Abstract
Purpose
Drusen are hallmarks of age-related macular degeneration (AMD). Amyloid-beta 1-40 (Aβ 1-40), a constituent of drusen, is known to stimulate inflammatory pathways in RPE; however, its effect in vivo is not known. The purpose of this study was to examine the effect of Aβ 1-40 on cytokine expression and inflammasome activation relevant to AMD in an animal model.
Methods
Wild-type rats received intravitreal injections of Aβ 1-40, and eyes were taken at days 1, 4, 14, and 49 postinjection. The RPE, neuroretina, and vitreous were analyzed for cytokine expression, inflammasome activation, and microglial response via RT-PCR, immunohistochemistry, and suspension array assay. Retinal cell loss was assessed via apoptotic markers and retinal thickness.
Results
Aβ 1-40 stimulated upregulation of IL-6, TNF-α, IL-1β, IL-18, caspase-1, NLRP3, and XAF1 genes in the RPE/choroid and the neuroretina. Increased IL-1β and IL-6 immunoreactivity was found in retinal sections, and elevated levels of IL-1β and IL-18 were found in the vitreous of Aβ-injected eyes. Aβ 1-40 induced a moderate increase in CD11b/c-reactive cells on day 1 postinjection only. No evidence of the proapoptotic XAF1 protein, p53, TUNEL immunoreactivity, or retinal thinning was observed.
Conclusions
These results confirm earlier in vitro work and support the proinflammatory role of drusen component Aβ 1-40 in the RPE and retina. Inflammasome activation may be responsible for this effect in vivo. This model is useful for understanding cellular triggers of inflammasome activation and proposed early inflammatory events in the outer retina associated with the etiology of AMD.
Age-related macular degeneration (AMD) is a multifactorial disease that is responsible for a significant proportion of visual impairment in the elderly in Western society.1,2 Early AMD is characterized by the presence of drusen and abnormal pigmentation of retinal pigment epithelium (RPE)3,4 and is associated with gradual vision loss.5 In some cases early AMD will progress to a late, more advanced form that causes profound loss of central vision.6 Little is known about the etiology of this disease or the processes that occur in the eye during the early stages of AMD. Identifying the molecular and cellular events that lead to symptomatic AMD and the development of treatment strategies that act at the early stages of the disease are therefore of high priority.7
One of the strongest predictors of AMD is the number and size of drusen.5 Amyloid-beta (Aβ), the peptide associated with neurodegenerative events in the brain in Alzheimer’s disease (AD), is an important constituent of drusen (Fig. 1).5,8 By analogy, the presence of Aβ in drusen raises the possibility that it may also contribute to neurodegenerative events in the retina in AMD.9,10 A study on donor eye tissue established a correlation between Aβ and geographic atrophy (GA).11 A recent clinical trial addressing the safety and efficacy of anti-Aβ treatment for AMD has begun, further emphasizing the need to understand the specific mechanisms underlying the effect of Aβ in the eye.7
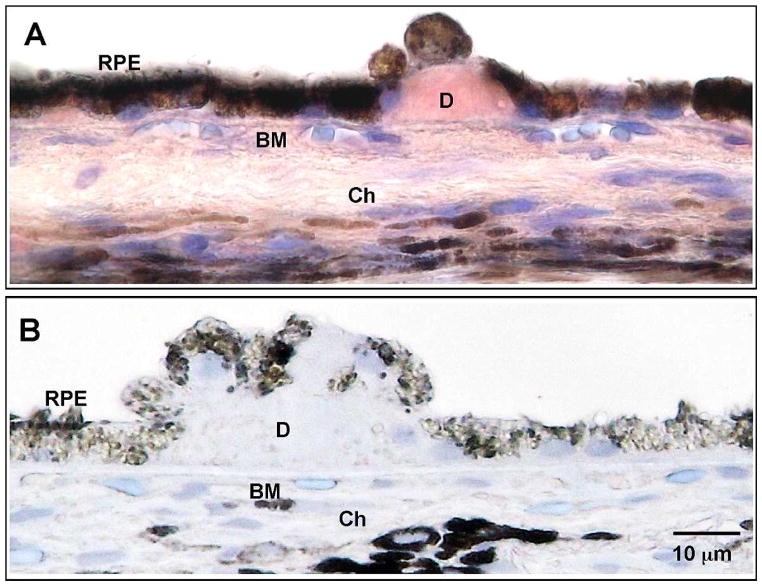
Figure 1.
Aβ 1-40 in drusen. Immunohistochemistry for Aβ 1-40 was developed in AEC (red) and counterstained with Mayer’s hematoxylin (blue). (A) Aβ 1-40 in drusen of a 72-year-old non-GA female postmortem donor eye. (B) Negative control using nonimmune rabbit IgG isotype antibody on the same donor eye section. RPE, retinal pigment epithelium; D, drusen; BM, Bruch’s membrane; Ch, choroid. Scale bar: 10 μm.
In contrast to past studies that used the more AD-specific, Aβ 1-42 peptide, we focused on the structurally similar Aβ 1-40 peptide in this study.12 Aβ 1-40, the more common and less toxic form, is present in drusen13,14 and is thus a relevant candidate to consider for AMD (Fig. 1). Using microarray analysis, Kurji et al.15 identified genes in the immune response and inflammation pathways as being highly upregulated by RPE cells in response to Aβ 1-40 stimulation in vitro. There is emerging evidence that these pathways may be mediated by inflammasome activation.16 Recent studies on AMD pathogenesis have concentrated on NLR family, pyrin domain containing 3 (NLRP3) inflammasome activation in the RPE. The inflammasome NLRP3 is an intracellular multiprotein complex that recruits caspase-1 to catalyze the formation of IL-1β and IL-18,17,18 along with other cytokines and growth factors such as IL-33 and fibroblast growth factor.19 However, important questions remain, such as whether inflammasome activation is involved in the retina’s response to Aβ and whether exposure to inflammatory mediators such as IL-1β and IL-18 leads to AMD-like pathology.
To elucidate the effect of Aβ 1-40 in vivo and investigate the sequelae of inflammatory mediators on the retina and RPE in the context of early AMD, we performed intravitreal injection of Aβ 1-40 in a rodent model and studied the expression profile of key genes involved in the putative pathways of AMD pathogenesis, with a focus on inflammation and apoptosis. Intravitreal injection was chosen because it achieves the same effect of delivering peptide to outer retina/RPE as subretinal injections without the associated retinal damage or detachment, which may itself cause inflammation and confound the results.20,21 We hypothesized that Aβ would stimulate inflammasome activation and overexpression of inflammatory mediators, including cytokines, in the retina and RPE; such a response to Aβ may occur in the outer retina of presymptomatic AMD patients. Thus, treatments to minimize Aβ’s effect in the eye may slow the progression of AMD, a strategy being investigated in clinical trials.22,23
Methods
Ethics
The postmortem tissue study was approved by the Clinical Research Ethics Board at the University of British Columbia. Methods for securing human tissue were in compliance with the Declaration of Helsinki. The animal procedures were carried out according to the protocol reviewed and approved by the University of British Columbia Animal Care Committee and conformed to the Canadian Council on Animal Care guidelines. All animal studies were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Donor Eye Tissue Immunohistochemistry
Human eyes were obtained from the Eye Bank of British Columbia. Eye tissues were fixed in 10% formalin and embedded in paraffin to obtain 6 μm sections through the pupil and optic nerve axis. Sections were deparaffinized and rehydrated by standard procedures. After antigen retrieval in 70% formic acid for 18 minutes at room temperature (RT), sections were blocked with 3% H2O2 for 15 minutes and 3% goat serum for 40 minutes. Sections were incubated in primary antibody specifically against Aβ 1-40 (Cell Signaling Technology, Beverly, MA; Table 1) overnight at 4°C. Primary antibody omission or nonimmune isotype antibodies were used as negative controls. Sections were then incubated in appropriate secondary antibodies and developed in the ABC–AEC system (Vector Laboratories, Burlingame, CA).
