Abstract
Hematopoietic stem cells (HSCs) reside in specialized microenvironments (niches) in the bone marrow. The stem cell niche is thought to provide signals that support key HSC properties, including self-renewal capacity and long-term multilineage repopulation ability. The stromal cells that comprise the stem cell niche and the signals that they generate that support HSC function are the subjects of intense investigation. Here we review the complex and diverse stromal cell populations that reside in the bone marrow and examine their contribution to HSC maintenance. We highlight recent data suggesting that perivascular CXCL12-expressing mesenchymal progenitors and endothelial cells are key cellular components of the stem cell niche in the bone marrow.
Keywords: hematopoietic stem cells, HSCs, stem cell niche, mesenchymal stem cells, osteoblast, CXCL12
The hematopoietic stem cell niche
Hematopoiesis is the process by which all mature blood cells are produced. It must balance enormous production needs (more than 500 billion blood cells are produced every day) with the need to precisely regulate the number of each blood cell type in the circulation. In vertebrates, the vast majority of hematopoiesis occurs in the bone marrow and is derived from a limited number of hematopoietic stem cells (HSCs) that are multipotent and capable of extensive self-renewal. In mammals, it is estimated that there are approximately 10,000 HSCs, of which, in humans, approximately 1,000 contribute hematopoiesis at any given time [1]. Prospective identification of HSCs using cell surface markers and flow cytometry is best described for murine HSCs. c-Kit+ Sca-1+ lineage− (KSL) CD150+ CD48− cells [2] and CD34− Flk2− KSL cells [3] represent the two most commonly used murine HSCs phenotypes, each containing approximately 50% HSCs based on long-term repopulating assays.
Key properties of HSCs are multipotency, self-renewal capacity, and quiescence. The bone marrow microenvironment appears to be uniquely adapted to support these, and other, HSC properties. As first proposed by Schofield in 1978 [4], the concept of a “stem cell niche” in the bone marrow has gained widespread popularity. In this model, specialized niche cells in the bone marrow are physically associated with HSCs and provide specific signals that help maintain their function. In this review, we highlight recent studies defining the bone marrow stromal cells that comprise the stem cell niche and the signals that they generate that contribute to HSC maintenance.
Anatomy of the Bone Marrow
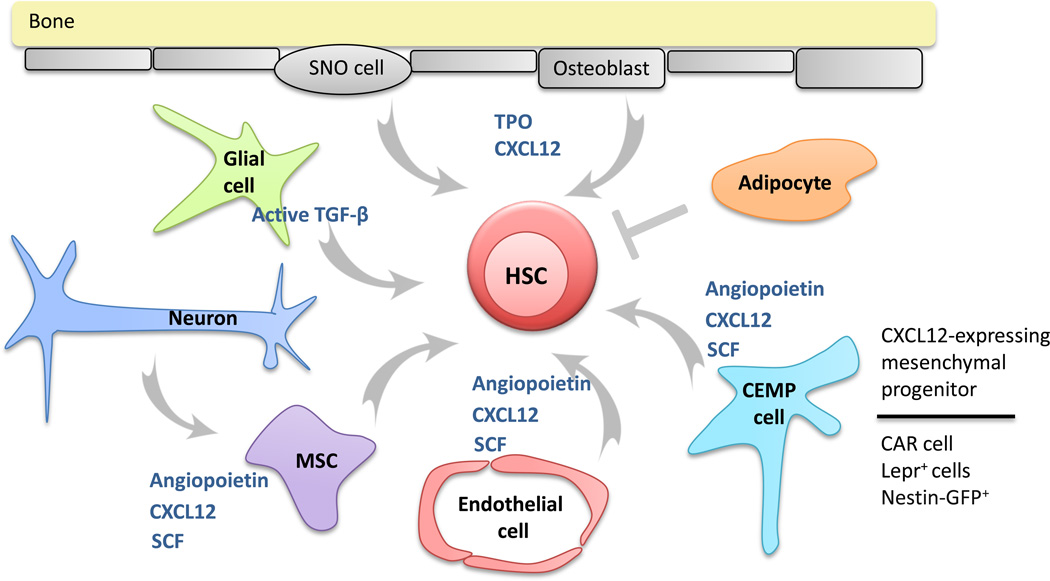
