergic control, though the concomitant lack of sympathetic control after blockade may have obscured this. However, a recent study directly explored the involvement of cholinergic control in cerebral autoregulation, and showed that muscarinic blockade (via glycopyrrolate) abolishes the increase in cerebral blood flow and cerebrovascular conductance in response to dynamic (cycling) and static (handgrip) exercise (Seifert et al., 2010). It should be noted that exercise involves changes to numerous determinants of cerebral blood flow other than autoregulation, such as brain metabolism and arterial gas concentrations (Querido & Sheel, 2007) and may, in fact, impair cerebral autoregulation (Ogoh et al., 2005). Therefore, the results of Seifert et al. (2010), though strongly suggestive of a cholinergic role in control of the cerebral circulation during exercise, did not explicitly define whether this role is specific to exercise. More recently, we demonstrated that systemic cholinergic blockade increases the linear relation (i.e., coherence) between the pressure-flow relationship within the time scales wherein cerebrovascular regulation is most active (0.03 - 0.07 Hz, or ∼15 - 30 second fluctuations) (Hamner et al., 2012). Thus, in addition to the sympathetic nervous control, cholinergic control plays a clear role in cerebral autoregulation in humans.
2. Interactions between Autoregulation and Other Effectors of Cerebral Blood Flow
Though cerebral autoregulation ensures relatively steady cerebral blood flow in the face of fluctuations in arterial pressure, it is important to note that alterations in arterial gas concentration and regional metabolic demand (i.e., cerebrovascular vasoreactivity and neurovascular coupling) also have a strong effect on cerebral blood flow. In fact, there are some data suggestive of interactions between autoregulation, vasoreactivity, and neurovascular coupling (described below). Therefore, while a detailed description of physiologic mechanisms that underlie cerebral vasoreactivity and neurovascular coupling is beyond the scope of this review, it is important to provide an overview of the possible interactions among the three effectors of cerebrovascular regulation.
Cerebral Autoregulation and Vasoreactivity
Arterial carbon dioxide is a potent vasodilator, and cerebral blood flow can be strongly influenced by alterations in arterial CO2 concentrations (cerebrovascular vasoreactivity). Moreover, there is some evidence that there may be an interaction between autoregulation and vasoreactivity, perhaps mediated via sympathetic neural pathways. For example, during ganglionic blockade in humans, changes in mean arterial pressure and those in partial CO2 demonstrate a strong positive correlation (r = 0.86) that is absent prior to blockade (Jordan et al., 2000). While it is unknown if a similar effect is observed in cerebrovascular resistance and/or conductance, this result suggests that the sympathetic system may restrain cerebral blood flow responses to CO2. On the other hand, neither agonists nor antagonists of the sympathetic system demonstrate any effect on cerebrovascular vasoreactivity (Moppett et al., 2004;Schroeder et al., 1991), Thus, while sympathetic pathways may play a role in the interaction between cerebral autoregulation and vasoreactivity, the specifics of this interaction remain largely unknown.
Neurovascular Coupling
Capillary endothelial cells, astrocytes, pericytes and neurons are tightly coupled – dubbed as neurovascular coupling, to modulate regional blood flow in response to local metabolic demand. This modulation ensures rapid spatial and temporal increases in cerebral blood flow in response to neuronal activation, despite the relatively constant global blood flow (i.e., despite autoregulation). However, it is not clear whether neurovascular coupling and cerebral autoregulation interact. It is generally thought that increased intracellular calcium in astrocytes in response to neural activity (Aguado et al., 2002;Cornell-Bell et al., 1990) leads to the formation and release of vasoactive signals that can alter the regional vascular tone (Chisari et al., 2004;Fellin & Carmignoto, 2004;Zonta et al., 2003). It was also suggested that other vasoactive signaling pathways, such as dopamine, may have a role in mediating activity-dependent vascular responses (Choi et al., 2006;Tan, 2009). It is possible that these pathways may alter vascular responses to fluctuations in arterial pressure. In addition, autonomic control of the vasculature may also play a role in neurovascular coupling. For example, one study demonstrated that neurovascular coupling (assessed by the increase in cerebral blood flow in response to a visual task) was impaired in individuals with autonomic dysfunction, and that neurovascular coupling worsens (i.e., visually evoked increases in cerebral blood flow are reduced) during orthostatic stress (standing) in the same individuals (Azevedo et al., 2011). Thus, and given the prominent role of autonomic pathways in cerebral autoregulation (above), it is likely that there may be some interplay between cerebral autoregulation and neurovascular coupling, mediated via autonomic nervous control. However, neurovascular coupling in Parkinson's patients does not appear to be different than in healthy controls, despite a ∼30% lower acetylcholinesterase activity (Rosengarten et al., 2010), indicating that the potential physiologic mechanism that may be common to both autoregulation and neurovascular coupling is unlikely to involve cholinergic pathways. Nonetheless, though these studies are suggestive, it remains unclear whether there is an interaction between autoregulation and neurovascular coupling, and if so, what common physiologic substrates may subserve this interaction.
3. Analytic Approaches to Cerebral Autoregulation
The data reviewed in the first section show that integrity of cerebral autoregulation clearly depends on intact autonomic (both sympathetic and cholinergic) control. This conclusion is mostly based on the analytic approaches that indicate the “absence” of cerebral autoregulation after pharmacologic blockades. However, their limitations (generally acknowledged in the studies that employed these approaches) can preclude precise description of the role autonomic effectors play in autoregulation. In this section, we briefly review these approaches and their limitations to help better evaluate the inferences drawn from the data. We refer the reader to several other reviews in the literature (Panerai, 1998;Panerai et al., 1999;Tzeng et al., 2012) for a more in-depth treatment of the analytic approaches and their strengths and weaknesses.
