Abstract
PURPOSE
To investigate the relationship between systemic cytokines, the complement factor H (CFH) Y402H polymorphism, drusen load, and subfoveal choroidal thickness in patients with dry age-related macular degeneration (AMD).
DESIGN
Cross-sectional study.
METHODS
Forty-four dry AMD patients under care of the Retina Service at the University of British Columbia were enrolled. Drusen load was measured with an automated software algorithm in spectral-domain optical coherence tomography; subfoveal choroidal thickness was measured manually using enhanced depth imaging. Bio-Plex suspension assays (Bio-Rad Laboratories) were used to analyze cytokines in plasma and CFH Y402H was genotyped. Statistical analyses included analysis of covariance and Pearson correlation, corrected for multiple comparisons.
RESULTS
The levels of 3 of 4 studied cytokines were significantly different among patients with CC, CT, or TT variants of the CFH Y402H polymorphism (P < .01). Patients with the at-risk CC variant had higher systemic levels of interleukin-6, interleukin-18, and tumor necrosis factor α than those with the CT variants, the TT variant, or both (P < .01). Interleukin-1β did not reach significance (P = .02), but did demonstrate a consistent trend. No correlation was found between plasma cytokines and drusen load or choroidal thickness (all P >.15).
CONCLUSIONS
The elevated systemic levels of selected proinflammatory cytokines, including those representing products of inflammasome activation, were associated with the CC at-risk variant of the Y402H polymorphism and suggest that genetic factors regulate the inflammatory status in dry AMD patients. Our data support the central role of inflammation in the pathogenesis of AMD and provide further evidence of a systemic involvement in AMD etiology.
Age-related macular degeneration (amd) constitutes the number 1 cause of blindness among the elderly in industrialized countries.1 The hallmark of AMD is the accumulation of extracellular deposits called drusen, which are located between the retinal pigmented epithelium (RPE) and Bruch membrane.2 Larger size and greater number of drusen confer higher risk of developing vision-loss due to geographic atrophy and choroidal neovascularization.3 In addition, choroidal abnormalities have been reported to be associated with drusen formation in dry AMD.4–6 The recent introduction of enhanced depth imaging and segmentation algorithm in spectral-domain optical coherence tomography (SD-OCT) allows for better quantification of drusen load and choroidal measurements.7,8 This noninvasive, reliable imaging method of measuring drusen load and choroidal thickness creates an opportunity to quantify these parameters of slow progression in patients with the early stage of dry AMD. Additionaly, this method has the potential to allow for an in-depth study of the underlying mechanisms in AMD pathogenesis.9
Although the clinical presentation of AMD mainly involves local processes within the retina, systemic factors in the circulating blood also may contribute to the pathogenesis of AMD via exchange between the choroid and the retina. For example, the homozygous CC variant of the Y402H polymorphism in the gene coding complement factor H (CFH), a regulator of the alternative complement pathway, is associated with a high incidence as well as progression to late AMD.10 Proposed mechanisms supporting this include the finding that circulating CFH at-risk proteins have a weaker capacity to bind oxydized phospholipids in situ, thereby modulating proinflammatory stress in the outer retina.11,12 Systemic activation of the complement cascade also was found in AMD patients.13,14 Together with the crosstalk between the complement activation products and proinflammatory cytokines reported in blood cells, these findings highlight the potential role of systemic inflammatory cytokines in the pathogenesis of AMD.15,16 However, several questions remain because few studies have addressed the relationship between the systemic cytokine levels and the CFH Y402H polymorphism in AMD patients, or whether systemic levels of cytokines are associated with the local ocular manifestations such as drusen load and choroidal thinning that occur in early dry AMD. Using a new SD-OCT tool to follow drusen enlargement and changes of choroidal thickness, 2 parameters used to assess disease progression, we conducted a pilot study to investigate the relationship of systemic cytokines with these 2 parameters as well as the CFH Y402H polymorphism in patients with dry AMD. We focused on 4 inflammation-related cytokines. Interleukin (IL)-1β, IL-6, IL-18, and tumor necrosis factor α (TNF-α) are classic proinflammatory cytokines. Previously, they were shown to affect RPE function and were implicated in AMD pathogenesis.17–23
METHODS
STUDY POPULATION
The design of this study was cross-sectional, and it was approved prospectively by Providence Health Care Research Ethics Board at the University of British Columbia and complied with the tenets of the Declaration of Helsinki. Forty-four patients under the care of the physicians at the Retina Service at University of British Columbia (D.A.A., F.F., A.K., and A.B.M.) were enrolled in this study with informed consent. The inclusion criteria were age 55 years or older, diagnosis of dry AMD in both eyes, and absence of any other disease affecting the macula that could compromise the ability to assess properly for drusen (eg, epiretinal membrane, vitreomacular traction, adult vitelliform dystrophy, etc). Exclusion criteria were exudative AMD or geographic atrophy in either eye; ocular media opacity that precluded adequate visualization or imaging of the macula; cancer within the previous 10 years (except for basal cell carcinoma); cardiovascular disease with complications (heart attack or stroke) within the previous year; diabetes with uncontrolled glucose level or complications (diabetic eye disease, diabetic kidney disease, or diabetic nerve damage); any other medical conditions that, in the opinion of the investigators, would affect the study results; and medications that could affect the levels of biomarkers in blood (eg, corticosteroids and NSAIDs).
