Abstract
Alzheimer’s disease (AD) is a growing health crisis around the world. While significant progress has been made in our understanding of AD pathogenesis, there is currently no effective treatment to delay the onset of or prevent the disease. The focus has now shifted to the identification and treatment of AD in the early clinical stages as well as before cognitive symptoms emerge – during the long preclinical stage. With this shift in focus, diagnosis of individuals with AD can be more accurate when clinical symptoms and signs are combined with biomarkers. Biomarkers can improve both the diagnostic and prognostic accuracy of AD and its differentiation from other neurodegenerative diseases. This review will discuss fluid and imaging biomarkers that have shown promise in such areas, as well as some of the current challenges that face the field.
Keywords: Alzheimer disease, biomarker, cerebrospinal fluid, neuroimaging, diagnosis, prognosis
Introduction
Alzheimer’s disease (AD) is a chronic, progressive neurodegenerative disease that slowly strips the person of their memories and other cognitive functions. The World Health Organization (WHO) estimated that as many as 35.6 million people worldwide were living with dementia in 2012 – the majority of whom had AD. It is estimated that those numbers will approximately double every 20 years without the aid of treatment or prevention (1). Clinical trials of AD therapeutics to date have been unsuccessful in reversing, halting or slowing cognitive decline. A widely held belief is that some of this failure is due to the exclusive enrollment of individuals who already exhibit mild or moderate dementia, stages of AD that are accompanied by robust neuronal cell death. At even earlier stages of the disease (very mild dementia and mild cognitive impairment due to AD), neuron loss in certain critical brain regions is already significant (2). Thus, it is important to diagnose individuals at very early disease stages - and enroll them in clinical trials - in order to identify and apply therapies that have the best chance of preserving normal cognitive function. This does not imply that there is no merit in treating symptomatic individuals. Indeed, since neurodegeneration continues to progress through advancing clinical stages, development of therapies that could block or slow this degeneration is also highly desirable. Given the prospect of long-term treatment, especially in asymptomatic cases and in elderly individuals who potentially exhibit other age-related co-morbid conditions, rigorous safety testing, as was performed in initial studies of anti-hypertensive and cholesterol-lowering medications, will be required.
Currently, a definitive diagnosis of AD requires postmortem identification of the pathological hallmarks of the disease: extracellular amyloid plaques composed mainly of aggregated amyloid-β (Aβ) peptides and neurofibrillary tangles composed mainly of hyperphosphorylated forms of the microtubule associated protein, tau (p-tau)(3). Clinical diagnosis of AD is based on guidelines established by the National Institute of Neurological Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINDS-ADRDA), although the sensitivity and specificity of such a diagnosis is lower than desirable (4). It is now widely accepted that AD starts with a “preclinical” stage beginning 10–20 years prior to the onset of cognitive decline. The addition of biomarkers to the diagnostic criteria for AD may increase the sensitivity and specificity of both the diagnostic and prognostic capabilities currently available through clinical and cognitive assessment. One study suggests that it may be possible for biomarkers to reliably be used as indicators of disease stage (5). One goal of studying biomarkers is to identify those with AD pathological changes before clinical signs emerge, as well as predict the odds that such individuals will clinically progress and at what rate. A recent workgroup cautions that biomarkers are not yet ready for clinical use (6); however, a small number of biomarkers for AD are currently used in clinical practice (see Current CSF biomarker utilization and challenges).
Current AD therapies are only available after a clinical diagnosis is made and are limited to symptomatic drugs which offer a modest, temporary improvement in or stabilization of cognitive decline in some patients. However, these drugs do not target the underlying pathology of the disease. Recent clinical trials have focused on anti-Aβ antibodies that bind either aggregated or soluble forms of Aβ and encourage the removal or neutralization of these species from the brain. Phase III trials of the anti-Aβ antibodies bapineuzumab and solanezumab in mild to moderate dementia believed to be due to AD ended in late 2012. Neither drug achieved its primary cognitive endpoint, and an increased risk of vasogenic edema was seen with bapineuzumab (7, 8). However, in the solanezumab trial, pre-specified analyses of individuals with mild dementia (MMSE 20–26) revealed a ~30% slowing of cognitive decline as measured by the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and significant improvement over placebo on some activities of daily living measures (9). An additional phase III trial of this compound in mild dementia is planned.
As the AD community moves toward a treatment, it seems certain that biomarkers will play a key role in the process. A biomarker is defined as any measurable characteristic that can be used as an indicator of a particular disease, and with such a broad definition, it is certain that many options will exist for any given disease. It is the goal of investigators to determine the most effective combination of biomarkers to enable identification, differentiation, and treatment of the disease in question. Cognitive measures can serve as a biomarker of disease; however, fluid (CSF, plasma, serum) and imaging (magnetic resonance imaging [MRI] and positron emission tomography [PET]) biomarkers as measures of underlying pathology and disease progression are the focus of this review. Each modality presents its own challenges for identifying and/or developing viable markers, test validation, and intra- and inter-lab consistency in measurement.
