Abstract
Immune Reconstitution Inflammatory Syndrome (IRIS) is a major adverse event of antiretroviral therapy (ART) in HIV infection, and paradoxically occurs as HIV viremia is suppressed and CD4 T cell numbers recover. IRIS reflects pathogenic immune responses against opportunistic infections acquired during the period of immunodeficiency, but little is understood about the mechanisms of inflammatory pathology. Here we show that IL-6 and CRP levels transiently rise at the time of the IRIS event in HIV-infected patients umasking Mycobacterium avium complex (MAC) infection after starting ART. To directly test the role of IL-6 in IRIS pathology, we employed a model of experimentally inducible IRIS in which M. avium infected T cell deficient mice undergo a fatal inflammatory disease after reconstitution with CD4 T cells. We find that IL-6 neutralization reduces CRP levels, alleviates wasting disease and extends host survival during experimental IRIS. Moreover, we show that combined blockade of IL-6 and IFNγ further reduces IRIS pathology, even after the onset of wasting disease. The combination of these clinical and experimental-model data show that the IL-6 pathway is not only a biomarker of mycobacterial IRIS but also a major mediator of pathology distinct from IFNγ, and may be a useful target for therapeutic intervention.
Introduction
HIV infection results in defects in CD4 T cell numbers and function leading to susceptibility to microbial infections, and control of viral replication with ART allows CD4 T cell responses to recover and restores normal host resistance. In most patients, this process leads to an improvement of clinical symptoms. However, some individuals, who harbor a microbial co-infection and have extremely low numbers of CD4 T cells at the time of ART initiation(1), experience a rapid deterioration within the first few weeks of treatment. This worsening of disease in ART treated patients is referred to as Immune Reconstitution Inflammatory Syndrome (IRIS) and occurs despite successful control of HIV viremia and recovery of circulating CD4 T cell numbers. Indeed, IRIS is thought to be mediated by dysregulated immune responses mounted against an underlying opportunistic infection OI. Many different pathogens have been associated with IRIS, but mycobacterial infections such as Mycobacterium tuberculosis and Mycobacterium avium complex (MAC) are frequent culprits. While the specific symptoms of IRIS depend on the particular microbial co-infection and the affected tissue, the manifestation of IRIS can be broadly categorized as either a paradoxical or unmasking presentation. Paradoxical IRIS occurs in patients already receiving treatment for an OI who then develop an exacerbation of inflammation associated with that co-infection when ART is initiated. Unmasking IRIS describes the appearance of pathology associated with an infection that only becomes recognized following ART due to the IRIS event itself.
The mechanisms of IRIS are poorly understood, and no targeted therapies exist for the treatment of IRIS. Although it may seem counterintuitive to broadly immunosuppress an HIV patient with a disease causing microbial co-infection, corticosteroid therapy is currently the best approach for reducing inflammation and pathology during IRIS(2-6). Therefore, a better understanding of the immunopathology of IRIS is needed for the development of targeted therapies. Many clinical studies have examined inflammatory cytokine profiles that are associated with IRIS. IFNγ levels(5-8) and IFNγ secreting T cells(9-13)have been found to be increased in individuals who develop IRIS following ART, suggesting that this cytokine may play an important role in IRIS pathology. There have also been indications that the IL-6 pathway may be engaged during IRIS. C-reactive protein, an acute phase reactant know to be down stream of IL-6 signaling, is one of the most often observed biomarkers of IRIS. While a few reports have failed to find an association between IL-6 and IRIS susceptibility(5, 14, 15), other studies covering diverse manifestations of IRIS have found associations between the risk of IRIS and increased levels of IL-6 preceding ART(16), at the time of the IRIS event(6, 16-19), and sometimes even for years following recovery from IRIS(16, 20, 21). It has also been shown that effective corticosteroid treatment of IRIS decreases IL-6 concentrations in the serum(4). Moreover, a single nucleotide polymorphism in the IL6 locus has been shown to associate with the susceptibility to MAC-IRIS(22). Thus, a large amount of clinical evidence acquired over the past decade has indicated that increased IL-6 production correlates with IRIS, but its role in directly driving the pathology of IRIS has not been addressed.
We have previously described a model of experimentally-inducible M. avium IRIS in mice(23, 24). In the system employed, T cell deficient mice harboring a disseminated M. avium infection are injected with CD4 T cells to mimic the ART induced recovery of T helper cells in a mycobacterial co-infected, T cell deficient AIDS patient. In this robust model of severe IRIS, adoptive transfer of CD4 T cells into chronically infected TCRαKO mice leads to a rapid, IFNγ-dependent wasting disease, and most of the mice succumb two to four weeks following T cell transfer. Here we perform both patient and animal model studies to examine the role of IL-6 in M. avium associated IRIS. We show that IL-6 levels rise sharply in HIV/MAC co-infected patients at the time of an unmasking IRIS event and use the experimental animal model to demonstrate that IL-6 blockade greatly reduces IRIS disease. Moreover, co-blockade of IFNγ and IL-6 in the experimental model further reduced IRIS associated disease, and was effective even when therapy was initiated after the onset of wasting. These data strongly support the hypothesis that IL-6 is a major mediator of disease during IRIS in M. avium co-infected HIV+ individuals.