The bone marrow is the major site of hematopoiesis and bone formation in most vertebrates. Thus, in addition to containing hematopoietic cells, the bone marrow contains cells that contribute to bone homeostasis, including mesenchymal stem cells (also called skeletal stem cells), osteoprogenitors, osteoblasts, osteocytes, and chondrocytes (Figure 1). To add further complexity, other stromal cell populations that reside in the bone marrow may regulate hematopoiesis, including neuronal cells, glial cells, and adipocytes. Recent advances in imaging technologies have greatly advanced our understanding of the organization of the bone marrow. It is clear that the bone marrow in both long bones (e.g., femur) and flat bones (e.g., calvaria) is highly vascular [5, 6]. In long bones, central longitudinal arteries give rise to radial arteries that in turn branch into arterioles near the endosteum (Figure 2) [5]. The transition from Sca-1+ arterioles to Sca-1− venous endothelium occurs in close proximity to the endosteum. Venous sinusoids extend back towards the central cavity where they coalesce into a large central sinus. Of note, the venous sinusoids, which contain numerous fenestra, are thought to be the major sites of leukocyte egress from the bone marrow into the circulation [7]. Osteoblasts and bone lining cells form a layer between mineralized bone and the bone marrow. Spindle-shaped N-cadherin+ osteoblasts (SNO cells), which may represent an immature osteoblast, also are localized near the endosteum [8]. There is a rich network of stromal cells interspersed between islands of hematopoietic cells and include mesenchymal stem cells, CXCL12-abundant reticular cells, and adipocytes.
Figure 1. Distinct stromal cell populations in the bone marrow contribute to HSC maintenance.
A complex and diverse group of stromal cells in the bone marrow have been implicated in HSC maintenance. Endothelial cells, mesenchymal stem cells (MSCs), and CXCL12-expressing mesenchymal progenitors (CEMP cells) are perivascular stromal cells that produce a number of factors that support HSCs, including CXCL12, angiopoietin, and stem cell factor (SCF). CEMP cells have been identified as CXCL12-abundant reticular (CAR) cells, leptin receptor+ stromal (Lepr+) cells, and Nestin-GFP+ cells; these stromal cell populations likely overlap considerably. Osteoblasts and spindle-shaped N-cadherin+ osteoblast (SNO cells) produce a number of factors that support HSCs, including thrombopoietin (TPO) and CXCL12. Sympathetic neurons indirectly regulate HSCs by targeting CXCL12 expression. Finally, glial cells, through production of active transforming growth factor-α TGF-α and adipocytes regulate HSCs.
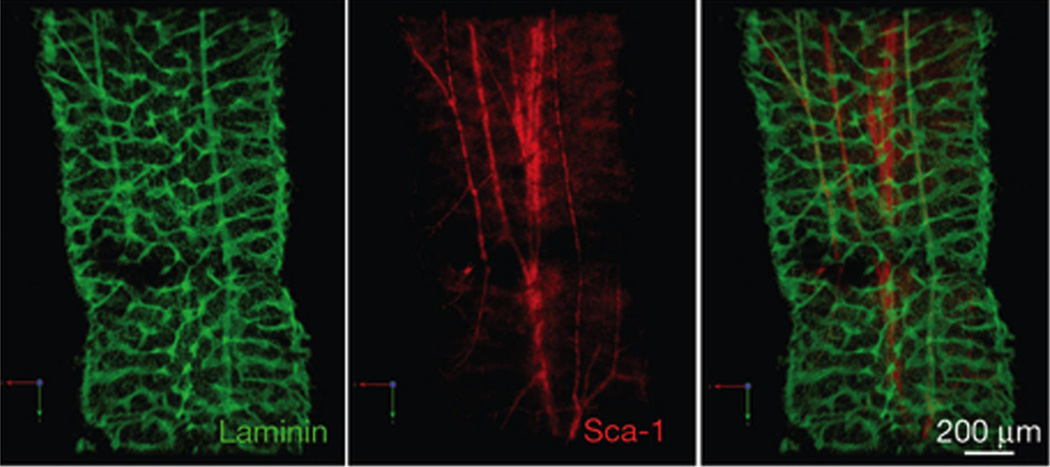
Figure 2. The bone marrow is highly vascularized.