Linear Methods in the Time-Domain
A common approach is to assess the correlation between arterial pressure or flow and cerebrovascular resistance (the ratio of pressure over cerebral blood flow) or conductance (the inverse of resistance) as surrogates for autoregulation (Edwards et al., 2002;Hughson et al., 2001;O'Leary et al., 2004;Ogoh et al., 2008). The rationale for this is that resistance (or conductance) is commonly conceptualized as the effector of changes in blood flow. However, the relation of pressure to resistance or conductance (ratios between flow and pressure) unavoidably contains an artifactual self-correlation: the two are a priori related due to pressure correlating with itself. This self-correlation may confound assessment of autoregulation. An alternative approach relies upon the idea that intact autoregulation produces a dissociation between flow and pressure, and as the autoregulation is lost, the linear correlation between pressure and flow fluctuations (either averaged over several seconds or beats, or beat-by-beat) should approach one. One well-known example of this approach is the “correlation coefficient index” (termed Mx) (Czosnyka et al., 1996;Czosnyka et al., 1999) which is derived from Pearson's correlation between arterial pressure and cerebral blood flow (sometimes also accounting for possible time-delays between the two signals). The computational ease of this approach may be advantageous in a clinical setting. For example, the correlation coefficient index has been shown to predict secondary clinical outcomes after subarachnoid hemorrhage with fairly high accuracy at the population level (Budohoski et al., 2012;Budohoski et al., 2013). In addition to its simplicity, some favored this approach partly because the index was found to be related to other cerebrovascular variables, such as intracranial pressure, critical closing pressure, and cerebral perfusion pressure (Czosnyka et al., 1996;Czosnyka et al., 1999;Reinhard et al., 2003;Reinhard et al., 2005). However, it is not yet clear whether this approach can be applied to quantify the (patho-)physiology of cerebral autoregulation at the individual level. Moreover, pressure and flow fluctuations, when explored without filtering, will contain all fluctuations, whereas autoregulation is effective in buffering slow, but not fast fluctuations in pressure. It is possible that the close relation between faster fluctuations may dominate the overall pressure – flow relation, and may obscure the relation between slower ones.
An alternative method that can avoid this limitation is to induce transient changes in arterial pressure and to observe resultant blood flow responses. For example, the release of bilateral ischemic thigh cuffs (or its analogue, sit-to-stand maneuver) causes a transient caudal shift in blood volume, and leads to a near step-wise drop in arterial pressure. Consequently, cerebral blood flow also drops, but returns to baseline values faster than the arterial pressure (Aaslid et al., 1982;Mahony et al., 2000;Tiecks et al., 1995), presumably due to engagement of autoregulatory mechanisms that quickly compensate for the drop in pressure. Thus, the relation between pressure and flow responses to thigh cuff maneuver may be used to assess cerebral autoregulation. In the 1990s, the “Autoregulatory Index” (ARI) was proposed as a standardized framework to describe this relation (Tiecks et al., 1995). Calculation of ARI entails the application of a model with several predefined parameters to generate a family of curves, and each of these curves is assigned a number from 0 (absent autoregulation) to 9 (intact autoregulation). Subsequently, measured arterial pressure and cerebral blood flow velocity responses to the thigh cuff maneuver are fit with a curve that is then compared to the theoretical ARI curves. Whichever ARI curve is closest to the measured curve is then assigned to the signal. It should be noted, however, that the purpose of this approach is to grade cerebral autoregulation and not to quantify underlying physiology.
Linear Methods in the Frequency-Domain
In the late 1990s, cross-spectral analysis was proposed as a statistical approach to examine characteristics of the pressure – cerebral blood flow relation (Zhang et al., 1998). Cross spectral analysis is based upon exploration of the relation between two signals (e.g., pressure and flow) in a frequency-dependent manner. That is, one can assess the relation separately at distinct frequencies of interest, and provide parametric estimates of autoregulation, such as coherence (a measure of the linear, frequency-dependent correlation), gain, and phase (i.e., time delay). Moreover, these parameters can be used to derive an ‘impulse function’ (or related ‘step function’), which describes the output response (cerebral blood flow) of a dynamic system (autoregulation) to a unit change in the input (arterial pressure) (Panerai, 1998;Panerai et al., 2001;Zhang et al., 1998). When the impulse function derived from the relation between spontaneous pressure and flow fluctuations was used to predict cerebral blood flow responses to thigh-cuff deflation, it was found that the average prediction was fairly close to the actual response at the population level (Zhang et al., 1998). This suggested that the cross-spectral parameters (coherence, gain, and phase) may be used to quantify the relation between spontaneous pressure – flow fluctuations, and may serve as quantitative metrics of cerebral autoregulation as an alternative to qualitative descriptions (e.g., ARI).