Cerebrospinal Fluid Biomarkers
CSF is considered a prime source for AD biomarkers because many proteins and metabolites in CSF directly reflect the internal milieu of the brain. A lumbar puncture (LP) is necessary for the collection of CSF which makes it somewhat more invasive than a blood draw. However, complications stemming from LP are not frequent (10, 11), and when the procedure is performed by experienced clinicians, it is usually not painful.
Established Core CSF Biomarkers
Three proteins are typically considered the gold standards for AD CSF biomarkers – Aβ42, total tau (tau) and p-tau. These proteins are the most abundant components of amyloid plaques and neurofibrillary tangles, respectively. Each of these analytes has been extensively studied and validated in a variety of cohorts world-wide and, while absolute concentrations of each marker may vary, similar results have been reported in multiple studies.
Amyloid β42
Aβ42 is a 42 amino acid peptide created from the processing of the amyloid precursor protein (APP). There are multiple lengths of Aβ peptides; the 42 amino acid form is the most abundant in amyloid plaques. Significantly reduced levels of CSF Aβ42 in individuals diagnosed with AD, compared to cognitively normal, age-matched individuals, is one signature of amyloid plaques (12). Studies have shown that CSF Aβ42 levels correlate inversely with amyloid plaque load in the brain as determined by postmortem histology (13) and concomitant in vivo plaque measurement using amyloid imaging (14–18). CSF Aβ42 is likely low in the presence of amyloid deposition due to its sequestration in plaques (19). Low CSF Aβ42 is useful as a marker that predicts future clinical disease progression and rate of cognitive decline, especially in the early clinical stages of the disease (20–22). However, Aβ42 alone is not a sufficient biomarker for AD diagnosis and prognosis (23, 24), nor does it mark the presence of other AD pathology.
Tau
Tau is a microtubule associated protein that is considered to be a biomarker of neurodegeneration in AD. High levels of tau in the CSF may reflect neuronal damage, as is suggested by increases in tau after acute neuronal injury such as stroke and traumatic brain injuries (25, 26). Phospho-tau levels correlate with total tau and also correlate closely with neurofibrillary tangle load (27). Tau is normally released by neurons in the absence of cell death. Evidence from wild type and P301S tau transgenic mice suggests tau is continuously secreted from healthy neurons into the brain interstitial fluid space (28). In addition, both soluble and aggregated forms of tau have been shown to be secreted by cultured cells (29). CSF tau and p-tau181 are significantly increased in AD and Mild Cognitive Impairment (MCI) (21, 27, 30). Levels of p-tau231 in CSF are also associated with disease progression in AD cases (31). Tau and p-tau are similar to Aβ42 in diagnostic and prognostic performance but are not sufficient biomarkers on their own.
Current CSF biomarker utilization and challenges
In current clinical practice, in the absence of a disease modifying therapy for AD, CSF is only obtained for the assessment of Aβ42, tau or p-tau181 by most clinicians under specific circumstances. For example, in very mildly symptomatic individuals, especially in individuals younger than 60, CSF assessment can be useful in differential diagnosis between other neurodegenerative diseases such as frontotemporal dementia (FTD) and even certain psychiatric disorders such as depression. It is worth noting that in addition to CSF, some clinicians obtain FDG-PET scans to aid in distinguishing possible FTD from AD since there is usually a different pattern of abnormalities between these disorders. The CSF profiles for common biomarkers in a selection of common disorders and neurodegenerative diseases are shown in the Table.
Table.
Comparison of the core AD CSF biomarkers in other neurodegenerative diseases
| Diagnosis | Aβ42 | tau | p-tau181 | Other |
|---|---|---|---|---|
| AD | ↓↓(20, 21, 86, 95, 96) | ↑(20, 21, 86, 95, 96) | ↑(20, 86, 95) | p-tau231: ↑(97) |
| FTD | —(21) | —(21) | —(21) | |
| DLB | ↓(21, 86, 95, 98) | ↑(86, 95, 98) | —(21, 86, 95) | |
| PDD | —(86, 95) | —/↓(86, 95) | —(86, 95) | |
| CJD | ↓(99) | ↑↑(12, 100) | ↑(12) | |
| Depression | ↑(96) | —(96) | —(96) | p-tau231: —(97) |
AD: Alzheimer’s disease, FTD: Frontotemporal dementia, DLB: Dementia with Lewy bodies, PDD: Parkinson’s disease with dementia, CJD: Creutzfeldt-Jakob disease. Comparisons are reported as difference from a normal CSF biomarker profile. ↑: increase, ↑↑: large increase, ↓: decrease, : ↓↓ large decrease, —: no difference.