Materials and Methods
Human subjects and sample collection
Plasma samples were obtained from seven HIV+ patients (5 male/ 2 female; median age of 45, range 27-50) with a median CD4 T-cell count of 24 (range 1-86) cells/μL recruited as part of a cohort study at the Clinical Center of the National Institutes of Health (NIH) and who developed unmasking Mycobacterium avium complex (MAC) IRIS after ART initiation. All participants provided written informed consent at the Clinical Center of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under an institutional review board-approved protocol (NCT NCT00286767). All patients were ART-naive or had interrupted ART for over 6 months after receiving less than 3 months of therapy (N=1) and had less than or equal to 100 CD4+ T cells/μl at baseline. IRIS was defined according to the AIDS Clinical Trials Group criteria. The median time to unmasking MAC IRIS was 19 days (range, 7-99 days).
Mice, bone marrow chimeras and M. avium infections
C57BL/6 and athymic nude mice were obtained from Taconic Farms, and IL-6-/- mice were purchased from The Jackson Laboratory. Ag85b-specific TCR Tg P25 mice(25) were bred in our animal facility. To generate bone marrow chimeric mice, animals were lethally irradiated with 950 rad and reconstituted with 8-10×106 BM cells isolated from WT or IL-6-/- mice the same day after irradiation. Thy1.2 depleting Ab (500 μg) was added to the bone marrow cell inoculum to ensure depletion of contaminating mature CD4 T cells. Mice were allowed to reconstitute for 10-12 weeks before they were exposed to M. avium. For bacterial infections, the mice were intravenously inoculated in the lateral tail-vein with 1×106 CFU of Mycobacterium avium SmT 2151. All Kaplan-Meier graphs of mouse survival represent mice that were found dead or reached moribundity and were euthanized. All animals were bred and maintained in an AAALAC-approved facility at the National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, MD) according to the Guide for Care and Use of Laboratory Animals, and all procedures were conducted according to an animal study proposal that was approved by the NIAID Animal Care and Use Committee.
CD4 T cell adoptive transfer and in vivo antibody treatments
Spleen and lymph node CD4 T cells were isolated from naïve WT, IL-6-/- or P25 TCR Tg mice as indicated using MACS magnetic beads according to the manufacturer’s instructions (Miltenyi Biotec). Cells were routinely >90% pure CD4+ cells after column separation. For adoptive transfer cells were suspended in saline and 2×106 T cells were injected intravenously in a volume of 300μl. Control Ab (clone HRPN), anti-IL-6 (clone MP5-20F3), anti-IL-6R (clone 15A7), anti-IFNγ (clone XMG1.2), and anti-Thy1.2 (clone 30H12) monoclonal antibodies were purchased from BioXCell. For in vivo blockade experiments 200-500 μg of Ab was administered every third day for the indicated amount of time. No difference was observed between mice given PBS or control Ab.
Flow Cytometry
CD4 T cell frequencies in blood were determined by flow cytometry. Blood was collected in EDTA tubes and RBC’s were lysed with ACK. In different experiments, cells were stained with various combinations of CD4, TCRβ, CD3ε, Vβ11, and fixable viability dye eFlour780. All antibodies and live/dead dyes were purchased from eBioscience and Biolegend. All samples were acquired on a BD LSR Fortessa and analyzed with Flowjo version 9.5.
Human and mouse cytokine measurements
Cytokine measurements were performed on cryopreserved human plasma samples or mouse serum samples stored at -80°C. Mouse CRP, mouse IL-6, mouse TNFα and human IL-6 was measured in with ELISA kits purchased from R&D systems. Human CRP and mouse IFNγ was measured with a high sensitivity ELISA purchased from eBioscience.
Weight loss and core body temperature measurements
Weight loss was represented as a percentage of initial weight before T cell transfer as follows: % weight change = ((current weight/pre-transfer weight) × 100) -100. Core body temperature was measure by rectal thermometry on non-anesthetized mice with a murine probe thinly coated with a petroleum-based lubricant.
Statistical analysis
The statistical differences between groups were analyzed by Student t test, and the difference between survival curves was calculated with a Log-rank Mantel-Cox test in GraphPad Prism software version 6. Groups were considered significantly different when P < 0.05 or less.
Results
IL-6 and CRP increase during unmasking IRIS in MAC/HIV co-infected individuals
Increases in the plasma concentrations of IL-6 and CRP after ART are frequently found to distinguish HIV patients who develop IRIS from those who do not. To characterize the dynamics of IL-6 and CRP expression during IRIS, we performed a detailed kinetic analysis of these proteins in a cohort of seven HIV infected individuals who developed unmasking MAC-IRIS. The patients developed IRIS at time points ranging from 2 to 10 weeks following ART. In patients #1, #2, #3, #5, and #6, CRP was at or close to normal concentrations (< 3mg/L) before ART and spiked at the time of the IRIS event (Figure 1a). This peak in CRP was then followed by a rapid decline afterwards. In patient #4, the plasma CRP concentration was high even before ART, but increased further at the time of the IRIS episode.
Figure 1. CRP and IL-6 levels are elevated during unmasking MAC-IRIS in HIV infected patients.