Confocal microscopic images of the femoral diaphysis stained with the pan-vasculature marker laminin (green) and the arterial specific marker, Sca-1 (red). A Sca-1+ central artery runs through the central marrow. The central artery branches off to smaller arterioles towards the endosteum. Reprinted by permission from Macmillan Publishers Ltd: Nature Cell Biology; 15 (5):533-43, copyright (2013).
The location of HSCs in the bone marrow is controversial. Initial studies using labeled HSC-enriched cell populations transplanted into recipients suggested a mostly endosteal location for HSCs [9–11]. This is consistent with data showing that long-term BrdU retaining cells (presumed to be HSCs) are preferentially localized next to SNO cells at the endosteum [8]. In contrast, using a rigorous definition of HSCs (CD150+ CD48− CD41− lineage− cells), Kiel et al showed that the majority of HSCs are in contact with sinusoidal endothelium at bone-distant sites [2]. This is consistent with studies showing that most HSCs are in direct contact with perivascular CXCL12-abundant reticular (CAR) cells [12] and nestin-GFP+ stromal cells [13]. Interestingly, a recent study suggested that both HSCs and lineage-committed hematopoietic progenitor cells (HPCs) were localized near endothelium with a preference for the endosteal region [5]. Collectively, these data suggest that the majority of HCS are perivascular and enriched in the highly vascular endosteal region.
Hypoxia and the stem cell niche
The prevailing view is that HSCs are maintained in a hypoxic niche in the bone marrow. As noted, HSCs are localized to the endosteal region, which previous studies characterized as having low perfusion and relative hypoxia [14–16]. Indeed, HSCs with lower cellular levels of reactive oxygen species have higher self-renewal potential [17]. Moreover, disruption of hypoxia-inducible factor-1 alpha (HIF-1 alpha) results in loss of HSC quiescence and repopulating activity [18], while stabilization of HIF-1 alpha induces HSC quiescence and enhances repopulating activity [18, 19]. However, as noted in the previous section, the endosteal region is actually highly vascularized and most HSCs are perivascular [5]; thus, HSCs are likely to be relatively well oxygenated. Interestingly, hematopoietic stem/progenitor cells (HSPCs) display a hypoxic profile (defined by strong retention of pimonidazole and expression of HIF-1α) regardless of their location in the bone marrow [5]. Indeed, even HSPCs in the peripheral circulation display a hypoxic profile. Thus, intrinsic differences in cellular metabolism rather than localization to a hypoxic microenvironment may define the hypoxic profile of HSCs.
Stromal cells in the Bone Marrow
The bone marrow contains several stromal cell populations implicated in the regulation of hematopoiesis. There is emerging data that specific stromal cell populations regulate distinct hematopoietic progenitor populations; both positive and negative regulators of hematopoiesis have been identified. In the following sections, we review studies that focus on the contribution of specific stromal cell populations in the regulation of hematopoiesis. However, it is important to keep in mind, that it is the sum of the complex signals generated by these different stromal cell populations that are sensed by HSCs and other hematopoietic progenitor cell populations in the bone marrow.
Osteolineage cells
The localization of HSCs to the endosteum supported studies of the role of osteoblast lineage cells (which we will refer to as osteolineage cells) in the stem cell niche. Osteolineage cells produce a number of cytokines implicated in HSC regulation, including G-CSF [20], thrombopoietin [21, 22], and CXCL12 [23]. Expansion of osteolineage cells through enforced expression of parathyroid hormone receptor 1 [24] or through conditional inactivation of bone morphogenic protein receptor-1 [8] is associated with an increase in HSCs. Conversely, conditional ablation of osteolineage cells is associated with a loss of HSCs in the bone marrow and extramedullary hematopoiesis [25].