However, spontaneous pressure fluctuations can be extremely inconsistent and small in amplitude (Taylor et al., 1998), and the resultant spontaneous flow fluctuations are expected to be similarly inconsistent, and even more so with intact autoregulation. Indeed, while the spontaneous pressure – flow relation entails periods of high correlation, these periods are interspersed with sections of extremely low correlation where fluctuations in blood flow may appear with no apparent arterial pressure drive (Giller & Mueller, 2003). As a result, overall coherence between beat-by-beat pressure and cerebral flow fluctuations tends to be low (Zhang et al., 1998). It is possible that this low coherence might indicate that cerebral vasculature buffers pressure changes (Narayanan et al., 2001;Zhang et al., 1998), but low coherence could also simply result from small amplitude random fluctuations (i.e. noise). To truly examine the relation between two signals, one needs fluctuations with sufficient amplitude, often absent in resting steady-state data. To generate sufficiently large fluctuations, OLBNP has been used. Standard application of negative pressure effectively distends the veins in the lower body, causing a caudal shift in blood volume proportional to the level of negative pressure. This allows study of cardiovascular responses to central blood volume shifts similar to that which occurs during standing, but in a controlled and graded manner without accompanying muscle contraction. Even mild negative pressure (as low as -5 mmHg or less) reduces central venous pressure, stroke volume, and cardiac output (Johnson et al., 1974), and moderate levels of OLBNP (-30 to -40 mmHg) results in pressure fluctuations that are about 15 – 20 mmHg in magnitude (Hamner et al., 2004). These fluctuations are not greater than those that occur during everyday activities. Thus, moderate OLBNP is a useful technique for augmenting arterial pressure oscillations at distinct frequencies to generate sufficiently large fluctuations, and to engage physiologic effectors of autoregulation (Birch et al., 2002;Brown et al., 2004;Hidaka et al., 2001;Levenhagen et al., 1994;Zhang et al., 2002).
Several studies combined this technique with cross-spectral analysis of the resultant pressure – flow fluctuations. These studies have shown that while both thigh cuff maneuver and OLBNP elicit transient changes in arterial pressure, the cerebrovascular responses to thigh-cuff maneuver does not appear to be consistent with those predicted from the pressure – flow relation derived during OLBNP. For example, while the group average impulse function derived from OLBNP up to 0.30 Hz produces a cerebral flow pattern that is not so different from the averaged thigh cuff response (Zhang et al., 1998), there is a considerable inter-individual variability in cerebrovascular responses that makes simple averaging inappropriate (Hamner et al., 2004). This raises the possibility that the cerebral blood flow response engendered by thigh cuff deflation does not consistently represent the same mechanism responsible for buffering pressure fluctuations. Baroreflex engagement, concomitant with the sudden caudal blood volume shift consequent to release of ischemic thigh cuffs could play some role in this discrepancy. In fact, the thigh cuff maneuver has been used by some to assess arterial baroreflex gain (Fadel et al., 2003). It is possible that OLBNP also engages the baroreflexes, which may play a role in cerebral blood flow responses. However, baroreflex engagement during OLBNP is inconsistent within and across subjects (Hamner et al., 2001) whereas cerebral blood flow responses to OLBNP are highly consistent (Hamner et al., 2004).
While some studies explore the pressure – flow relation at distinct frequency bands (typically, the very low frequency: < 0.07 Hz, low frequency: 0.07-0.15 Hz, and high frequency: 0.15-0.4 Hz), studies based on cross-spectral analysis consistently demonstrate that autoregulation manifests itself as a lack of coherence (i.e., lack of linear dependence) between slow (< 0.1 Hz) arterial pressure and cerebral blood fluctuations (Hamner et al., 2004;Tzeng & Ainslie, 2013;Zhang et al., 1998). In fact, partly based on this evidence, recent studies have suggested that the pressure – flow relation in the very low frequency range (<0.07 Hz) is more likely to be reflective of cerebral autoregulation compared to the low and high frequency ranges (Tzeng & Ainslie, 2013;Zhang et al., 2009). But, ironically, the low coherence also limits the utility of cross-spectral analysis at the low frequencies where autoregulation is most active: if the relation does not display a high coherence, one cannot assess the relationship with any amount of certainty (Giller & Mueller, 2003). The use of cross-spectral gain or phase when coherence is low is analogous to reporting a regression slope despite the fact that regression did not achieve statistical significance. This is demonstrated in Figure 2, where the confidence intervals for coherence were calculated based on the estimation parameters, and those for gain and phase were calculated based on coherence (Koopmans, 1995). This example demonstrates that at low (< 0.05 Hz) frequencies, confidence on the estimated relations can span a wide range.
Figure 2.
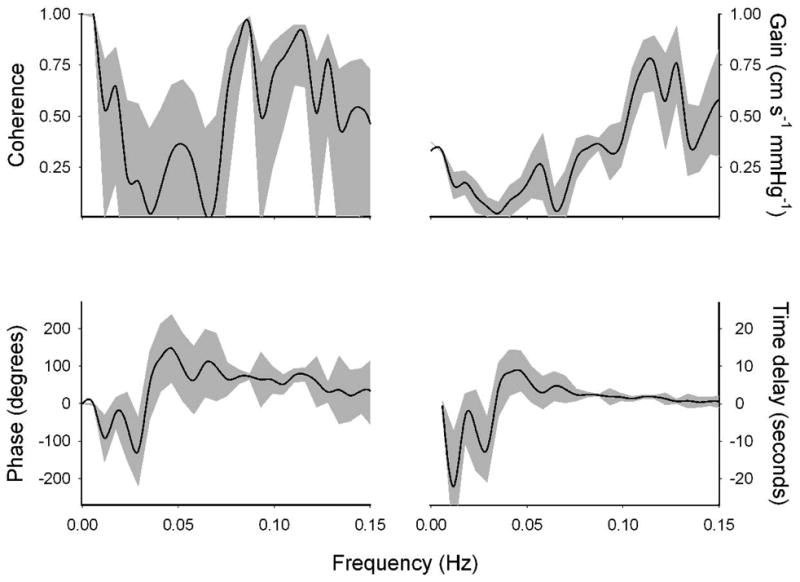
Cross-spectral coherence, gain, and phase relations between arterial pressure and cerebral blood flow fluctuations in one young healthy individual during 15 minutes of -30 mmHg OLBNP at 0.03 Hz. Solid lines show the mean values, and shaded regions show the 95% confidence intervals. Confidence intervals for the gain and phase were calculated based on the mean coherence. Note the relatively wide confidence intervals for coherence and phase at slow fluctuations (< 0.1 Hz; i.e., slower than 10 seconds).