Table 1.
Primary Antibodies Used in Immunohistochemistry
| Antigen | Primary Antibody | Type | Dilution | Specificity | Source and Catalog No. (Clone No.) |
|---|---|---|---|---|---|
| Amyloid-beta (Aβ) 1-40 | Rabbit antihuman | Polyclonal | 1:200 | Recognizes human Aβ 1-40 isoform and crossreacts with mouse, rat, and monkey isoforms | Cell Signaling Technology, Beverly, MA; 9682 |
| Amyloid-beta (Aβ) | Mouse antihuman | Monoclonal | 1:500 | Recognizes amino acid residues 17 to 24 of Aβ, crossreacts with mouse isoform | Covance, Princeton, NJ; SIG-39220 (4G8) |
| Interleukin-1 beta (IL-1β) | Goat antirat | Polyclonal | 1:400 | Detects rat IL-1β/IL-1F2 in cultured cells or tissue sections | R&D Systems, Inc., Minneapolis, MN; AF-501-NA |
| Interleukin-6 (IL-6) | Rabbit antirat | Polyclonal | 1:400 | Detects recombinant and native IL-6 secreted into body fluids and/or cell supernatants in rabbits, humans, mice, rats, and pigs | Abcam, Cambridge, UK; ab6672 |
| OX-42 (CD11b/c equivalent antibody) | Mouse antirat | Monoclonal | 1:300 | Detects rat CD11 b/c in macrophages/dendritic cells with morphology of microglia in the brain | Cedarlane, Hornby, ON; CL042AP (MRC OX-42) |
| X-linked inhibitor of apoptosis protein- associated factor 1 (XAF1) | Rabbit antirat | Polyclonal | 1:500 | Detects cytoplasmic, mitochondrial, and nuclear XAF1 in rabbits, humans, mice, and rats | Abcam, Cambridge, UK; ab81353 |
Aβ Oligomerization
Aβ 1-40 (American Peptide, Sunnyvale, CA) was prepared as previously described.15 Briefly, lyophilized Aβ peptide was dissolved in hexa-fluoroisopropanol and agitated at RT for 48 hours. Oligomerization was confirmed with atomic force microscopy and dot blot assay. Stock Aβ was diluted in phosphate-buffered saline (PBS, pH 7.4) to yield a final concentration of 1.4 μg/μL. Aliquots of this solution were kept at −80°C until use. Human reverse peptide Aβ 40-1 (American Peptide) was prepared in an identical manner.
Animal Model and Treatment
Five-month-old male Long-Evans rats (Charles River Laboratory, Wilmington, MA) were raised on standard rodent diet and kept in an enclosure with environmental enrichment and a 12-hour light cycle. On day 0, under inhalational anesthesia, intravitreal injections were performed under a dissecting microscope (Stereo dissection microscope, SMZ 1000; Nikon, Tokyo, Japan) using a 32-gauge Hamilton needle and syringe (Hamilton, Reno, NV) to deliver 5 μL oligomeric Aβ 1-40 peptide (7 μg) with 0.1 μL 10% sodium fluorescein as dye (Akorn, Buffalo Grove, IL). This dosage yields an Aβ intravitreal concentration of approximately 30 μM, higher than that used in the in vitro experiment15 and comparable to in vivo doses in other studies.21,24,25 Age-matched control animals received 5 μL intravitreal injection of reverse peptide Aβ 40-1 (1.4 μg/μL) or vehicle (PBS). After the procedure, animals were given oxygen and were monitored in a recovery enclosure until they resumed baseline activity level. Animals were kept for 1, 4, 14, and 49 days postinjection (n = 7 per treatment group), and euthanasia was performed with CO2 inhalation. Eyes were immediately enucleated and frozen or preserved in 4% paraformaldehyde in Dulbecco’s phosphate-buffered saline (DPBS; Invitrogen, Carlsbad, CA).
Real-Time PCR
The anterior segment of the frozen eye was removed to expose the posterior eye cup. Separate samples of neuroretina or RPE/choroid were obtained from the eye cup by surgical dissection under a dissecting microscope (Stereo dissection microscope, SMZ 1000; Nikon). Total RNA was isolated from tissue using the RNAqueous-4PCR Kit (Ambion, Austin, TX). RNA quantity and quality were assessed using the Nanodrop 2000c spectrophotometer (Fisher Thermo Scientific, Wilmington, DE). RNA (750 ng) from each tissue was reverse transcribed into cDNA using the High Capacity RNA-to-cDNA Master Mix (Invitrogen). PCR primer sequences are listed in Table 2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as endogenous control. All reactions were carried out on the Applied Biosystems 7500 Fast SDS (Applied Biosystems, Carlsbad, CA) using Power SYBR Green (Applied Biosystems). Each sample was repeated in triplicate with near-identical results. Cycling conditions were as follows: 95°C for 15 seconds, 58°C for 30 seconds, 60°C for 45 seconds, 40 cycles. Melting curve analysis was automatically performed immediately after amplification. Gene product was quantified relative to GAPDH using the 2−ΔΔCT method.
Table 2.
RT-PCR Primer Sequences
| Gene | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| IL-6 | TCAACTCCATCTGCCCTTCAG | AAGGCAACTGGCTGGAAGTCT | Sanchez et al., 200364 |
| TNF-α | AAATGGGCTCCCTCTCATCAGTTC | GCTTGGTGGTTTGCTACGAC | Peinnequin et al., 200465 |
| IL-1β | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC | Peinnequin et al., 200465 |
| IL-18 | AGAAGGCTCTTGTGTCAAC | CTTCCTTTTGGCAAGCTAG | |
| Caspase-1 | AGAGAAGAGAGTCCTGAAC | TCTCTGAGGTCAACATCAG | |
| NLRP3 | CAGAAGGCATGTGAGAAG | ACAGGATCTTGCAGACTG | |
| XAF1 | GGAGAGGAGACAGCCTATG | CCTGGTGCTCATTTAGAA | |
| VEGF | TGCAGACCAAAGAAAGATAGAAC | GGATCTTGGACAAACAAATGC | Mor et al., 200466 |
| GAPDH | GAACATCATCCCTGCATCCA | CCAGTGAGCTTCCCGTTCA | Medhurst et al., 199967 |
Retinal Tissue Immunohistochemistry
Rat paraffin tissue sections cut at a thickness of 4 μm were prepared through standard procedures as described in our previous publication.26 Sections of retina within 200 μm from the optic disc were known to be of even thickness regardless of embedding orientation27 and thus were selected for processing.
To identify the localization of intravitreally injected Aβ 1-40 peptide, anti-Aβ antibody (4G8; Covance, Princeton, NJ; Table 1) was applied to tissue sections for 1 hour at RT and subsequently left overnight at 4°C, following antigen retrieval in 70% formic acid and blocking steps (3% H2O2, 5% goat serum). Incubation in primary antibody was followed by standard biotinylated secondary antibody incubation. These sections were developed with VIP chromogen (Vector Laboratories) and counterstained with methyl green.