Despite these findings, the contribution of osteolineage cells, particularly mature osteoblasts, to HSC maintenance is controversial. A recent study suggests that parathyroid hormone treatment increases short-term HSCs, not through osteoblast expansion, but through Wnt ligand production by T cells [26]. Moreover, an increase in osteoblasts is not sufficient to expand HSCs. Treatment of mice with the bone anabolic agent, strontium, leads to mature osteoblast expansion but has no effect on HSC number or function [27]. Conversely, in mice with chronic inflammatory arthritis, resulting in osteoblast suppression, HSCs are normal [28]. Finally, as discussed in more detail in a later section, conditional deletion of Cxcl12 [29, 30] or stem cell factor (SCF, Kitl) [31] from mature osteoblasts has no effect on HSCs. These apparently discrepant data may be due, at least in part, the heterogeneity of osteolineage cells. In particular, a recent study showed that more primitive osteolineage cells express higher levels of SCF and CXCL12 and support the long-term repopulating activity of HSCs better than more differentiated osteolineage cells [32]. Thus, while mature osteoblasts appear not to play a major role in vivo, immature osteolineage cells may contribute to HSC maintenance, a concept we will explore in a later section.
The role of N-cadherin in the stem cell niche
The contribution of N-cadherin to HSC maintenance remains unclear. Cadherins are calcium-dependent homotypic adhesion molecules that form adherens junctions. N-cadherin is expressed on a subset of osteolineage cells, with higher expression observed on immature osteolineage cells [32]. As discussed in the previous section, at least a subset of HSCs are localized near SNO cells [8, 10, 11], and N-cadherin has been implicated in the adhesion of HSCs to osteolineage cells [32]. Inhibition of N-cadherin signaling through knock-down of N-cadherin or expression of a dominant-negative N-cadherin in HSCs inhibits their repopulating activity [33, 34]. On the other hand, conditional deletion of Cdh2 (encoding for N-cadherin) in HSCs has no effect on HSC number or function [35]. Moreover, conditional deletion of Cdh2 in osteolineage cells has no effect on HSC number, trafficking, cell cycle status, or repopulating activity [36, 37]. Thus, the preponderance of evidence, suggests that N-cadherin is not required for normal HSC function. It is also important to note that these results do not discount a role for SNO cells in the regulation of HSCs. SNO cells are suggested to be immature osteoblasts, and N-cadherin may simply mark an earlier developmental stage of osteoblasts important for niche maintenance.
Perivascular CXCL12-expressing stromal cells
The perivascular location of most HSCs has focused recent attention on the stromal cells that reside in the perivascular region as candidate niche cells. Besides endothelial cells, the perivascular region contains mesenchymal stem cells and a heterogeneous population of stromal cells characterized by very high CXCL12 expression. CXCL12 (stromal-derived factor-1, SDF-1) is a chemokine that plays a crucial role in maintaining HSC function. Three perivascular stromal cell populations that express high levels of CXCL12 have been identified: CXCL12-abundant reticular (CAR) cells, nestin-GFP+ stromal cells, and leptin receptor+ stromal cells. These stromal cell populations are defined by transgene expression using defined stromal-specific promoters, and it likely that there is considerable overlap.
CAR cells were identified using mice with GFP knocked into the Cxcl12 locus as perivascular stromal cells with very high GFP expression [12, 38]. CAR cells are mesenchymal progenitors that have both adipogenic and osteogenic potential in vitro [39]. HSPCs and certain lymphoid progenitors directly contact CAR cells in the bone marrow [12, 38]. Conditional ablation of CAR cells using transgenic mice expressing the diphtheria toxin receptor (DTR) under control of Cxcl12 regulatory elements (Cxcl12-Dtr mice) leads to a reduction in HSCs and HSC long-term repopulating activity but increased HSC quiescence [39]. CAR cells are the major source of SCF and CXCL12 in the bone marrow, and conditional ablation is associated with a marked loss of bone marrow SCF and CXCL12. Of note, although no obvious toxicity was observed in endothelial cells or osteoblasts, these cells express CXCL12, and it is possible that their function was altered after conditional ablation in Cxcl12-Dtr mice.
