Abstract
Lyme neuroborreliosis (LNB) affects both the central and peripheral nervous systems. In a rhesus macaque model of LNB we had previously shown that brains of rhesus macaques inoculated with Borrelia burgdorferi release inflammatory mediators, and undergo oligodendrocyte and neuronal cell death. In vitro analysis of this phenomenon indicated that while B. burgdorferi can induce inflammation and apoptosis of oligodendrocytes per se, microglia are required for neuronal apoptosis. We hypothesized that the inflammatory milieu elicited by the bacterium in microglia or oligodendrocytes contributes to the apoptosis of neurons and glial cells, respectively, and that downstream signaling events in NFkB and/or MAPK pathways play a role in these phenotypes. To test these hypotheses in oligodendrocytes, several pathway inhibitors were used to determine their effect on inflammation and apoptosis, as induced by B. burgdorferi. In a human oligodendrocyte cell line (MO3.13), inhibition of the ERK pathway in the presence of B. burgdorferi markedly reduced inflammation, followed by the JNK, p38 and NFkB pathway inhibition. In addition to eliciting inflammation, B. burgdorferi also increased total p53 protein levels, and suppression of the ERK pathway mitigated this effect. While inhibition of p53 had a minimal effect in reducing inflammation, suppression of the ERK pathway or p53 reduced apoptosis as measured by active caspase-3 activity and the TUNEL assay. A similar result was seen in primary human oligodendrocytes wherein suppression of ERK or p53 reduced apoptosis. It is possible that inflammation and apoptosis in oligodendrocytes are divergent arms of MAPK pathways, particularly the MEK/ERK pathway.
Keywords: B. burgdorferi, oligodendrocytes, inflammation, apoptosis, MAPK
Introduction
Lyme borreliosis (LB), caused by the spirochete Borrelia burgdorferi, is the most frequently reported tick-borne disease in the United States [1,2]. A primary infection manifests itself as a red rash (erythema migrans, EM) at the site of inoculation in about 80% of infected individuals. This early-localized phase is followed by dissemination to multiple organs that may include distal skin, joints, eyes, heart, peripheral nerves and the central nervous system (CNS). Involvement of the latter, termed Lyme neuroborreliosis (LNB), is the most morbid form of LB due to its debilitating neurological sequelae. It affects both the CNS and the peripheral nervous system (PNS) and occurs in about 15% of patients with EM [3]. Signs and symptoms range from radiculoneuropathy, sensory loss, weakness and facial palsy in the case of PNS neuroborreliosis, to meningitis, encephalomyelitis and encephalopathies in those with CNS involvement [4].
LNB as well as other manifestations of LB, are inflammatory in nature. Perivascular cellular infiltrates have been found localized to peripheral nerves, meninges, brain, and other tissues in both human patients as well as in animal models of LB [5-10]. Production of inflammatory mediators such as CCL2, IL-6, CXCL8, IL-1β, IFNγ, TNF, and several others have also been recorded during B. burgdorferi infection in vitro and in vivo, involving several cell types, tissues, or animal models, as well as LNB patients [11-18]. Production of such chemokines and cytokines has been shown to play key roles in neurodegenerative diseases and CNS injury [19-23]. We have hypothesized, by analogy, that such mediators could lead to loss of neurons or other glial cells, chiefly by apoptosis, and that this process would underlie the pathogenesis of LNB. In support of this hypothesis, brain sections of rhesus macaques exposed ex vivo to B. burgdorferi showed an upregulation of IL-6, CXCL8, IL-1β and CXCL13 as well as apoptosis of neurons and oligodendrocytes [24]. Similarly, intrathecal inoculation of B. burgdorferi into the cisterna magna of rhesus macaques resulted in elevated levels of IL-6, CXCL8, CCL2 and CXCL13 in the CSF, multifocal leptomeningitis, radiculitis and inflammatory lesions in the dorsal root ganglia (DRG) with concomitant neuronal and satellite cell death through apoptosis [25]. Subsequent in vitro studies have indicated that apoptosis of CNS neurons occurs only in the presence of microglia and B. burgdorferi [26] while oligodendrocyte apoptosis can occur in the presence B. burgdorferi alone with no other cell involvement [27]. In both these studies, an intense inflammatory environment was present, again supporting the hypothesis that neuronal or glial loss occurs in the context of an inflammatory milieu. Moreover, both inflammation and apoptosis of oligodendrocytes was mitigated in vitro, in the presence of the anti-inflammatory drug dexamethasone [27]. In order to expand our knowledge of the pathogenesis of LNB, the current study was undertaken to delineate the molecular cell signaling mechanisms underlying inflammation and apoptosis in human oligodendrocytes during exposure to B. burgdorferi, using both immortalized and primary human oligodendrocytes. Our results indicate a predominant role for MAPK pathways, particularly the MEK/ERK pathway in inflammation and apoptosis along with mitochondrial involvement through the p53 molecule.
Materials and Methods
Bacterial strain and culture
B. burgdorferi strain B31 (clone 5A19) was used for all infection assays. B. burgdorferi was routinely cultured under microaerophilic conditions in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma Aldrich, St. Louis-MO) supplemented with Amphotericin (0.25 mg/mL), Phosphomycin (193 mg/mL) and Rifampicin (45.4 mg/mL) for about 5-7 days. At the end of the time period and on the day of infection, bacterial concentration was determined using a dark-field microscope. Required numbers of bacteria were harvested at 2095 × g for 30 minutes at room temperature (RT) without brakes and resuspended in experimental medium containing DMEM-high glucose (Invitrogen/Life Technologies, Inc., Grand Island-NY) and 100 nM phorbol myristate acetate (PMA) (Sigma Aldrich, St. Louis-MO) and diluted further to the required multiplicity of infection (MOI).
Cell culture
Cells from the human oligodendrocyte cell line MO3.13 (CELLutions Biosystems Inc., Ontario, Canada) were cultured according to the manufacturer's protocol. Briefly, cells were grown in complete medium containing DMEM (high glucose) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) at 37 C, 5% CO2. After confluency, cells were trypsinized, collected, and seeded at the required density (0.8 × 104/ well for 6-well plates, 1 × 105/T-75 flask and 0.5 × 104/well for 2-well chamber slides). After day 3, cells were allowed to differentiate for 3 days further by replacing the complete medium with medium devoid of serum and supplemented with 100 nM PMA and 1% P/S (differentiation medium). Cells grown accordingly, as per the manufacturer, stain positive for markers such as myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG), which are phenotypic markers of mature, myelinating oligodendrocytes [28]. Such differentiated and mature oligodendrocytes were used for all of the experiments described. For all of the experiments with B. burgdorferi, differentiation medium without P/S (termed experimental medium) was used.
Human oligodendrocyte precursor cells (HOPCs) were obtained from ScienCell Laboratories (Carlsbad, CA) and grown according to the manufacturer's protocol. Briefly, cells were seeded at a density of ≥ 10,000 cells/cm2 in 2-well chamber slides in oligodendrocyte precursor cell medium supplemented with oligodendrocyte precursor cell growth supplement and 1 × P/S. The chambers were coated with poly-l-lysine according to the manufacturer's recommendations, prior to the seeding of HOPCs. The medium was replenished after 24 h and refreshed every third day for 10 days and replaced with oligodendrocyte precursor cell differentiation medium (OPCDM) supplemented with FBS, P/S and OPCDM supplement for 3-4 days. This allows the precursor cells to differentiate to the oligodendrocyte lineage. On day 14, infection assays with B. burgdorferi were carried out in OPCDM without P/S and processed for ELISA and TUNEL as described in the following paragraphs.
Infection assays and pathway inhibitors
For the infection assays, B. burgdorferi diluted to the appropriate MOI in either experimental medium or in OPCDM without P/S was added to the mature, differentiated MO3.13 cells or to the primary oligodendrocytes respectively, for various time intervals. Viability of B. burgdorferi after 48 h in experimental medium was confirmed by re-culturing in BSK-H medium as well as by live/dead bacterial viability assay kit (Invitrogen/Life Technologies, Inc., Grand Island-NY). To determine the role of various pathways, several pathway inhibitors were added 2 h prior to bacterial addition and co-incubated for the duration of infection. The following inhibitors (EMD Millipore, Billerica, MA) were used: p38 (SB203580), MEK1/2 (U0126), JNK (SP600125), NFkB p65 (JSH 23), IKK-1/2 (inhibitor XII), p53 (pifithrins α and μ). Supernatants were collected at specified intervals, centrifuged at 2095 × g, 10 minutes at 4 C to remove bacteria and cellular debris, aliquoted and stored at −20 C until analysis by Multiplex Enzyme-Linked Immuno Sorbent Assay (ELISA) for chemokines and cytokines.