One consequence of this within-subject uncertainty in estimated relations is perhaps best exemplified by the apparent lack of convergence in different linear metrics of autoregulation. Only a few metrics derived via linear cross-spectral (gain and phase) and time-domain (ARI) approaches exhibit statistical associations with each other, and for those that do, the association appears to be less than 30% (Tzeng et al., 2012). While this lack of convergence could be evidence of a lack of common functional basis (Tzeng & Ainslie, 2013), this requires creating a physiologic construct around the lack of agreement. Generally, disagreement between metrics simply reflects a lack of construct validity. That is, disagreement may simply show that the uncertainty in some estimated metrics is too large to quantify the phenomenon under investigation. In fact, this was implicitly acknowledged in one of the earliest studies describing the utility of linear cross-spectral analysis to probe autoregulation: “the coherence function was high in the frequency range of 0.10-0.30 Hz, suggesting linear associations between the changes in pressure and velocity and, therefore, reliable transfer function estimates [i.e., gain and phase]. In contrast, the low coherence at the low frequencies <0.10 Hz may suggest a fundamentally nonlinear relationship between the two variables” (Zhang et al., 1998). In other words, while linear approaches may indicate the presence or absence of cerebral regulation as ‘all-or-none,’ they are mathematically inadequate to quantify the characteristics of this inherently nonlinear phenomenon.
Nonlinear Approaches
There have been attempts to account for nonlinearities inherent in the relationship of cerebral blood flow to arterial pressure. For example, Novak et al. (2004) evaluated the pressure – flow relation using an empirical multi-modal approach. This approach entails decomposition of the blood pressure waveform during the Valsalva maneuver into nine “empirical modes”. Each mode represents a distinct frequency modulation of the observed signal, and the mode deemed to best represent the blood pressure waveform is visually identified. Subsequently, the instantaneous phase of this identified mode and its relation to cerebral blood flow is calculated via the Hilbert-Huang transform. This transform can provide a detailed description of temporal variations and thus, a reliable estimate of the phase relations between arterial pressure and cerebral blood flow. However, the main drawback of this method is that it relies on visual inspection, rather than using a standardized algorithm. Reliance on visual inspection invariably introduces intra- and inter-observer variation. Another approach is the use a variant of the general Volterra-Wiener approach to reveal the nonlinearities inherent in autoregulation (Marmarelis, 1993;Marmarelis, 1997;Mitsis et al., 2002;Mitsis et al., 2004). On the one hand, this approach attains a remarkable performance in explaining the pressure – flow relation, and reveals the existence of significant nonlinearities in this relation. On the other hand, this approach demonstrated a poor predictive performance when the parameters obtained from a group of individuals were used to predict flow responses to pressure in another group (Panerai et al., 1999). It has been argued that this apparent poor generalizability may be a result of the nonlinear term fitting noise rather than the autoregulatory mechanisms (Panerai et al., 1999). Moreover, the nonlinearity in this model is primarily represented by a second-order (i.e., quadratic) term, which may obscure a straightforward physiological interpretation of the pressure – flow relation.
One approach that we have been recently exploring attempts to concurrently overcome the limitations of linear approaches and the complexity of the nonlinear approaches (Tan, 2012). It is based on projection pursuit regression which is a simple nonlinear, nonparametric, atheoretical method wherein a model is not posited a priori, but derived directly from the variables of interest (i.e., from arterial pressure and cerebral blood flow). This approach revealed a dominant nonlinearity in the pressure – flow relation that was consistent across individuals as well as within individuals across different study sessions. Further parametrization of the dominant nonlinearity showed points where the pressure – flow relation change and ranges within which this relation is approximately linear. This allowed derivation of a “gain” of the relation within each region (Figure 3) as a measure of the effectiveness of autoregulation within that region. Thus, this approach may allow identification of the characteristic nonlinear pressure – flow relation to permit straightforward physiologic interpretation of any alterations in this relation. Although this has only a short history of use in this field, more recent work (Tan et al., 2013) found an advantage in using it to explore cerebral autoregulation in the presence of calcium channel blockade. Though linear cross-spectral analysis did not show any effect of blockade on the pressure -- flow relation, projection pursuit regression showed that blockade halved the range of pressures for which cerebral autoregulation was effective, and reduced the effectiveness of autoregulation within this range. That is, while the nonlinearity of autoregulation remained, its characteristics were changed by calcium blockade. Thus, although the advantages of this approach over other traditional techniques remain to be fully demonstrated, the ability of projection pursuit regression to quantify the nonlinear pressure – flow relation in an interpretable way may afford a novel method to quantify cerebral autoregulation.
Figure 3.