Nestin-GFP+ cells are defined as perivascular stromal cells that express high levels of GFP under control of the nestin (Nes) promoter [13]. Nestin-GFP+ stromal cells are enriched for mesenchymal stem cell activity with approximately 1% of cells having colony-forming units-fibroblast (CFU-F) activity. Nestin-GFP+ cells express several genes implicated in HSC maintenance, including CXCL12, SCF, and angiopoietin. Transplanted HSCs preferentially home near nestin-GFP+ cells. Finally, conditional ablation of nestin-expressing stromal cells results in a modest loss of HSCs.
Lineage mapping using Cre-recombinase expressed under the control of leptin receptor (Lepr) regulatory elements identified a perivascular stromal cell population in the bone marrow [31]. Similar to CAR cells, leptin receptor+ stromal cells express high levels of CXCL12 and stem cell factor, suggesting that CAR cells and leptin-receptor+ cells identify largely overlapping stromal cell populations. Indeed, we observed high level leptin receptor expression in sorted CAR cells [30]. This also is consistent with data showing that CAR cells have adipogenic (as well as osteogenic) capacity in vitro [39]. However, lineage mapping studies using Lepr:Cre showed no targeting of osteoblasts [31], raising the possibility that a subpopulation of leptin-receptor-negative CAR cells with osteogenic capacity exists. In any case, deletion of stem cell factor (Kitl) from leptin-receptor+ stromal cells results in a loss of HSCs, indicating that these cells are an important cellular component of the stem cell niche.
Mesenchymal stem cells
A rigorous definition of MSCs is a cell with self-renewal capacity that is able to generate all mesenchymal cells within a skeletal element, including osteoblasts, chondrocytes, adipocytes, and fibroblasts [40]. Although there is currently no prospective way to identify MSCs on a per cell basis, several groups have reported methods to identify MSC-enriched cell populations. In mice, approximately 4% of CD45− lineage− PDGFRα+ Sca+ (PαS) cells have CFU-F activity [41] and approximately 1% of nestin-GFP+ stromal cells have CFU-F activity [13]. We recently showed that the Prx1-targeted subset of PaS is further enriched for MSC activity, with greater than 10% of these cells having CFU-F activity [30]. Surprisingly, we observed that neither Prx1-Cre targeted PaS cells nor CAR cells express nestin [30]. One potential explanation for the disparate results is that the nestin-GFP transgene results in aberrant expression of GFP that does not accurately reflect nestin expression. We suggest that nestin-GFP+ expression identifies a heterogeneous stromal cell population that includes MSCs and CAR cells.
In human bone marrow, CD146-expressing stromal cells identifies an MSC-enriched cell population [42]. Recently, Pinho and colleagues showed that PDGFRα and CD51 expression define a bone marrow stromal cell population in both mice and humans that is highly enriched for MSCs and can support HSPC expansion in vitro [43].
Endothelial cells, adipocytes, neuronal, and glial cells
Hemogenic endothelium in the dorsal aorta gives rise to the first definitive HSCs during embryonic development [44, 45]. Bone marrow endothelial cells express several genes implicated in HSC maintenance, including CXCL12, SCF, and angiopoietin, and they support the proliferation of HSPCs in vitro [46]. Regeneration of sinusoidal endothelial cells is required for hematopoietic recovery from myeloablation [47, 48]. Moreover, deletion of the endothelial specific adhesion molecule, E-selectin, results in increased HSC quiescence, suggesting that endothelial cells regulate HSC proliferation [49]. Finally, endothelial cells contribute to HSC maintenance through production of SCF, as deletion of Kitl specifically from endothelial cells results in the loss of HSCs [31]. Collectively, these data show that endothelial cells are a key component of the stem cell niche.
