Abstract
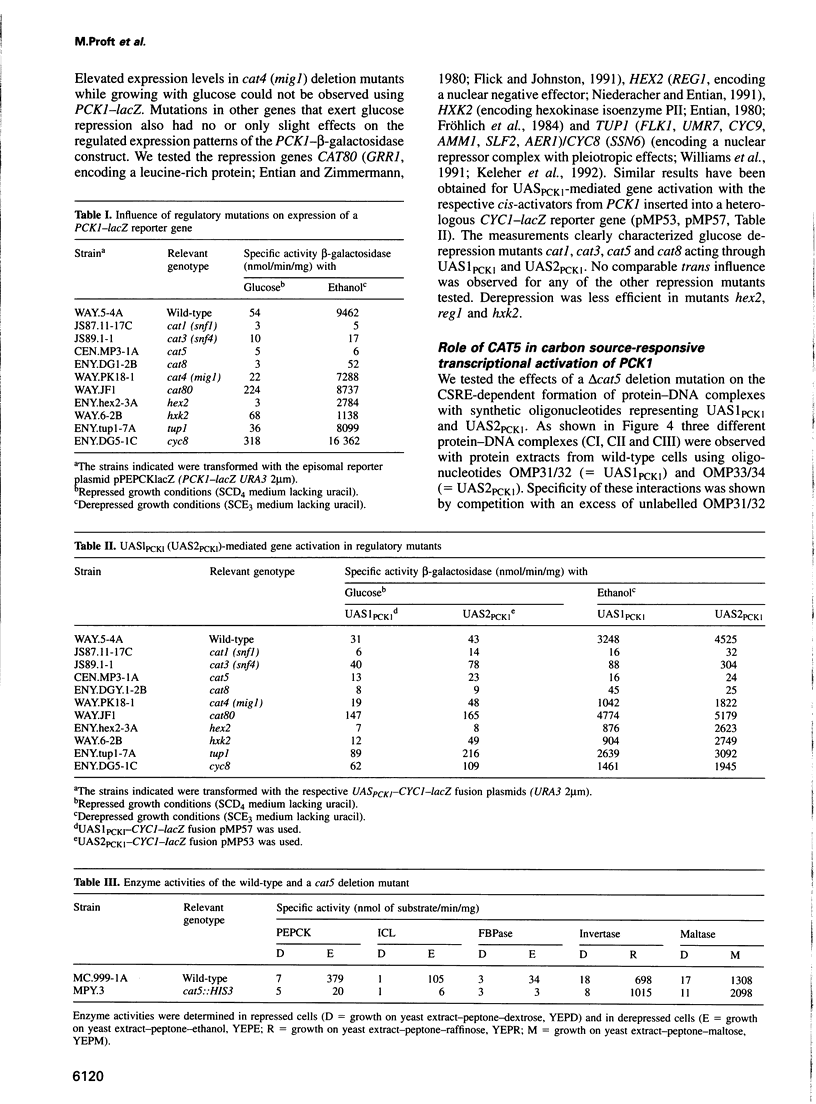
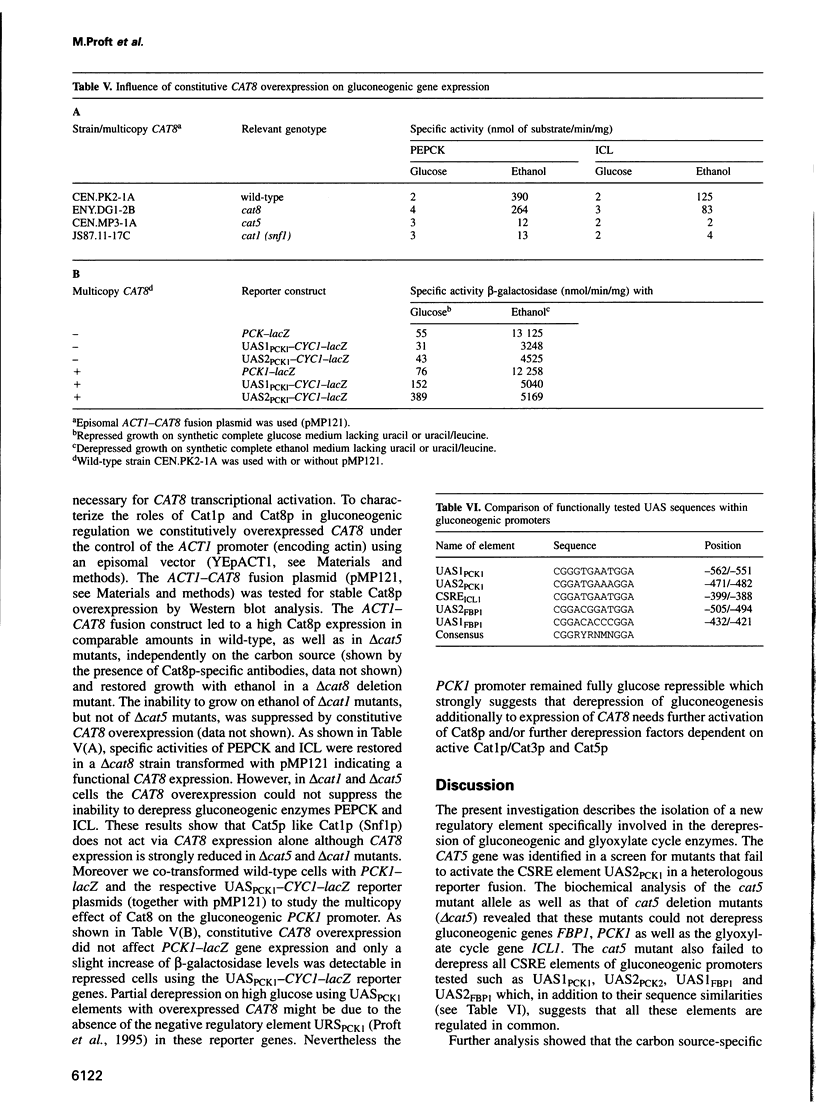
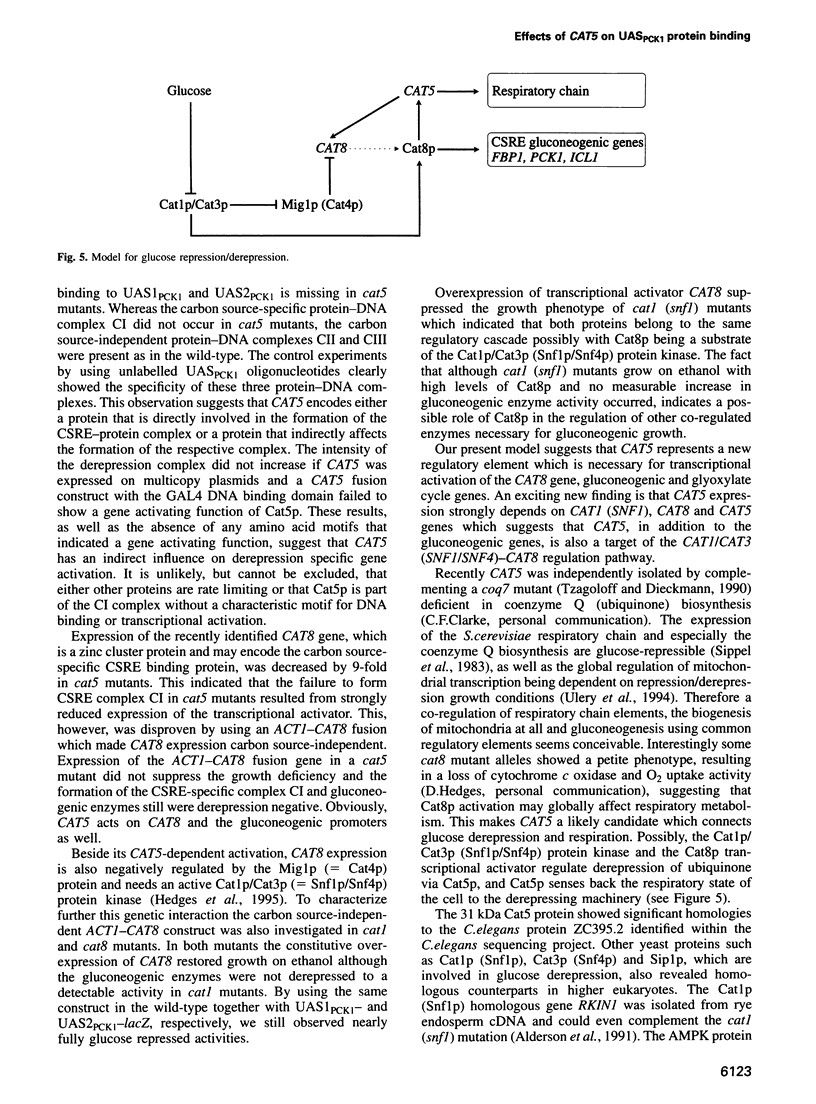
PCK1 encoding phosphoenolpyruvate carboxykinase is transcriptionally regulated by two upstream activating elements. By screening for mutants that failed to derepress a UAS2PCK1-CYC1-lacZ reporter gene we isolated the new recessive derepression mutation cat5. The CAT5 gene encodes a protein of 272 amino acids showing a 42% identity to the ZC395.2 gene product of Caenorhabditis elegans whose function is unknown. Deletion of CAT5 caused a complete loss of glucose derepression affecting gluconeogenic key enzymes. Respiration, but not mitochondrial cytochrome c oxidase activity, was also affected. CAT5 expression is 5- to 6-fold repressed by glucose, and CAT5 transcriptional activation was dependent on CAT1 (SNF1), CAT8 and CAT5 itself. The CAT5 gene is necessary for UAS1PCK1 and UAS2PCK1 protein binding since a carbon source-specific interaction was no longer detectable in cat5 mutants. Glucose derepression of gluconeogenesis depends on the active Cat1 (Snf1) protein kinase and the Cat8 zinc cluster activator. Mig1p-independent overexpression of CAT8 did not stimulate activation of gluconeogenic promoters in cat1 and in cat5 mutants. Since Cat8p multicopy expression suppresses the ethanol growth deficiency in cat1 (snf1) mutants, these results indicate that activation of Cat8p by the Cat1p (Snf1p) kinase and the Cat5p protein might be necessary for release from glucose repression.