A current challenge in the fluid biomarker field is protocol standardization. A world-wide quality control study has reported significant inter- and intra-lab variability in the measurement of the three core CSF markers, even when using the same assay kits and lot numbers (32, 33). Generally, intra-lab is lower than inter-lab variability. One goal of the ongoing quality control initiative is to determine accurate “cutoff” values that can identify an individual as biomarker-positive or –negative that has utility for both clinical trial design/evaluation and disease diagnosis. However, current CSF biomarkers are continuous variables and clinicians should be aware that treating them as such may be helpful in day to day practice – for instance, if a patient presents with a clinical syndrome consistent with early AD but with biomarker values that are borderline, strict cutoff values are not likely to be as useful as clinical judgment.
Animal Models of AD
The discovery of human genetic mutations in certain families with an autosomal-dominant pattern of AD phenotypic expression facilitated the critical development of transgenic mouse models that are widely used in AD research today. Significant effort has been dedicated to investigation of the pathologic similarities between such models and the actual human disease. For example, in the Tg2576 APP transgenic mouse model, levels of CSF Aβ42 decrease as plaques begin to deposit in the brain (34). This mirrors what is seen in the humans carrying such disease-causing mutations, where decreases in CSF Aβ42 were seen in mutation carriers at least 15 years prior to their estimated age of symptom onset (35).
Novel Cerebrospinal Fluid Biomarkers
Because it is likely that no single biomarker will perform satisfactorily on its own, identification and development of additional CSF biomarkers that do not directly reflect AD pathology (plaques and tangles), but instead reflect more general processes such as neurodegeneration and inflammation might be very useful. Proteomics and multi-analyte profiling (MAP) are two common techniques used to identify novel fluid biomarkers. These non-biased approaches can be used to identify proteins and lipids in human fluids that have differential expression between groups of individuals. MAP analysis relies on bead-based immunoassay techniques or microscopy/flow cytometry to quantify a large number of analytes from a relatively small volume of sample. This method is useful for a fast assessment of novel biomarkers, though is often limited to panels of analytes chosen by the manufacturers of MAP kits. A challenge presented by unbiased approaches to biomarker identification has been in identifying biomarkers that have high enough sensitivity and specificity to warrant investigation in large cohorts.
Of the novel CSF biomarkers proposed to date, few have been validated in large, independent cohorts. One candidate is visinin-like protein 1 (VILIP-1) – a neuron-specific intracellular calcium sensor protein. Increases in CSF VILIP 1 have been observed in AD and is a strong predictor of future cognitive decline in individuals with MCI/very mild dementia and in cognitively normal controls (36–38). Another candidate with a similar degree of validation in CSF is chitinase-3-like protein 1 (YKL-40) – an astrocytic protein that is upregulated in neuroinflammatory conditions (39). In two reports, YKL-40 performed nearly as well as the core biomarkers for both diagnosis and prognosis in AD vs. controls (5, 40). However these results were not replicated in a subsequent study (41). There are countless additional novel CSF biomarkers for AD, though few have been validated to the extent of VILIP-1 or YKL-40 (42).
It is possible that antibody-free assays, such as mass spectrometry, will provide more reliable means to measure current and novel biomarkers for AD. Some studies have shown promising differences between preclinical AD or MCI and control groups using mass spectrometry to measure non-protein metabolites such as F2-isoprostanes (43, 44) and lipids such as sulfatides (45). Such data have yet to be reported in large cohorts comparable to other promising biomarkers exploring CSF, plasma or imaging markers.
Blood Biomarkers
Identification of blood biomarkers (plasma and serum) for AD has been disappointing. Possible contributing factors include low expression of target biomarker proteins in the periphery that could make quantification of central nervous system (CNS)-derived analytes difficult, as well as the relatively higher levels of total protein in plasma and serum compared to CSF that could interfere with analyte detection.
Plasma Amyloid-β42
Findings from the many published studies of plasma Aβ species have been contradictory (46). Some groups report slightly higher plasma levels of either Aβ42 or Aβ40 in AD, although with broad overlap between AD and control groups, whereas most studies find no change. Some studies report that a high level of plasma Aβ42, or a high Aβ42/Aβ40 ratio, is an indicator of increased risk for future AD; however, other studies have reported the opposite. While levels of CSF Aβ42 are negatively correlated with plaque load, as evidenced by amyloid imaging, levels of plasma Aβ species are not (47).
Plasma Tau
There are conflicting reports as to the behavior of tau in the periphery – some report that plasma tau decreases in AD (48), while another finds tau increases in AD (49). These differences are likely due to variability created by extremely low levels of tau in plasma. Considering the behavior of tau in CSF it seems plausible that, as the disease progresses, tau would become more abundant in plasma. Ultrasensitive and other assays are being developed to address the usefulness of measuring tau in the blood (49).
Novel Blood Biomarkers
There are few well-characterized biomarkers in blood compared to CSF and imaging. Novel serum candidates include C-Reactive Protein (50) and the presence of antibodies in serum that recognize selective peptoid ligands in AD vs. control samples (51). Novel plasma biomarkers, at least recently, have been presented as panels rather than individual biomarkers (see The Potential of Biomarker Panels). More work needs to be done before blood biomarkers can be considered useful for AD diagnosis and prognosis.