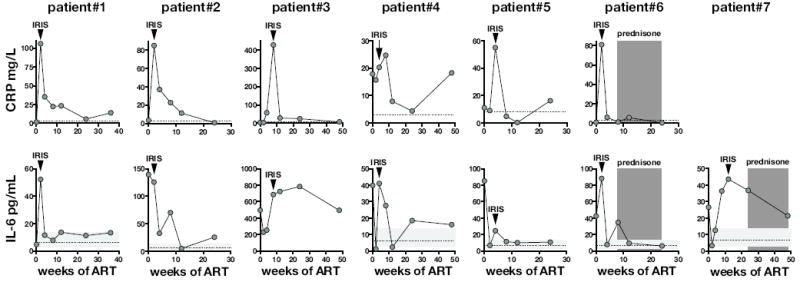
CRP (A) and IL-6 (B) concentrations in plasma were measured before and at several time points following ART. Arrow heads represent the time points when the IRIS event occurred. The dotted line in (A) represents a normal CRP value for reference. The horizontal light grey shaded region and dotted line in (B) represents the range and the geometric mean of IL-6 values in healthy donors. The dark grey bars in Patient 6 and 7 represents the time points were prednisone was administered to control inflammatory disease.
We next measured IL-6 in these patient samples. In patient #1, the concentration of plasma IL-6 was within the range found in healthy donors but then rapidly increased at the time of the IRIS event at week two and then dropped back to normal by week four (Figure 1b). Moreover, in patients #3, #4, #5, #6, and #7 the IL-6 concentrations increased between the time point immediately preceding and the IRIS event. Interestingly, in patients #3, #4, #5, and #7 the concentration of IL-6 initially dropped in the first weeks following ART, but then rose sharply during IRIS. In patient #2, the concentration of IL-6 was high before ART and remained elevated until after the patient developed IRIS at week 2. These data indicate that CRP and IL-6 increase in HIV infected individuals at the time of unmasking MAC-IRIS.
IL-6 increases and drives CRP expression during experimentally induced M. avium-IRIS
To model IRIS in mice we developed a system that recapitulates the immunological scenario underlying IRIS in HIV infected individuals: T cell reconstitution of a lymphopenic host harboring a microbial infection. TCRαKO mice are infected with M. avium, and after at least 60 days of infection the mice are injected with purified CD4 T cells (Figure 2a). Although CD4 T cells are normally critical for control of mycobacterial infections, in this setting of immune reconstitution CD4 T cell transfer triggers a rapid wasting disease that results in the death of most of the animals(24 and Figure 3). We found that CD4 T cell transfer into M. avium infected TCRαKO mice induces increases in the serum concentration of IL-6 (Figure 2b). IFNγ production by the CD4 T cells was not required for IL-6 induction since transfer of IFNγ KO CD4 T cells induced similar amounts of IL-6 compared to WT T cells (Figure 2b). Serum CRP was elevated in M. avium infected TCRαKO mice compared to naïve animals, and during IRIS following adoptive transfer of CD4 T cells CRP increased further (Figure 2c). CRP expression can be induced by IL-6, and CRP and IL-6 concentrations positively correlated in the serum (Figure 2d). Therefore, we next examined the role of IL-6 in inducing CRP expression during IRIS. IL-6 neutralization with mAbs decreased serum IL-6 back down to the concentrations seen in uninfected mice (Figure 2c). IFNγ deficient CD4 T cells also failed to drive increases in CRP above what is found in TCRαKO mice that did not receive T cells (Figure 2c). Collectively, these data indicate that IL-6 and CRP increase during experimentally induced M. avium-IRIS. Furthermore, these commonly observed biomarkers of IRIS are directly linked as elevated CRP expression requires IL-6 and IFNγ.
Figure 2. IL-6 increases during experimentally-induced IRIS and drives CRP expression.
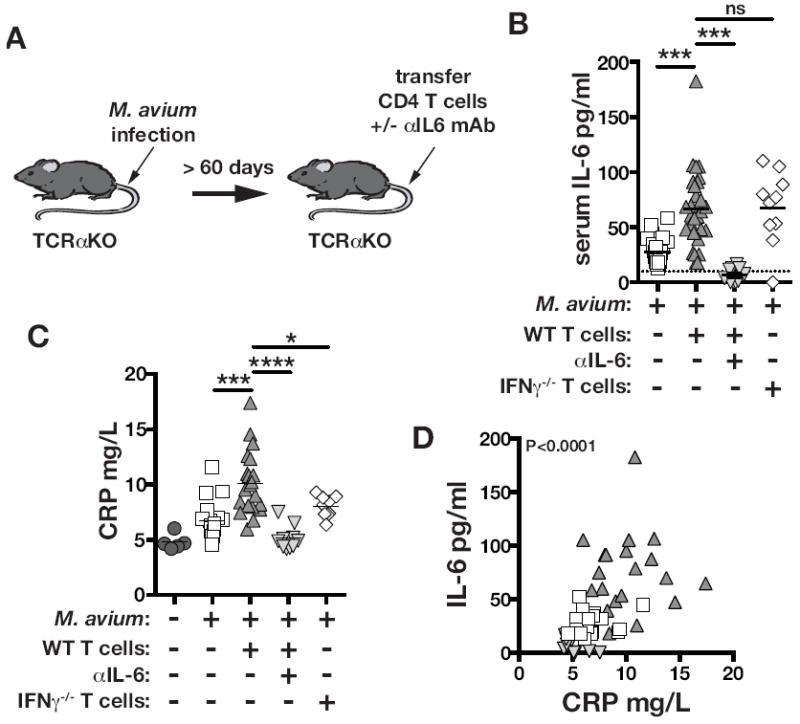
(A) TCRαKO mice were intravenously injected with 1×106 CFU of M. avium. After at least 2 months post-infection, 2×106 CD4 T cells isolated WT or IFNγ-/- mice were adoptively transferred intravenously, and some mice were then either untreated or administered 200μg of IL-6 neutralizing mAb every third day intraperitoneally as indicated. The concentrations of CRP (B) and IL-6 (C) in serum were measured by ELISA. (D) Correlation between IL-6 and CRP from same measurements, r=0.54. There was no significant correlation between CRP and IFNγ. Data in (B-D) are pooled from 2 independent experiments from day 11-12 post- CD4 T cell transfer.