Preparation of cell lysates
Cell lysates were extracted according to the manufacturer's protocols (Cell Signaling Technology, Boston, MA). Briefly, medium was removed, oligodendrocytes were washed once with ice-cold PBS and incubated on ice for 5 minutes in lysis buffer containing 1mM phenyl methyl sulfonyl fluoride, a serine protease inhibitor. Cells were scraped, briefly sonicated, and lysates were centrifuged at 14,000 × g for 10 minutes at 4 C, collected and stored at −80 C until analysis.
ELISAs for chemokines and cytokines, total p53 and cleaved caspase- 3
Multiplex ELISAs for various chemokines and cytokines were carried out at the Pathogen Detection and Quantification Core, Tulane National Primate Research Center using custom Milliplex kits from EMD Millipore (Billerica, MA), and performed according to the manufacturer's protocols. Total p53 and cleaved caspase-3 protein levels were measured using Pathscan kits from Cell Signaling Technology with 50 μg of protein from the cell lysates prepared as described above. Protein measurement was performed with a standard Bradford assay using Bradford dye reagent and standards (Biorad Hercules, CA).
Cell viability assay
Cell viability was assessed by a cytotoxicity assay using the tetrazolium dye MTT as a substrate and conducted according to manufacturer's protocols (Sigma Aldrich, St. Louis-MO). Briefly, 5 mg/mL MTT was added at a volume corresponding to 10% of the volume of medium in the wells and incubated at 37°C, 5% CO2 for 2 h. Cells were solubilized using the solubilization solution and the optical density of the resulting colored solution was read at 570 nm. Viability of cells due to various treatments was normalized to that of medium controls.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
The MO3.13 and primary human oligodendrocyte cells were fixed and stained for MBP according to Ramesh G. et al [27], and the TUNEL assay was performed thereafter following the manufacturer's instructions (EMD Millipore Apoptag Fluorescein kit). In brief, at the end of the treatment period the supernatants were removed and the cells were fixed in cold 2% paraformaldehyde in phosphate buffered saline (PBS) for 10 minutes, washed in PBS and permeabilized with an ethanol:acetic acid (2:1) mix, and kept in PBS at 4 C until the next steps were performed. Cells were then re-permeabilized in PBS containing 0.1% Triton-X-100, blocked with 10% normal goat serum (NGS) for 30 minutes, followed by staining for MBP with a primary anti-human MBP rabbit polyclonal antibody (1:100; Millipore) and a secondary goat anti-rabbit IgG antibody conjugated to Alexa Fluor 568 (1:1000; Invitrogen) in PBS containing 10% NGS for 45 min-1 h each. The nuclei were stained with TOPRO-3 iodide (1:1000; Invitrogen). The cells were re-blocked with 10% NGS and then the TUNEL assay was performed, according to the manufacturer's protocol.
Microscopy
TUNEL-positive cells were visualized with a Leica DMRE Fluorescent microscope (Leica microsystems, Buffalo Grove-IL) and Lumecor SOLA GUI software (Lumencor, Beaverton-OR), and imaged using Nuance Multispectral Imaging System (CRi, PerkinElmer, Waltham-MA). To quantify differences between treatment groups, a total of 500-1000 cells (MO3.13) or 2000-3000 cells (DHOPC) were counted over 10 fields or more for each well along with TUNEL positive cells in each field/frame. Percent apoptosis was calculated as TUNEL positive cells/total cells × 100 and graphed using Microsoft Excel® software. Adobe® Photoshop CS3 software was used to assemble the images.
Statistics
The Student's t-test was used to determine the statistical significance of an experimental outcome. P values < 0.05 were deemed statistically significant.
Results
MAPK inhibitors, especially the MEK1/2 inhibitor, suppress inflammation induced by B. burgdorferi in MO3.13 oligodendrocytes
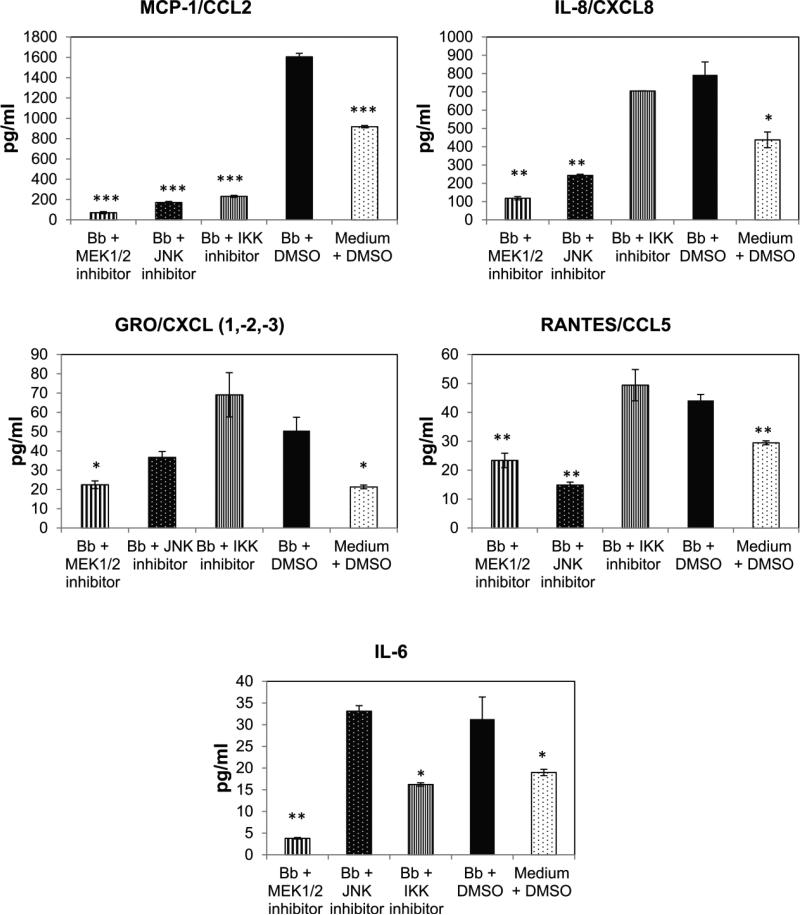
In previous studies, B. burgdorferi has been shown to elicit CCL2/MCP-1, IL-6, and CXCL8/IL-8 production from mature oligodendrocytes [27]. To make a more comprehensive analysis of the repertoire of mediators produced by mature MO3.13 oligodendrocytes, we assessed the production of additional cytokines and chemokines by Multiplex analysis in response to B. burgdorferi (MOI 10:1). The results showed that in addition to CCL2, IL-6, and CXCL8, these cells also produce statistically significant amounts of CXCL(1,2,3)/GRO and CCL5/RANTES at 48 h of co-culture with B. burgdorferi (Fig. 1). There was no significant presence of the following mediators in the culture supernatants: IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12 p40 or p70, IL-13, IL-17α, IL-23, BCA-1, EGF, FGF-2, I-309, IP-10, CCL8/MCP-2, CCL13/MCP-4, CCL4/MIP 1β, SDF1α + β, TNF or TRAIL (data not shown).
Fig. 1.
Cytokine/chemokine release from MO3.13 cells treated with various inhibitors at a 10 μM concentration or solvent control in the presence of B. burgdorferi (Bb, MOI 10:1). Supernatants from the mature oligodendrocytes were collected after 48 h and analyzed by a multiplex ELISA. Values represent mean +/− standard deviation for a representative experiment. Experiments were repeated 2-3 times. Statistical significance was evaluated for each condition compared to Bb + DMSO. * p < 0.05; ** p < 0.01; *** p < 0.001
Next, we investigated the signaling pathway(s) that were likely to be involved in eliciting the production of these pro-inflammatory mediators. Since the NFkB and MAPK pathways have been documented to induce inflammatory mediator release from other glia, endothelial cells and monocytes [29-32], we evaluated the role of these pathways in mediating inflammation using pathway inhibitors. Most of the inhibitors suppressed the production of at least two chemokines/cytokines by MO3.13 in the presence of B. burgdorferi. However, the MEK1/2 inhibitor U0126, which suppresses the ERK pathway upstream of ERK activation, had the most profound effect on inflammatory mediator release, followed by the JNK inhibitor, the p38 inhibitor and the IKK α and β kinase inhibitor, and finally the NFkB p65 transactivation inhibitor (Fig. 1, Table 1). In summary, the MEK 1/2 inhibitor significantly suppressed the production of all five of the pro-inflammatory mediators produced by MO3.13 oligodendrocytes, while the JNK pathway inhibitor had the most marked effect on CCL2, CXCL8, and CCL5 secretion. The p38 inhibitor and the IKK inhibitor each suppressed production of two mediators (CCL2, CXCL(1,2,3) and CCL2, IL-6, respectively) while the NFkB p65 transactivation inhibitor had the least effect in our study (Table 1). CCL2 was the most commonly suppressed chemokine indicating that multiple pathways are involved in its synthesis and release, a possibility that also accounts for its abundance over other inflammatory mediators. The release of other chemokines/cytokines involved at least 2 pathways, with the MEK pathway being one of those in all cases (Table 1). This effect of inhibitors on chemokine/cytokine release was on the cells alone, and did not involve an effect on the bacteria, as the pattern of suppression of constitutively expressed pro-inflammatory mediators was similar to that seen in the presence of bacteria. In addition, the MAPK pathway inhibitors had no deleterious effect on B. burgdorferi viability (data not shown).