Full text
PDF
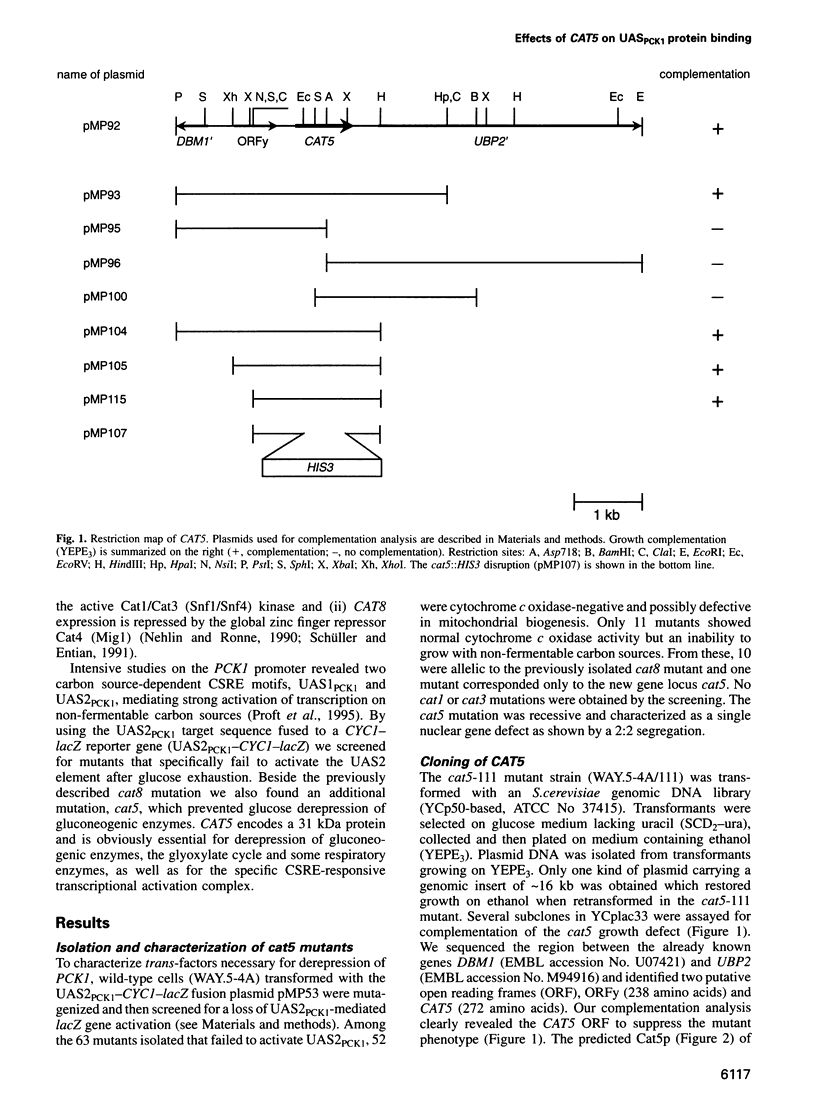
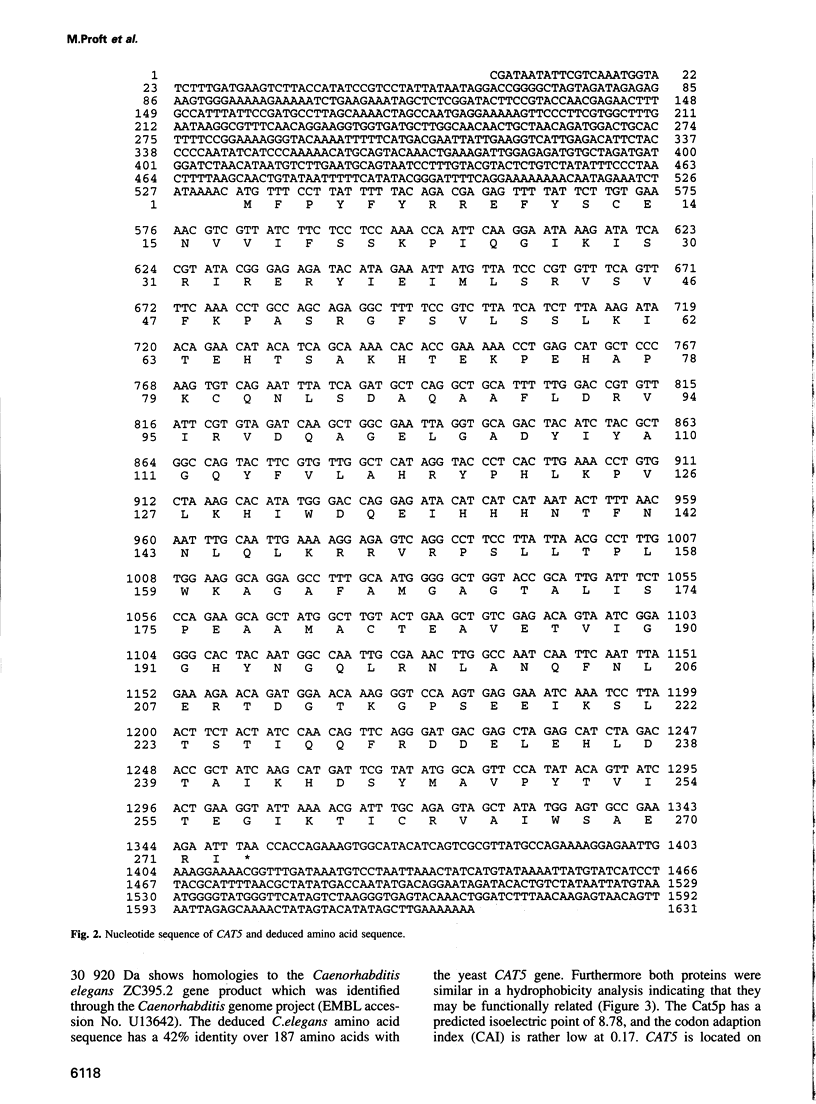
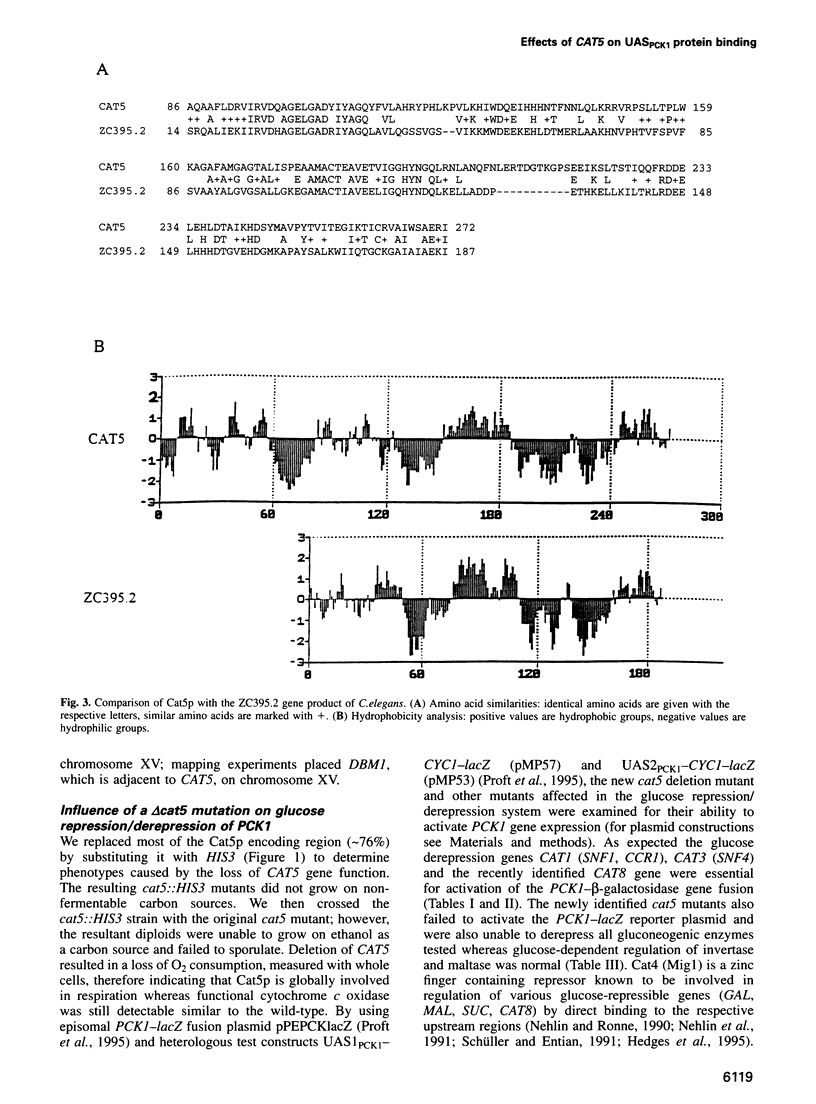




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson A., Sabelli P. A., Dickinson J. R., Cole D., Richardson M., Kreis M., Shewry P. R., Halford N. G. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8602–8605. doi: 10.1073/pnas.88.19.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlini N., Morandi S., Pellegrini R., Tortora P., Guerritore A. Studies on the degradative mechanism of phosphoenolpyruvate carboxykinase from yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1989 Nov 20;1014(2):153–161. doi: 10.1016/0167-4889(89)90028-1. [DOI] [PubMed] [Google Scholar]
- Carling D., Aguan K., Woods A., Verhoeven A. J., Beri R. K., Brennan C. H., Sidebottom C., Davison M. D., Scott J. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem. 1994 Apr 15;269(15):11442–11448. [PubMed] [Google Scholar]
- Carlson M., Osmond B. C., Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981 May;98(1):25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol. 1989 Nov;9(11):5034–5044. doi: 10.1128/mcb.9.11.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. Structure and expression of the SNF1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):54–60. doi: 10.1128/mcb.4.