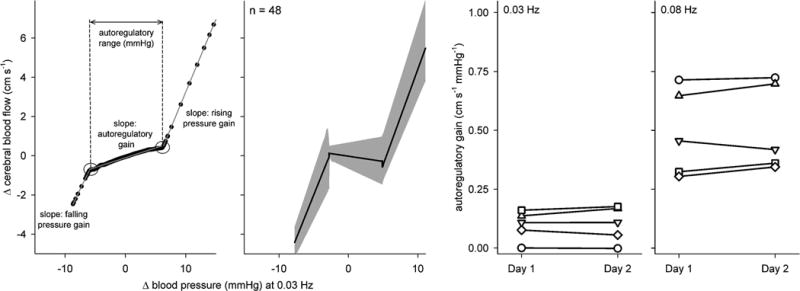
Characteristic nonlinear relation between arterial pressure and cerebral blood flow fluctuations. First panel shows the relation at 0.03 Hz in a young, healthy subject. Black points show the relation, and gray piecewise linear lines show the result of parametrization. The linear slope of autoregulatory range (i.e., autoregulatory gain) represents the effectiveness of cerebral autoregulation. Second panel shows the relation at 0.03 Hz across 48 data sets. Shaded areas show 95% confidence intervals. Third and fourth panels show the autoregulatory gain assessed from the same 5 individuals at 0.03 Hz and 0.08 Hz during two separate but identical experimental sessions. Data with the same symbols on both panels show the data from the same individuals. Data from Tan (2012).
4. Perspectives and Suggestions for Future Research
Since the first observation almost a century ago that cerebrovasculature is able to maintain constant global cerebral blood flow despite variations in regional blood flow and systemic arterial pressure, there has been significant progress in our understanding of cerebral autoregulation and its physiologic substrates. Numerous studies have shown that slow (< 0.1 Hz) arterial pressure – cerebral blood flow fluctuations demonstrate markedly low coherence, with changes in pressure and flow occurring asynchronously (higher phase) and with dampening (lower gain), compared to at those at faster fluctuations. Thus, we now know that fast fluctuations in pressure are transmitted to the cerebral circulation almost linearly (high coherence and gain, and low phase), whereas slow pressure fluctuations are effectively buffered against. Moreover, several studies have conclusively shown that blockade of sympathetic and cholinergic mechanisms impairs this relation at slow frequencies. That is, the integrity of cerebral autoregulation clearly requires intact autonomic (both sympathetic and cholinergic) control. The natural next step should be to consolidate available and future data into a comprehensive model of cerebral autoregulation and its underlying physiology.
Earlier research relied on a wide range of experimental and analytic approaches to characterize cerebral autoregulation. These approaches provided valuable insight into cerebral autoregulation, and all have their strengths and weaknesses that should be taken into account when interpreting their outcome. In terms of experimental approaches, reliance on spontaneous pressure and flow fluctuations is appealing, especially in a clinical setting, because of simplicity, but the small amplitude of spontaneous fluctuations risks random fluctuations (i.e. noise) dominating the observed relation. Oscillatory negative pressure and thigh cuff deflation are two common experimental techniques to engage autoregulatory mechanisms, but clearly, these require specialized equipment and cannot be applied easily in a clinical setting. In terms of analytical methods, there are a variety of linear approaches (e.g., correlation coefficient index or cross-spectral analysis) that can provide an easy and intuitive way assess the relation between pressure – flow fluctuations. However, due to the nonlinear nature of cerebral autoregulation (manifested as a low coherence between pressure and flow fluctuations), measures obtained from linear approaches (e.g., gain and phase) come with an associated uncertainty that can be substantial, especially at low (< 0.1 Hz) frequencies. One possibility to account for this uncertainty is to assess the precision of the estimated metrics for each subject, and to use it to account for the uncertainty during statistical analysis, e.g., by statistical weighting (Gommer et al., 2010;Hamner et al., 2010;Hamner et al., 2012;Oeinck et al., 2013). An obvious alternative to avoid this uncertainty would be to quantify the nonlinearities inherent in the cerebral autoregulation. However, the complexity of many nonlinear approaches precludes a straightforward physiologic interpretation of the observed relationships. While one exception may be projection pursuit regression, only time and further application of this method can demonstrate its advantages for accurate quantification of cerebral autoregulation. Of course, the lack of a broad consensus on a “gold standard” method to quantify cerebral autoregulation is a limitation for all analytical approaches. Nonetheless, it is important to emphasize that all existing approaches can be (and have been) indispensable tools to understand autoregulation as long as their underlying assumptions are taken into account when interpreting the data. This is critical to drawing the correct inferences from observations.
New findings.
-
What is the Topic of this review?
This review focuses on the autonomic control of the cerebral vasculature in health and disease from an integrative physiologic and computational perspective.
-
What advances does it highlight?
This review highlights recent studies exploring autonomic effectors of cerebral autoregulation as well as recent advances in experimental and analytic approaches to understand cerebral autoregulation,
Acknowledgments
Funding: This research was supported by National Heart, Lung, and Blood Institute Grant R01 HL093113.
Footnotes
Competing interests: Authors have no competing interests to declare.