EYE EXAMINATION, DRUSEN AREA, AND CHOROIDAL THICKNESS MEASUREMENTS
Patients underwent a full ophthalmic examination including measurement of best-corrected visual acuity, intraocular pressure, bio-microscopy, and dilated fundus examination. Cirrus SD-OCT (version 6 software; Carl Zeiss Meditec, Inc., Dublin, California, USA) was used for drusen area and choroidal thickness measurements. Each eye was scanned once using a cube scan protocol (200 × 200 cube scan). In the same session, each eye also was scanned a minimum of twice with the enhanced depth imaging raster scan. Each scan was centered on the fovea automatically and covered a 6 × 6-mm area. The minimum required signal strength for every scan was 6 of 10.
The drusen areal measurements were generated automatically by the Cirrus SD-OCT software algorithm from the cube scans. For measurement of subfoveal choroidal thickness, 3 independent investigators (A.K., K.P.-V., F.F.) measured the choroidal thickness beneath the fovea manually from the enhanced depth imaging raster images using the program’s caliper function during 2 different sessions. The scan with the best signal strength score was chosen for each eye. Each researcher was blinded to the measurements of others and to their own previous sessions.
CYTOKINE ANALYSIS
Nonfasting blood specimens were obtained at the time of the study visit. Ethylene-diamine-tetraacetic acid plasma from patients was prepared into aliquots within 2 hours of phlebotomy and was stored at −80°C until analysis. Cytokines of ethylene-diamine-tetraacetic acid plasma from patients were measured by Bio-Plex Pro human cytokine, chemokine, and growth factor assays as described by the manufacturer (Bio-Rad Laboratories, Hercules, California, USA). The assays use xMAP technology (Luminex, Austin, Texas, USA), which permits the quantification of multiple cytokines in a single well with 50 μL of diluted sample (1:4). In our experiments, the premixed multiplex beads of the Bio-Plex human cytokine 27-plex assay and an additional 2-plex were used. They included the following cytokines: Interleukin (IL)-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, IL-18, chemokine (C-C motif) ligand (CCL)11/eotaxin, basic fibroblast growth factor (basic FGF), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), chemokine (C-X-C motif) ligand (CXCL)10/interferon gamma-induced protein 10 (IP-10), CCL2/monocyte chemoattractant protein (MCP)-1, CCL3/macrophage inflammatory protein (MIP)-1α, CCL4/MIP-1β, platelet-derived growth factor (PDGF)-BB, CCL5/regulated on activation, normal T cell expressed and secreted (RANTES), CXCL12/stromal cell-derived factor (SDF)-1α, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF).
