Abstract
Noonan syndrome (NS) is a genetic disorder caused by mutations altering proteins relevant to RAS/mitogen-activated protein kinase (MAPK) signal transduction. Cardiac involvement is common, most prevalently pulmonary valve stenosis and hypertrophic cardiomyopathy. Since abnormal MAPK signaling contributes to the aortopathy in Marfan syndrome and with rare reports of aortic aneurysm in NS, we undertook a retrospective study of ascending aortic anatomy in 37 individuals with NS and without confounding medical conditions. Age ranged from 0.6 to 32 years. Based on the most recent echocardiogram, the aortic annulus and root were dilated in the cohort (mean Z-scores of 1.14 and 0.98, respectively; p<0.005) but the sinotubular junction and ascending aorta were not (mean Z-scores of 0.05 and 0.19, respectively). The aortic root was aneurysmal (>2 Z-scores) in 8 subjects (21.6%). PTPN11 mutations were present in 14 subjects, whose aortic status was similar to the cohort overall. Comparison of age and Z-scores revealed a modest tendency for the aortic annulus and root to dilate over time. Among 13 subjects with multiple imaging studies over an average of 6.8 years, the average Z-score increased 0.78 and 0.39 for the aortic annulus and root, respectively. Multivariate analysis revealed that age accounted for 7.0% and 11.0% of the variance in the aortic annular and root diameters, respectively. In conclusion, we found that aortic annular dilation and aortic root aneurysm are prevalent in NS, often presenting during childhood and progressing over time. Further study is needed to identify potential risks associated with these abnormalities.
Keywords: Noonan syndrome, aortic aneurysm
Noonan syndrome (NS) is a genetically heterogeneous, pleiomorphic autosomal dominant disorder.1 NS with multiple lentigines (formerly LEOPARD syndrome), Costello syndrome and cardiofaciocutaneous syndrome, are closely related phenotypically.2 Gene mutations underlying NS and the related disorders, termed the RASopathies, alter several proteins involved in the RAS/mitogen-activated protein kinase (MAPK) signal transduction pathway. In NS, 80–90% of affected individuals exhibit cardiovascular involvement, comprising morphologic defects, particularly pulmonary valve stenosis,3 and early-onset hypertrophic cardiomyopathy.4,5 Marfan syndrome, an unrelated genetic disorder caused by FBN1 mutations, is associated with aortic aneurysm, a result of excess transforming growth factor β (TGFβ) signaling.6 In a mouse model of Marfan syndrome, the MAPK, Erk1/2, is hyperactivated in aortic aneurysm and inhibition of RAS pathway is therapeutic.7,8 Since the RASopathies are characterized by excessive signaling through ERK1/2, we questioned whether aortic aneurysm is an unrecognized part of the phenotype. A literature review revealed a few case reports of aortic aneurysm at the aortic root or ascending aorta in NS (Table 1).9–17 For the other, rarer RASopathies, one case of aortic root aneurysm in an adolescent with Costello syndrome was identified.18 The combination of clinically relevant aortic issues in several NS patients and evidence suggesting the involvement of MAPK pathways in the development of aortic aneurysms led us to investigate the prevalence of aortic dilation in NS patients using a retrospective study design.
Table 1.
Reported cases of aortic aneurysm in Noonan syndrome
| Age | Sex | Aortic Pathology | Treatment | Genotype | Reference |
|---|---|---|---|---|---|
| 8 | F | Root, annulus | Atenolol | PTPN11 R501K | Jeffries, 9 |
| Adult | M | Ascending, dissection | - | - | Kretschmar, 10 |
| 42 | M | Root | - | - | Lin, 11 |
| 18 | F | Ascending (aortitis) | Surgical replacement | None for PTPN11, KRAS, BRAF | Menon, 12 |
| 42 | M | Root | - | - | Morgan, 13 |
| 8 | M | Root | - | PTPN11 E76D | Power, 14 |
| 49 | M | Root | Surgical replacement | - | Purnell, 15 |
| Adult | M | Ascending, dissection | Surgical replacement | - | Shachter, 16 |
| 41 | M | Ascending; abdominal, dissection | Surgical replacement | - | Tousimis, 17 |
-, no information available
Methods
With IRB approval, medical records and echocardiographic data were reviewed retrospectively for all patients with NS at the Icahn School of Medicine at Mount Sinai. Cases were identified through searches of an echocardiographic database covering 1993-present and a clinical database for the Cardiovascular Genetics Program in which affected individuals are frequently assessed and followed. The inclusion criterion was any individual diagnosed with NS clinically, generally using established criteria,19 or through genetic testing. The exclusionary criteria were aortic valve disease, subaortic stenosis and prior surgery with aortic cannulation.