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriacy M. Isolation and characterization of yeast mutants defective in intermediary carbon metabolism and in carbon catabolite derepression. Mol Gen Genet. 1977 Jul 20;154(2):213–220. doi: 10.1007/BF00330840. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Barnett J. A. Regulation of sugar utilization by Saccharomyces cerevisiae. Trends Biochem Sci. 1992 Dec;17(12):506–510. doi: 10.1016/0968-0004(92)90341-6. [DOI] [PubMed] [Google Scholar]
- Entian K. D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in carbon catabolite repression in yeast. Mol Gen Genet. 1980;178(3):633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980 Jan;177(2):345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. New genes involved in carbon catabolite repression and derepression in the yeast Saccharomyces cerevisiae. J Bacteriol. 1982 Sep;151(3):1123–1128. doi: 10.1128/jb.151.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Flick J. S., Johnston M. GRR1 of Saccharomyces cerevisiae is required for glucose repression and encodes a protein with leucine-rich repeats. Mol Cell Biol. 1991 Oct;11(10):5101–5112. doi: 10.1128/mcb.11.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich K. U., Entian K. D., Mecke D. Cloning and restriction analysis of the hexokinase PII gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1984;194(1-2):144–148. doi: 10.1007/BF00383509. [DOI] [PubMed] [Google Scholar]