COMPLEMENT FACTOR H Y402H GENOTYPING
Genomic DNA was extracted from ethylene-diamine-tetraacetic acid-contained whole blood using QIAsymphony SP system (Qiagen, Toronto, Canada) and was eluted in 200 μL volume. The extracted DNA was quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Burlington, Canada) to make sure that a sufficient concentration (> 50ng/μL) of genomic DNA was obtained. The subsequent polymerase chain reaction (PCR) amplification and sequencing analysis were performed as previously described.24 Briefly, amplicons of 660 bp containing the CFH Y402H polymorphism (rs1061170) were produced in PCR reaction using the forward and reverse primers of 5′ AGTAACTTTAGTTCGTCTTCAG 3′ and 5′ ATCTTCTTGGTGTGAGATAACG 3′, respectively. After being purified with the QIAquick PCR Purification Kit (Qiagen), the PCR products were mixed with sequencing primer at suitable concentrations as required and then sent to GENEWIZ (South Plainfield, New Jersey, USA) for sequencing analysis. The CFH sequencing primer was 5′ ACTTTAGTTCGTCTTCAG 3′.
STATISTICAL ANALYSIS
Means were calculated between the 2 eyes for drusen area, as well as between the 2 eyes and the 3 measurements for choroidal thickness. We used a mean value to compare with the single measurements obtained from the cytokines. All analyses were conducted with SPSS Statistics version 20 (IBM, Armonk, New York, USA). Our previous study showed that SD OCT-based drusen area and drusen volume, 2 parameters of drusen, are highly correlated (r = 0.86).25 For this reason, we decided to use only drusen area in our analysis.
In the first analysis, we examined the differences in plasma levels of 4 cytokines among the 3 variants of the CFH Y402H polymorphism in our study population. We conducted a series of analyses of covariance using the genotype groups (CC, CT, TT) as the between-subjects factor and individual cytokine, drusen area, and choroidal thickness as the dependent variables. We also included age as a covariate because previous work showed the possible relationship between cytokines and age.26–28 We used a Bonferroni correction to account for multiple comparisons (P < .01) and post hoc comparisons were conducted after a significant omnibus result. For the post hoc comparisons, we used a priori contrasts between the CC and CT variants and between the CC and TT variants, because we were most interested in these contrasts.
In the second analysis, we examined the correlation between each selected cytokine and drusen area or choroidal thickness. We conducted a series of Pearson correlations using cytokines, drusen area, and choroidal thickness as variables. We again used a Bonferroni correction to account for multiple comparisons (P < .01).
RESULTS
SUBJECT CHARACTERISTICS
Participants for this study were recruited between February 2012 and May 2012. The characteristics of the 44 subjects were described previously.25 Briefly, each subject had bilateral dry AMD. None of our subjects were current smokers. The mean age of all subjects was 75.77 years (standard deviation, 8.42 years). The mean drusen area between the 2 eyes was 1.44 mm2 (standard deviation, 1.22 mm2), with 1.57 mm2 (standard deviation, 1.64 mm2) for right eyes and 1.30 mm2 (standard deviation, 1.16 mm2) for left eyes. The mean choroidal thickness between the 2 eyes was 230.73 μm (standard deviation, 53.23 μm), with 232.00 μm (standard deviation, 53.44 μm) for right eyes and 229.47 μm (standard deviation, 59.58 μm) for left eyes. In terms of the CFH Y402H polymorphism, 17 patients (38.6%) had the CC variant, 14 patients (31.8%) had the CT variant, and 13 patients (29.5%) had the TT variant (Table 1).
TABLE 1.
Characteristics of the Recruited Patients with Dry Age-Related Macular Degeneration
| Sample Characteristics | Mean (SD) or No. (%) | 95% CI |
|---|---|---|
| Age (y) | 75.77 (8.42) | 73.28 to 78.26 |
| Drusen area (mm2) | 1.44 (1.22) | 1.08 to 1.80 |
| Right eyes | 1.57 (1.64) | 1.09 to 2.05 |
| Left eyes | 1.30 (1.16) | 0.96 to 1.65 |
| Choroidal thickness (μm) | 230.73 (53.23) | 215.00 to 246.46 |
| Right eyes | 232.00 (53.44) | 216.21 to 247.79 |
| Left eyes | 229.47 (59.58) | 211.86 to 247.07 |
| CFH Y402H genotypea | ||
| CC | 17 (38.6%) | |
| CT | 14 (31.8%) | |
| TT | 13 (29.5%) | |
CFH = complement factor H; CI = confidence interval; SD = standard deviation.
CC, CT, and TT are 3 genotype variants of CFH Y402H polymorphism.