Table 1.
Average fold changea (fold down-regulation) in chemokine/cytokine release from MO3.13 in response to various pathway inhibitors in the presence of B. burgdorferi (MOI 10:1) at 48 h of co-culture
| Inhibitor (10 μM) | CCL2 | CXCL8 | CXCL(1,2,3) | CCL5 | IL-6 |
|---|---|---|---|---|---|
| MEK 1/2 | 18.1175 (± 2.648) | 6.385 (± 0.996) | 1.86 (± 0.148) | 2.1125 (± 0.239) | 5.9775 (± 0.978) |
| JNK | 4.2875 (± 0.705) | 2.57 (± 0.305) | 0.86 (± 0.175) | 2.725 (± 0.375) | 0.551 (± 0.140) |
| p38 | 1.71 (± 0.159) | 1.44 (± 0.178) | 1.1806 (± 0.073) | 0.906 (± 0.143) | 1.0788 (± 0 .089) |
| IKK | 4.09 (± 0.508) | 0.928333 (± 0.179) | 0.74 (± 0.01) | 0.927 (± 0.037) | 1.536667 (±0 .240) |
| NFkB p65 | Inconclusive | 0.825 (± 0.275) | 0.955 (± 0.235) | 0.7515 (± 0.032) | Inconclusive |
Fold changes were calculated for each condition with respect to that of B. burgdorferi with solvent alone. Values that were statistically significant in multiple experiments are indicated in bold and underlined and standard error of the mean (SEM) is within parentheses
Since the MEK/ERK pathway was predominantly involved in release of inflammatory mediators from MO3.13 cells, we then evaluated the effect of inhibitor doses 2 to 4-fold lower than the 10-μM concentration used. The results show that even a 4-fold lower dose was sufficient in reducing chemokine/cytokine production significantly from these cells in the presence of B. burgdorferi (MOI 10:1) for 48 h (Table 2).
Table 2.
Effectiveness of lower doses of MEK1/2 inhibitor in dampening inflammationa from MO3.13 cells in the presence of B. burgdorferi (MOI 10:1) at 48 h of co-culture
| Inhibitor | CCL2 | CXCL8 | CXCL(1,2,3) | CCL5 | IL-6 |
|---|---|---|---|---|---|
| MEK 1/2 2.5 μM | 11.425 (± 2.665) | 8.625 (± 2.835) | 2.607 (± 0.563) | 1.732 (± 0.098) | 3.3385 (± 0.462) |
| MEK 1/2 5 μM | 13.66 (± 0.14) | 9.91 (± 2.04) | 3.04 (± 0.47) | 2.09 (± 0.45) | 4.765 (± 0.675) |
| MEK 1/2 10 μM | 18.1175 (± 2.648) | 6.385 (± 0.996) | 1.86 (± 0.148) | 2.1125 (± 0.239) | 5.9775 (± 0.978) |
Values represent average fold decrease in comparison to B. burgdorferi with solvent alone.
SEM value is indicated within parenthesis. The fold decrease in response to 10 μM MEK 1/2 inhibitor is the same as in Table 1. The average fold-change in response to the 2.5-μM and 5-μM doses was calculated from two independent experiments, while the 10-μM dose values were from four independent experiments
The P53 pathway is involved in the response of oligodendrocytes to B. burgdorferi
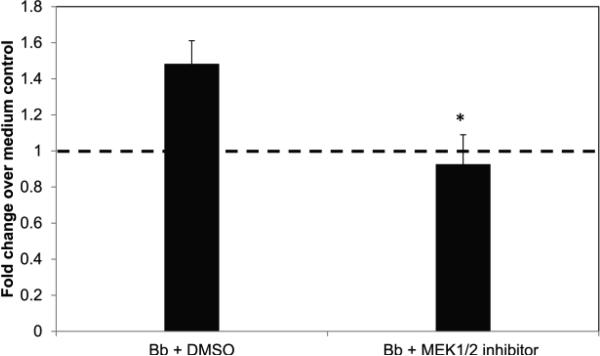
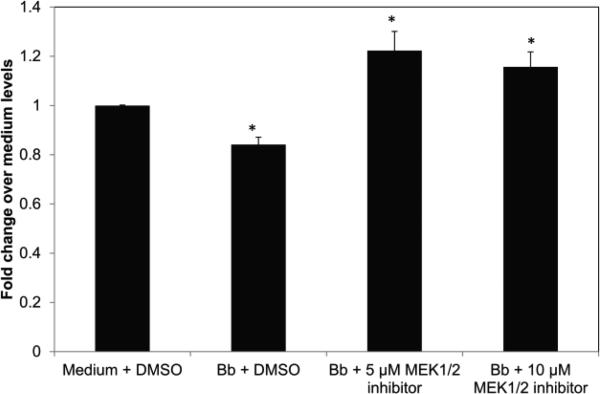
The P53 pathway has been shown to partake in multiple processes within eukaryotic cells including survival, apoptosis, autophagy, and senescence [33-35]. In our previous work we have shown that neuronal cells undergoing apoptosis in the presence of microglia and B. burgdorferi induced the expression of p53-related genes [26]. Therefore, in our current study we assessed the role of the P53 protein in the response of human oligodendrocytes to B. burgdorferi. We found that co-culture with B. burgdorferi increased the level of total p53 protein in these cells by about 40%, as determined by ELISA for the endogenous protein in total cell lysates at 48 h of co-culture (Fig. 2). Treatment with the MEK 1/2 inhibitor in the presence of the bacteria suppressed this upregulation to control levels (dotted line in Fig. 2).
Fig. 2.
Endogenous levels of total p53 protein in mature MO3.13 cells 48 h after infection with B. burgdorferi (Bb, MOI-10:1) with or without the MEK1/2 inhibitor U0126 (10 μM). Cell lysates were obtained as described in Materials and Methods, and a sandwich ELISA was carried out using 50 μg of total protein. Fold change was calculated over medium control containing solvent alone. Values represent averages from three experiments with bars representing SEM. * p < 0.01
As the MEK/ERK pathway mediated inflammation most significantly in these cells and also suppressed p53 levels, we then tested for the role of p53 itself in inflammation. Two types of inhibitors were used, an inhibitor that suppresses the transactivation of p53 to the nucleus (pifithrin-α) and inhibits p53 mediated gene expression, and pifithrin-μ that inhibits p53 from binding to mitochondrial anti-apoptotic proteins like Bcl2, thereby preventing apoptosis. Both inhibitors were independently confirmed to suppress p53 levels (data not shown). With either inhibitor, the contribution of the p53 pathway in mediating inflammation was not as significant as that mediated by the MEK 1/2 inhibitor (Table 3 and Table 1). Inhibition of the transactivation function of p53 suppressed the production of CCL2 and IL-6 while suppressing the mitochondrial arm of p53 had an effect only on CCL5 production- but only at 0.1 μM concentration. There was no effect on inflammation when pifithrin-μ was used at 0.5 μM. (higher doses of pifthrin-μ were toxic to cells). In summary, while total p53 levels are elevated during infection with B. burgdorferi, they play a minor role in inflammation, in comparison to the MEK/ERK pathway, which seems to influence both inflammation and p53 levels.
Table 3.