Echocardiographic data were extracted from reports, which routinely included Z-scores normalized for body surface area for the diameters of the aortic annulus, aortic root, sinotubular junction, and ascending aorta. The sites for those measurements were as previously described.20 Z-scores for those echocardiographic parameters from each subject’s most recent study were compared statistically using T-testing to the population norm. For subjects with 2 or more echocardiograms performed over time, multivariable regression models were constructed with aortic root or annulus diameter Z-score as the dependent variable and ln(age) and dummy variables for individuals as the independent variables using IBM SPSS Statistics 20 (Armonk, NY).
Results
We identified 37 individuals with NS who fit our inclusion and exclusion criteria. Among those, the underlying mutation was known in 16 cases, 14 altering PTPN11. The remaining 2 were mutations in SOS1 and SHOC2. The ages of the subjects ranged from 0.6 to 32 years (mean 10.7, median 7.0) and 57% were male.
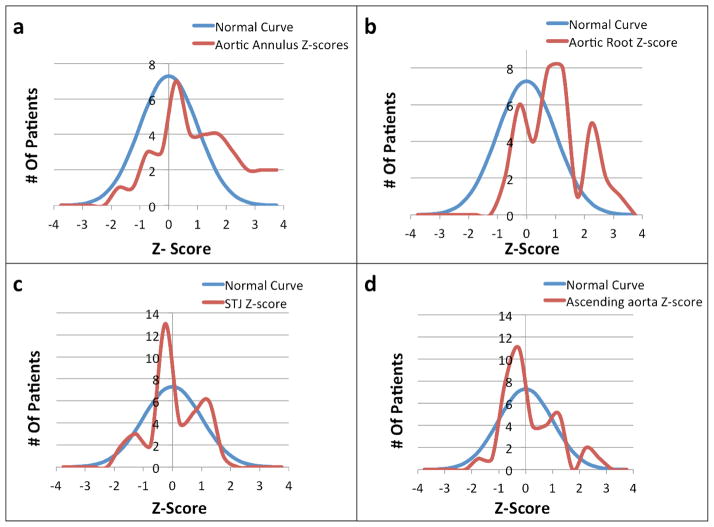
The mean Z-scores from the subjects’ most recent echocardiogram for the aortic annulus and root were 1.14 and 0.98, respectively, which were significantly greater than the population norm (p <0.005). The distribution of Z-scores for the aortic annulus and root (Fig. 1a and b) were similar to the expected normal distribution but shifted towards larger values. Of the 37 NS subjects, 10 (27.0%) had aortic annulus Z-scores >2, the threshold for declaring annular dilation. Similarly, 8 (21.6%) had aortic root Z-scores >2, qualifying as aortic root aneurysm. The numbers of subjects with Z-scores >2 was significantly more than expected (p <0.01 and <0.05, respectively).
Figure 1.
Comparisons of the distribution of aortic annulus (a), aortic root (b), sinotubular junction (c), and ascending aorta diameter (d) Z-scores to normally distributed values. For all graphs, Z-scores are indicated on the X-axis and the number of subjects on the Y-axis.
The mean Z-scores for the sinotubular junction and ascending aortic diameters were 0.05 and 0.19, respectively, which were not different from the general population. The distribution of Z-scores for these aortic anatomic sites was similar to the expected normal distribution (Fig. 1c and d). No individual had dilation of the sinotubular junction and two subjects (5.4%) had ascending aortic Z-scores >2, not significantly more than the expected 2.5%.
The 14 subjects with a PTPN11 mutation had average aortic annulus and aortic root diameter z-scores of 1.103 and 0.86, respectively, which were significantly greater than the population (p<0.05), but not different from the NS cohort overall. The mean Z-scores for the sinotubular junction and ascending aortic diameters were −0.07 and 0.20, respectively, which were not different from the general population or the NS cohort as a whole.