CYTOKINES, DRUSEN AREA, CHOROIDAL THICKNESS, AND GENOTYPE VARIANTS
To examine if there is a relationship between systemic cytokines, drusen area, or choroidal thickness and genotype of the CFH Y402H polymorphism, 4 cytokines were selected for analysis: IL-1β, IL-6, IL-18, and TNF-α. IL-1β and IL-18 were both products of inflammasome activation, a pathway recently studied for its association with both geographic atrophy and choroidal neovascularization development.22,23 IL-18 was known to induce RPE degeneration in mice.22 IL-6 and TNF-α are classic proinflammatory cytokines that have been shown to affect RPE function in vitro.17–21
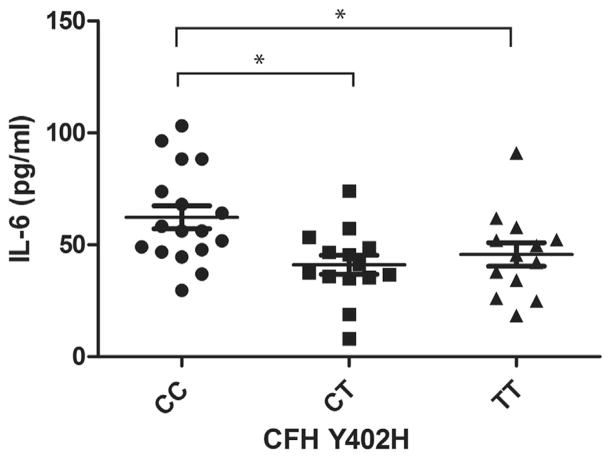
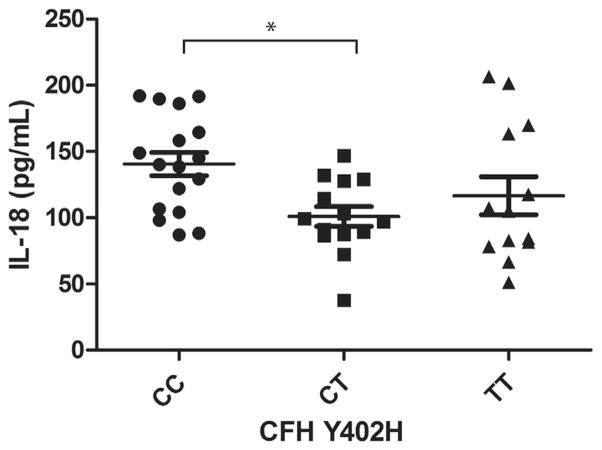
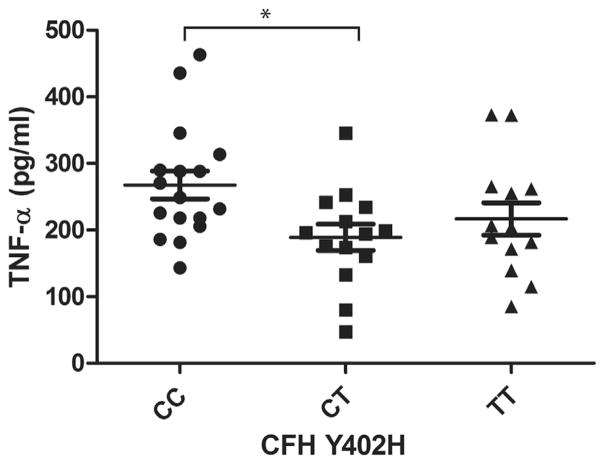
Our analysis of covariance results showed that 3 of the 4 cytokines were significantly different among patients with the CC, CT, or TT variants of the CFH Y402H genotype (P < .01), when corrected for age (Table 2). Patients with the CC variant had a higher level of IL-6 than those with the CT or TT variant (P < .01). The level of IL-6 in patients with the CC variant was greater than that in patients with the TT variant (36%) or the CT (52%) variant (Figure 1). The levels of IL-18 and TNF-α in patients with the CC variant were significantly higher only compared with those with the CT variant, with a 39% and 42% increase, respectively (Figures 2 and 3). There was a trend for group differences in IL-1β levels, but it did not reach significance when corrected for multiple comparisons (P = .02; Figure 4).
TABLE 2.