Immunohistochemistry was also performed to evaluate the product of selected upregulated genes in the retina. Sections first underwent antigen retrieval, removal of endogenous peroxidase, and normal serum blocking by sequential incubation in protease K (20 μg/mL, pH 8.0; Sigma-Aldrich, St. Louis, MO), 0.3% H2O2, and 3% normal horse serum. Sections were then incubated overnight at 4°C with monoclonal antibodies including interleukin-6 (IL-6), interleukin-1 beta (IL-1β), X-linked inhibitor of apoptosis protein-associated factor 1 (XAF1), and CD11b/c, a cell surface marker for microglial cells (Table 1). After development with VIP chromogen, sections were photographed at ×20 and ×60 magnification using a brightfield microscope (Eclipse Ni U; Nikon) with a digital camera attachment (DS-Fi2 camera and DS-L3 monitor; Nikon). Microscopic scoring was conducted by scanning whole retinal sections under ×20 magnification in 1000 μm increments (diameter of the field) and averaging the number of immunoreactive cells per increment. The final mean immunoreactive cell count was the averaged score of a minimum of two to four retinal sections per animal at each time point.
Retinal thickness was measured from the internal limiting membrane to the photoreceptor outer segments/RPE junction, and the mean value was derived from measurements of two to four retinal sections per animal at each time point.
Suspension Array Assay for Secreted Cytokines
Rat vitreous was collected and aliquoted for cytokine analysis using suspension 23-plex cytokine array plate (Bio-Rad Laboratories, Hercules, CA) as described by the manufacturer. Fifty microliters each of cytokine standards, samples (pooled vitreous fluids), and blanks (water only) was incubated with 25 μL anticytokine conjugated beads in 96-well filter plates for 30 minutes at RT with agitation (1100 rpm for 30 seconds, then 300 rpm for 30 minutes). Plates were subsequently washed three times by vacuum filtration with 100 μL Bio-Plex (Bio-Rad Laboratories) wash buffer per well using the Bio-Plex Pro Wash Station (Bio-Rad Laboratories). This was followed by the addition of 25 μL diluted biotinylated detection antibody and further incubation with agitation at RT. After three filter washes (described above), 25 μL streptavidin-phycoerythrin (SAPE) was added, and the plates were incubated for 10 minutes at RT with agitation. Finally, plates were washed by vacuum filtration three times. Beads were subsequently resuspended in 125 μL Bio-Plex assay buffer, vortexed for 30 seconds at 1100 rpm, and further incubated for 2 minutes at 300 rpm. Standards, samples, and blanks were analyzed using the Bio-Plex 200 Suspension Array System, and subsequent raw median fluorescent intensity (MFI) data were captured and analyzed using Bio-Plex Manager software 4.1 (Bio-Rad Laboratories) at a standard high photomultiplier tube (PMT) setting.
Statistical Analysis
Statistical analyses on RT-PCR and immunohistochemical studies were performed between the Aβ 1-40 group and the reverse peptide group and/or the vehicle control group using Student’s t-test with unequal variance. Suspension array data statistics were performed using Bio-Plex Manager software 4.1 (Bio-Rad Laboratories). Standard curves were developed using Brendon’s Five Point logistic regression analysis, and a recovery range of 70% to 130% was established for the determination of a statistically valid standard curve.28 RT-PCR and suspension array data are expressed as mean ± SE; immunohistochemical data are expressed as mean ± SD with P < 0.05 set as threshold for statistical significance.
Results
Aβ 1-40 Is a Component of Drusen
We first used a specific antibody to verify that Aβ 1-40, the more prevalent but less toxic form of Aβ peptide, is a component of human drusen (Fig. 1A). Using nonimmune isotype antibody yielded no Aβ 1-40 immunoreactivity (Fig. 1B). This is consistent with previous studies of drusen composition.8
Presence of Aβ 1-40 in All Retinal Layers after Intravitreal Injection
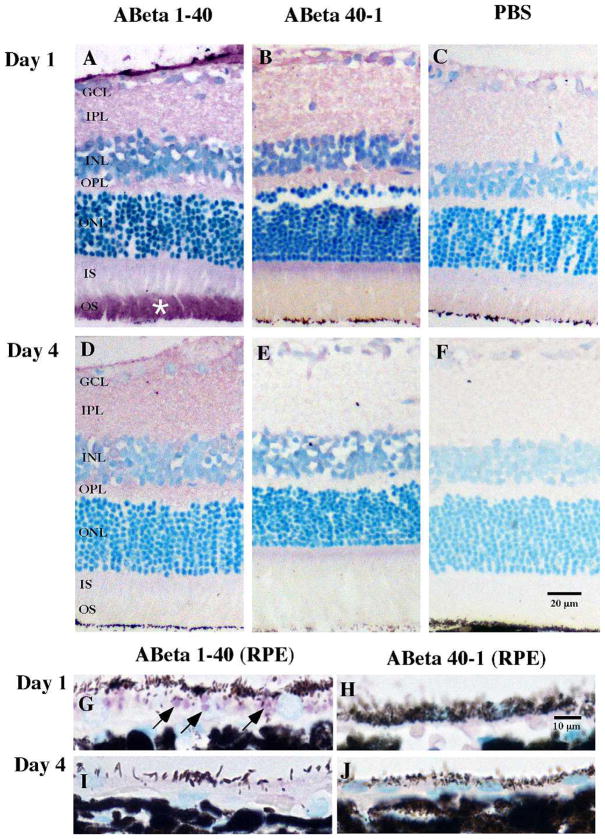
To confirm the effectiveness of intravitreal injection in delivering peptide to the outer retina, we probed the retinal tissue sections with 4G8 antibody that binds residues 17 to 24 in the human Aβ peptide. In Aβ 1-40-injected eyes, Aβ immunoreactivity was detected throughout retinal layers on day 1; the greatest intensity was localized to the photoreceptor outer segment (OS), while the RPE also demonstrated marked immunoreactivity (Figs. 2A, 2G). This immunoreactive pattern was less intense on day 4 (Figs. 2D, 2I). Preferential accumulation of Aβ in the OS has been previously reported in both mice and humans.29 Our results were in keeping with previous reports21 and verified the penetration of intravitreally injected peptide through the retina and reaching the RPE. The reverse peptide Aβ 40-1 sections showed significantly less 4G8 immunoreactivity in the retina (Figs. 2B, 2E, 2H, 2J); this was expected as the 4G8 antibody is specific to the forward (1-40) peptide. We observed background levels of immunoreactivity in vehicle-injected eyes, confirming minimal nonspecific binding of the antibody (Figs. 2C, 2F).
Figure 2.
Localization of Aβ 1-40 or reverse peptide Aβ 40-1 demonstrated in retinal tissue on days 1 and 4 following intravitreal injections. Immunoreactivity for Aβ 1-40 and Aβ 40-1 was undertaken with 4G8 antibody, visualized with VIP chromogen (pink/purple), and counterstained with methyl green. VIP chromogen revealed immunoreactivity in the neuropil and extracellular compartments throughout all retinal layers on day 1 and less on day 4 in the Aβ group (A, D). Strong immunoreactivity was detected in the OS (star) in Aβ-injected eyes. Tissues from eyes receiving reverse peptide injection demonstrated significantly less immunoreactivity than the forward peptide group (B, E), as did the vehicle-injected eyes (C, F). Focusing on the RPE, there are discrete intracellular vesicles (arrows) possibly representing phagocytosed outer segment discs in the Aβ group compared to the reverse peptide group in day 1 sections (G, H). Day 4 sections show no vesicles but rather a diffuse and faint layer of VIP chromogen at the RPE in the Aβ group compared to the reverse peptide group (I, J). GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS, inner segment; OS, outer segment. Scale bar: 20 μm (A–F), 10 μm (G–J).