Figure 3. IL-6 drives experimentally induced M. avium-IRIS.
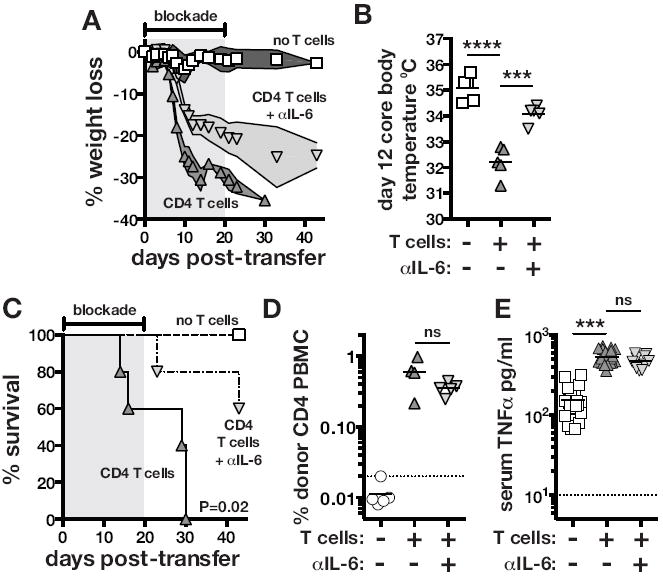
TCRαKO mice were intravenously injected with 1×106 CFU of M. avium. After at least 2 months post-infection, 2×106 WT CD4 T cells were adoptively transferred intravenously, and where indicated mice were then either untreated or administered IL-6 neutralizing mAb. (A) Weight loss was normalized to the day of T cell transfer. The shaded region indicates the duration of IL-6 blockade. (B) Core body temperature was measured on day 12 after T cell transfer. Uninfected TCRαKO mice displayed a body temp of 37-37.5°C. (C) Mortality was monitored following CD4 T cell reconstitution. The shaded region indicates the duration of IL-6 blockade. Data are representative of six independent experiments. (D) The frequency of donor CD4 T cells in the blood was measured on day 10 post-transfer. The data are representative of two independent experiments. (E) TNF serum concentrations were measured on day 11-12 post-transfer of CD4 T cells. The data are pooled from 4 independent experiments.
IL-6 blockade prevents wasting disease and prolongs survival of experimentally-induced M. avium-IRIS
Given the association of IL-6 with disease during M. avium-IRIS in both humans and mice, we sought to directly evaluate the role of IL-6 in the pathogenesis of IRIS. M. avium infected TCRαKO mice were reconstituted with purified CD4 T cells and treated with αIL-6 neutralizing mAb starting on the day of T cell transfer. Mice that received CD4 T cells alone developed a severe wasting disease starting around day 7 post-transfer and lost greater than 30% of their body weight by day 12 (Figure 3a). In contrast, mice that received CD4 T cells and αIL-6 first began to show signs of weight loss around day 10 post-transfer, and by day 20 when blockade was stopped the mice had lost ~20% of their body weight (Figure 3a). CD4 T cell transfer also induced a dramatic drop in core body temperature. TCRαKO mice harboring a chronic M. avium infection were ~35° C and on day 12 post-transfer of CD4 T cells, untreated control mice had dropped to ~32° C while mice receiving CD4 T cells and αIL-6 had dropped to ~34° C (Figure 3b). Moreover, IL-6 blockade significantly extended the survival of mice following CD4 T cell transfer (Figure 3c). The protective effect of IL-6 blockade was not due to differences in the degree of the expansion of the donor CD4 T cells as untreated and αIL-6 treated mice had similar percentages of CD4 T cells in their PBMC (Figure 3d). TNFα can sometimes also be a major mediator of wasting disease, and we have previously shown that it partially contributes to pathology in our model of murine IRIS(23). IL-6 blockade also had no effect the induction of TNF following CD4 T cell reconstitution (Figure 3e), and conversely TNF blockade had no effect on IL-6 induction (data not shown). Taken together, these data indicate that IL-6 production not only correlates with IRIS in humans and mice, but also directly contributes to the pathology seen in M. avium-infected T cell deficient mice following CD4 T cell reconstitution. Interestingly, the protective effect of IL-6 blockade in experimentally induced IRIS is even greater than what we previously reported with TNF neutralization.
Radio-resistant cells are the major source of pathogenic IL-6
Cells of both hematopoietic and non-hematopoietic origin can produce IL-6, so we next examined the cell types involved in mediating IL-6 dependent disease in experimentally induced IRIS. To test the role of CD4 T cell derived IL-6 in IRIS pathogenesis, we transferred either WT or IL-6 KO CD4 T cells into M. avium infected TCRαKO mice. WT and IL-6 KO CD4 T cells induced identical courses of wasting diseases, indicating that CD4 T cells are not a significant source of IL-6 in murine IRIS (Figure 4a).
Figure 4. Radio-resistant cells mediate the IL-6 driven pathology of IRIS after reconstitution of M. avium infected T cell deficient mice.