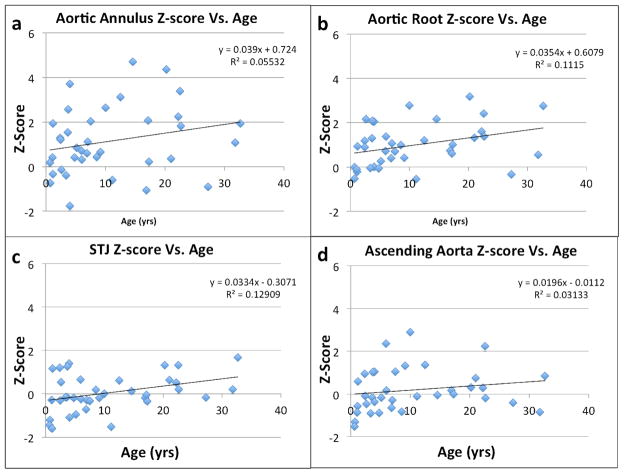
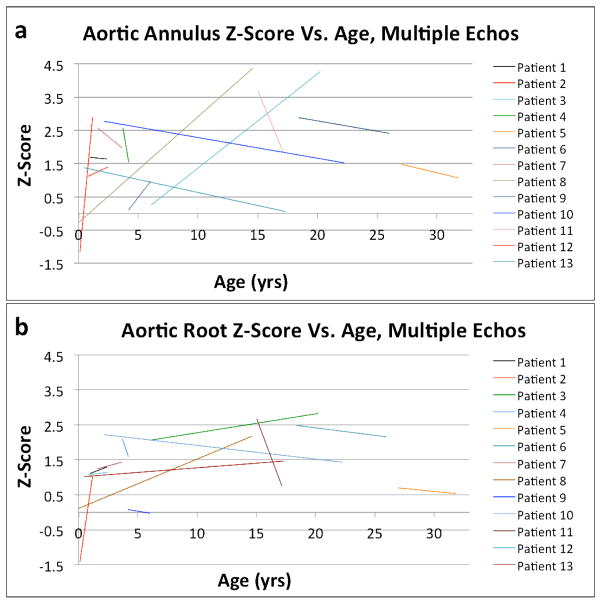
Next, we sought to determine if the aortic dilation was progressive in NS. To do that, we first looked at the effect of age on aortic annular and root sizes. As shown in Fig. 2a and b, there was a modest tendency for Z-scores to increase with age. Similar effects were seen for the sinotubular junction and ascending aortic Z-scores. More strikingly, we studied subjects who had undergone two or more echocardiograms (Fig. 3). Among 13 such individuals in our cohort, 5 (38.5%) had progressive aortic annular dilation and 7 (53.8%) had progressively dilating aortic roots over an average follow-up interval of 6.8 years. The average increase in Z-score for the aortic annulus and root were 0.78 and 0.39, respectively. Multivariable models of Z-scores in subjects who had undergone 2 or more echocardiograms revealed that ln(age) accounted for 11.0% of the variation in the aortic root Z-score (p=0.002) and for 7.0% of the variation in the aortic annulus Z-score (p=0.001).
Figure 2.
Plots of the aortic annulus (a), aortic root (b), sinotubular junction (c), and ascending aorta (d) diameter Z-scores (Y-axis) versus the subject’s age at the most recent echocardiogram (X-axis).
Figure 3.
Lines of best fit for Z-scores for the aortic annulus (a) and aortic root (b) vs. age to demonstrate the trajectory of aortic annular and root growth for subjects observed serially.
Discussion
Aortic aneurysm has been considered a rare complication of NS in adults,21 although it has been observed as early as at age 8.14 Our retrospective review of aortic imaging in individuals with NS suggests that dilated aortic annulus and root are prevalent in this condition, often presenting in childhood. The distribution of Z-scores for these 2 anatomic sites was notably different from the expected population norm, and more than one-quarter of the patients studied had frankly abnormal annular size, aortic root aneurysm or both. The observation that the diameters of the sinotubular junction and ascending aorta were normal in this cohort was important in two respects. First, this showed anatomic specificity to the dilation, as is generally observed in other conditions with aortopathy. Second, it provided an internal control, eliminating concern that abnormal growth associated with NS might somehow distort the calculation of body surface area needed to assess the aortic diameters. Our data, both cross-sectional and serial, suggest that the dilation of the aortic annulus and root can be progressive.