Descriptives Statistics and Analysis of Covariance Results from Plasma Cytokine Levels in Dry Age-Related Macular Degeneration Patients with Different Genotypes of the Complement Factor H Y402H Polymorphism
|
CFH Y402H Polymorphisma
|
P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC
|
CT
|
TT
|
||||||||
| Mean | SD | SEM | Mean | SD | SEM | Mean | SD | SEM | ||
| IL-1β | 22.22 | 6.21 | 8.92 | 16.89 | 5.54 | 7.17 | 17.61 | 6.17 | 7.09 | .02 |
| IL-6 | 62.35 | 21.22 | 13.53 | 41.03 | 15.99 | 10.26 | 45.70 | 18.99 | 10.49 | <.005 |
| IL-18 | 140.56 | 36.32 | 23.32 | 101.00 | 28.06 | 19.07 | 116.61 | 51.81 | 16.20 | .01 |
| TNF-α | 267.86 | 85.75 | 28.93 | 189.14 | 73.65 | 22.04 | 216.77 | 87.81 | 23.13 | .01 |
CFH = complement factor H; IL = interleukin; SD = standard deviation; SEM = standard error of the mean; TNF = tumor necrosis factor.
CC, CT, and TT are 3 genotype variants of the CFH Y402H polymorphism.
FIGURE 1.
Graph showing plasma levels of interleukin-6 (IL-6) in patients with dry age-related macular degeneration (AMD) stratified by 3 genotype variants of complement factor H (CFH) Y402H polymorphism. Plasma IL-6 level in dry AMD patients with homozygous CC variant is 36% higher than homozygous TT variant and 52% higher than heterozygous CT variant (mean ± standard error of the mean; *P <.01, post hoc).
FIGURE 2.
Graph showing plasma levels of interleukin-18 (IL-18) in patients with dry age-related macular degeneration (AMD) stratified by 3 genotype variants of complement factor H (CFH) Y402H polymorphism. Plasma IL-18 level in dry AMD patients with homozygous CC variant is 39% higher than heterozygous CT variant (mean ± standard error of the mean; *P <.01, post hoc).
FIGURE 3.
Graph showing plasma levels of tumor necrosis factor-α (TNF-α) in patients with dry age-related macular degeneration (AMD) stratified by 3 genotype variants of complement factor H (CFH) Y402H polymorphism. Plasma TNF-α level in dry AMD patients with homozygous CC variant is 42% higher than heterozygous CT variant (mean ± standard error of the mean; *P <.01, post hoc).
FIGURE 4.
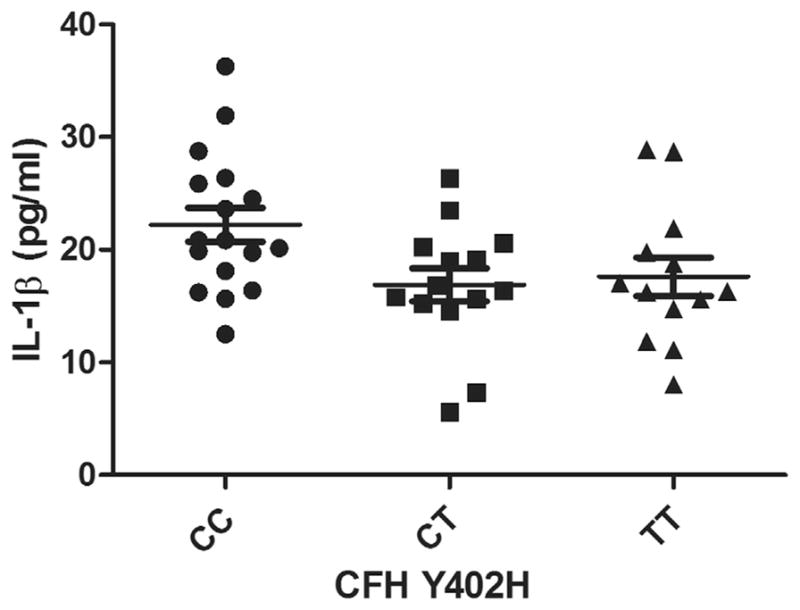
Graph showing plasma levels of interleukin-1β (IL-1β) in patients with dry age-related macular degeneration (AMD) stratified by 3 genotype variants of complement factor H (CFH) Y402H polymorphism. There is a trend for IL-1β levels to be different among dry AMD patients with CC, CT, and TT variants, but it does not reach significance when corrected for multiple comparisons (mean ± standard error of the mean; *P = .02).