Imaging Biomarkers
Imaging biomarkers capture a broad range of AD-associated processes, from brain size and structure to the presence of protein aggregation. Imaging biomarkers can be non-invasive or moderately invasive based on the modality used (i.e. MRI vs. PET with radioactivity, respectively). A particular advantage for imaging biomarkers is the continual improvement of equipment/software and discovery of new radioimaging ligands that either improve upon or add to the pool of available proteins that can be imaged in vivo. As with fluid biomarkers, inter-lab standardization is paramount but can be quite difficult due to the use of different makes and models of scanners, each with their own idiosyncrasies (52). In studies such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI), use of software to analyze scans and implementation of a phantom – a small, standardized object that contains filling readable by MRI – has helped reduce some variability.
MRI Biomarkers
Volumetric MRI is one of the most studied imaging biomarkers. The measurement of the size of a brain region at a single time point and within individuals longitudinally allows for detection of atrophy in either whole brain or targeted areas (53–55). In many studies, a marked decrease in volume is observed in AD – this is seen both in normalized whole brain volume and in specific areas such as the hippocampus and entorhinal cortex (56, 57). Volumetric MRI performs as well as the CSF gold standard biomarkers for diagnosis and prognosis, especially in the more advanced clinical stages of the disease (58, 59). Currently, however, few diagnostic software programs for volumetric MRI have Food and Drug Administration (FDA) approval, and the process of analyzing such scans is labor intensive and time consuming.
Both task-based and resting state functional MRI (fMRI) are promising imaging biomarkers for AD (60). The difference in magnetization between oxygen-rich and oxygen-poor blood can be measured using fMRI to detect changes in connectivity between areas of the brain while an individual is performing a task or resting. Some studies have shown fMRI differences between individuals with MCI vs. controls on task-based assessments (61). Of particular interest in resting state fMRI assessments is the default mode network (DMN), a network of brain regions that is most active when a person is not engaged in a specific cognitive task and is deactivated when an individual is externally stimulated or is engaged in a specific task. In AD, DMN resting connectivity is weakened, while in some cases other networks have increased activity (62, 63). This change in activity has been shown to have diagnostic and prognostic ability in small cohorts (64). Standardization and validation in large AD cohorts is needed (65), although a recent large study of 500 individuals showed progressive decline in resting state functional connectivity across multiple networks with disease progression (66). Whether fMRI will prove useful in differential diagnosis, prognosis, or in clinical trials awaits further studies.
PET Biomarkers
18Fluorodeoxyglucose PET, or FDG PET, is a highly studied radioligand that acts as an indicator of glucose metabolism and, by proxy, neuronal activity. Reductions in FDG PET are observed in AD in vulnerable areas such as the posterior cingulate and medial temporal lobe early in the disease process, and gradually expand to areas including the frontal association cortices, providing both diagnostic and prognostic capabilities (67, 68). However, these changes are not typically seen until the symptomatic phase of AD (69).
Use of amyloid imaging PET biomarkers began with development of the first radioligand specific to fibrillar Aβ, Pittsburgh Compound B (PiB), in the mid 2000’s (70). Other amyloid imaging radioligands have since become available that utilize 18F instead of 11C (e.g., florbetapir, flutemetamol, florbetaben and AZD4694 (71)), allowing for studies to be carried out in locations that do not have access to a cyclotron (72, 73). In AD, levels of fibrillar Aβ are significantly increased when measured by amyloid PET (16). These increases correlate inversely with CSF Aβ42 levels and offer some value for diagnosis and prognosis in early stages of the disease, including the preclinical period (18, 74–76). Florbetapir (also known as Amyvid™) was recently approved by the FDA for use in patients being evaluated for AD and other causes of cognitive decline (77). The widespread use of these agents outside of clinical trials will probably await approval of the first effective disease modifying treatment. Molecular imaging of other disease related aggregated proteins, such as tau and synuclein, is a critical need in the field and is currently under development.
The Potential of Biomarker Panels
An ideal biomarker panel will: 1) have clear cutoff values for biomarker positivity; 2) identify AD preclinically and reflect the different stages of the disease throughout its course; 3) offer differentiation between AD and other dementias; and 4) be simple and not costly, such as might be afforded by a blood test. Such a panel will likely need to include markers of AD pathology as well as supplementary markers of more general neuroinflammatory and neurodegenerative processes.