Adipocyte number in the bone marrow increases with age (especially in humans). Naveiras et al. showed that the hematopoietic activity of distinct regions of the mouse skeleton correlated inversely with adiposity [50]. That is, adipocyte-rich bone marrow, such as the vertebrae of mice, has a decreased number of HSCs compared with adipocyte-poor bone marrow. In mice with impaired adipogenesis, hematopoietic activity is increased in bone marrow sites that are normally adipocyte-rich. Of potential clinical relevance, pharmacological inhibition of adipogenesis results in enhanced hematopoietic recovery following stem cell transplantation in mice [50]. These data suggest that adipocytes play an inhibitory role in HSC maintenance. Elegant studies by the Frenette group show that the sympathetic nervous system contributes to HSC trafficking from the bone marrow [51]. Signals from the sympathetic nervous system coordinate the circadian egress of HSCs into the circulation by regulating local production of CXCL12. Genetic or pharmacological ablation of adrenergic signaling inhibits G-CSF-induced HSPC mobilization [52].
A recent study also implicates glial cells, specifically nonmyelinating Schwann cells, in the regulation of HSCs [53]. Transforming growth factor β (TGF-β) is an important regulator of HSC function. It induces HSC quiescence in vitro [54] and loss of TGF-β signaling in HSCs results in impaired long-term repopulating activity [53]. While the latent form of TGF-β is produced by many cell types, Yamazaki and colleagues provide data suggesting that nonmyelinating Schwann cells are the major source of active TGF-β in the bone marrow. Importantly, surgical disruption of sympathetic nerves resulting in loss of Schwann cells is associated with decreased active TGF-β expression and the loss of HSCs.
Distinct contributions of Cxcl12-producing niche cells to HSC maintenance CXCL12 plays a crucial role in maintaining HSC function, including retention in the bone marrow [55–58], quiescence [59, 60], and repopulating activity [60]. To test the hypothesis that CXCL12 production from different stromal cell populations would have distinct effects on HSCs, the Morrison group and our group independently deleted Cxcl12 from candidate niche cells [29, 30]. Loss of CXCL12 from mature osteoblasts had no effect on HSC number or function. Loss of CXCL12 from CAR cells using osterix (Osx)-Cre results in HSPC mobilization, and a modest (50%) reduction in HSCs [30]. Deletion of Cxcl12 from leptinreceptor+ stromal cells also results in constitutive HSPC mobilization, but no defect in HSC function was observed [31]. These observations show that CXCL12 expression from CAR cells or leptin-receptor+ stromal cells is essential for efficient retention of HSPCs in the bone marrow. Both groups showed that deletion of Cxcl12 using Prx1-Cre resulted in a significant loss of HSCs, long-term repopulating activity, and HSC quiescence. Like Osx-Cre, Prx1-Cre targets CAR cells and osteoblasts but also targets PaS mesenchymal progenitors [30]. A modest decrease in long-term repopulating activity (but not HSC quiescence) also was observed in mice with Cxcl12 deleted from endothelial cells. Together, these data suggest that CXCL12 expression from perivascular stromal cells is required for HSC maintenance. Whereas CAR cells and endothelial cells are minor contributors, mesenchymal progenitors are the major source of CXCL12 that supports HSCs.