The analysis of covariance results showed that there were no significant differences between the CFH Y402H variants on variables such as drusen area (F (degrees of freedom, 2, 39) = 0.79; P = .46) or choroidal thickness (F (2, 39) = 0.06; P = .94) when corrected for age. This suggests that the elevation of cytokines in the CC variant is not the result of drusen area or choroidal thickness.
CYTOKINES, CHOROIDAL THICKNESS, AND DRUSEN AREA
In our earlier study, drusen area and choroidal thickness were related inversely in dry AMD patients, and both may reflect, to some degree, the severity of disease.25 In the current study, to explore the relationship of the ocular pathologic features and the systemic cytokine levels, we investigated the relationship of drusen area or choroidal thickness with cytokine levels in plasma. However, we did not see any relationship between the systemic levels of cytokines studied and drusen area or choroidal thickness (all P > .15).
DISCUSSION
In this pilot study, we showed that patients with the homozygous at-risk CC variant for the CFH Y402H polymorphism had higher systemic cytokine levels than those with only 1 risk allele (CT) or without any risk alleles (TT). The cytokines examined in our study are proinflammatory and are known to affect the function of retinal cells in the outer retina. They can work alone or synergically to induce further cytokine production and adhesion molecules from RPE, thereby enhancing the local proinflammatory status. Other effects include increasing the permeability of the endothelium, a process involved in blood-retina barrier breakdown, and neovascularization.29–34 The association of patients with the CC variant and the significant elevated IL-18 levels and the trend demonstrated by IL-1β (Table 2) suggests that there may be systemic inflammasome activation in these patients. IL-1β and IL-18 are both products of inflammasome activation and are known to induce local eye changes associated with both geographic atrophy and choroidal neovascularization development.22,23 With the access to the outer retina via the choroidal vasculature, the higher cytokines in the blood may predispose the local retinal environment to a proinflammatory status, which may explain why patients with a CC variant have a higher risk for AMD progression than those with a TT variant.
The CFH Y402H polymorphism is considered to be the most salient genetic factor to confer an increased risk for AMD incidence as well as progression, although the details of the underlying mechanisms are still under investigation.10,35–39 CFH is the main inhibitor of the complement alternative pathway. By interactions with C3 and inactivation of its activation products, CFH keeps the spontaneous activation of the alternative pathway in check.40 With the CFH Y402H risk variant, the inhibitory function of CFH may be altered. Earlier studies showed that the CFH Y402H polymorphism may be associated with an increased activation of complement cascade not only systemically, but also in local tissues.13,41,42 The complement activation products such as C3a and C5a may regulate the expression of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α in blood cells.16,43–45 It therefore is possible that the elevation of the cytokines shown in this pilot study is secondary to the increased complement activation associated with the CC risk variant. In addition, the CFH Y402H variant also may affect systemic cytokine levels because of its altered binding properties. Previous studies showed that the at-risk Y402H variant of CFH binds poorly to molecules such as C-reactive protein and malondialdehyde.11,41 The efficient formation of C-reactive protein-CFH complex on cell surfaces is important to dampen complement activation and reduce the secretion of the proinflammatory cytokine TNF-α,46 which may explain our finding that at-risk variant carriers have a higher TNF-α level. Similarly, malondialdehyde, a common lipid peroxidation product, when inefficiently bound to CFH (because of the CC variant), can induce macrophages or monocytes to secrete many proinflammatory cytokines, including IL-6.47 Our data support the relationship of elevated systemic cytokine levels and CFH Y402H at-risk variants in patients with dry AMD and suggest that systemic cytokines also may be associated with genetic factors, and thus may contribute to disease manifestations in the ageing macula.
Our finding that dry AMD patients with the CC variant had higher IL-6 levels than patients with the TT variant was consistent with an earlier study that focused on an older general population.48 In addition, our study was also consistent with that of Moorijaart and associates in that no differences were observed between individuals with the CT variant compared with those with the TT variant.48 However, it is interesting that the IL-6 level found in our study population (median, 48.3 pg/mL) generally is higher than in a general older population (median, 11 pg/mL), as shown by Moorijaart and associates. The difference in the level of IL-6 between 2 studies may be related to 2 factors. The participants in our study were all diagnosed with dry AMD, whereas the earlier study focused on a general population. Methods to quantify IL-6 levels from plasma included a suspension array in our study, whereas Moorijaart and associates used an enzyme-linked immunosorbent assay.