Statistical Methods in Biomarker Analysis
Depending on the specific scientific objectives of analyzing biomarker data, a wide range of analytic tools can be used. One approach is to compare biomarkers across well-established clinical stages, and identify biomarker cutoffs that best discriminate clinical stages by optimizing well-established measures of diagnostic accuracy (e.g., Youden index or the distance of Receiver Operating Characteristic [ROC] curve/surface) to the ideal diagnosis (78). These cutoffs can then be applied to individuals who are cognitively normal to define those with preclinical AD. Statistical methods that combine multiple biomarkers to optimize the diagnostic accuracy in both a parametric (79) and nonparametric setting (80) can be used to improve the diagnostic accuracy. Another potential use of biomarkers is the prediction of the longitudinal course of the disease. Standard ‘survival’ analysis such as those based on Cox proportional hazards models (81) and generalized illness-death models (82) can be implemented.
Current Biomarker Panels
Of the fluid biomarkers, small panels that assess CSF Aβ42 with tau and/or p-tau181 are good diagnostic tools for predicting cognitive decline in individuals with cognitive impairment (22, 83), as well as in cognitively normal individuals who have preclinical AD (20, 22, 83, 84). The sensitivity and specificity for Aβ42, tau, p-tau181 alone range from 80–90% (12). Some studies have shown that the tau(s)/Aβ42 ratios have outstanding sensitivity (at 80% specificity) in identifying groups of individuals with amyloid deposition in the brain, ranging from 92–98%, and perform better than Aβ42 alone (sensitivity 85–89%) (85). Other small panels of novel biomarkers have been studied by groups around the world, some showing differential diagnostic sensitivity and specificity as high as 90% and 80%, respectively, for AD vs. dementia with Lewy bodies (DLB) and Parkinson’s disease with dementia (PDD) (86). The threshold for biomarker sensitivity and specificity considered acceptable for individual diagnosis remains unclear.
A panel of 18 plasma biomarkers was found suitable for diagnosis of AD both in the preclinical and clinical stages (87); however, these results were not replicated in a subsequent study (88). Other studies have reported panels of plasma biomarkers, including apoE, B-type natriuretic peptide, C-reactive protein, and pancreatic polypeptide, that identify individuals with AD dementia from normal controls or accurately reflect a diagnosis of AD in a discovery cohort (87, 89, 90). This indicates the need for replication and the use of large, independent sample sets. Such information will also help provide sensitivity and specificity measures for blood biomarkers, as is currently reported in studies of CSF.
Some studies have explored panels combining multiple biomarker modalities such as fluid and imaging. In these cases, biomarkers in combination panels have been reported to add significant value to one another – sensitivity increased from 83/84% for individual MRI/CSF Aβ42, respectively, to 89% when combined, and specificity increased from 90/79% for CSF MRI/Aβ42, respectively, to 95% when combined (91). It will be important to determine if the combination of fluid biomarkers with imaging biomarkers adds significant value for diagnosis, prognosis, and/or assessment of response to therapy in large varied cohorts before considering their widespread application in clinical or research settings.
Conclusions
An important challenge in AD biomarker research is the quality control of biomarkers. Aβ42 is plagued by inter-laboratory variability when using immunoassays and imaging by technological variability (32). The silver lining is that there are consistent trends for AD signatures as defined by the most highly validated biomarkers, including: 1) low CSF Aβ42 and high tau concentration; 2) positive amyloid imaging; 3) decreased glucose utilization in specific regions; and 4) hippocampal and whole brain atrophy.
Currently, CSF and imaging biomarkers are playing important roles in clinical trials that target AD in the preclinical and mildly symptomatic stages of the disease. In the Dominantly Inherited Alzheimer Network Trial Unit (DIAN TU) and the Alzheimer’s Prevention Initiative (API) (92, 93), two prevention trials in families with dominantly inherited AD-causing mutations, these markers will serve as measures of drug efficacy but will also double as measures of underlying pathology at different disease stages. Other trials, such as the Anti-amyloid treatment in Asymptomatic Alzheimer’s trial (A4) (94), will enroll cognitively normal older adults with evidence of amyloid pathology via amyloid imaging. Both applications of biomarkers represent a step forward in improving the diagnostic and prognostic capabilities in research settings and will help provide evidence that biomarkers can be reliably used in diagnosis of AD and for tracking therapeutic efficacy.
AD biomarkers have reached a critical stage in their development. As novel biomarkers are validated across large cohorts, it is likely that a panel of biomarkers will someday assist in the diagnosis of preclinical and symptomatic AD, to the exclusion of other forms of dementia. Should current or future clinical trials of AD drugs provide evidence that symptoms of the disease can be halted or delayed, such a diagnosis will be critical in giving the right patients the right therapies at the right time.