References
- Aaslid R, Blaha M, Sviri G, Douville CM, Newell DW. Asymmetric dynamic cerebral autoregulatory response to cyclic stimuli. Stroke; a journal of cerebral circulation. 2007;38:1465–1469. doi: 10.1161/STROKEAHA.106.473462. [DOI] [PubMed] [Google Scholar]
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol. 2005;566:613–624. doi: 10.1113/jphysiol.2005.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo E, Castro P, Santos R, Freitas J, Coelho T, Rosengarten B, Panerai R. Autonomic dysfunction affects cerebral neurovascular coupling. Clin Auton Res. 2011;21:395–403. doi: 10.1007/s10286-011-0129-3. [DOI] [PubMed] [Google Scholar]
- Birch AA, Neil-Dwyer G, Murrills AJ. The repeatability of cerebral autoregulation assessment using sinusoidal lower body negative pressure. Physiol Meas. 2002;23:73–83. doi: 10.1088/0967-3334/23/1/307. [DOI] [PubMed] [Google Scholar]
- Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2006:CD001190. doi: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- Brown CM, Dutsch M, Ohring S, Neundorfer B, Hilz MJ. Cerebral autoregulation is compromised during simulated fluctuations in gravitational stress. Eur J Appl Physiol. 2004;91:279–286. doi: 10.1007/s00421-003-0965-5. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol. 2013;9:152–163. doi: 10.1038/nrneurol.2013.11. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, Pickard JD, Kirkpatrick PJ. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke; a journal of cerebral circulation. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- Busija DW, Heistad DD. Effects of cholinergic nerves on cerebral blood flow in cats. Circ Res. 1981;48:62–69. doi: 10.1161/01.res.48.1.62. [DOI] [PubMed] [Google Scholar]
- Chisari M, Salomone S, Laureanti F, Copani A, Sortino MA. Modulation of cerebral vascular tone by activated glia: involvement of nitric oxide. J Neurochem. 2004;91:1171–1179. doi: 10.1111/j.1471-4159.2004.02782.x. [DOI] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Cobb S, Talbott JH. Studies in Cerebral Circulation II: A quantitative study of cerebral capillaries. Tr A American Physicians. 1927;42 [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Richards HK, Whitehouse HE, Pickard JD. Relationship between transcranial Doppler-determined pulsatility index and cerebrovascular resistance: an experimental study. J Neurosurg. 1996;84:79–84. doi: 10.3171/jns.1996.84.1.0079. [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Piechnik S, Al-Rawi PG, Kirkpatrick PJ, Matta BF, Pickard JD. Critical closing pressure in cerebrovascular circulation. J Neurol Neurosurg Psychiatry. 1999;66:606–611. doi: 10.1136/jnnp.66.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alecy LG, Rose CJ. Parasympathetic cholinergic control of cerebral blood flow in dogs. Circ Res. 1977;41:324–331. doi: 10.1161/01.res.41.3.324. [DOI] [PubMed] [Google Scholar]
- Donders FC. Die Bewegung des Gehirns und die Verinderungen der Gefissfiillung der Pia Mater. Schmidts Jahrb. 1851;69:16. [Google Scholar]
- Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl. 1975;427:1–35. [PubMed] [Google Scholar]
- Edwards MR, Shoemaker JK, Hughson RL. Dynamic modulation of cerebrovascular resistance as an index of autoregulation under tilt and controlled PET(CO(2)) Am J Physiol Regul Integr Comp Physiol. 2002;283:R653–R662. doi: 10.1152/ajpregu.00452.2001. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Stromstad M, Wray DW, Smith SA, Raven PB, Secher NH. New insights into differential baroreflex control of heart rate in humans. Am J Physiol Heart Circ Physiol. 2003;284:H735–H743. doi: 10.1152/ajpheart.00246.2002. [DOI] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. The Journal of physiology. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA, Mueller M. Linearity and non-linearity in cerebral hemodynamics. Med Eng Phys. 2003;25:633–646. doi: 10.1016/s1350-4533(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Gommer ED, Shijaku E, Mess WH, Reulen JP. Dynamic cerebral autoregulation: different signal processing methods without influence on results and reproducibility. Med Biol Eng Comput. 2010;48:1243–1250. doi: 10.1007/s11517-010-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Tierney N, Schaller F, Raven PB, Smith SA, Shi X. Cerebral autoregulation is preserved during orthostatic stress superimposed with systemic hypotension. J Appl Physiol. 2006;100:1785–1792. doi: 10.1152/japplphysiol.00690.2005. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Sagawa K. Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. Am J Physiol. 1961;200:1157–63. doi: 10.1152/ajplegacy.1961.200.6.1157. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol. 2004;559:965–973. doi: 10.1113/jphysiol.2004.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner JW, Morin RJ, Rudolph JL, Taylor JA. Inconsistent link between low-frequency oscillations: R-R interval responses to augmented Mayer waves. J Appl Physiol. 2001;90:1559–1564. doi: 10.1152/jappl.2001.90.4.1559. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke; a journal of cerebral circulation. 2010;41:102–109. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner JW, Tan CO, Tzeng YC, Taylor JA. Cholinergic control of the cerebral vasculature in humans. The Journal of physiology. 2012;590:6343–6352. doi: 10.1113/jphysiol.2012.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Marcus ML. Evidence that neural mechanisms do not have important effects on cerebral blood flow. Circ Res. 1978;42:295–302. doi: 10.1161/01.res.42.3.295. [DOI] [PubMed] [Google Scholar]