Effect of p53 suppression on inflammatory mediator releasea from MO3.13 oligodendrocytes in the presence of B. burgdorferi (MOI 10:1) at 48 h
| Inhibitor/Mediator | CCL2 | CXCL8 | CXCL(1,2,3) | CCL5 | IL-6 |
|---|---|---|---|---|---|
| Pifithrin-α 10 μM | 1.83 (± 0.547) | 1.076667 (± 0.216) | 0.996667 (± 0.222) | 0.933333 (± 0.182) | 2.4 (± 0.420) |
| Pifithrin-μ 0.1 μM | 0.9905 (± 0.021) | 0.815 (± 0.175) | 0.8375 (± 0.153) | 1.595 (± 0.045) | 0.76 (± 0.29) |
| Pifithrin-μ 0.5 μM | 0.98 (± 0.03) | 0.805 (± 0.005) | 0.89 (± 0.17) | 1.015 (± 0.045) | 0.7 (± 0.03) |
Fold-decreases were calculated over B. burgdorferi with solvent alone. Mean fold-change from two experiments each is indicated with SEM within parenthesis. Numbers in bold represent significant down-regulation of the specific chemokine/cytokine
The MEK/ERK pathway and the mitochondrial arm of the p53 pathway play a role in apoptosis of MO3.13 oligodendrocytes during exposure to B. burgdorferi
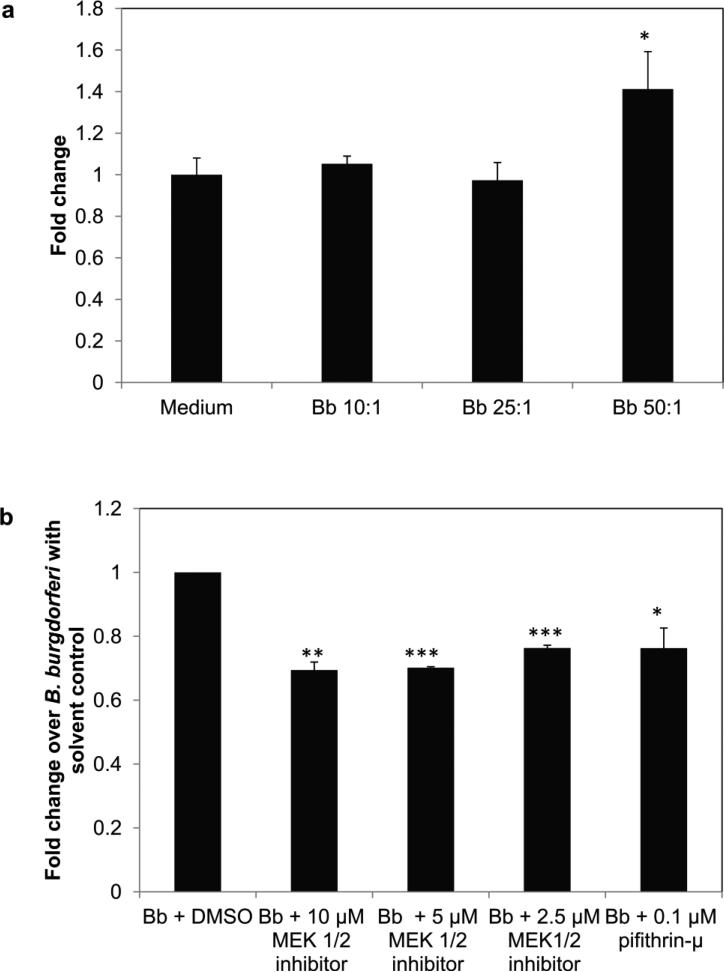
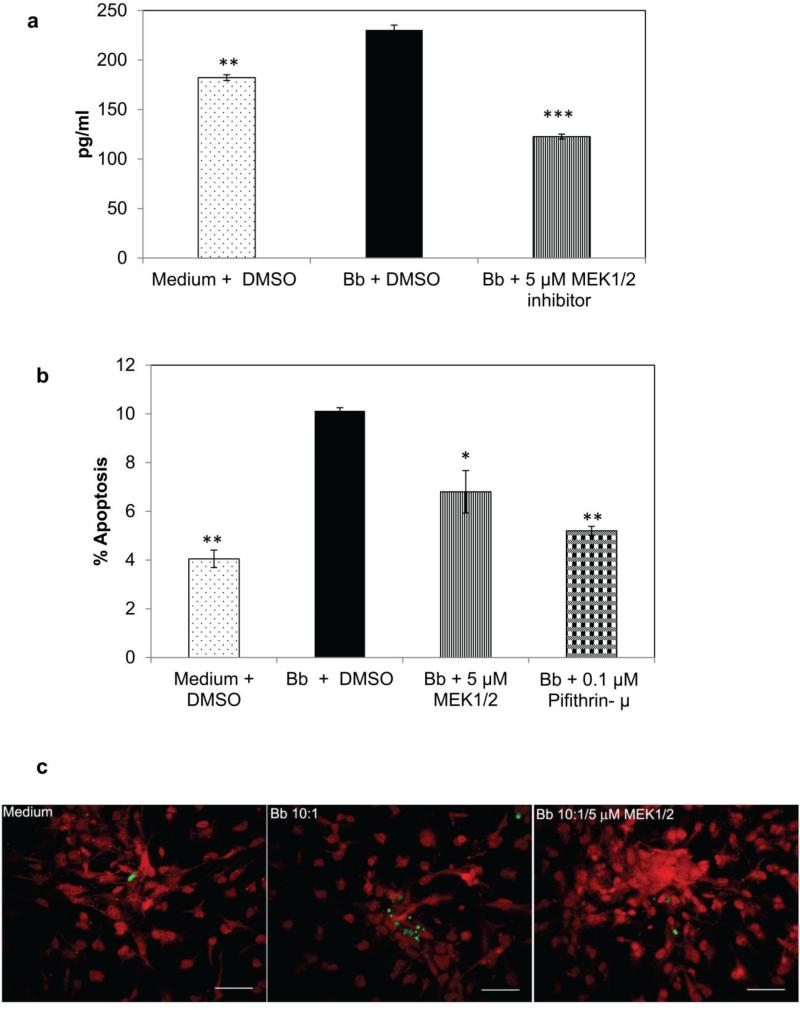
Exposure of oligodendrocytes to B. burgdorferi causes apoptosis of these cells in vivo and in vitro [24,27]. We have hypothesized that the inflammatory milieu elicited during infection results in apoptosis of neural cells. Since the MEK/ERK pathway markedly affected inflammatory chemokine and cytokine production by oligodendrocytes in our current study, we evaluated the role of this pathway in influencing apoptosis. This was determined quantitatively through measurement of endogenous levels of cleaved (active) caspase-3, as well as semi-quantitatively by TUNEL assay. As seen in Fig. 3a, there was an upregulation of active caspase - 3 at 48 h in response to B. burgdorferi infection at an MOI of 50:1 but not at lower doses. There was an average 1.4 fold increase in active caspase-3 production in MO3.13 cells at this MOI. As seen in Fig. 3b, inhibition of the MEK/ERK pathway suppressed caspase- 3 activation at 48 h of co-incubation with B. burgdorferi at all of the inhibitor doses tested. Inhibition of p53 using the pifithrin-μ inhibitor also suppressed caspase-3 activation. There was a significant reduction (ranging from 22% -30%) in endogenous levels of the cleaved caspase-3 protein when either the MEK/ERK pathway or the mitochondrial arm of the p53 pathway was suppressed, indicating that while the p53 protein likely influences apoptosis, as induced by B. burgdorferi in mature MO3.13 human oligodendrocytes, the MEK/ERK pathway affects inflammation, p53 levels, and apoptosis.
Fig. 3.