Gene Expression Changes after Aβ 1-40 Intravitreal Injections
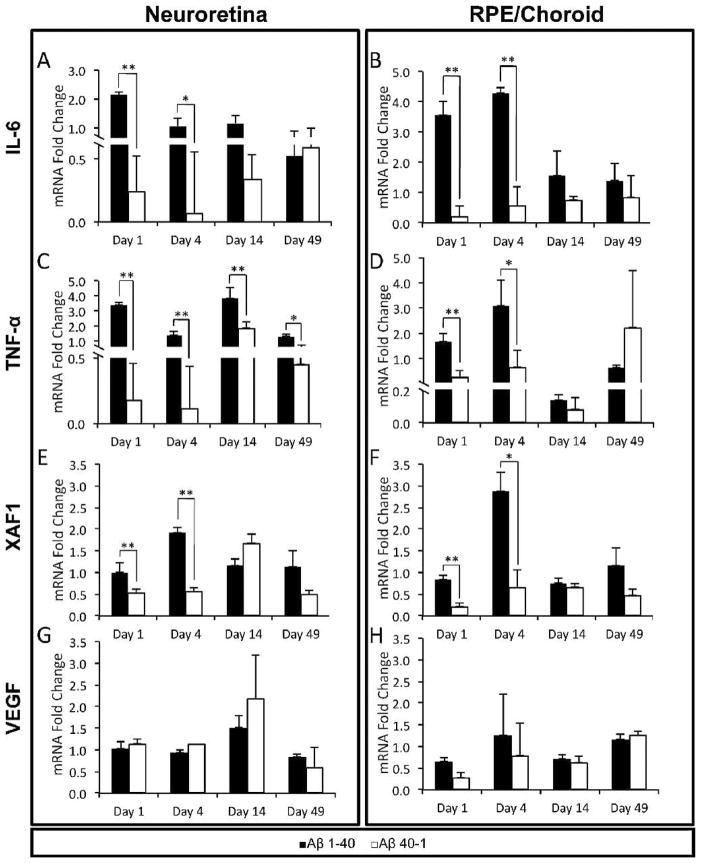
To assess whether the proinflammatory effect of Aβ on RPE extrapolates to an in vivo setting, we first screened for expression of inflammatory mediators by examining two cytokines. IL-6 is a known inducer of acute-phase protein production, and its serum level has been associated with progression of AMD.30 In the Aβ group, there was significant upregulation of IL-6 gene on days 1 and 4 compared to reverse peptide (Figs. 3A, 3B). The RPE/choroid exhibited a greater fold change than the neuroretina. No significant IL-6 upregulation was found in either tissue at the later time points.
Figure 3.
RT-PCR of selected genes in the RPE/choroid and neuroretina. The relative mRNA quantity (normalized to endogenous control GAPDH and expressed as fold change over vehicle control PBS) in the Aβ 1-40 group was compared to that in the reverse peptide group. Genes were examined on days 1, 4, 14, and 49 to capture both immediate and later changes after intravitreal injection at day 0: (A, B) IL-6, (C, D) TNF-α, (E, F) XAF1, (G, H) VEGF. Error bars denote standard error. *P < 0.05; **P < 0.01.
TNF-α is a prototypic cytokine with a potent effect on the retina and RPE.20,31 The Aβ group showed significantly higher levels of TNF-α mRNA than the reverse peptide group in the neuroretina at all time points, with a relatively greater response on days 1 and 14 (Fig. 3C). In contrast, TNF-α in the RPE/choroid from the Aβ group was significantly upregulated on day 1 and increased further on day 4, but was no longer significant at days 14 and 49 (Fig. 3D).
Apoptosis is a putative mechanism of RPE and photoreceptor loss in AMD, but its exact trigger and onset remain unknown. XAF1, a proapoptotic gene, was markedly overexpressed in RPE stimulated by Aβ 1-4015; therefore it was examined here. Compared to reverse peptide and vehicle, Aβ caused significantly higher fold change in XAF1 expression in the neuroretina and RPE/choroid on days 1 and 4 (Figs. 3E, 3F). The magnitude of change was slightly greater in the RPE/choroid than in the neuroretina. No significant fold change in XAF1 expression was found on days 14 and 49 in either tissue.
Given the central role of vascular endothelial growth factor (VEGF) in stimulating choroidal neovascularization (CNV) in late AMD, we assessed the expression profile of VEGF. VEGF upregulation has been observed in RPE cells treated with Aβ 1-4032 and in vivo after an intravitreal injection with Aβ 1-42.24 Our results, however, showed no significant upregulation of VEGF in either neuroretina or RPE/choroid in the Aβ 1-40 group compared to the reverse peptide group throughout the time course of the study (Figs. 3G, 3H). This is consistent with our earlier in vitro observations using Aβ 1-40.15
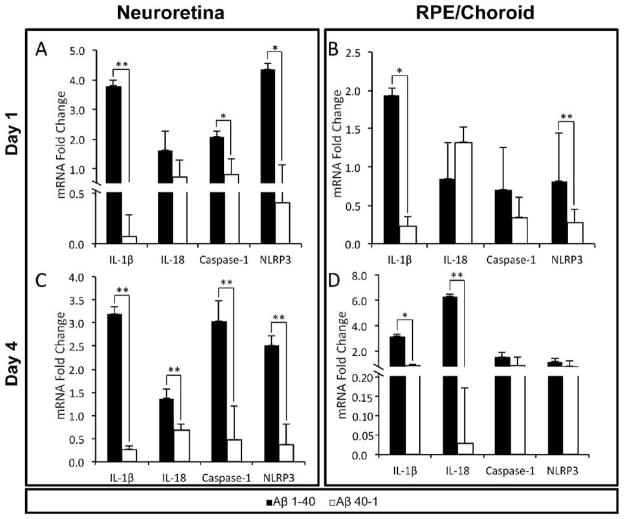
Recently, inflammasome NLRP3 activation has been proposed as a potential mechanism promoting inflammation in AMD.17 The increased levels of inflammatory cytokines in response to Aβ in our animals raised the possibility that NLRP3 could mediate cytokine upregulation in this setting. The expression profiles of IL-6 and TNF-α indicated that the strongest inflammatory response occurred on days 1 and 4 postinjection; therefore we sought evidence of inflammasome activation at these time points using IL-1β, IL-18, caspase-1, and NLRP3 genes. IL-1β and IL-18, two proinflammatory cytokines, are products of NLRP3 inflammasome processing,33 and IL-1β was markedly elevated in RPE upon Aβ 1-40 stimulation in vitro.15 Caspase-1 is part of the NLRP3 inflammasome complex and is required for the cleavage of IL-1β and IL-18 propeptides.16 In our study, IL-1β transcription was significantly elevated in the Aβ group on day 1; the neuroretina showed a greater fold change than the RPE/choroid (Figs. 4A, 4B). In contrast, IL-18 was not significantly upregulated on day 1 in the Aβ group. Caspase-1 expression was significantly higher in the neuroretina but not RPE/choroid on day 1 (Fig. 4A). NLRP3 was markedly upregulated in the Aβ group, with neuroretina showing a greater response than RPE/choroid (Figs. 4A, 4B). The trend for inflammasome marker was similar on day 4. Of note is the significant upregulation of IL-18 at this later time point, with the RPE/choroid showing the largest fold increase (Figs. 4C, 4D).
Figure 4.
Inflammasome gene RT-PCR data. The relative mRNA quantity (normalized to endogenous control GAPDH and expressed as fold change over vehicle control PBS) in the Aβ 1-40 group was compared to that in the reverse peptide group. (A, B) Day 1, (C, D) day 4. Error bars denote standard error. *P < 0.05; **P < 0.01.