- GANCEDO C., SALAS M. L., GINER A., SOLS A. RECIPROCAL EFFECTS OF CARBON SOURCES ON THE LEVELS OF AN AMP-SENSITIVE FRUCTOSE-1,6-DIPHOSPHATASE AND PHOSPHOFRUCTOKINASE IN YEAST. Biochem Biophys Res Commun. 1965 Jun 18;20:15–20. doi: 10.1016/0006-291x(65)90944-7. [DOI] [PubMed] [Google Scholar]
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol. 1971 Aug;107(2):401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C., Schwerzmann K. Inactivation by glucose of phosphoenolpyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol. 1976 Sep 1;109(3):221–225. doi: 10.1007/BF00446632. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M. Carbon catabolite repression in yeast. Eur J Biochem. 1992 Jun 1;206(2):297–313. doi: 10.1111/j.1432-1033.1992.tb16928.x. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hansen R. J., Hinze H., Holzer H. Assay of phosphoenolpyruvate carboxykinase in crude yeast extracts. Anal Biochem. 1976 Aug;74(2):576–584. doi: 10.1016/0003-2697(76)90240-2. [DOI] [PubMed] [Google Scholar]
- Hedges D., Proft M., Entian K. D. CAT8, a new zinc cluster-encoding gene necessary for derepression of gluconeogenic enzymes in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1995 Apr;15(4):1915–1922. doi: 10.1128/mcb.15.4.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Keleher C. A., Redd M. J., Schultz J., Carlson M., Johnson A. D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992 Feb 21;68(4):709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Lederer B., Vissers S., Van Schaftingen E., Hers H. G. Fructose 2,6-bisphosphate in yeast. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1281–1287. doi: 10.1016/0006-291x(81)90261-8. [DOI] [PubMed] [Google Scholar]