Endogenous levels of cleaved caspase-3 in MO3.13 cells at 48 h, in response to different MOI of B. burgdorferi (a), while (b) shows the levels of cleaved caspase-3 at a B. burgdorferi MOI of 50:1, alone and in the presence of MEK1/2 inhibitor at different concentrations, or 0.1 μM p53 inhibitor (pifithrin-μ). A representative experiment is shown in (a) while in (b) mean value from two experiments is shown, with bars representing SEM. * p < 0.05; ** p < 0.01; *** p < 0.001
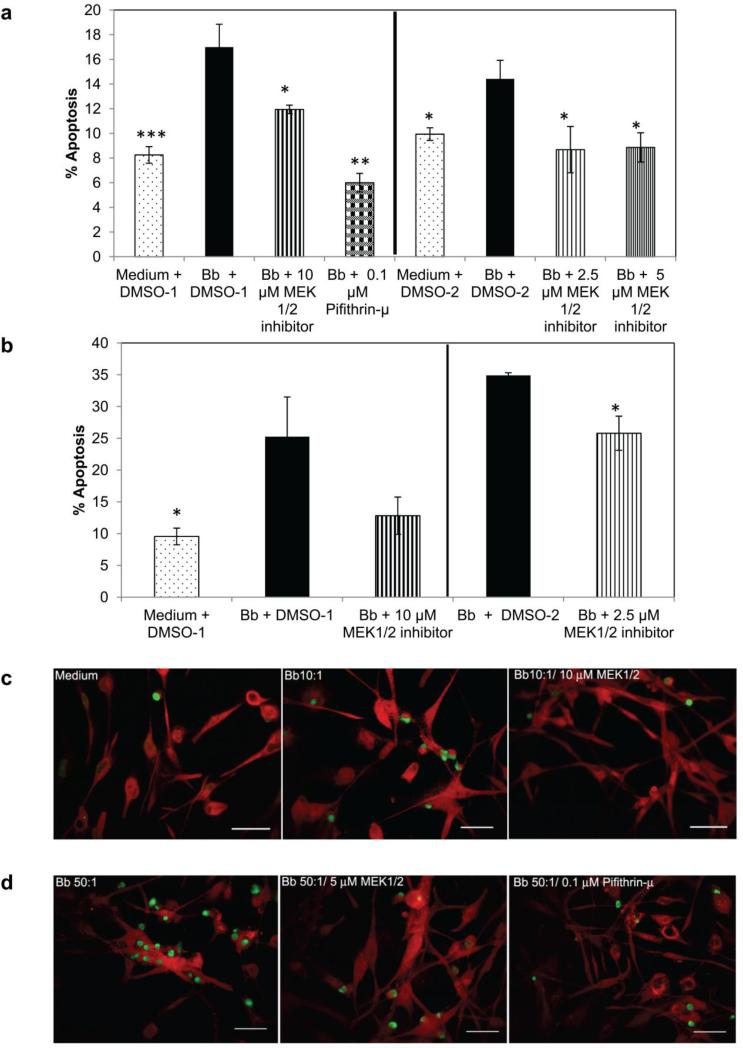
This effect on apoptosis by MEK and p53 inhibitors was confirmed by TUNEL assay, wherein percentage of TUNEL positive cells was determined microscopically. As seen in Fig. 4 a and b, the proportion of apoptotic cells was decreased significantly with all doses of the MEK1/2 inhibitor and pifithrin-μ at 0.1 μM. At the lower MOI of 10:1 (Fig. 4a), the oligodendrocytes with MEK 1/2 inhibitor showed a ~ 4 (10 μM) - 6 (2.5 and 5 μM) reduction in the percent of apoptotic cells on average while the p53 inhibitor had the most profound effect by reducing the percent apoptosis by about 10 (Fig. 4a). At the higher MOI of 50:1, the lowest dose of MEK 1/2 inhibitor (2.5 μM) reduced the percentage of apoptotic cells by ~ 10 while the higher dose (10 μM) reduced the percent apoptosis by 12, but this difference was not significant due to the higher dispersion of the data (Fig. 4b). MEK 1/2 inhibitor at 5 μM and pifithrin-μ had a significant effect and reduced the percent apoptosis by about 16-17 respectively (data not shown). A microscopic visualization of these results is shown in Fig. 4c and 4d, where TUNEL positive (apoptotic, green) cells are increased in samples exposed to B. burgdorferi alone in comparison to other conditions.
Fig. 4.
Effect of the MEK1/2 inhibitor U0126, and the p53 inhibitor pifithrin-μ on apoptosis of MO3.13 cells during exposure to B. burgdorferi. The TUNEL assay was used to quantify apoptosis at two different MOI (10:1 in 4a; 50:1 in 4b). a) represents mean values from 2-5 experiments for each condition while a representative experiment is shown in b). * p < 0.05; ** p < 0.01; *** p < 0.001. DMSO-1 and DMSO-2 represent the volumes of the solvent used in concert with the concentration of inhibitors. 4c and 4d show MO3 cells stained with MBP (red) and TUNEL positive cells (green) in response to various treatments. Bar represents 50 μm (c ) – 100 μm (d )
The MEK1/2 inhibitor affects overall cell growth and viability of MO3.13 cells
Culture wells with MO3.13 cells co-incubated with B. burgdorferi and the higher doses of the MEK1/2 inhibitor U0126 (5 μM and 10 μM) exhibited a change in culture medium color from pink to slight orange, to orange, indicative of rapid cell growth, while wells with oligodendrocytes plus B. burgdorferi and 2.5-μM MEK1/2 inhibitor or pifithrin-μ (0.1 μM) remained pink- as did wells with oligodendrocytes and B. burgdorferi alone or medium controls (data not shown). So an MTT assay was conducted to determine cell viability of MO3.13 oligodendrocytes in the presence or absence of the MEK1/2 inhibitor during infection with B. burgdorferi, and as shown in Fig. 5, cell viability increased often beyond the medium control levels in the wells containing 5-μM and 10-μM concentrations of the MEK1/2 inhibitor -in accordance with the color change in medium. The 2.5-μM concentration of MEK1/2 inhibitor also increased the viability of cells in comparison to cells containing B. burgdorferi alone, even though the medium did not change color very dramatically. A similar pattern of improved cell viability in the presence of MEK inhibitor was also seen when assessed by trypan blue cell viability assay in the presence of bacteria (data not shown).
Fig. 5.
Viability of MO3.13 cells was assessed by an MTT-based assay at 72 h of co-incubation with B. burgdorferi (MOI 10:1) either in the presence or absence of MEK 1/2 inhibitor. A representative experiment is shown with bars representing standard deviation. Fold change values and statistical significance were calculated over medium control containing solvent alone. * p < 0.05
The MEK/ERK pathway plays a role in inflammation and apoptosis of primary oligodendrocytes
Since the MEK pathway played a significant role in oligodendrocyte inflammation and apoptosis when modeled by the human oligodendrocyte cell line MO3.13, its role in primary human oligodendrocytes during B. burgdorferi infection was also determined. Inhibition of the MEK/ERK pathway suppressed the production of CXCL8 (Fig. 6a) as induced by B. burgdorferi, while its effect on CCL2 or CXCL(1,2,3) was inconclusive (data not shown). Also, the primary cells produced a lower concentration of these chemokines, especially CXCL8 and CXCL(1,2,3), compared to the MO3.13 cells (Fig. 6a and data not shown). In addition, the primary cells, contrary to MO3.13 cells, do not secrete any significant amount of IL-6 or CCL5 during exposure to B. burgdorferi. But similar to the effect seen in MO3.13, both the MEK/ERK inhibitor U0126, and pifithrin-μ significantly downregulated apoptosis (Fig. 6b) in the presence of the bacteria. As seen in Fig. 6c, MBP (red)-stained differentiated human oligodendrocyte precursor cells (DHOPC) show a higher number of TUNEL-positive (green) cells when exposed to B. burgdorferi compared to cells exposed to either medium (with solvent) alone or in the presence of MEK inhibitor. But, as with inflammation, the overall percentage of apoptosis that was induced by B. burgdorferi in primary cells was more limited in comparison to that of MO3.13 cells. Finally, MEK suppression improved the cell viability of DHOPC during infection in a manner that was similar to MO3.13 cells (data not shown). In summary, MEK/ERK suppression in primary human oligodendrocytes diminished CXCL8 production and apoptosis, and improved cell viability during infection, similar to the effects seen in the oligodendrocyte cell line, although these phenotypes were not as robust as those observed in the MO3.13 cells.
Fig. 6.
Effect of MEK pathway inhibition on inflammation and apoptosis of primary oligodendrocytes exposed to B. burgdorferi (MOI 10:1). 6a) B. burgdorferi-mediated IL-8 release from primary oligodendrocytes is suppressed by the inhibition of MEK pathway. A representative experiment is shown with bars representing standard deviation; ** p < 0.01;*** p < 0.001. 6b) and (6c) show the results of apoptosis as evaluated by TUNEL assay in primary human oligodendrocytes in response to B. burgdorferi ± inhibitors. After 48 h of exposure to B. burgdorferi, cells were stained with MBP (red) and processed for TUNEL as described in Materials and Methods. Nearly 2000 (or more) cells were counted/well/treatment over 13-20 microscope fields along with TUNEL positive cells (green) in each field. 6b) shows the mean apoptotic values from 2 independent but identical experiments with bars representing SEM. * p < 0.05; ** p < 0.01. Panels in (6c) show apoptosis (green) in differentiated HOPC cells (red) for various treatment groups. Bar represents 100 μm
Discussion
In this manuscript, we show that MAPK pathways, particularly the MEK/ERK pathway, play a predominant role in mediating release of pro-inflammatory chemokines and cytokines from human oligodendrocytes, as well as in apoptosis of these cells, during exposure to B. burgdorferi. In the mature oligodendrocytes, as modeled by the MO3.13 cells, co-culture with B. burgdorferi elicited marked release of CCL2, CXCL8, CXCL(1,2,3), CCL5, and IL-6, in decreasing order of concentration (Fig. 1), in agreement with our previously published results [27], while adding to the repertoire of secreted molecules. The primary oligodendrocytes, which are mature but likely at an earlier point in the developmental process compared to MO3.13 oligodendrocytes (based on morphology and time allowed for growth) produced a more limited amount of CXCL8 (Fig. 6a) (and CXCL(1,2,3), in addition to CCL2), in response to B. burgdorferi, but no CCL5 or IL-6. This suggests that the process of maturation towards myelin-producing cells perhaps also affects the production/secretion machinery for additional mediator release; in addition, there could be limited mRNA production for these chemokines in primary cells at this stage. Alternatively, since the HOPC are obtained from human tissues, these differences could simply be the consequence of natural variability among individuals. However, in both these cell types, the MEK pathway played a role in eliciting inflammatory mediators, wherein blocking the MEK pathway reduced the amounts of produced mediators to basal levels even in the presence of spirochetes. For MO3.13 cells this effect was also apparent for the JNK and p38 pathways, where repression of either one also resulted in significant down-regulation of some pro-inflammatory mediators (Fig. 1 and Table 1). While suppression of IKK α and β kinases of the NFkB pathway decreased the release of CCL2 and IL-6 during B. burgdorferi infection, suppression of the transactivation function of RelA (p65) by the NFkB p65 inhibitor had a minimal effect, with inconclusive results. It is possible that other transcription factors in the NFkB family might play a role. Together, these results suggest that multiple pathways are involved in inducing release of inflammatory mediators from oligodendrocytes in the presence of B. burgdorferi, but most notably so for CCL2 release, whose production was mediated by all three MAPK and NFkB pathways for its secretion (Table 1). This pathway promiscuity possibly explains the abundance of CCL2. It should be noted that although the p38 inhibitor and the IKK inhibitor significantly downregulated CXCL (1,2,3) and IL-6 respectively, it remains to be determined whether these downregulations are biologically relevant and affect physiological functions, especially considering the modest fold downregulation values in comparison to those elicited by the MEK inhibitor (Table 1).