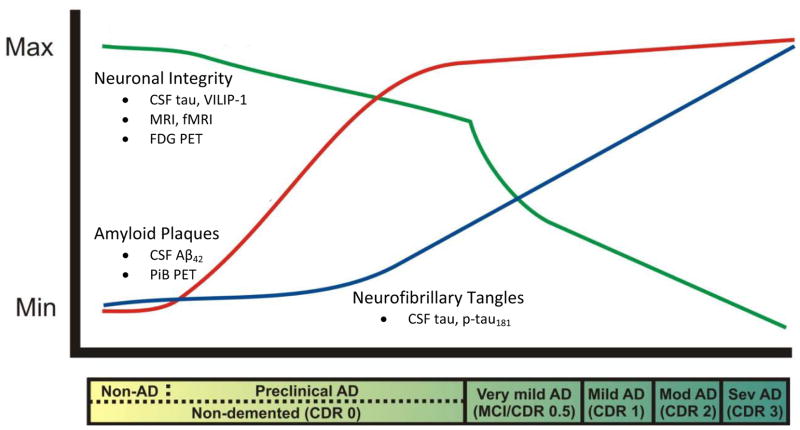
Figure 1.
Proposed biomarker trajectories in Alzheimer’s disease.
The clinical stages of Alzheimer’s disease are classified as “very mild AD/mild cognitive impairment (MCI),” “mild,” “moderate,” and “severe.” These stages correspond to scores of 0.5, 1, 2, and 3, respectively, on the Clinical Dementia Rating (CDR) scale (represented in the bar below the plot). All clinical stages are associated with the presence of amyloid plaques, but the plaque buildup begins in the preclinical stage, ~15 years prior to the onset of cognitive symptoms, and can be measured reliably using CSF Aβ42 and PET imaging with Pittsburgh Compound B (PiB) (red line). Neurofibrillary tangles seem to develop gradually later in the preclinical stage, the severity of which correlates well with CSF tau and p-tau181 levels (blue line). Synaptic and neuronal loss occurs robustly in association with the onset of dementia, and may be represented by CSF tau and/or VILIP-1 and MRI, fMRI and FDG PET (green line). The trajectories of inflammatory markers such as CSF YKL-40 are unknown at this time. The goal of AD biomarker research is to identify those preclinical individuals who will go on to develop dementia due to AD.
(Modified with permission from Perrin et al., Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature, 2009, 461:916–22).
Acknowledgments
Supported by the National Institutes of Health (P50-AG05681, P01-AG03991, P01– 26276). The authors thank Drs. Tammie Benzinger and Chengjie Xiong for helpful comments.
Footnotes
Financial Disclosures: Courtney L. Sutphen reports no biomedical financial interests or potential conflicts of interest. Anne M. Fagan reports being a member of the advisory board for Roche and Eli Lilly. David M. Holtzman co founded C2N Diagnostics, LLC and is on the scientific advisory board. He consults for Astra Zeneca, Genentech, and Bristol Myers Squibb. His laboratory receives research grant support from Astra Zeneca, Eli Lilly, and Biogen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization and Alzheimer’s Disease International. Dementia: A public health priority 2012 [Google Scholar]
- 2.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 3.Montine T, Phelps C, Beach T, Bigio E, Cairns N, Dickson D, et al. National Institute on Aging Alzheimer’s guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. ACTA Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handels R, Aalten P, Wolfs C, OldeRikkert M, Scheltens P, Visser P, et al. Diagnostic and economic evaluation of new biomarkers for Alzheimer’s disease: The research protocol of a prospective cohort study. BMC Neurol. 2012;12:72. doi: 10.1186/1471-2377-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrin RJ, Craig Schapiro R, Malone JP, Shah AR, Gilmore P, Davis AE, et al. Identification and validation of novel cerebrospinal fluid biomarkers for staging early Alzheimer’s disease. PLoS ONE. 2011;6:e16032. doi: 10.1371/journal.pone.0016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eli Lilly and Company. Lilly announces detailed results of the phase III solanezumab EXPEDITION studies following a presentation of the independent analyses by the Alzheimer’s disease cooperative study (ADCS) 2012 [Google Scholar]
- 8.Pfizer Inc. Phizer announces co primary clinical endpoints not met in second phase III bapineuzumab study in mild-to-moderate Alzheimer’s disease patients who do not carry the Apoe4 genotype 2012 [Google Scholar]
- 9.Eli Lilly and Company. Lilly provides update on next steps for solanezumab 2012 [Google Scholar]
- 10.Blennow K, Wallin AOH. Low frequency of post-lumbar puncture headache in demented patients. ACTA Neurol Scand. 1993;88:221–223. doi: 10.1111/j.1600-0404.1993.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 11.Peskind E, Nordberg A, Darreh-Shori T, Soininen H. Safety of lumbar puncture procedures in patients with Alzheimer’s disease. Curr Alzheimer Res. 2009;6:290–292. doi: 10.2174/156720509788486509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 13.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 14.Fagan AM, Mintun MA, Mach RH, Lee S-Y, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 15.Grimmer T, Riemenschneider M, Förstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta amyloid in Alzheimer’s disease: Increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiat. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapiola T, Alafuzoff I, Herukka S-K, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg A, Almkvist O, Engler H, Wall A, Langstrom B, Nordberg A. High PIB retention in Alzheimer’s disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;7:55–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 19.Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, et al. Dynamic analysis of β-amyloid protein in behaving mice reveals opposing changes in ISF versus parenchymal Aβ during age-related plaque formation. J Neurosci. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 22.Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor MD, Karlawish JH, Arnold SE, Khachaturian AS, Khachaturian ZS, Lee VMY, et al. Advancing alzheimer’s disease diagnosis, treatment, and care: Recommendations from the Ware Invitational Summit. Alzheimers Dement. 2012;8:445–452. doi: 10.1016/j.jalz.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling R, Johnson K. Biomarkers of Alzheimer disease: Current and future applications to diagnostic criteria. CONTINUUM: Lifelong Learning in Neurology. 