Concluding remarks
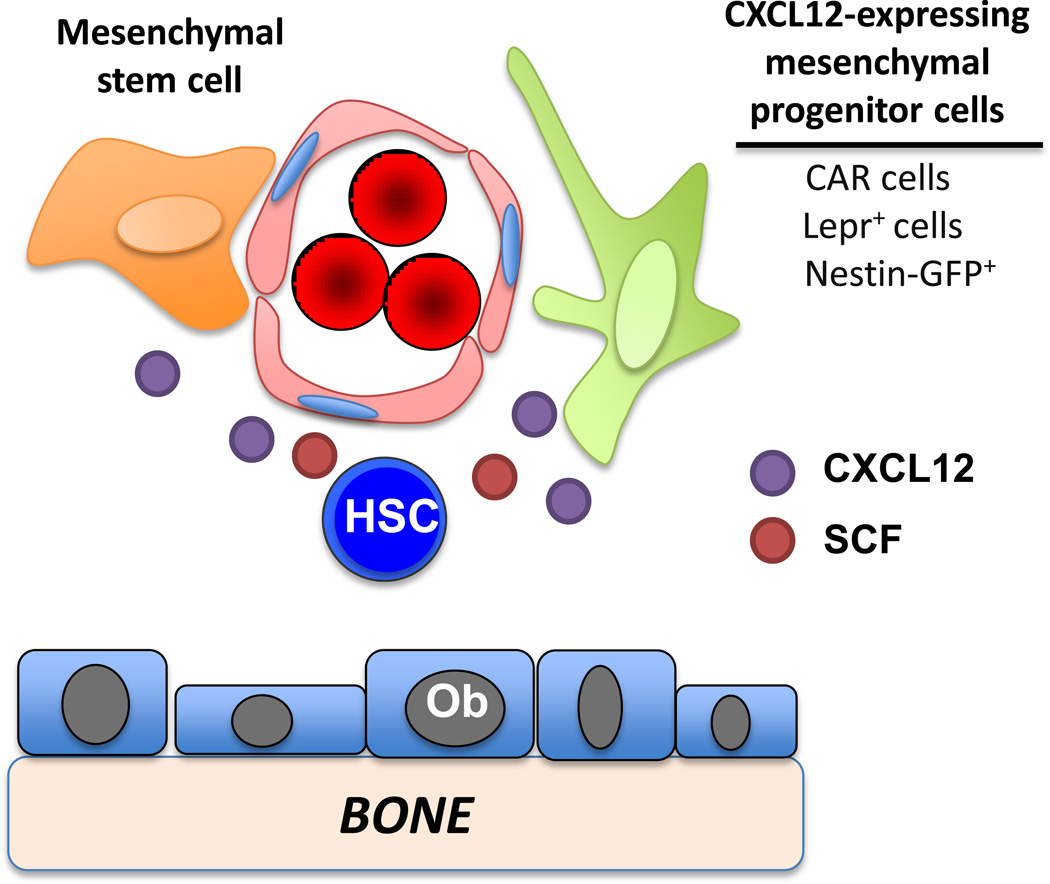
A model of the murine stem cell niche incorporating recent findings is shown in Figure 3. Current evidence supports a perivascular location for most HSCs, with a preference for the endosteal region. Key components of the perivascular niche include endothelial cells, mesenchymal progenitors, and CXCL12-expressing stromal cells (including CAR cells, leptin receptor+ cells, and Nestin-GFP+ cells). These perivascular stromal cells provide signals, including CXCL12, SCF, and angiopoietin that maintain HSC quiescence and self-renewal capacity. The sympathetic nervous system and associated glial cells and adipocytes also contribute to HSC maintenance. There are several important unresolved issues in field (Box 1). A better understanding of the interaction of HSCs with stromal cells in the bone marrow may provide potential therapeutic targets for manipulation to modulate HSC and their progeny. Moreover, since the stem cell niche may provide signals that support certain malignancies [61, 62], drugs that target the niche hold promise as a way to sensitize cancer cells to chemotherapy.
Figure 3. Model of murine HSC niche.
Conditional deletion of two key HSC maintenance genes, Kitl (stem cell factor, SCF) and Cxcl12, in candidate niche cells has emphasized the importance of stromal cells in the perivascular region. SCF production from endothelial cells and leptin receptor+ stromal (Lepr+) cells but not osteoblasts (Ob) is required for HSC maintenance. CXCL12 production from mesenchymal stem cells (and to a lesser extent endothelial cells) is required for HSC maintenance, while CXCL12 production form CXCL12 abundant reticular (CAR) cells or Lepr+ cells is required for efficient retention of hematopoietic progenitors in the bone marrow. Nestin-GFP+ stromal cells likely overlap with CAR cells and Lepr+ cells.
Outstanding areas for future study.