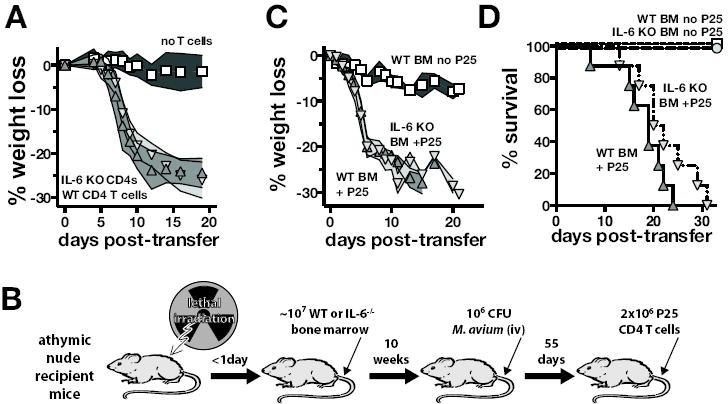
(A) WT or IL-6-/- CD4 T cells were adoptively transferred into TCRα-/- mice harboring a chronic M. avium infection as described in Figure 2a, and weight loss was measured. (B) To study IRIS in M. avium infected T cell deficient mice selectively lacking IL-6 from hematopoietic cells, athymic nude mice were lethally irradiated and constituted with bone marrow from WT or IL-6-/- mice. After at least 10 weeks of reconstitution, the mice were infected with M. avium and the infection was allowed to progress for at least an additional 55 days. To induce IRIS, the mice were then injected with Ag85b-specific TCR Tg CD4 T cells (P25 cells). (C) Weight loss was monitored after T cell injection. Data are representative of 2 independent experiments. (D) Survival after T cell injection into the nude chimeric animals was followed. The data are pooled from two independent experiments that gave similar results. The survival curve comparison between the WT and IL-6-/- BM recipients was not statistically significant (P=0.1). In panels (C – D) triangles pointing up represent WT BM recipients and triangles pointing down represent IL-6 KO BM recipients.
We next tested the role of hematopoietic cell derived IL-6 in IRIS. Athymic nude mice were lethally irradiated and then reconstituted with BM from either WT or IL-6 KO mice (Figure 4a). Thy1.2 depleting mAb was added to the bone marrow cell inoculum to ensure that any mature CD4 T cells contaminating the bone marrow donor cells were removed. Ten weeks after BM reconstitution the mice were injected with M. avium and the infection was allowed to progress for an additional 2 months. Although the injected BM was inherently capable of producing T cells, the use of athymic nude mice recipients prevented the development of T cells allowing us to later study the impact of CD4 T cell reconstitution. This approach yielded T cell deficient mice that harbor a chronic M. avium infection and selectively lack IL-6 in bone marrow derived cells. Lastly, TCR Tg CD4 T cells specific for Ag85b (P25 cells) were adoptively transferred to induce IRIS. Interestingly, CD4 T cell reconstitution induced identical wasting disease in both WT and IL-6 KO bone marrow recipient nude mice (Figure 4c). Although there was a slight trend towards a two to three day delay in mortality of the IL-6 KO BM recipients, the difference in the survival of WT or IL-6 KO BM recipients during IRIS did not reach statistical significance, and all mice eventually succumbed by day 31 post-transfer (Figure 4d). Moreover, serum IL-6 concentrations were similar between WT and IL-6 KO BM recipient mice undergoing IRIS (data not shown). These data indicate that radio-resistant cells are the predominant source of IL-6 that drives wasting disease and death during experimentally induced IRIS in M. avium infected T cell deficient mice.
Prophylactic and therapeutic coblockade of IL-6 and IFNγ greatly alleviates IRIS pathology
We previously showed that IFNγ produced by the reconstituting CD4 T cells is a major mediator of disease in the murine model of M. avium-IRIS(23), so we next asked if IL-6 blockade affects the production of IFNγ in this system. M. avium infected TCRαKO mice were injected with CD4 T cells and treated with αIL-6 blocking mAb, and IFNγ was measured in the serum. CD4 T cell reconstitution induced elevations in serum concentrations of IFNγ equally in mice treated or not with IL-6 blockade (Figure 5a). Thus, IL-6 and IFNγ both contribute to IRIS pathology but do not affect the expression of each other in this system.
Figure 5. Combined IL-6 and IFNγ blockade further alleviates IRIS wasting and prolongs survival.