Cytokine Levels in the Retina and Vitreous following Aβ 1-40 Intravitreal Injection
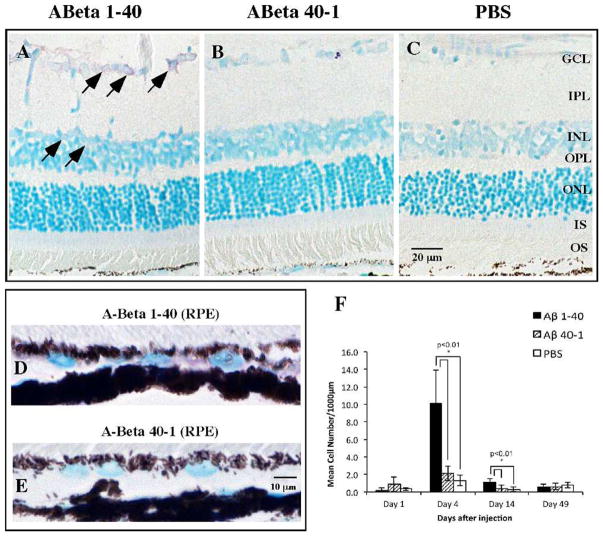
Based on the overexpression of inflammatory mediator genes in both neuroretina and RPE/choroid, we performed immunohistochemistry on retinal sections to further study the distribution of selected mediators. IL-6 immunoreactivity in the Aβ-injected eyes was distributed predominantly in the ganglion cell layer (GCL) and inner nuclear layer (INL) (Figs. 5A–C), but the RPE also demonstrated significantly greater immunoreactivity compared to that in the reverse peptide group (Figs. 5D, 5E). The overall number of IL-6-immunoreactive cells was greater in the Aβ group than in the reverse peptide or vehicle control group on day 4 (Fig. 5F), corresponding with the peak in IL-6 mRNA level (Figs. 3A, 3B). This difference was diminished on day 14 but still significant. By day 49, however, there was no significant difference between the groups.
Figure 5.
IL-6 immunoreactivity in the retina and RPE. (A–C) Representative retinal micrographs of IL-6 immunoreactivity on day 4. In the Aβ group, IL-6 immunoreactivity was mostly detected in cellular profiles in the GCL and INL (arrows). In contrast, the reverse peptide group shows very sparse IL-6 immunoreactivity, while the vehicle injection group identifies background level of IL-6 immunoreactivity. (D, E) The Aβ group also demonstrates more intense IL-6 immunoreactivity in the RPE than the reverse peptide group. (F) Bar graph of mean cell count of IL-6-immunoreactive cells at four time points, showing significantly greater IL-6 immunoreactivity in the Aβ group on days 4 and 14 than in the reverse peptide or vehicle control group. Micrographs were examined at ×20 and ×60 magnification. Scale bar: 20 μm (A–C), 10 μm (D, E). Error bars denote standard deviation. *P < 0.01.
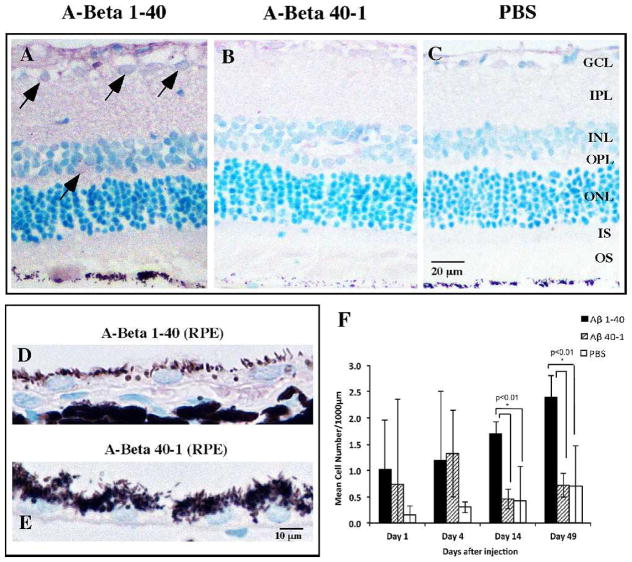
IL-1β immunoreactivity was primarily detected in the GCL and INL in the Aβ group, with sparse immunoreactivity in the reverse peptide and vehicle control groups (Figs. 6A–C). The RPE also demonstrated greater IL-1β immunoreactivity in the Aβ group than in the reverse peptide group (Figs. 6D, 6E). Comparing the cell counts among the groups, we noted more IL-1β-immunoreactive cells in both the Aβ and reverse peptide groups than in vehicle control on days 1 and 4, but these differences did not reach significance threshold (Fig. 6F). On days 14 and 49, the Aβ group, compared to the reverse peptide and vehicle control groups, had significantly more IL-1β-immunoreactive cells.
Figure 6.
IL-1β immunoreactivity in the retina and RPE. (A–C) Representative retinal micrographs of IL-1β immunoreactivity on day 14. Marked IL-1β immunoreactivity is observed in the Aβ group and is predominantly localized to cellular profiles in GCL and INL (arrows) and in the neuropil IPL and OPL. Reverse peptide group demonstrates weak IL-1β immunoreactivity without any foci of distribution. Vehicle injection group demonstrates background IL-1β immunoreactivity. (D, E) Aβ group shows greater IL-1β immunoreactivity in the RPE than the reverse peptide group. (F) Bar graph of mean cell count of IL-1β-immunoreactive cells at four time points, showing significantly more IL-1β immunoreactivity in the Aβ group on days 14 and 49 compared to the reverse peptide or control group. Micrographs were examined at ×20 and ×60 magnification. Scale bar: 20 μm (A-C), 10 μm (D, E). Error bars denote standard deviation. *P < 0.01.
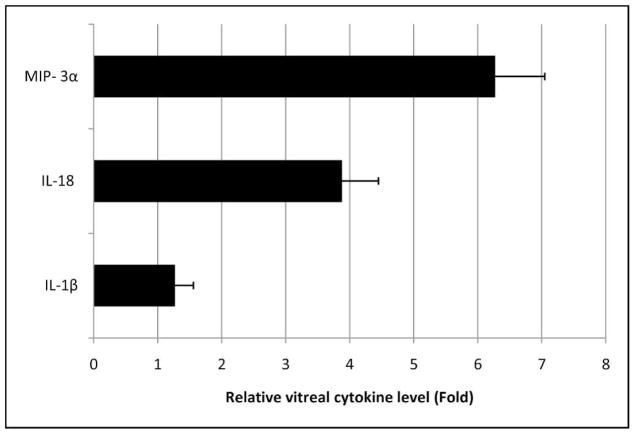
Increased vitreal levels of inflammatory cytokines are present in patients with retinal pathologies including proliferative diabetic retinopathy34 and central retinal vein occlusion.35 Few studies have assessed the cytokines in the vitreous of AMD patients. In our study, the elevated amount of IL-1β immunoreactivity in the retina exposed to Aβ prompted us to look further for evidence of cytokines in the vitreous in this model. Using ELISA-based assay, we found significantly higher levels of IL-1β and IL-18 in the vitreous of Aβ-injected eyes (13,920 ± 433 pg/mL and 1126 ± 264 pg/mL, respectively) than in reverse peptide controls (9803 ± 280 pg/mL and 440 ± 56 pg/mL, respectively) (Fig. 7). Of special note, Macrophage Inflammatory Protein 3 alpha (MIP-3α), a lymphocyte and dendritic cell chemokine, showed the greatest increase in vitreal concentration in the Aβ group (759 ± 54 pg/mL) compared to the reverse peptide group (199 ± 21 pg/mL) (Fig. 7).
Figure 7.