The relationship between CAR cells, nestin-GFP+ stromal cells, and leptin receptor+ stromal cells is not clear. Although they likely overlap considerably, it will be important to define precisely what cell types are included in each cell population.
The genes expressed by niche cells that contribute to HSC maintenance have not been fully defined.
The signals that regulate stromal cells in the niche are poorly understood. For example, the pro-inflammatory cytokine G-CSF induces HSC mobilization, at least in part, by disrupting the stem cell niche. Of particular interest are the signals that determine osteoblastic versus adipogenic differentiation of mesenchymal progenitors. Strategies that suppress adipogenic differentiation might reverse the age-associated loss of hematopoietic activity.
Acknowledgements
The authors thank Dr. Leslie E. Silberstein for sharing a figure generated in his lab and recently published. This work is supported by the National Institutes of Health grant HL60772 (DCL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose
REFERENCES
- 1.Catlin SN, et al. The replication rate of human hematopoietic stem cells in vivo. Blood. 2011;117:4460–4466. doi: 10.1182/blood-2010-08-303537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Osawa M, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 4.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 5.Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature cell biology. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell F. Ultrastructural studies of transmural migration of blood cells in the bone marrow of rats, mice and guinea pigs. American Journal of Anatomy. 1972;135:521–536. doi: 10.1002/aja.1001350406. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson SK, et al. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes & development. 2004;18:2747–2763. doi: 10.1101/gad.313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 12.Sugiyama T, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levesque JP, et al. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25:1954–1965. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- 15.Winkler IG, et al. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–385. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 16.Parmar K, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takubo K, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell stem cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Forristal CE, et al. Pharmacologic stabilization of HIF-1alpha increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121:759–769. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- 20.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. The Journal of experimental medicine. 1994;179:1677–1682. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian H, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell stem cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara H, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell stem cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 25.Visnjic D, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 26.Li JY, et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood. 2012;120:4352–4362. doi: 10.1182/blood-2012-06-438531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lymperi S, et al. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 28.Ma YD, et al. Defects in osteoblast function but no changes in long-term repopulating potential of hematopoietic stem cells in a mouse chronic inflammatory arthritis model. Blood. 2009;114:4402–4410. doi: 10.1182/blood-2008-12-196311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding L, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116:1422–1432. doi: 10.1182/blood-2009-08-239194. [DOI] [PubMed] [Google Scholar]
- 33.Hosokawa K, et al. Cadherin-based adhesion is a potential target for niche manipulation to protect hematopoietic stem cells in adult bone marrow. Cell stem cell. 2010;6:194–198. doi: 10.1016/j.stem.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Hosokawa K, et al. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood. 2010;116:554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 35.Kiel MJ, et al. Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell stem cell. 2009;4:170–179. doi: 10.1016/j.stem.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromberg O, et al. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood. 2012;120:303–313. doi: 10.1182/blood-2011-09-377853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenbaum AM, et al. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood. 2012;120:295–302. doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokoyoda K, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Omatsu Y, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa S, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. The Journal of experimental medicine. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 43.Pinho S, et al. PDGFRalpha and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 45.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 46.Chute JP, et al. Molecular profile and partial functional analysis of novel endothelial cell-derived growth factors that regulate hematopoiesis. Stem Cells. 2006;24:1315–1327. doi: 10.1634/stemcells.2005-0029. [DOI] [PubMed] [Google Scholar]
- 47.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 49.Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 50.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 52.Mendez-Ferrer S, et al. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 53.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki S, et al. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- 55.Ara T, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 56.Bonig H, et al. PTX-sensitive signals in bone marrow homing of fetal and adult hematopoietic progenitor cells. Blood. 2004;104:2299–2306. doi: 10.1182/blood-2004-04-1605. [DOI] [PubMed] [Google Scholar]
- 57.Kawabata K, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci U S A. 1999;96:5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 59.Nie Y, et al. CXCR4 is required for the quiescence of primitive hematopoietic cells. The Journal of experimental medicine. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tzeng YS, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 61.Uy GL, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiozawa Y, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]