2013;19:325–338. doi: 10.1212/01.CON.0000429181.60095.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 26.Schraen Maschke S, Sergeant N, Dhaenens C-M, Bombois S, Deramecourt V, Caillet-Boudin M-L, et al. Tau as a biomarker of neurodegenerative diseases. Biomark Med. 2008;2:363–384. doi: 10.2217/17520363.2.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buerger K, Ewers M, Pirttilä T, Zinkowski R, Alafuzoff I, Teipel SJ, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 28.Yamada K, Cirrito J, Stewart F, Jiang H, Finn M, Holmes B, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blom E, Giedraitis V, Zetterberg H, Fukumoto H, Blennow K, Hyman B, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the Apoe ε4/ε4 genotype. Dement Geriatr Cogn. 2009;27:458–464. doi: 10.1159/000216841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buerger K, Teipel S, Zinkowski R, Blennow K, Arai H, Engel R, et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2004;63:1144. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 32.Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, et al. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395. e6. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattsson N, Andreasson U, Persson S, Carrillo MC, Collins S, Chalbot S, et al. CSF biomarker variability in the Alzheimer’s Association quality control program. Alzheimers Dement. 2013;9:251–261. doi: 10.1016/j.jalz.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. NEJM. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J-M, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–1623. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, et al. Visinin-like protein-1: Diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70:274–285. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarawneh R, Lee J-M, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78:709–719. doi: 10.1212/WNL.0b013e318248e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Østergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 is elevated in cerebrospinal fluid from patients with purulent meningitis. Clin Vaccine Immunol. 2002;9:598–604. doi: 10.1128/CDLI.9.3.598-604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, et al. Ykl-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiat. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattsson N, Tabatabaei S, Johansson P, Hansson O, Andreasson U, Månsson J-E, et al. Cerebrospinal fluid microglial markers in Alzheimer’s disease: Elevated chitotriosidase activity but lack of diagnostic utility. Neuromol Med. 2011;13:151–159. doi: 10.1007/s12017-011-8147-9. [DOI] [PubMed] [Google Scholar]
- 42.Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med. 2012;6:455–476. doi: 10.2217/bmm.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kester MI, Scheffer PG, Koel-Simmelink MJ, Twaalfhoven H, Verwey NA, Veerhuis R, et al. Serial CSF sampling in Alzheimer’s disease: Specific versus non-specific markers. Neurobiol Aging. 2012;33:1591–1598. doi: 10.1016/j.neurobiolaging.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann Neurol. 2003;54:115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- 46.Mayeux R, Schupf N. Blood-based biomarkers for Alzheimer’s disease: Plasma Aβ40 and Aβ42, and genetic variants. Neurobiol Aging. 2011;32(Supplement 1):S10–S19. doi: 10.1016/j.neurobiolaging.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ingelson M, Blomberg M, Benedikz E, Wahlund LO, Karlsson E, Vanmechelen E, et al. Tau immunoreactivity detected in human plasma, but no obvious increase in dementia. Dement Geriatr Cogn. 1999;10:442–445. doi: 10.1159/000017187. [DOI] [PubMed] [Google Scholar]
- 49.Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, et al. A blood-based screening tool for Alzheimer’s disease that spans serum and plasma: Findings from TARC and ADNI. PLoS ONE. 2011;6:e28092. doi: 10.1371/journal.pone.0028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, et al. Identification of candidate IgG biomarkers for Alzheimer’s disease via combinatorial library screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement. 2012;8:S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox N, Warrington E, Freeborough P, Hartikainen P, Kennedy A, Stevens J, et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 54.Bobinski M, de Leon M, Convit A, De Santi S, Wegiel J, Tarshish C, et al. MRI of entorhinal cortex in mild Alzheimer’s disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- 55.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clifford R, Jack J, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csernansky J, Wang L, Joshi S, Miller J, Gado M, Kido D, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology. 2000;55:1636–1643. doi: 10.1212/wnl.55.11.1636. [DOI] [PubMed] [Google Scholar]