Day 14 vitreous cytokine suspension array assay. Only statistically significant results (P < 0.05) are shown. Inflammasome product IL-1β was significantly elevated in Aβ-injected vitreous (13,920 ± 433 pg/mL) compared to reverse peptide–injected vitreous (9803 ± 280 pg/mL). Similarly, IL-18 showed significantly higher concentration in Aβ-injected vitreous (1126 ± 264 pg/mL) than in reverse peptide–injected vitreous (440 ± 56 pg/mL). MIP-3α showed the greatest difference between the Aβ group (759 ± 54 pg/mL) and the reverse peptide group (199 ± 21 pg/mL). Other cytokines including IL-6 and TNF-α did not reach threshold of significance (P < 0.05). Error bars denote standard error.
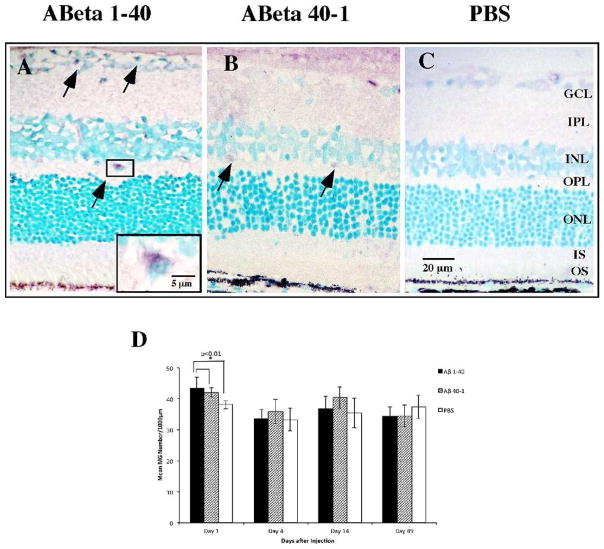
Microglia Activation Was Significant at Day 1
Microglia are known to take up Aβ and generate an inflammatory response that affects RPE function36,37; this has been proposed as a possible mechanism of AMD pathogenesis. To test this hypothesis we evaluated the microglial response to Aβ 1-40 using OX-42 antibody against CD11b/c antigen, a cell surface marker representing total microglia (active and resting) (Figs. 8A–C). The majority of CD11b/c-positive cells were localized to the GCL and INL, and some demonstrated evidence of activation based on their rounded morphology (Fig. 8A, inset). We observed a statistically significant but marginal increase in CD11b/c-positive cells in the Aβ group compared to reverse peptide and vehicle control on day 1 postinjection (Fig. 8D). No significant difference in microglial count was observed among the groups at other time points.
Figure 8.
Microglia response to Aβ 1-40 stimulation. Retinal sections were reacted with OX-42 antibody against cell surface marker CD11b/c, representative of total microglia. (A–C) Representative immunoreactivity pattern of CD11b/c labeling in the retinal sections on day 1. Note that the Aβ section and the reverse peptide section both contain CD11b/c-positive cells within the GCL and INL (arrows), with the vehicle group demonstrating significantly lower CD11b/c immunoreactivity. Some CD11b/c-positive cells demonstrate rounded morphology and are intimately associated with cell bodies of retinal neurons (inset). (D) Bar graph of mean CD11b/c immunoreactivity profiles in three groups, with significance demonstrated at day 1 between the Aβ 1-40 forward peptide group and the reverse peptide and vehicle control groups. Micrographs were examined at ×20 and ×60 magnification. Scale bar: 20 μm; 5 μm (inset). Error bars: standard deviation. *P < 0.01.
Cell Death and Neovascularization Were Not Associated with Aβ 1-40
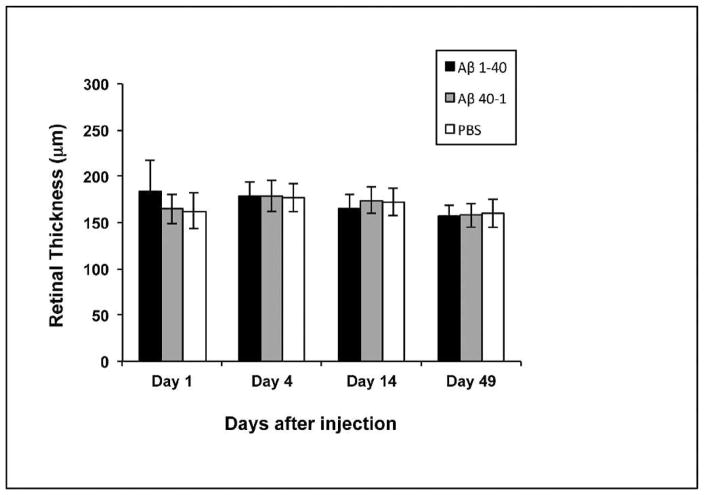
Geographic atrophy represents a late stage of AMD and is characterized by extensive RPE and photoreceptor loss that is thought to occur via apoptosis. Cytokines including TNF-α have been linked to photoreceptor apoptosis20; therefore it is important to establish whether apoptosis is a feature in this model of early AMD. We first looked for protein expression of the proapoptotic factor XAF1. Despite upregulation of XAF1 gene, we did not detect higher XAF1 immunoreactivity in the retina in the Aβ group compared to controls (data not shown). Additional immunohistochemical analyses of the tumor suppressor gene p53, as well as terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining, were undertaken, but no significant differences were observed between the groups (data not shown). To further evaluate retinal degeneration and preclude cell loss via nonapoptotic mechanisms,38 we compared the neuroretinal thickness between Aβ-injected and control eyes. Neuroretinal thickness was preserved at all time points (Fig. 9), and we did not observe any evidence of Bruch’s membrane disruption or angiogenic activities.
Figure 9.
Retinal thickness measurements. Retinal thickness was measured from internal limiting membrane to photoreceptor outer segment/RPE junction in parafoveal sections. No significant difference in retinal thickness was observed in any group throughout the experiment. Error bars denote standard deviation.
Discussion
A compelling role for local, chronic inflammation in the pathogenesis of AMD has been established by studies on drusen5,39 and gene polymorphisms.40 Cytokines, key drivers of inflammation, have gained much interest for their potential role in AMD pathophysiology; for instance, high serum IL-6 level has been found to be an independent predictor for the progression of AMD.30 Analysis of postmortem human eyes from AMD patients revealed elevated eotaxin and IP-10,41 as well as increased IL-1β immunoreactivity in association with large drusen.42 Finding a trigger for cytokine expression is clearly important for targeted intervention, and we hypothesized that Aβ 1-40, the principal form of the Aβ peptide found in the eye43 and in drusen,13 may be responsible for promoting cytokine production and inflammatory pathways.15 Our results demonstrated that intravitreal injection of Aβ 1-40 caused marked upregulation of cytokines in the RPE and neuroretina. This model is consistent with the early phases of age-related maculopathy according to the hypothesis of Hageman et al.5 proposing that RPE dysfunction generates a proinflammatory milieu in early AMD pathogenesis.