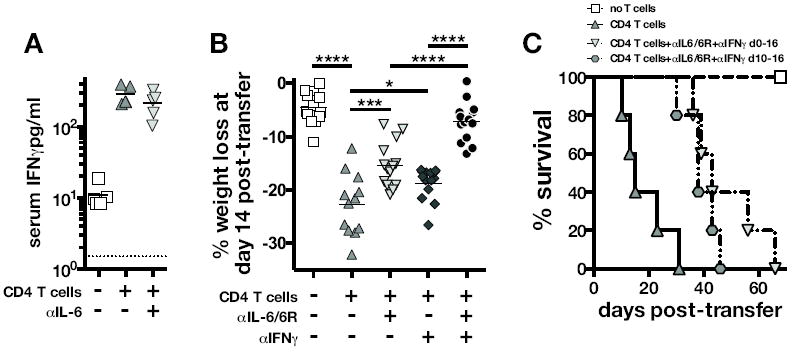
(A) Concentration of IFNγ in serum 10 days after injection of WT CD4 T cells into TCRα-/- mice harboring a chronic M. avium infection. Where indicated the mice were treated with IL-6 blocking mAb starting on day 0 of T cell transfer. The dotted line indicates the level of detection. Data are representative of 2 independent experiments. (B) WT CD4 T cells were injected into M. avium chronically infected TCRα-/- mice as before and treated with the indicated cytokine blocking antibodies. Weight change on day 14 after T cell transfer was measured. Data are pooled from 3 independent experiments. (C) After transferring CD4 T cells to induce IRIS as done in (A-B), mice were treated with a mixture of αIL-6+αIL-6R+αIFNγ mAbs either from day 0 to 16 or from 10-16 post-transfer of T cells and survival was monitored. Data are representative of two independent experiments. Treatment at day 0 and day 10 both significantly extended survival: P=0.001 for day 0 co-blockade group, and P=0.006 for day 10 blockade group compared to isotype control treated CD4 T cell recipients.
These observations indicated that IL-6 and IFNγ are independent mediators of wasting and mortality during M. avium-IRIS, so we next tested the therapeutic efficacy of combined IL-6 and IFNγ blockade. M. avium infected TCRαKO mice were injected with CD4 T cells and treated with αIL-6/αIL-6R, αIFNγ, or a mixture of αIL-6/αIL-6R and αIFNγ starting on the day of T cell reconstitution, and weight loss was measured on day 14 post-transfer. As expected, individual blockade of each pathway with αIL-6/αIL-6R or αIFNγ alleviated the extent of wasting (Figure 5b), but IL-6 blockade was slightly more effective than IFNγ blockade. When αIL-6/αIL-6R and αIFNγ were administered simultaneously at the beginning of T cell transfer only a slight decrease in weight loss was detected on day 14 post-transfer (Figure 5b) compared to mice that did not receive T cells, indicating that IL-6 and IFNγ represent two major mediators of IRIS pathology. Moreover, co-blockade of IL-6 and IFNγ had no effect on serum concentrations of TNF indicating that the protective effects of this treatment is not likely to be due to suppression of TNF (data not shown).
Finally, we tested the ability of combined IL-6 and IFNγ blockade to treat IRIS after the onset of wasting disease. To do so, IRIS was induced as before and T cell recipients were treated with a cocktail of αIL-6+αIL-6R+αIFNγ mAbs from day 0 to 16 or from day 10 to 16. Survival after withdrawal of the blocking Abs was monitored. Untreated CD4 T cell recipients showed a median survival time (MST) of 15 days, while combined cytokine blockade administered day 0-16 of T cell transfer extended the MST to 43 days (Figure 5c). Importantly, mice that began cytokine blockade on day 10 had already undergone ~20% weight loss (data not shown), but still showed an increased MST of 38 days (Figure 5c). These data indicate that even after the onset of a rapidly progressing wasting disease, blockade of these two effector pathways can dramatically extend survival of this severe M. avium associated IRIS.
Discussion
Many studies of HIV patients have sought to find correlations between inflammatory mediators and susceptibility to IRIS. Although several pathways have been associated with mycobacterial IRIS, the lack of a pre-clinical model has made it difficult to ascribe a role of these molecules to the immunopathology of IRIS. This is the first study to combine human data and a pre-clinical model to evaluate the potential therapeutic efficacy of targeting an inflammatory mediator for the treatment of IRIS. We show that elevations in serum IL-6 concentrations are associated with disease onset in HIV/MAC co-infected individuals who develop unmasking IRIS following ART and use the mouse model of experimentally induced M. avium-IRIS to directly test the role of IL-6 in driving IRIS pathology. In fact, the system of experimentally induced IRIS employed here can even be considered a form of unmasking IRIS similar to the patients described, as the mice are not treated with antimycobacterial drugs and display no overt symptoms resulting from the M. avium infection until after CD4 T cell reconstitution. We find that IL-6 not only correlates with disease but also is a major mediator of IRIS pathology in M. avium infected T cell deficient mice. Moreover, we find that IL-6 also likely contributes to the increased levels of the acute phase reactant CRP, which are so often observed in IRIS patients, indicating that these two biomarkers of IRIS are likely indicative of the same pathway. Although the studies performed here were limited to M. avium associated IRIS, the observation of increased IL-6 and CRP in IRIS associated with M. tuberculosis and C. neoformans infections suggests that the mechanisms delineated here may be of general relevance to multiple manifestations of the syndrome.
Numerous studies have examined many different inflammatory pathways in HIV/TB co-infected individuals during ART, and we have experimentally addressed the role of several key cytokines that have been associated with IRIS susceptibility. We showed that IFNγ and to a lesser TNF drive IRIS pathology in M. avium infected mice, while IL-4 and IL-17 have no role(23). Moreover, we have found that IL-7R, IL-1R1, GM-CSF, M-CSF, G-CSF blockade has no therapeutic effect in this murine model of IRIS, indicating that these pathways may play little role in the induction of mycobacterial immune reconstitution disease (data not shown). Therefore, while cytokine biomarkers may be found to associate with IRIS, it is critical to functionally examine the role of each molecule in experimental models. Moreover, the accumulating negative data on other inflammatory pathways we are observing emphasizes the major role of IL-6 in IRIS pathology.