The MAPK/NFkB pathways that were elicited in vitro by exposure of cells to B. burgdorferi have also been assessed in a few other cell types. Their roles vary depending on the cell type utilized. In mature THP-1 macrophage-like cells, upregulation of CXCL8 by B. burgdorferi lysates was shown to occur preferentially via the ERK pathway, although, unlike with oligodendrocytes, this pattern was not statistically significant. The p38 pathway also had no role in this process [36]. Unlike with our results, the IkB kinase inhibitor did have a role in CXCL8 production. Although a different inhibitor was used (inhibitor VII vs. inhibitor XII in this study), this disparate outcome is likely due to the cell type utilized. In a mouse macrophage cell line RAW264.7, both the p38 and the JNK pathways have been implicated in the production of TNF [37,38] in response to B. burgdorferi lysates, and it was later demonstrated that the p38α MAP kinase regulated phosphorylation of RelA [39] in response to B. burgdorferi antigens. In human chondrocytes all of the three MAPK pathways were shown to influence TNF production in response to B. burgdorferi. However, in our study, we saw no secretion of TNF into the cell culture supernatants of MO3.13 cells in response to B. burgdorferi (MOI 10:1) at any time (2 h, 6 h, 24 h, 48 h- data not shown). Whether this cytokine is intracellularly produced or secreted in response to a higher infection dose in human oligodendrocytes is currently unknown. In a more recent study, bone marrow macrophages (BMM) derived from transgenic mice with a dominant-negative form of p38 MAPK produced significantly lower levels of CCL2 in comparison to BMMs from controls in response to B. burgdorferi [40]. This is similar to our results, where suppression of the p38 MAPK pathway significantly reduced CCL2 production in MO3.13 oligodendrocytes. No other pathways were tested, so the possibility that other kinases may influence outcomes to a similar or greater degree in murine BMM is not known.
Since the MEK/ERK pathway emerged as the most predominant mediator of pro-inflammatory molecule production/release, we next examined the mechanisms by which this kinase could mediate apoptosis as well, since the hypothesis of our study is that inflammation and apoptosis are causally related. Since CCL2 was the most abundant molecule produced, treatment of cells with a recombinant version of this protein and its ability to cause cell death was initially tested. However, due to inconclusive results with carrier-free protein and dominant effects of the carrier due to its high concentration in the recombinants, this approach was abandoned. Hence we looked at other mechanisms by which the ERK pathway could mediate apoptosis. In our previous study of microglia and B. burgdorferi-mediated neuronal cell death, p53-dependent genes were upregulated in apoptotic neurons [26], and p53 is well known for its role in cell cycle, apoptosis, autophagy, and senescence [33-35]. P53 is normally maintained in cells at low levels due to its low half-life. However, its levels can be stabilized, leading to its accumulation and p53-mediated effects [33,41]. We found that p53 levels were enhanced in MO3.13 oligodendrocytes in response to B. burgdorferi at 48 h, and that inhibition of the MEK/ERK pathway downregulated this effect (Fig. 2). ERK has been shown to bind p53 in vitro, stabilize the molecule by phosphorylation of serine 15 or threonine 55, and promote its activity in vitro [42-44]. Alternatively, the effect could be indirect, as in the case of c-myc for instance, which is stabilized by ERK phosphorylation of serine 62 and which in turn promotes the apoptotic function of p53 [45-47]. However, in preliminary studies, we did not see an increase in serine 15 phosphorylation of p53 in response to B. burgdorferi infection indicating that increased levels via stabilization is not likely through this effect (data not shown).
Also in concurrence with the above-mentioned studies on the effect of ERK on p53, and of p53 on apoptosis, suppression of both the MEK/ERK pathway and p53 suppressed apoptosis as measured by both caspase-3 activation (cleaved caspase-3) and the TUNEL assay (Fig. 3b, 4, 6b, and 6c). In our current study, caspase-3 activation was seen only at an MOI of 50:1, in contrast to a previous study from our lab, where statistically significant caspase-3 activation (albeit at low levels) could be seen at an MOI of 10:1 [27]. This could be a result of differences in techniques used (flow cytometry vs. ELISA on total cell lysates). However, due to its ease of use with multiple samples concurrently and to avoid time lags between samples, we decided to proceed with the ELISA assay at the MOI of 50:1. The assay showed that both MEK and p53 inhibitors suppressed caspase-3 activation in oligodendrocytes co-incubated with B. burgdorferi at this MOI. This result was confirmed with the TUNEL assay, which showed the same effect on apoptosis by both inhibitors not only at 50:1 but also at 10:1 MOI. This confirmed that suppression of both the MEK pathway and p53 inhibits apoptosis of human oligodendrocytes as induced by B. burgdorferi, and that the MEK-mediated effect on apoptosis is, at least partly, through p53 (Fig. 2, 4, and 6b). As MEK/ERK mediated inflammation as well as apoptosis, the latter likely primarily through p53, we tested to see if p53 can mediate release of inflammatory mediators as well. This was tested through two different p53 suppressors, pifithrin-α that inhibits the transactivation of p53 to the nucleus [48] and pifithrin-μ that inhibits the mitochondrial arm of the p53 pathway, such that p53 interaction with anti-apoptotic molecules in the mitochondria is inhibited [49,50]. Neither had a profound effect on inflammation in MO3 oligodendrocytes (Table 3), indicating either that inflammation and apoptosis are two separate arms of the ERK pathway, or that the ERK pathway's effect on inflammation occurs prior to its effect on apoptosis, i.e. inflammation precedes p53 upregulation. These results are consistent with those of our previous study, in which treatment of MO3.13 oligodendrocytes with glucocorticoids such as dexamethasone suppressed both inflammation and apoptosis due to B. burgdorferi [27]. Although glucocorticoids exert their anti-inflammatory effect through multiple mechanisms [51], one of the well documented means is through inhibition of MAPK pathways [52,53]. Thus it is possible that some of the dexamethasone effects documented in that study occur through the mechanisms described herein.
An additional effect of the MEK inhibitor that was seen in both the MO3.13 and DHOPC was an increase in cell numbers in the wells. This was initially noticed as a change in color of the medium with increasing concentration of U0126 (data not shown). Light microscopy and an MTT-based assay revealed an increase in cell number even in the presence of B. burgdorferi, for MO3.13 cells (Fig. 5) as well as primary oligodendrocytes (data not shown). Although it is possible that some of the MEK/ERK-mediated effect seen on apoptosis is due to an increase in cell number and therefore a decrease in percentage of apoptosis, this is not the entire explanation as the MEK1/2 inhibitor also had an effect on active caspase-3 levels, as measured by ELISA. These data on cell viability and apoptosis affected by the MEK/ERK inhibitor are similar to the results obtained by Tang D. et al. [54]. In that study, in the presence of DNA-damaging agents like etoposide, which leads to apoptosis, both ERK activation and upregulation of p53 levels were seen in NIH3T3 cells (a mouse fibroblast cell line). However, inhibition of the ERK pathway had no effect on p53 levels at 2 h, and ERK activation also led to cell-cycle arrest independently of p53. In these cells, ERK activation and p53 upregulation were parallel processes, unlike in our study, where MEK/ERK activation was found to be upstream of p53 (at 48 h). As with our data, however, inhibition of the MEK/ERK pathway increased the cell viability in the presence of agents leading to apoptosis. In contrast, in another study, while ERK activation was involved in the production of IL-1β, IL-6 and CXCL8 from chicken macrophages in response to H9N2 avian influenza virus, it actually suppressed TNF-family mediated apoptosis in these cells due to the virus [55]. Thus it is important to delineate the specific mechanisms underlying individual conditions/diseases in the particular cell types involved, as clearly there is no general explanation for all.