- Lenz A. G., Holzer H. Rapid reversible inactivation of fructose-1,6-bisphosphatase in Saccharomyces cerivisiae by glucose. FEBS Lett. 1980 Jan 14;109(2):271–274. doi: 10.1016/0014-5793(80)81103-3. [DOI] [PubMed] [Google Scholar]
- Müller D., Holzer H. Regulation of fructose-1,6-bisphosphatase in yeast by phosphorylation/dephosphorylation. Biochem Biophys Res Commun. 1981 Dec 15;103(3):926–933. doi: 10.1016/0006-291x(81)90899-8. [DOI] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991 Nov;10(11):3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L., Carlson M. Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae. Genetics. 1984 Dec;108(4):845–858. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederacher D., Entian K. D. Characterization of Hex2 protein, a negative regulatory element necessary for glucose repression in yeast. Eur J Biochem. 1991 Sep 1;200(2):311–319. doi: 10.1111/j.1432-1033.1991.tb16187.x. [DOI] [PubMed] [Google Scholar]
- Niederacher D., Entian K. D. Isolation and characterization of the regulatory HEX2 gene necessary for glucose repression in yeast. Mol Gen Genet. 1987 Mar;206(3):505–509. doi: 10.1007/BF00428892. [DOI] [PubMed] [Google Scholar]