Given the size of our cohort and the length of follow up, we cannot adequately address the natural history of or risks associated with this aortic disease in NS. While we believe it would be prudent to continue surveillance for affected individuals with aortic root aneurysm, we cannot advocate medical therapy or know when to intervene surgically, beyond common-sense recommendations like doing so if significant aortic insufficiency develops.
A subset of our NS cohort harbored PTPN11 mutations, which is the most common gene altered for this trait.1 Based on that small group, the aortopathy associated with NS-causing PTPN11 mutations seemed typical. Knowing that important genotype-phenotype associations exist for NS, including ones relevant to the cardiac involvement,1 future work is needed to determine whether individuals with NS due to mutations in other prevalent genes like SOS1, RAF1 and RIT1 are at similar risk for aortic disease.
This study focused only on proximal aortic involvement in NS. A review of the literature, however, revealed case reports of patients with NS who presented with aneurysms of coronary arteries,22 intracranial arteries (including one presenting with hemorrhagic stroke),23–25 the main pulmonary artery26 and the descending aorta.27 These findings suggest that the vascular involvement in NS can be broad. More information is needed to determine if screening for aneurysm such as with magnetic resonance angiography is appropriate for individuals with NS in general, those with particular mutations, or those with aortic root aneurysm.
Finally, the shared biology among the RASopathies raises the question of whether individuals with the NS-related disorders are also at risk for vascular involvement. A review of the literature identified one report of aortic root aneurysm in an individual with Costello syndrome18 but none with cardiofaciocutaneous syndrome. Coronary aneurysms and polyaneurysms have been reported in individuals with Noonan syndrome with multiple lentigines.28 Of note, these three disorders are much rarer than NS so the paucity of prior reports of thoracic aortic aneurysm cannot be viewed as definitive. On the other hand, the types and extent of organ involvement vary among the RASopathies, believed to result from the specific effects on RAS-MAPK signaling or signaling through other pathways engendered by the molecular lesions underlying them. Thus, we cannot assume that the aortic disease in NS will inevitably be found in patients with other RASopathies.
Acknowledgments
This work was supported in part by a grant to B.D.G. from the National Heart, Lung, and Blood Institute (HL071207).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab. 2011;25:161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart diseases in children with Noonan syndrome: An expanded cardiac spectrum with high prevalence of atrioventricular canal. J Pediatr. 1999;135:703–706. doi: 10.1016/s0022-3476(99)70088-0. [DOI] [PubMed] [Google Scholar]
- 4.Hickey EJ, Mehta R, Elmi M, Asoh K, McCrindle BW, Williams WG, Manlhiot C, Benson L. Survival implications: hypertrophic cardiomyopathy in Noonan syndrome. Congenit Heart Dis. 2011;6:41–47. doi: 10.1111/j.1747-0803.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson JD, Lowe AM, Salbert BA, Sleeper LA, Colan SD, Cox GF, Towbin JA, Connuck DM, Messere JE, Lipshultz SE. Outcomes in children with Noonan syndrome and hypertrophic cardiomyopathy: A study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2012;164:442–448. doi: 10.1016/j.ahj.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferies JL, Belmont JW, Pignatelli R, Towbin JA, Craigen WJ. PTPN11 mutation associated with aortic dilation and hypertrophic cardiomyopathy in a pediatric patient with Noonan syndrome. Pediatr Cardiol. 2010;31:114–116. doi: 10.1007/s00246-009-9537-8. [DOI] [PubMed] [Google Scholar]
- 10.Kretschmar K, Witkowski R. Dissecting aortic aneurysm in a man with symptoms of Turner’s syndrome. Z Gesamte Inn Med. 1982;37:278–281. [PubMed] [Google Scholar]