- 58.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 59.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: Predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 63.Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiol Aging. 2012;33:1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. PNAS. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J Neurosci. 2012;32:8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karow DS, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jennings RG, Brewer JB, et al. Relative capability of MR imaging and FDG pet to depict changes associated with prodromal and early Alzheimer disease. Radiology. 2010;256:932–942. doi: 10.1148/radiol.10091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, Wolk DA, Reddin JS, Korczykowski M, Martinez PM, Musiek ES, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 71.Mathis CA, Mason NS, Lopresti BJ, Klunk WE. Development of positron emission tomography β-amyloid plaque imaging agents. Semin Nucl Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klunk WE, Mathis CA. The future of amyloid-beta imaging: A tale of radionuclides and tracer proliferation. Curr Opin Neurol. 2008;21:683–687. doi: 10.1097/WCO.0b013e3283168e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, et al. Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Någren K, et al. Conversion of β-amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology. 2009;73:754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeng F, Goodman MM. Fluorine-18 radiolabeled heterocycles as PET tracers for imaging β - amyloid plaques in Alzheimer’s disease. Curr Top Med Chem. 2013;13:909–919. doi: 10.2174/1568026611313080004. [DOI] [PubMed] [Google Scholar]
- 78.Luo J, Xiong C. Youden index and associated cutoff points for three ordinal diagnostic groups. Commun Stat Simulat. 2012;42:1213–1234. doi: 10.1080/03610918.2012.661906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiong C, McKeel DW, Miller JP, Morris JC. Combining correlated diagnostic tests: Application to neuropathologic diagnosis of Alzheimer’s disease. Med Decis Making. 2004;24:659–669. doi: 10.1177/0272989X04271046. [DOI] [PubMed] [Google Scholar]
- 80.Pepe MS, Thompson ML. Combining diagnostic test results to increase accuracy. Biostatistics. 2000;1:123–140. doi: 10.1093/biostatistics/1.2.123. [DOI] [PubMed] [Google Scholar]
- 81.Cox D. Regression models and life-tables. J Roy Stat Soc B Met. 1972;34:187–220. [Google Scholar]
- 82.Commenges D. Inference for multi-state models from interval-censored data. Stat Methods Med Res. 2002;11:167–182. doi: 10.1191/0962280202sm279ra. [DOI] [PubMed] [Google Scholar]
- 83.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 84.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 85.Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, et al. Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1-42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol. 2011;68:1137–1144. doi: 10.1001/archneurol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or Parkinsonian disorders. Arch Neurol. 2012;69:1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 87.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 88.Björkqvist M, Ohlsson M, Minthon L, Hansson O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer’s disease. PLoS ONE. 2012;7:e29868. doi: 10.1371/journal.pone.0029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu WT, Holtzman DM, Fagan AM, Shaw LM, Perrin R, Arnold SE, et al. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM, et al. Plasma biomarkers associated with the apolipoprotein e genotype and Alzheimer disease. Arch Neurol. 2012;69:1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westman E, Muehlboeck JS, Simmons A. Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. Neuroimage. 2012;62:229–238. doi: 10.1016/j.neuroimage.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 92.Bateman R, Aisen P, De Strooper B, Fox N, Lemere C, Ringman J, et al. Autosomal-dominant Alzheimer’s disease: A review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reiman EM, Langbaum JBS, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s prevention initiative: A plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26:321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sperling RA, Jack CR, Aisen PS. Testing the right target and right drug at the right stage. Sci Trans Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Andersson M, Zetterberg H, Minthon L, Blennow K, Londos E. The cognitive profile and CSF biomarkers in dementia with Lewy bodies and Parkinson’s disease dementia. Int J Geriatr Psychiatry. 2011;26:100–105. doi: 10.1002/gps.2496. [DOI] [PubMed] [Google Scholar]
- 96.Reis T, Brandão CO, Freire Coutinho ES, Engelhardt E, Laks J. Cerebrospinal fluid biomarkers in Alzheimer’s disease and geriatric depression: Preliminary findings from Brazil. CNS Neurosci Ther. 2012;18:524–529. doi: 10.1111/j.1755-5949.2012.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Buerger K, Zinkowski R, Teipel SJ, Arai H, DeBernardis J, Kerkman D, et al. Differentiation of geriatric major depression from Alzheimer’s disease with CSF tau protein phosphorylated at threonine 231. Am J Psychiat. 2003;160:376–379. doi: 10.1176/appi.ajp.160.2.376. [DOI] [PubMed] [Google Scholar]
- 98.Schoonenboom NSM, Reesink FE, Verwey NA, Kester MI, Teunissen CE, van de Ven PM, et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78:47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 99.Van Everbroeck B, Green AJE, Pals P, Martin JJ, Cras P. Decreased levels of amyloid-β 1-42 in cerebrospinal fluid of Creutzfeldt-Jakob disease patients. J Alzheimers Dis. 1999;1:419–424. doi: 10.3233/jad-1999-1606. [DOI] [PubMed] [Google Scholar]
- 100.Otto M, Wiltfang J, Tumani H, Zerr I, Maria L, Kornhuber J, et al. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurosci Lett. 1997;225:210–212. doi: 10.1016/s0304-3940(97)00215-2. [DOI] [PubMed] [Google Scholar]