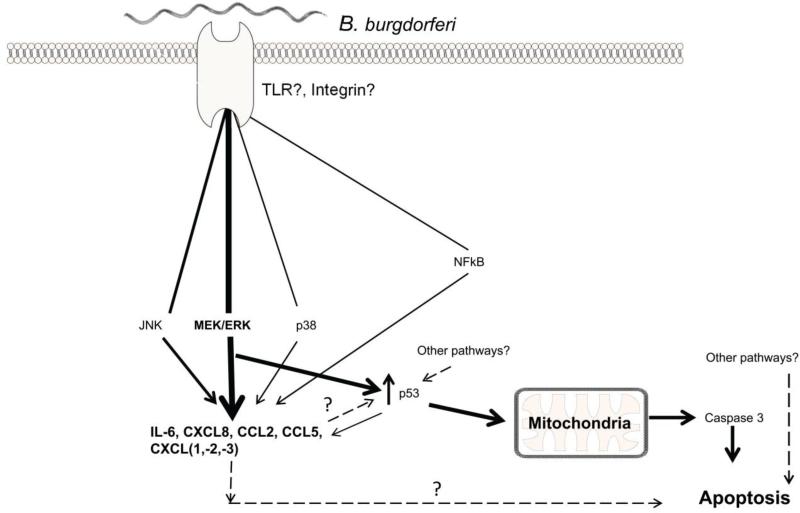
We show here that MEK/ERK pathway plays an important role in mediating inflammation and apoptosis of human oligodendrocytes in the presence of B. burgdorferi, and that apoptosis occurs at least partially through p53. We also show that in mature oligodendrocytes, as modeled by the MO3.13 cells, inflammation and apoptosis are either sequentially elicited post-MEK/ERK stimulation, or diverge thereafter. The latter option would falsify our hypothesis that inflammation is causally related to apoptosis in oligodendrocytes. These notions are depicted in a proposed model of signaling events in these cells (Fig. 7). In the primary oligodendrocytes, as with MO3.13 cells, suppression of either MEK/ERK or p53 suppresses apoptosis. However, it remains to be determined whether these are parallel processes independent of each other or are sequential, as seen in MO3.13 cells.
Fig. 7.
The proposed model for the signaling events in mature, human oligodendrocytes in response to B. burgdorferi. Upon exposure to the bacteria, signaling events mediated by an as yet unknown receptor(s) lead to activation of various pathways, but predominantly the MEK/ERK pathway, resulting in release of specific inflammatory mediators. Thickness of the lines for MAPK/NFkB pathways depicts the attributed signal strength. Activation of the MEK/ERK pathway also leads to increased p53 levels, with the mitochondrial arm of p53 playing a role in apoptosis. Dashed lines depict undetermined possibilities
While this study has provided a pivotal molecular mechanism underlying inflammation and apoptosis of human oligodendrocytes as stimulated by B. burgdorferi, it also provides data to suggest other areas that should be further explored. These include the roles of other MAPK pathways in apoptosis, other caspases (e.g. caspase 8), the effect of p53 on extrinsic caspase pathways, cell toxicity of chemokines and, importantly, caspase-independent cell death. Nevertheless, our data convincingly show that suppression of MEK/ERK and p53 molecules by use of inhibitors lead to a decrease in oligodendrocyte death. Since oligodendrocytes are vital to neuronal survival these results provide a blueprint for the evaluation of such inhibitors as therapies for Lyme neuroborreliosis in animal models and perhaps also in humans.
Acknowledgements
This study was supported by the National Institute of Neurologic Disorders and Stroke through grant NS048952, and by the National Center for Research Resources/Office of Research Infrastructure Programs of the National Institutes of Health through grant P51RR000164/P51OD011104. We thank the TNPRC Pathogen Detection and Quantification Core Laboratory for help with the multiplex ELISA assays. Ms. Robin Rodriguez of the TNPRC Media Laboratory is gratefully acknowledged for her assistance with graphics.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest
References
- 1.Jaenson TG. The epidemiology of lyme borreliosis. Parasitol Today. 1991;7(2):39–45. doi: 10.1016/0169-4758(91)90187-s. [DOI] [PubMed] [Google Scholar]
- 2.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992-2006. MMWR Surveill Summ. 2008;57(10):1–9. [PubMed] [Google Scholar]
- 3.Steere AC. Lyme disease. The New England journal of medicine. 2001;345(2):115–125. doi: 10.1056/NEJM200107123450207. doi:10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 4.Halperin JJ. Neuroborreliosis: central nervous system involvement. Seminars in neurology. 1997;17(1):19–24. doi: 10.1055/s-2008-1040908. doi:10.1055/s-2008-1040908. [DOI] [PubMed] [Google Scholar]
- 5.Vallat JM, Hugon J, Lubeau M, Leboutet MJ, Dumas M, Desproges-Gotteron R. Tick-bite meningoradiculoneuritis: clinical, electrophysiologic, and histologic findings in 10 cases. Neurology. 1987;37(5):749–753. doi: 10.1212/wnl.37.5.749. [DOI] [PubMed] [Google Scholar]
- 6.Benach JL, Garcia-Monco JC. Aspects of the pathogenesis of neuroborreliosis. In: Schutzer S, editor. Lyme disease; Molecular and immunological approaches. New York. Cold Spring Harbor Laboratory Press; Plainview: 1992. pp. 1–10. [Google Scholar]
- 7.Philipp MT, Aydintug MK, Bohm RP, Jr., Cogswell FB, Dennis VA, Lanners HN, Lowrie RC, Jr., Roberts ED, Conway MD, Karacorlu M, Peyman GA, Gubler DJ, Johnson BJ, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infection and immunity. 1993;61(7):3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts ED, Bohm RP, Jr., Cogswell FB, Lanners HN, Lowrie RC, Jr., Povinelli L, Piesman J, Philipp MT. Chronic lyme disease in the rhesus monkey. Laboratory investigation; a journal of technical methods and pathology. 1995;72(2):146–160. [PubMed] [Google Scholar]
- 9.England JD, Bohm RP, Jr., Roberts ED, Philipp MT. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Annals of neurology. 1997;41(3):375–384. doi: 10.1002/ana.410410313. doi:10.1002/ana.410410313. [DOI] [PubMed] [Google Scholar]
- 10.Cadavid D, Bai Y, Hodzic E, Narayan K, Barthold SW, Pachner AR. Cardiac involvement in non-human primates infected with the Lyme disease spirochete Borrelia burgdorferi. Laboratory investigation; a journal of technical methods and pathology. 2004;84(11):1439–1450. doi: 10.1038/labinvest.3700177. doi:10.1038/labinvest.3700177. [DOI] [PubMed] [Google Scholar]
- 11.Beck G, Habicht GS, Benach JL, Coleman JL, Lysik RM, O'Brien RF. A role for interleukin-1 in the pathogenesis of Lyme disease. Zentralblatt fur Bakteriologie, Mikrobiologie, und Hygiene Series A, Medical microbiology, infectious diseases, virology, parasitology. 1986;263(1-2):133–136. doi: 10.1016/s0176-6724(86)80114-6. [DOI] [PubMed] [Google Scholar]
- 12.Straubinger RK, Straubinger AF, Harter L, Jacobson RH, Chang YF, Summers BA, Erb HN, Appel MJ. Borrelia burgdorferi migrates into joint capsules and causes an up-regulation of interleukin-8 in synovial membranes of dogs experimentally infected with ticks. Infection and immunity. 1997;65(4):1273–1285. doi: 10.1128/iai.65.4.1273-1285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns MJ, Sellati TJ, Teng EI, Furie MB. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted [corrected] IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infection and immunity. 1997;65(4):1217–1222. doi: 10.1128/iai.65.4.1217-1222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashenkov M, Teleshova N, Kouwenhoven M, Smirnova T, Jin YP, Kostulas V, Huang YM, Pinegin B, Boiko A, Link H. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. Journal of neuroimmunology. 2002;122(1-2):106–116. doi: 10.1016/s0165-5728(01)00451-9. [DOI] [PubMed] [Google Scholar]
- 15.Brown CR, Blaho VA, Loiacono CM. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J Immunol. 2003;171(2):893–901. doi: 10.4049/jimmunol.171.2.893. [DOI] [PubMed] [Google Scholar]
- 16.Kondrusik M, Swierzbinska R, Pancewicz S, Zajkowska J, Grygorczuk S, Hermanowska-Szpakowicz T. [Evaluation of proinflammatory cytokine (TNF-alpha, IL-1beta, IL-6, IFN-gamma) concentrations in serum and cerebrospinal fluid of patients with neuroborreliosis]. Neurologia i neurochirurgia polska. 2004;38(4):265–270. [PubMed] [Google Scholar]
- 17.Grygorczuk S, Pancewicz S, Zajkowska J, Kondrusik M, Rwierzbinska R, Hermanowska-Szpakowicz T. Concentrations of macrophage inflammatory proteins MIP-1alpha and MIP-1beta and interleukin 8 (il-8) in lyme borreliosis. Infection. 2004;32(6):350–355. doi: 10.1007/s15010-004-3110-4. doi:10.1007/s15010-004-3110-4. [DOI] [PubMed] [Google Scholar]
- 18.Grygorczuk S, Zajkowska J, Swierzbinska R, Pancewicz S, Kondrusik M, Hermanowska-Szpakowicz T. Concentration of interferon-inducible T cell chemoattractant and monocyte chemotactic protein-1 in serum and cerebrospinal fluid of patients with Lyme borreliosis. Rocz Akad Med Bialymst. 2005;50:173–178. [PubMed] [Google Scholar]