- 11.Lin AE, Garver KL, Allanson J. Aortic-root dilatation in Noonan’s syndrome. N Engl J Med. 1987;317:1668–1669. doi: 10.1056/NEJM198712243172616. [DOI] [PubMed] [Google Scholar]
- 12.Menon S, Pierpont ME, Driscoll D. Giant cell aortitis and Noonan syndrome. Congenit Heart Dis. 2008;3:291–294. doi: 10.1111/j.1747-0803.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JM, Coupe MO, Honey M, Miller GA. Aneurysms of the sinuses of Valsalva in Noonan’s syndrome. Eur Heart J. 1989;10:190–193. doi: 10.1093/oxfordjournals.eurheartj.a059462. [DOI] [PubMed] [Google Scholar]
- 14.Power PD, Lewin MB, Hannibal MC, Glass IA. Aortic root dilatation is a rare complication of Noonan syndrome. Pediatr Cardiol. 2006;27:478–480. doi: 10.1007/s00246-006-1210-x. [DOI] [PubMed] [Google Scholar]
- 15.Purnell R, Williams I, Von Oppell U, Wood A. Giant aneurysms of the sinuses of Valsalva and aortic regurgitation in a patient with Noonan’s syndrome. Eur J Cardiothorac Surg. 2005;28:346–348. doi: 10.1016/j.ejcts.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Shachter N, Perloff JK, Mulder DG. Aortic dissection in Noonan’s syndrome (46 XY turner) Am J Cardiol. 1984;54:464–465. doi: 10.1016/0002-9149(84)90228-5. [DOI] [PubMed] [Google Scholar]
- 17.Tousimis E, Deshmukh N. Noonan’s syndrome associated with spontaneous rupture of the ascending aorta: A case report. Vasc Endovasc Surg. 2000;34:623–627. [Google Scholar]
- 18.Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, Dobyns WB, Hudson C, Johnson J, Tenconi R, Graham GE, Sousa AB, Heller R, Piccione M, Corsello G, Herman GE, Tartaglia M, Lin AE. Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet A. 2011;155A:706–716. doi: 10.1002/ajmg.a.33884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet. 1994;53:187–191. doi: 10.1002/ajmg.1320530213. [DOI] [PubMed] [Google Scholar]
- 20.Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 21.Lin AE, Basson CT, Goldmuntz E, Magoulas PL, McDermott DA, McDonald-McGinn DM, McPherson E, Morris CA, Noonan J, Nowak C, Pierpont ME, Pyeritz RE, Rope AF, Zackai E, Pober BR. Adults with genetic syndromes and cardiovascular abnormalities: clinical history and management. Genet Med. 2008;10:469–494. doi: 10.1097/GIM.0b013e3181772111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loukas M, Dabrowski M, Kantoch M, Ruzyllo W, Waltenberger J, Giannikopoulos P. A case report of Noonan’s syndrome with pulmonary valvar stenosis and coronary aneurysms. Med Sci Monit. 2004;10:CS80–83. [PubMed] [Google Scholar]
- 23.Dineen RA, Lenthall RK. Aneurysmal sub-arachnoid haemorrhage in patients with Noonan syndrome: a report of two cases and review of neurovascular presentations in this syndrome. Neuroradiology. 2004;46:301–305. doi: 10.1007/s00234-004-1185-3. [DOI] [PubMed] [Google Scholar]
- 24.Hara T, Sasaki T, Miyauchi H, Takakura K. Noonan phenotype associated with intracerebral hemorrhage and cerebral vascular anomalies: case report. Surg Neurol. 1993;39:31–36. doi: 10.1016/0090-3019(93)90106-b. [DOI] [PubMed] [Google Scholar]
- 25.McAnena O, Padilla JR, Buckley TF. Intracranial aneurysm in association with Noonan’s syndrome. Ir Med J. 1984;77:140–141. [PubMed] [Google Scholar]
- 26.Brown JR, Plotnick G. Pulmonary artery aneurysm as a cause for chest pain in a patient with Noonan’s syndrome: a case report. Cardiology. 2008;110:249–251. doi: 10.1159/000112408. [DOI] [PubMed] [Google Scholar]
- 27.Pac M, Kibar AE, Oflaz MB, Pac FA. Two cases of Noonan syndrome: aortic coarctation causing a giant aneurysm of the descending aorta. Turk Kardiyol Dern Ars. 2011;39:629. doi: 10.5543/tkda.2011.01603. [DOI] [PubMed] [Google Scholar]
- 28.Yagubyan M, Panneton JM, Lindor NM, Conti E, Sarkozy A, Pizzuti A. LEOPARD syndrome: a new polyaneurysm association and an update on the molecular genetics of the disease. J Vasc Surg. 2004;39:897–900. doi: 10.1016/j.jvs.2003.11.030. [DOI] [PubMed] [Google Scholar]