RPE Plays a Major Role in Responding to Aβ
Our data suggest that both neuroretina and RPE/choroid upregulate inflammatory pathway genes in response to Aβ stimulation. Relative to the neuroretina, the RPE demonstrated a comparable magnitude of gene expression changes despite fewer total cells, indicating a high sensitivity to Aβ stimulation. The RPE is chiefly responsible for the maintenance and homeostasis of the outer retina and photoreceptor, but it is also known to actively participate in immune response via cytokine production.44,45 In contrast, it is mostly glial cells,46 and principally microglia, that secrete cytokines in the neuroretinal layers.10,37 Based on immunohistochemistry data, the microglial response was minimal and transient in the Aβ group. Previous studies have induced marked microglial activation using intravitreal Aβ 1-42,10,21 likely due to the greater neurotoxicity associated with Aβ 1-42 compared to Aβ 1-40.14 The increase in microglia we observed on day 1 did not correlate with cytokine gene expression at other time points, nor was the magnitude of change large enough to convincingly demonstrate an association with the differential expression of inflammatory mediators seen. Combined with the fact that Aβ induces RPE to upregulate inflammatory pathways in vitro,15 it is likely that the RPE, not microglia, was the predominant source of cytokine in this study. The choroid was considered as a potential portal for systemic cytokines entering the retina; however, given that the blood–retinal barrier was intact after intravitreal injections, it is unlikely that Aβ oligomers (dimer: 8.6 kDa) and cytokines (all larger than Aβ) were exchanged between the choroidal vasculature and the retina.
A Role for Inflammasome NLRP3 Activation?
We observed a time-dependent overexpression of IL-6 and TNF-α in eyes treated with Aβ 1-40. IL-1β, a cytokine product of inflammasome NLRP3, was concurrently upregulated on days 1 and 4 in the neuroretina and RPE. IL-18 was also upregulated on day 4. The associations between Aβ, inflammasome NLRP3, and RPE have been described separately: Halle et al.16 reported Aβ-induced NLRP3 activation in microglia while Kauppinen et al.18 showed NLRP3-dependent cytokine synthesis in human RPE under oxidative stress in vitro. The temporal pattern that emerged from our data is strongly suggestive of a role for NLRP3 in response to Aβ in the eye, a novel finding. We propose that, upon Aβ exposure, NLRP3 inflammasome is initially activated in the RPE and neuroretina, as illustrated by the overexpression of NLRP3 gene on day 1. The activation of NLRP3 recruits caspase-1 and catalyzes the formation of IL-1β initially, followed shortly by IL-18. IL-1β in turn stimulates the RPE in an autocrine manner,47 causing overexpression of TNF-α and IL-6 to further propagate a local proinflammatory state.48 This novel association highlights the key function of NLRP3 inflammasome in coordinating cytokine expression in the eye. In our model, cytokine expression mostly diminished to baseline levels by 14 days postinjection. This observation presumably reflects clearance of Aβ, as microglia are known to remove Aβ from the retina.10 Interestingly, we observed high TNF-α mRNA levels and IL-1β immunoreactivity on days 14 and 49 that might indicate some degree of persistent inflammatory pathway activation in the eye. Whether this represents the establishment of chronic inflammation is unclear and should be investigated in a future study that extends the observation period beyond the 49 days studied here. We demonstrated that Aβ 1-40 stimulates cytokine production in healthy RPE and retina and establishes a proinflammatory environment in the posterior segment of the rodent eye. It can be inferred that accumulation of Aβ in drusen may generate an increasing level of cytokines that primes the outer retina for subsequent damage, prior to the onset of clinical evidence of AMD.
Cytokines are integral to the host’s immune defense, but their imbalances are often implicated in diseases. TNF-α, IL-6, and IL-1β are also elevated in retinal detachment,20 proliferative vitreoretinopathy,47 and ischemia.49 These cytokines also play an important part in the pathophysiology of AMD based on evidence from clinical and epidemiologic studies.30 For instance, cytokines are known to stimulate chemotaxis, and our vitreous assay revealed an elevated level of chemokine MIP-3α (Fig. 7). MIP-3α mediates migration of dendritic cells and lymphocytes via CCR6 receptor as part of mucosal defense against microbes.50 Human ARPE-19 produces MIP-3α in response to IL-17A.51 RPE has also been known to increase production of IL-8, another chemokine, when stimulated by Aβ 1-40.15 In light of these observations, chemotaxis may constitute an important part of the response to Aβ in the eye and warrants further investigation.
To the best of our knowledge, our study showed, for the first time, that IL-18 is upregulated in the retina, RPE, and vitreous following Aβ stimulation. IL-18 is thought to be involved in late stages of AMD. Doyle et al.52 showed that IL-18 inhibits angiogenesis in CNV, while Tarallo et al.17 reported that IL-18 promotes RPE death in dry AMD. Neither event was observed in this study, but our model assessed the effects only up to 49 days following a single Aβ injection. It is likely that multiple Aβ injections and follow-up beyond 49 days may better simulate the chronic, local proinflammatory environment thought to be present in the retina of patients with early AMD and thus may be helpful to define the role of IL-18 in AMD.
Cell Loss Is Not a Feature of This Model of Early AMD
There is an established connection between inflammation and apoptosis. Both IL-1β and TNF-α are known to increase expression of apoptotic factors in the RPE.53–55 Apoptosis has been proposed as a mechanism contributing to degenerative events in AMD.56 The present study saw a significant upregulation of the XAF1 gene by RPE/choroid in Aβ-injected eyes (Figs. 3E, 3F), in keeping with our previous in vitro observations.15 XAF1 directly activate caspase-dependent apoptosis in conjunction with TNF-α.56,57 Surprisingly, there was no significant change in the immunoreactivity of XAF1 in the retinal tissue sections. Such mismatch between XAF1 mRNA and protein was previously reported in other tissue types as well.58 We suspect that antiapoptotic processes might have interfered with the synthesis of XAF1 protein. Absence of significant p53 or TUNEL immunoreactivity and preservation of retinal thickness in Aβ-injected eyes strongly suggest that cell death was not a feature of the present study. This is in contrast to intravitreal Aβ 1-42-induced retinal neuron degeneration in AD models.24,25 The difference is likely due to the inherently greater neurotoxicity of Aβ 1-42 compared to Aβ 1-40.14 On the other hand, existing animal models of AMD capture many features of late disease: Malek et al.59 demonstrated drusen-like deposits, RPE changes, and CNV in the apoE4-HFC diet mouse model. Both the Ccl2/Cx3cr1 double-knockout mouse model and the Ccl2- or Ccr2- knockout mouse model produced subretinal deposits and RPE/photoreceptor atrophy.60–62 However, these models do not directly or independently rely on the local proinflammatory retinal environment thought to be present in presymptomatic AMD. Using intravitreal Aβ 1-40 injection, we induced a proinflammatory response in the healthy retina without causing cell loss. This model creates the opportunity to study how inflammatory cytokines affect the homeostasis of the outer retina and to evaluate the effectiveness of interventions that control the proinflammatory environment, hypothesized to be one of the earliest changes associated with the etiology of AMD.
In conclusion, the present study verified the proinflammatory effects of drusen component Aβ 1-40 in vivo in the rat retina. Cytokine genes including IL-6, TNF-α, IL-1β, and IL-18 are upregulated by the RPE and neuroretina after Aβ stimulation. The activation of NLRP3 inflammasome may play a central part in mediating this response and is thus a potential novel target for AMD treatment. Aβ 1-40 is likely to be an important contributor to AMD by promoting the release of cytokines and establishing a background level of chronic, local inflammation that promotes dysfunction of cells of the outer retina including the RPE. The absence of retinal cell apoptosis in this model makes it suitable for studying the early proinflammatory events that may prime the retina toward AMD development. Our results are useful for future studies requiring an in vivo platform for testing therapeutics that target and suppress inflammasome activation and cytokine production in the retina. Current literature on the use of anti-inflammatory agents in AMD derives from studies that focus on late stages of the disease63; however, our study prompts a closer look at the possible benefit of such an approach in early AMD with the goal to minimize disease progression and prevent vision loss.
Acknowledgments
Supported by Canadian Institute of Health Research (CIHR) Grant MOP 97806 (JAM).
We thank Idris Samad, Eleanor To, and Eric Wang for technical support and assistance throughout this study.