- Niederacher D., Schüller H. J., Grzesitza D., Gütlich H., Hauser H. P., Wagner T., Entian K. D. Identification of UAS elements and binding proteins necessary for derepression of Saccharomyces cerevisiae fructose-1,6-bisphosphatase. Curr Genet. 1992 Nov;22(5):363–370. doi: 10.1007/BF00352437. [DOI] [PubMed] [Google Scholar]
- Proft M., Grzesitza D., Entian K. D. Identification and characterization of regulatory elements in the phosphoenolpyruvate carboxykinase gene PCK1 of Saccharomyces cerevisiae. Mol Gen Genet. 1995 Feb 6;246(3):367–373. doi: 10.1007/BF00288610. [DOI] [PubMed] [Google Scholar]
- Ronne H. Glucose repression in fungi. Trends Genet. 1995 Jan;11(1):12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler A., Schüller H. J. A carbon source-responsive promoter element necessary for activation of the isocitrate lyase gene ICL1 is common to genes of the gluconeogenic pathway in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1994 Jun;14(6):3613–3622. doi: 10.1128/mcb.14.6.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller H. J., Entian K. D. Extragenic suppressors of yeast glucose derepression mutants leading to constitutive synthesis of several glucose-repressible enzymes. J Bacteriol. 1991 Mar;173(6):2045–2052. doi: 10.1128/jb.173.6.2045-2052.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller H. J., Entian K. D. Molecular characterization of yeast regulatory gene CAT3 necessary for glucose derepression and nuclear localization of its product. Gene. 1988 Jul 30;67(2):247–257. doi: 10.1016/0378-1119(88)90401-5. [DOI] [PubMed] [Google Scholar]