Given the fact that a systemic complement activation was found in AMD patients and a large drusen area confer higher risk for the late stage of AMD,3,15,49 we hypothesized that drusen load and chroidal thickness measured by OCT may demostrate a relationship with systemic levels of inflammatory cytokines associated with complement activation. However, we did not find any relationship between the cytokines we analyzed and drusen load or choroidal thickness. This in part may be a result of the small sample size, which is a limitation of our study. The standard deviation of drusen load is 1.22 mm2, relatively large with regard to the mean of 1.4 mm2, and the range of drusen load is from 0 to 4.25 mm2, which is relatively small compared with that reported in previous cohort studies.50,51 Therefore, future studies should be conducted with a larger cohort to assess the relationship between systemic cytokines and drusen load or choroidal thickness. We were constrained from analyzing all 29 tested cytokines and growth factors with multiple testing correction because of our relatively small sample size in this pilot study. Therefore, we focused on 4 cytokines that are known to play a significant role in the development of AMD. Future research should conduct a larger study to evaluate the effect of all 27 cytokines and whether there are any differences or similarities between them.
In conclusion, our study demonstrated that the systemic levels of selected proinflammatory cytokines, including those representing products of inflammasome activation, were higher in dry AMD patients with the CC at-risk variant of the CFH Y402H polymorphism. Our data support the central role of inflammation in the pathogenesis of AMD and provide new evidence of a systemic involvement in AMD etiology. Future work will assess the potential interactions of inflammasome activation at the systemic and local retinal level and the usefulness of systemic cytokine biomarkers as indicators of AMD progression.
Acknowledgments
This research was supported by a New Researcher Grant from the Canadian National Institute for the Blind, Toronto, ON, Canada, and by Grant CIHR MOP 97806 from the Canadian Institute of Health Research, Ottawa, ON, Canada. The authors thank Carl Zeiss Meditec, Inc, for use of the Cirrus SD OCT machine and software.
Footnotes
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest and none were reported.
Involved in Concept and design of study (A.K., F.F., J.A.M., S.C.); Recruitment of patients (A.B.M., D.A.A., A.W.K.); Conduct of study (A.K., A.W., J.Z.C., K.P.-V., S.C.); Analysis and interpretation of data (A.W., J.A.M., M.P., S.C.); Statistical expertise (M.P.); Obtaining funding (F.F., J.A.M.); Writing article (J.A.M., S.C.); Critical revision of article (A.K., F.F., J.Z.C., M.P.); and Final approval of article (J.A.M., F.F.).
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 4.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SW, Oh J, Kwon SS, Yoo J, Huh K. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31(9):1904–1911. doi: 10.1097/IAE.0b013e31821801c5. [DOI] [PubMed] [Google Scholar]
- 7.Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology. 2011;118(7):1373–1379. doi: 10.1016/j.ophtha.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehoshua Z, Rosenfeld PJ, Gregori G, Penha F. Spectral domain optical coherence tomography imaging of dry age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S6–S14. doi: 10.3928/15428877-20101031-19. [DOI] [PubMed] [Google Scholar]
- 10.Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multi-state models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130(9):1169–1176. doi: 10.1001/archophthalmol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weismann D, Hartvigsen K, Lauer N, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw PX, Zhang L, Zhang M, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109(34):13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholl HP, Charbel Issa P, Walier M, et al. Systemic complement activation in age-related macular degeneration. PloS One. 2008;3(7):e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smailhodzic D, Klaver CC, Klevering BJ, et al. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology. 2012;119(2):339–346. doi: 10.1016/j.ophtha.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Wei L, Meyerle C, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Kimura Y, Fang C, et al. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110(1):228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123(6):774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 18.Jonas JB, Tao Y, Neumaier M, Findeisen P. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol. 2012;90(5):e381–e388. doi: 10.1111/j.1755-3768.2012.02414.x. [DOI] [PubMed] [Google Scholar]
- 19.Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40(9):1891–1898. [PubMed] [Google Scholar]