- 19.Benveniste EN. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. The American journal of physiology. 1992;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 20.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends in neurosciences. 1996;19(8):331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 21.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain research Brain research reviews. 1999;30(1):77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 22.Raivich G, Jones LL, Werner A, Bluthmann H, Doetschmann T, Kreutzberg GW. Molecular signals for glial activation: pro- and anti-inflammatory cytokines in the injured brain. Acta neurochirurgica Supplement. 1999;73:21–30. doi: 10.1007/978-3-7091-6391-7_4. [DOI] [PubMed] [Google Scholar]
- 23.Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. Journal of neuroimmunology. 2010 doi: 10.1016/j.jneuroim.2010.05.010. doi:10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, Lackner AA, Philipp MT. Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. The American journal of pathology. 2008;173(5):1415–1427. doi: 10.2353/ajpath.2008.080483. doi:10.2353/ajpath.2008.080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh G, Borda JT, Gill A, Ribka EP, Morici LA, Mottram P, Martin DS, Jacobs MB, Didier PJ, Philipp MT. Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. Journal of neuroinflammation. 2009;6:23. doi: 10.1186/1742-2094-6-23. doi:10.1186/1742-2094-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers TA, Kaushal D, Philipp MT. Microglia are mediators of Borrelia burgdorferi-induced apoptosis in SH-SY5Y neuronal cells. PLoS pathogens. 2009;5(11):e1000659. doi: 10.1371/journal.ppat.1000659. doi:10.1371/journal.ppat.1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. Journal of neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. doi:10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological reviews. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 29.Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158(7):3285–3292. [PubMed] [Google Scholar]
- 30.Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, Goebel UB, Weber JR. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998;22(3):295–305. doi: 10.1002/(sici)1098-1136(199803)22:3<295::aid-glia8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck BS. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infection and immunity. 1999;67(8):3824–3829. doi: 10.1128/iai.67.8.3824-3829.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annual review of immunology. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. doi:10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 33.Amaral JD, Castro RE, Steer CJ, Rodrigues CM. p53 and the regulation of hepatocyte apoptosis: implications for disease pathogenesis. Trends in molecular medicine. 2009;15(11):531–541. doi: 10.1016/j.molmed.2009.09.005. doi:10.1016/j.molmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Vousden KH. Functions of p53 in metabolism and invasion. Biochemical Society transactions. 2009;37(Pt 3):511–517. doi: 10.1042/BST0370511. doi:10.1042/BST0370511. [DOI] [PubMed] [Google Scholar]
- 35.Vousden KH, Ryan KM. p53 and metabolism. Nature reviews Cancer. 2009;9(10):691–700. doi: 10.1038/nrc2715. doi:10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 36.Sadik CD, Hunfeld KP, Bachmann M, Kraiczy P, Eberhardt W, Brade V, Pfeilschifter J, Muhl H. Systematic analysis highlights the key role of TLR2/NF-kappaB/MAP kinase signaling for IL-8 induction by macrophage-like THP-1 cells under influence of Borrelia burgdorferi lysates. The international journal of biochemistry & cell biology. 2008;40(11):2508–2521. doi: 10.1016/j.biocel.2008.04.014. doi:10.1016/j.biocel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Anguita J, Barthold SW, Persinski R, Hedrick MN, Huy CA, Davis RJ, Flavell RA, Fikrig E. Murine Lyme arthritis development mediated by p38 mitogen-activated protein kinase activity. J Immunol. 2002;168(12):6352–6357. doi: 10.4049/jimmunol.168.12.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izadi H, Motameni AT, Bates TC, Olivera ER, Villar-Suarez V, Joshi I, Garg R, Osborne BA, Davis RJ, Rincon M, Anguita J. c-Jun N-terminal kinase 1 is required for Toll-like receptor 1 gene expression in macrophages. Infection and immunity. 2007;75(10):5027–5034. doi: 10.1128/IAI.00492-07. doi:10.1128/IAI.00492-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson CM, Hedrick MN, Izadi H, Bates TC, Olivera ER, Anguita J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infection and immunity. 2007;75(1):270–277. doi: 10.1128/IAI.01412-06. doi:10.1128/IAI.01412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley K, Navasa N, Olson CM, Jr., Bates TC, Garg R, Hedrick MN, Conze D, Rincon M, Anguita J. Macrophage p38 mitogen-activated protein kinase activity regulates invariant natural killer T-cell responses during Borrelia burgdorferi infection. The Journal of infectious diseases. 2012;206(2):283–291. doi: 10.1093/infdis/jis332. doi:10.1093/infdis/jis332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi P, Polson A, Reisman D. Elevated transcription of the p53 gene in early S-phase leads to a rapid DNA-damage response during S-phase of the cell cycle. Apoptosis : an international journal on programmed cell death. 2011;16(9):950–958. doi: 10.1007/s10495-011-0623-z. doi:10.1007/s10495-011-0623-z. [DOI] [PubMed] [Google Scholar]
- 42.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. The Journal of biological chemistry. 2000;275(46):35778–35785. doi: 10.1074/jbc.M004267200. doi:10.1074/jbc.M004267200. [DOI] [PubMed] [Google Scholar]
- 43.She QB, Chen N, Dong Z. ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. The Journal of biological chemistry. 2000;275(27):20444–20449. doi: 10.1074/jbc.M001020200. doi:10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- 44.Yeh PY, Chuang SE, Yeh KH, Song YC, Chang LL, Cheng AL. Phosphorylation of p53 on Thr55 by ERK2 is necessary for doxorubicin-induced p53 activation and cell death. Oncogene. 2004;23(20):3580–3588. doi: 10.1038/sj.onc.1207426. doi:10.1038/sj.onc.1207426. [DOI] [PubMed] [Google Scholar]
- 45.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes & development. 2000;14(19):2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavin MF, Gueven N. The complexity of p53 stabilization and activation. Cell death and differentiation. 2006;13(6):941–950. doi: 10.1038/sj.cdd.4401925. doi:10.1038/sj.cdd.4401925. [DOI] [PubMed] [Google Scholar]
- 47.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. The FEBS journal. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. doi:10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 48.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 49.Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV. Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nature chemical biology. 2006;2(9):474–479. doi: 10.1038/nchembio809. doi:10.1038/nchembio809. [DOI] [PubMed] [Google Scholar]
- 50.Speidel D. Transcription-independent p53 apoptosis: an alternative route to death. Trends in cell biology. 2010;20(1):14–24. doi: 10.1016/j.tcb.2009.10.002. doi:10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Pelaia G, Vatrella A, Cuda G, Maselli R, Marsico SA. Molecular mechanisms of corticosteroid actions in chronic inflammatory airway diseases. Life sciences. 2003;72(14):1549–1561. doi: 10.1016/s0024-3205(02)02446-3. [DOI] [PubMed] [Google Scholar]
- 52.Pelaia G, Cuda G, Vatrella A, Grembiale RD, De Sarro G, Maselli R, Costanzo FS, Avvedimento VE, Rotiroti D, Marsico SA. Effects of glucocorticoids on activation of c-jun N-terminal, extracellular signal-regulated, and p38 MAP kinases in human pulmonary endothelial cells. Biochemical pharmacology. 2001;62(12):1719–1724. doi: 10.1016/s0006-2952(01)00791-2. [DOI] [PubMed] [Google Scholar]
- 53.Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(12):4805–4820. doi: 10.1096/fj.12-216382. doi:10.1096/fj.12-216382. [DOI] [PubMed] [Google Scholar]
- 54.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. The Journal of biological chemistry. 2002;277(15):12710–12717. doi: 10.1074/jbc.M111598200. doi:10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 55.Xing Z, Cardona CJ, Anunciacion J, Adams S, Dao N. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. The Journal of general virology. 2010;91(Pt 2):343–351. doi: 10.1099/vir.0.015578-0. doi:10.1099/vir.0.015578-0. [DOI] [PubMed] [Google Scholar]