- Hidaka I, Ando S, Shigematsu H, Sakai K, Setoguchi S, Seto T, Hirooka Y, Takeshita A, Yamamoto Y. Noise-enhanced heart rate and sympathetic nerve responses to oscillatory lower body negative pressure in humans. J Neurophysiol. 2001;86:559–564. doi: 10.1152/jn.2001.86.2.559. [DOI] [PubMed] [Google Scholar]
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, Burns A, Dening T, Findlay D, Holmes C, Hughes A, Jacoby R, Jones R, Jones R, McKeith I, Macharouthu A, O'Brien J, Passmore P, Sheehan B, Juszczak E, Katona C, Hills R, Knapp M, Ballard C, Brown R, Banerjee S, Onions C, Griffin M, Adams J, Gray R, Johnson T, Bentham P, Phillips P. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- Hu HH, Kuo TB, Wong WJ, Luk YO, Chern CM, Hsu LC, Sheng WY. Transfer function analysis of cerebral hemodynamics in patients with carotid stenosis. J Cereb Blood Flow Metab. 1999;19:460–465. doi: 10.1097/00004647-199904000-00012. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Edwards MR, O'Leary DD, Shoemaker JK. Critical analysis of cerebrovascular autoregulation during repeated head-up tilt. Stroke; a journal of cerebral circulation. 2001;32:2403–2408. doi: 10.1161/hs1001.097225. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–24. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension. 2000;36:383–388. doi: 10.1161/01.hyp.36.3.383. [DOI] [PubMed] [Google Scholar]
- Jorgensen LG. Transcranial Doppler ultrasound for cerebral perfusion. Acta Physiol Scand Suppl. 1995;625:1–44. [PubMed] [Google Scholar]
- Koopmans LH. The spectral analysis of time series. Academic Press; New York: 1995. [Google Scholar]
- Lam JM, Hsiang JN, Poon WS. Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J Neurosurg. 1997;86:438–445. doi: 10.3171/jns.1997.86.3.0438. [DOI] [PubMed] [Google Scholar]
- Landau WM, Freygang WH, Jr, Roland LP, Sokoloff L, Kety SS. The local circulation of the living brain; values in the unanesthetized and anesthetized cat. Trans Am Neurol Assoc. 1955:125–129. [PubMed] [Google Scholar]
- Larsen FS, Olsen KS, Ejlersen E, Hansen BA, Paulson OB, Knudsen GM. Cerebral blood flow autoregulation and transcranial Doppler sonography in patients with cirrhosis. Hepatology. 1995;22:730–6. [PubMed] [Google Scholar]
- Larsen FS, Olsen KS, Hansen BA, Paulson OB, Knudsen GM. Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke; a journal of cerebral circulation. 1994;25:1985–8. doi: 10.1161/01.str.25.10.1985. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Levenhagen DK, Evans JM, Wang M, Knapp CF. Cardiovascular regulation in humans in response to oscillatory lower body negative pressure. Am J Physiol. 1994;267:H593–H604. doi: 10.1152/ajpheart.1994.267.2.H593. [DOI] [PubMed] [Google Scholar]
- Mahony PJ, Panerai RB, Deverson ST, Hayes PD, Evans DH. Assessment of the thigh cuff technique for measurement of dynamic cerebral autoregulation. Stroke; a journal of cerebral circulation. 2000;31:476–480. doi: 10.1161/01.str.31.2.476. [DOI] [PubMed] [Google Scholar]
- Marmarelis VZ. Identification of nonlinear biological systems using Laguerre expansions of kernels. Ann Biomed Eng. 1993;21:573–589. doi: 10.1007/BF02368639. [DOI] [PubMed] [Google Scholar]
- Marmarelis VZ. Modeling methodology for nonlinear physiological systems. Ann Biomed Eng. 1997;25:239–251. doi: 10.1007/BF02648038. [DOI] [PubMed] [Google Scholar]
- Mitsis GD, Poulin MJ, Robbins PA, Marmarelis VZ. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans Biomed Eng. 2004;51:1932–1943. doi: 10.1109/TBME.2004.834272. [DOI] [PubMed] [Google Scholar]
- Mitsis GD, Zhang R, Levine BD, Marmarelis VZ. Modeling of nonlinear physiological systems with fast and slow dynamics. II. Application to cerebral autoregulation. Ann Biomed Eng. 2002;30:555–565. doi: 10.1114/1.1477448. [DOI] [PubMed] [Google Scholar]
- Moppett IK, Wild MJ, Sherman RW, Latter JA, Miller K, Mahajan RP. Effects of ephedrine, dobutamine and dopexamine on cerebral haemodynamics: transcranial Doppler studies in healthy volunteers. Br J Anaesth. 2004;92:39–44. doi: 10.1093/bja/aeh014. [DOI] [PubMed] [Google Scholar]
- Narayanan K, Collins JJ, Hamner J, Mukai S, Lipsitz LA. Predicting cerebral blood flow response to orthostatic stress from resting dynamics: effects of healthy aging. Am J Physiol Regul Integr Comp Physiol. 2001;281:R716–R722. doi: 10.1152/ajpregu.2001.281.3.R716. [DOI] [PubMed] [Google Scholar]
- Nasr N, Pavy-Le TA, Larrue V. Baroreflex sensitivity is impaired in bilateral carotid atherosclerosis. Stroke; a journal of cerebral circulation. 2005;36:1891–1895. doi: 10.1161/01.STR.0000177890.30065.cb. [DOI] [PubMed] [Google Scholar]
- Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke; a journal of cerebral circulation. 1994;25:793–7. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- Novak V, Yang AC, Lepicovsky L, Goldberger AL, Lipsitz LA, Peng CK. Multimodal pressure-flow method to assess dynamics of cerebral autoregulation in stroke and hypertension. Biomed Eng Online. 2004;3:39. doi: 10.1186/1475-925X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD, Shoemaker JK, Edwards MR, Hughson RL. Spontaneous beat-by-beat fluctuations of total peripheral and cerebrovascular resistance in response to tilt. Am J Physiol Regul Integr Comp Physiol. 2004;287:R670–R679. doi: 10.1152/ajpregu.00408.2003. [DOI] [PubMed] [Google Scholar]