- 20.Lin T, Walker GB, Kurji K, et al. Parainflammation associated with advanced glycation end product stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine. 2013;62(3):369–381. doi: 10.1016/j.cyto.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122(7):1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 22.Tarallo V, Hirano Y, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149(4):847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JCC, Wang A, Gao J, et al. Isolation of total DNA from postmortem human eye tissues and quality comparison between iris and retina. Mol Vis. 2012;18(311):3049–3056. [PMC free article] [PubMed] [Google Scholar]
- 25.Ko A, Cao S, Pakzad-Vaezi K, et al. Optical coherence tomography-based correlation between choroidal thickness and drusen load in dry age-related macular degeneration. Retina. 2013;33(5):1005–1010. doi: 10.1097/IAE.0b013e31827d266e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ershler WB, Sun WH, Binkley N, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12(4):225–230. [PubMed] [Google Scholar]
- 27.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 28.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102(2–3):199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 29.Bamforth SD, Lightman S, Greenwood J. The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol. 1996;91(6):624–632. doi: 10.1007/s004010050476. [DOI] [PubMed] [Google Scholar]
- 30.Bamforth SD, Lightman SL, Greenwood J. Ultrastructural analysis of interleukin-1 beta-induced leukocyte recruitment to the rat retina. Invest Ophthalmol Vis Sci. 1997;38(1):25–35. [PubMed] [Google Scholar]
- 31.Duchini A, Govindarajan S, Santucci M, Zampi G, Hofman FM. Effects of tumor necrosis factor-alpha and interleukin-6 on fluid-phase permeability and ammonia diffusion in CNS-derived endothelial cells. J Investig Med. 1996;44(8):474–482. [PubMed] [Google Scholar]
- 32.Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64(6):819–825. [PubMed] [Google Scholar]
- 33.Elner VM, Scales W, Elner SG, Danforth J, Kunkel SL, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992;54(3):361–368. doi: 10.1016/0014-4835(92)90048-w. [DOI] [PubMed] [Google Scholar]
- 34.Nagineni CN, Detrick B, Hooks JJ. Synergistic effects of gamma interferon on inflammatory mediators that induce interleukin-6 gene expression and secretion by human retinal pigment epithelial cells. Clin Diagn Lab Immunol. 1994;1(5):569–577. doi: 10.1128/cdli.1.5.569-577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 36.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 38.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Priya RR, Chew EY, Swaroop A. Genetic studies of age-related macular degeneration: lessons, challenges, and opportunities for disease management. Ophthalmology. 2012;119(12):2526–2536. doi: 10.1016/j.ophtha.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradley DT, Zipfel PF, Hughes AE. Complement in age-related macular degeneration: a focus on function. Eye. 2011;25(6):683–693. doi: 10.1038/eye.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson DH, Radeke MJ, Gallo NB, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retina Eye Res. 2010;29(2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93(4):565–567. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171(3):715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takabayashi T, Vannier E, Clark BD, et al. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol. 1996;156(9):3455–3460. [PubMed] [Google Scholar]
- 45.Takabayashi T, Vannier E, Burke JF, Tompkins RG, Gelfand JA, Clark BD. Both C3a and C3a(desArg) regulate interleukin-6 synthesis in human peripheral blood mononuclear cells. J Infect Dis. 1998;177(6):1622–1628. doi: 10.1086/515316. [DOI] [PubMed] [Google Scholar]
- 46.Lauer N, Mihlan M, Hartmann A, et al. Complement regulation at necrotic cell lesions is impaired by the age-related macular degeneration-associated factor-H His402 risk variant. J Immunol. 2011;187(8):4374–4383. doi: 10.4049/jimmunol.1002488. [DOI] [PubMed] [Google Scholar]
- 47.Shanmugam N, Figarola JL, Li Y, Swiderski PM, Rahbar S, Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57(4):879–888. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mooijaart SP, Koeijvoets KM, Sijbrands EJ, Daha MR, Westendorp RG. Complement factor H polymorphism Y402H associates with inflammation, visual acuity, and cardiovascular mortality in the elderly population at large. Exp Gerontol. 2007;42(11):1116–1122. doi: 10.1016/j.exger.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon gamma-inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(8):4226–4236. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- 50.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003;121(4):519–526. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 51.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]