- Sedivy J. M., Fraenkel D. G. Fructose bisphosphatase of Saccharomyces cerevisiae. Cloning, disruption and regulation of the FBP1 structural gene. J Mol Biol. 1985 Nov 20;186(2):307–319. doi: 10.1016/0022-2836(85)90107-x. [DOI] [PubMed] [Google Scholar]
- Sippel C. J., Goewert R. R., Slachman F. N., Olson R. E. The regulation of ubiquinone-6 biosynthesis by Saccharomyces cerevisiae. J Biol Chem. 1983 Jan 25;258(2):1057–1061. [PubMed] [Google Scholar]
- Stapleton D., Gao G., Michell B. J., Widmer J., Mitchelhill K., Teh T., House C. M., Witters L. A., Kemp B. E. Mammalian 5'-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994 Nov 25;269(47):29343–29346. [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulery T. L., Jang S. H., Jaehning J. A. Glucose repression of yeast mitochondrial transcription: kinetics of derepression and role of nuclear genes. Mol Cell Biol. 1994 Feb;14(2):1160–1170. doi: 10.1128/mcb.14.2.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F. E., Varanasi U., Trumbly R. J. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol Cell Biol. 1991 Jun;11(6):3307–3316. doi: 10.1128/mcb.11.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann F. K., Kaufmann I., Rasenberger H., Haubetamann P. Genetics of carbon catabolite repression in Saccharomycess cerevisiae: genes involved in the derepression process. Mol Gen Genet. 1977 Feb 28;151(1):95–103. doi: 10.1007/BF00446918. [DOI] [PubMed] [Google Scholar]