- Oeinck M, Neunhoeffer F, Buttler KJ, Meckel S, Schmidt B, Czosnyka M, Weiller C, Reinhard M. Dynamic Cerebral Autoregulation in Acute Intracerebral Hemorrhage. Stroke; a journal of cerebral circulation. 2013 doi: 10.1161/STROKEAHA.113.001913. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke; a journal of cerebral circulation. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–H1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998;19:305–38. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Dawson SL, Eames PJ, Potter JF. Cerebral blood flow velocity response to induced and spontaneous sudden changes in arterial blood pressure. Am J Physiol Heart Circ Physiol. 2001;280:H2162–H2174. doi: 10.1152/ajpheart.2001.280.5.H2162. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol. 1999;277:H1089–H1099. doi: 10.1152/ajpheart.1999.277.3.H1089. [DOI] [PubMed] [Google Scholar]
- Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Roth M, Guschlbauer B, Harloff A, Timmer J, Czosnyka M, Hetzel A. Dynamic cerebral autoregulation in acute ischemic stroke assessed from spontaneous blood pressure fluctuations. Stroke; a journal of cerebral circulation. 2005;36:1684–9. doi: 10.1161/01.STR.0000173183.36331.ee. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Roth M, Muller T, Czosnyka M, Timmer J, Hetzel A. Cerebral autoregulation in carotid artery occlusive disease assessed from spontaneous blood pressure fluctuations by the correlation coefficient index. Stroke; a journal of cerebral circulation. 2003;34:2138–44. doi: 10.1161/01.STR.0000087788.65566.AC. [DOI] [PubMed] [Google Scholar]
- Roman GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, Moline M, Kumar D, Schindler R, Posner H. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke; a journal of cerebral circulation. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten B, Dannhardt V, Burr O, Pohler M, Rosengarten S, Oechsner M, Reuter I. Neurovascular coupling in Parkinson's disease patients: effects of dementia and acetylcholinesterase inhibitor treatment. J Alzheimers Dis. 2010;22:415–421. doi: 10.3233/JAD-2010-101140. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa K, Carrier O, Guyton AC. Elicitation of theoretically predicated feedback oscillation in arterial pressure. Am J Physiol. 1962;203:141–146. doi: 10.1152/ajplegacy.1962.203.1.141. [DOI] [PubMed] [Google Scholar]
- Sagawa K, Guyton AC. Pressure-flow relationships in isolated canine cerebral circulation. Am J Physiol. 1961;200:711–714. doi: 10.1152/ajplegacy.1961.200.4.711. [DOI] [PubMed] [Google Scholar]
- Schmidt CF, Hendrix JP. The action of chemical substances on cerebral blood vessels. A Res Nerv & Ment Dis Proc. 1938;18:229. [Google Scholar]
- Schroeder T, Schierbeck J, Howardy P, Knudsen L, Skafte-Holm P, Gefke K. Effect of labetalol on cerebral blood flow and middle cerebral arterial flow velocity in healthy volunteers. Neurol Res. 1991;13:10–12. doi: 10.1080/01616412.1991.11739958. [DOI] [PubMed] [Google Scholar]
- Seifert T, Fisher JP, Young CN, Hartwich D, Ogoh S, Raven PB, Fadel PJ, Secher NH. Glycopyrrolate abolishes the exercise-induced increase in cerebral perfusion in humans. Exp Physiol. 2010;95:1016–1025. doi: 10.1113/expphysiol.2010.054346. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke; a journal of cerebral circulation. 2000;31:1672–8. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Strandgaard S, Sigurdsson ST. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol. 2008;105:1366–1367. doi: 10.1152/japplphysiol.90597.2008a. [DOI] [PubMed] [Google Scholar]
- Tan CO. Anticipatory changes in regional cerebral hemodynamics: a new role for dopamine? J Neurophysiol. 2009;101:2738–2740. doi: 10.1152/jn.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CO. Defining the Characteristic Relationship between Arterial Pressure and Cerebral Flow. J Appl Physiol. 2012;113:1194–1200. doi: 10.1152/japplphysiol.00783.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CO, Hamner JW, Taylor JA. The role of myogenic mechanisms in human cerebrovascular regulation. The Journal of physiology. 2013 doi: 10.1113/jphysiol.2013.259747. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke; a journal of cerebral circulation. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- Tzeng YC, Ainslie PN. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur J Appl Physiol. 2013 doi: 10.1007/s00421-013-2667-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, Smirl JD, Horsman HM, Rickards CA. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol. 2012;303:H658–H671. doi: 10.1152/ajpheart.00328.2012. [DOI] [PubMed] [Google Scholar]
- Tzeng YC, Willie CK, Atkinson G, Lucas SJ, Wong A, Ainslie PN. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension. 2010;56:268–273. doi: 10.1161/HYPERTENSIONAHA.110.152066. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Shoemaker JK, Kozak R, Lee TY, Gelb AW. Reflex-mediated reduction in human cerebral blood volume. J Cereb Blood Flow Metab. 2005;25:136–143. doi: 10.1038/sj.jcbfm.9600015. [DOI] [PubMed] [Google Scholar]
- Zhang R, Behbehani K, Levine BD. Dynamic pressure-flow relationship of the cerebral circulation during acute increase in arterial pressure. The Journal of physiology. 2009;587:2567–2577. doi: 10.1113/jphysiol.2008.168302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