Given that IL-6 and IFNγ represent two major pathways that drive IRIS, we also examined the interplay between these two cytokines. Here we found that single neutralization of IL-6 or IFNγ does not impact the expression of the other, and simultaneous blockade of both cytokines synergistically alleviates wasting disease. Therefore, IL-6 and IFNγ represent two major and independent pathways of IRIS pathology. Indeed, the pathogenic sources of these two cytokines are different cell types. During experimentally induced IRIS the pathogenic IFNγ is derived from CD4 T cells. It is possible that many cell types produce IL-6 during IRIS, but our nude mouse bone marrow chimera experiments show that the pathogenic IL-6, which contributes to IRIS, is largely produced by radio-resistant cells. While we cannot exclude the possibility that IL-6 is derived from a long lived myeloid cell population, our T cell transfers were performed 18-20 weeks following BM reconstitution of the nude mice, so it is more likely that non-hematopoietic cell types are key producers of the pathogenic IL-6 in experimentally induced IRIS. Indeed, multiple non-hematopoietic cell types such as adipocytes, myocytes, fibroblasts, and endothelial cells have all been shown to produce IL-6 in response to a variety of stimuli. Since CD4 T cell transfer induces IL-6 expression, this raises the possibility that the communication between the repopulating CD4 T cells and non-hematopoietic tissues may be a key factor driving the systemic disease during mycobacterial IRIS. Further experiments will be required, however, to determine the relevant non-hematopoietic sources of IL-6 and the mechanisms through which CD4 T cells drive its expression. Likewise, the cell types that are targeted by IL-6 and the downstream activities of IL-6R signaling that lead to pathology will require further investigation.
While the data indicate that IL-6 is a key determinant of IRIS pathology associated with M. avium infection, the clinical feasibility of blocking IL-6 to treat IRIS HIV patients undergoing ART is not clear. It is possible that IL-6 blockade could exacerbate pathogen replication in HIV patients harboring MAC co-infections, as IL-6 blocking Abs have been shown to impair control of M. avium infections in mice(26, 27). There are, however, indications that IL-6 blockade can be beneficial for the treatment of other inflammatory disorders. For example, the mAb against the IL-6 receptor tocilizumab is prescribed for the treatment of rheumatoid arthritis(28). Our data indicate that IL-6 is a major mediator of the cachexia associated with M. avium associated IRIS in mice, and IL-6 has also been shown to play a major role in cancer cachexia(29). In a recent case report, a patient suffering from cachexia with an IL-6 overexpressing lung cancer was treated with tocilizumab(30). The elevated CRP in the patient quickly returned to normal concentrations and the patient’s weight loss and other symptoms were greatly alleviated. Cachexia is also frequently observed in HIV patients experiencing mycobacterial-IRIS during ART, and it is likely that treatments to prevent wasting would greatly reduce morbidity and mortality in individuals that experience a severe episode of IRIS.
In conclusion, we have shown that CRP/IL-6 elevations occur both in HIV+ persons who develop unmasking MAC-IRIS after ART initiation and in M. avium infected TCRα KO mice after CD4 T cell transfer. In the mouse model we showed that both CD4 T cell derived IFNγ and IL-6 produced by radio-resistant cells have independent pathogenic roles and that blocking both cytokines was synergistically beneficial. These data suggest that the IL-6 pathway may be a reasonable target in therapeutic interventions in HIV+ patients with mycobacterial IRIS.
Acknowledgments
The authors thank Sandra Oland, Sara Heiny and Keith Kauffman for technical assistance.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
Author Contributions
D.L.B. designed the research, performed the experiments, analyzed the data, made the figures, and wrote the manuscript. B.B.A. designed the research and performed the experiments. C.M. performed the research. I.S. and A.S. designed the research and wrote the manuscript.
Conflict-of-interest disclosure:
The authors declare no competing financial interests.
References
- 1.French MAH. Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. Med J Aust. 2012;196:318–321. doi: 10.5694/mja12.10089. [DOI] [PubMed] [Google Scholar]
- 2.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;1 doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, Moreno-Reyes R, Mehta M, Martineau AR, Wilkinson RJ, Colebunders R, Wilkinson KA. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55:1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Tadokera R, Conesa-Botella A, Seldon R, Rangaka MX, Rebe K, Pepper DJ, Morroni C, Colebunders R, Maartens G, Wilkinson RJ. Corticosteroid-modulated Immune Activation in the Tuberculosis Immune Reconstitution Inflammatory Syndrome. Am J Resp Crit Care Med. 2012;186:369–377. doi: 10.1164/rccm.201201-0094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant PM, Komarow L, Lederman MM, Pahwa S, Zolopa AR, Andersen J, Asmuth DM, Devaraj S, Pollard RB, Richterman A, Kanthikeel S, Sereti I. Elevated Interleukin 8 and T-Helper 1 and T-Helper 17 Cytokine Levels Prior to Antiretroviral Therapy in Participants Who Developed Immune Reconstitution Inflammatory Syndrome During ACTG A5164. J Infect Dis. 2012;206:1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadokera R, Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Seldon R, Chegou NN, Maartens G, Rangaka MX, Rebe K, Walzl G, Wilkinson RJ. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. European Respiratory Journal. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, Huffam S, Oelrichs R, Sophea P, Saphonn V, Kaldor J, Cooper DA, Chhi Vun M, French MA. Immunopathogenesis and Diagnosis of Tuberculosis and Tuberculosis Associated Immune Reconstitution Inflammatory Syndrome during Early Antiretroviral Therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 8.Marais S, Meintjes G, Pepper DJ, Dodd LE, Schutz C, Ismail Z, Wilkinson KA, Wilkinson RJ. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2013;56:450–460. doi: 10.1093/cid/cis899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonelli LRV, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, Greenwald JH, Roby G, Mican J, Sher A, Roederer M, Sereti I. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–3827. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahnke YD, Greenwald JH, DerSimonian R, Roby G, Antonelli LRV, Sher A, Roederer M, Sereti I. Selective expansion of polyfunctional pathogen-specific CD4+ T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, Marriott D, Pett S, Nanan R, Cooper DA, Zaunders JJ, Kelleher AD. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 12.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 13.Bourgarit A, Carcelain G, Samri A, Parizot C, Lafaurie M, Abgrall S, Delcey V, Vicaut E, Autran B the PARADOX Study Group. Tuberculosis-Associated Immune Restoration Syndrome in HIV-1-Infected Patients Involves Tuberculin-Specific CD4 Th1 Cells and KIR-Negative T Cells. The Journal of Immunology. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 14.Haddow LJ, Dibben O, Moosa M-YS, Borrow P, Easterbrook PJ. Circulating inflammatory biomarkers can predict and characterize tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2011;25:1163–1174. doi: 10.1097/QAD.0b013e3283477d67. [DOI] [PubMed] [Google Scholar]
- 15.Zaidi I, Peterson K, Jeffries D, Whittle H, de Silva T, Rowland-Jones S, Jaye A, de Jong BC. Immune reconstitution inflammatory syndrome and the influence of T regulatory cells: a cohort study in The Gambia. PLoS ONE. 2012;7:e39213. doi: 10.1371/journal.pone.0039213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone SF, Price P, Brochier J, French MA. Plasma bioavailable interleukin-6 is elevated in human immunodeficiency virus-infected patients who experience herpesvirus-associated immune restoration disease after start of highly active antiretroviral therapy. J Infect Dis. 2001;184:1073–1077. doi: 10.1086/323599. [DOI] [PubMed] [Google Scholar]
- 17.Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, Bohjanen PR, Novak RM, Neaton JD, Sereti I for the INSIGHT Study Group. Higher Levels of CRP, D-dimer, IL-6, and Hyaluronic Acid Before Initiation of Antiretroviral Therapy (ART) Are Associated With Increased Risk of AIDS or Death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morlese JF, Orkin CM, Abbas R, Burton C, Qazi NA, Nelson MR, Imami N, Gazzard BG. Plasma IL-6 as a marker of mycobacterial immune restoration disease in HIV-1 infection. AIDS. 2003;17:1411–1413. doi: 10.1097/00002030-200306130-00025. [DOI] [PubMed] [Google Scholar]
- 19.Worsley CM, Suchard MS, Stevens WS, Van Rie A, Murdoch DM. Multi-analyte profiling of ten cytokines in South African HIV-infected patients with Immune Reconstitution Inflammatory Syndrome (IRIS) AIDS Res Ther. 2010;7:36. doi: 10.1186/1742-6405-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price P, Mathiot N, Krueger R, Stone S, Keane NM, French MA. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. J Clin Virol. 2001;22:279–287. doi: 10.1016/s1386-6532(01)00200-1. [DOI] [PubMed] [Google Scholar]
- 21.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 22.Price P, Morahan G, Huang D, Stone E, Cheong KYM, Castley A, Rodgers M, McIntyre MQ, Abraham LJ, French MA. Polymorphisms in cytokine genes define subpopulations of HIV-1 patients who experienced immune restoration diseases. AIDS. 2002;16:2043–2047. doi: 10.1097/00002030-200210180-00009. [DOI] [PubMed] [Google Scholar]
- 23.Barber DL, Mayer-Barber KD, Antonelli LRV, Wilson MS, White S, Caspar P, Hieny S, Sereti I, Sher A. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol. 2012;10:150–156. doi: 10.1038/nrmicro2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura T. The role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic model. International Immunology. 2004;16:1691–1699. doi: 10.1093/intimm/dxh170. [DOI] [PubMed] [Google Scholar]
- 26.Appelberg R. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology. 1994;191:520–525. doi: 10.1016/S0171-2985(11)80458-4. [DOI] [PubMed] [Google Scholar]
- 27.Appelberg R, Castro AG, Pedrosa J, Minóprio P. Role of interleukin-6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–364. [PMC free article] [PubMed] [Google Scholar]
- 28.Genovese MC, Rubbert-Roth A, Smolen JS, Kremer J, Khraishi M, Gómez-Reino J, Sebba A, Pilson R, Williams S, van Vollenhoven R. Longterm Safety and Efficacy of Tocilizumab in Patients with Rheumatoid Arthritis: A Cumulative Analysis of Up to 4.6 Years of Exposure. J Rheumatol. 2013 doi: 10.3899/jrheum.120687. [DOI] [PubMed] [Google Scholar]
- 29.Guttridge KHFDGD, Glass DJ, Guttridge DC. Cancer Cachexia: Mediators, Signaling, and Metabolic Pathways. Cell Metabolism. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Ando K, Takahashi F, Motojima S, Nakashima K, Kaneko N, Hoshi K, Takahashi K. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. Journal of Clinical Oncology. 2013;31:e69–72. doi: 10.1200/JCO.2012.44.2020. [DOI] [PubMed] [Google Scholar]