Footnotes
Disclosure: R.T. Liu, None; J. Gao, None; S. Cao, None; N. Sandhu, None; J.Z. Cui, None; C.L. Chou, None; E. Fang, None; J.A. Matsubara, None
References
- 1.The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335–610. [PubMed] [Google Scholar]
- 3.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 4.Hogg RE, Stevenson MR, Chakravarthy U, Beirne RO, Anderson RS. Early features of AMD. Ophthalmology. 2007;114:1028. doi: 10.1016/j.ophtha.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 7.Yehoshua Z, Rosenfeld PJ, Albini TA. Current clinical trials in dry AMD and the definition of appropriate clinical outcome measures. Semin Ophthalmol. 2011;26:167–180. doi: 10.3109/08820538.2011.577132. [DOI] [PubMed] [Google Scholar]
- 8.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruban J, Glotin AL, Dinet V, et al. Amyloid-beta(1-42) alters structure and function of retinal pigmented epithelial cells. Aging Cell. 2009;8:162–177. doi: 10.1111/j.1474-9726.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DT, Bresciani L, Saunders D, et al. Amyloid beta peptide causes chronic glial cell activation and neuro-degeneration after intravitreal injection. Neuropathol Appl Neurobiol. 2005;31:491–502. doi: 10.1111/j.1365-2990.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 11.Dentchev T, Milam AH, Lee V, Trojanowski JQ, Dunaief JL. Amyloid-β is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–190. [PubMed] [Google Scholar]
- 12.Kayed R, Head E, Thompson JL, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 13.Isas JM, Luibl V, Johnson LV, et al. Soluble and mature amyloid fibrils in drusen deposits. Invest Ophthalmol Vis Sci. 2010;51:1304–1310. doi: 10.1167/iovs.09-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlgren KN, Manelli AM, Stine WB, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 15.Kurji KH, Cui JZ, Lin T, et al. Microarray analysis identifies changes in inflammatory gene expression in response to amyloid-β stimulation of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:1151–1163. doi: 10.1167/iovs.09-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halle A, Hornung V, Petzold GC, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarallo V, Hirano Y, Gelfand B, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—implications for age-related macular degeneration (AMD) Immunol Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Keller M, Rüegg A, Werner S, Beer H. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Nakazawa T, Kayama M, Ryu M, et al. Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Invest Ophthalmol Vis Sci. 2011;52:1384–1391. doi: 10.1167/iovs.10-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howlett D, Bate S, Collier S, et al. Characterisation of amyloid-induced inflammatory responses in the rat retina. Exp Brain Res. 2011;214:185–197. doi: 10.1007/s00221-011-2819-4. [DOI] [PubMed] [Google Scholar]
- 22.Landa G, Butovsky O, Shoshani J, Schwartz M, Pollack A. Weekly vaccination with copaxone (glatiramer acetate) as a potential therapy for dry age-related macular degeneration. Curr Eye Res. 2008;33:1011–1013. doi: 10.1080/02713680802484637. [DOI] [PubMed] [Google Scholar]
- 23.Pfizer. Efficacy, safety and tolerability study of RN6G in subjects with geographic atrophy secondary to age-related macular degeneration. [Accessed January 2, 2013];ClinicalTrials.gov. 2012 Available at: http://www.clinicaltrials.gov/ct2/show/NCT01577381.
- 24.Anderson P, Watts H, Hille C, et al. Glial and endothelial blood-retinal barrier responses to amyloid-β in the neural retina of the rat. Clin Ophthalmol. 2008;2:801–816. doi: 10.2147/opth.s3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh DT, Montero RM, Bresciani LG, et al. Amyloid-beta peptide is toxic to neurons in vivo via indirect mechanisms. Neurobiol Dis. 2002;10:20–27. doi: 10.1006/nbdi.2002.0485. [DOI] [PubMed] [Google Scholar]
- 26.Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-β deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–5143. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo L, Normando EM, Nizari S, Lara D, Cordeiro MF. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Invest Ophthalmol Vis Sci. 2010;51:6504–6513. doi: 10.1167/iovs.10-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis D, Zhang A, Etienne C, Huang I, Malit M. Tech Note 2861: Principles of Curve Fitting for Multiplex Sandwich Immunoassays. Hercules, CA: Bio-Rad; 2012. [Google Scholar]
- 29.Hoh Kam J, Lenassi E, Jeffery G. Viewing ageing eyes: diverse sites of amyloid beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS One. 2010;5:e13127. doi: 10.1371/journal.pone.0013127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 31.Bian Z, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1β and TNF-α-induced chemokine expression in human retinal pigment epithelial cells. Exp Eye Res. 2001;73:111–121. doi: 10.1006/exer.2001.1019. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Ohno-Matsui K, Ichinose S, et al. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115:2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 34.Elner S, Elner V, Jaffe G, Stuart A, Kunkel S, Strieter R. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 35.Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93. doi: 10.1016/j.ophtha.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Ohno-Matsui K, Yoshida T, et al. Amyloid-beta up-regulates complement factor B in retinal pigment epithelial cells through cytokines released from recruited macrophages/microglia: another mechanism of complement activation in age-related macular degeneration. J Cell Physiol. 2009;220:119–128. doi: 10.1002/jcp.21742. [DOI] [PubMed] [Google Scholar]
- 37.Ma W, Zhao L, Fontainhas AM, Fariss RN, Wong WT. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: a potential cellular interaction relevant to AMD. PLoS One. 2009;4:e7945. doi: 10.1371/journal.pone.0007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum DM, Degterev A, David J, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- 40.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mo FM, Proia AD, Johnson WH, Cyr D, Lashkari K. Interferon γ–inducible protein-10 (IP-10) and eotaxin as biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4226–4236. doi: 10.1167/iovs.09-3910. [DOI] [PubMed] [Google Scholar]
- 42.Jiang K, To E, Cui J, Cao S, Gao J, Matsubara J. Drusen and pro-inflammatory mediators in the post-mortem human eye. J Clin Exp Ophthalmol. 2012;3:1–9. doi: 10.4172/2155-9570.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakasam A, Muthuswamy A, Ablonczy Z, et al. Differential accumulation of secreted AβPP metabolites in ocular fluids. J Alzheimers Dis. 2010;20:1243–1253. doi: 10.3233/JAD-2010-100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20:29–48. doi: 10.1016/s1350-9462(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 45.Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993;12:205–212. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- 46.Nakazawa T, Matsubara A, Noda K, et al. Characterization of cytokine responses to retinal detachment in rats. Mol Vis. 2006;12:867–878. [PubMed] [Google Scholar]
- 47.Jaffe GJ, Van Le L, Valea F, et al. Expression of interleukin-1α, interleukin-1β, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp Eye Res. 1992;55:325–335. doi: 10.1016/0014-4835(92)90197-z. [DOI] [PubMed] [Google Scholar]
- 48.Elner VM, Scales W, Elner SG, Danforth J, Kunkel SL, Strieter RM. Interleukin-6 (IL-6) gene expression and secretion by cytokine-stimulated human retinal pigment epithelial cells. Exp Eye Res. 1992;54:361–368. doi: 10.1016/0014-4835(92)90048-w. [DOI] [PubMed] [Google Scholar]
- 49.Hangai M, Yoshimura N, Honda Y. Increased cytokine gene expression in rat retina following transient ischemia. Ophthalmic Res. 1996;28:248–254. doi: 10.1159/000267910. [DOI] [PubMed] [Google Scholar]
- 50.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bian ZM, Elner SG, Khanna H, Murga-Zamalloa CA, Patil S, Elner VM. Expression and functional roles of caspase-5 in inflammatory responses of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:8646–8656. doi: 10.1167/iovs.11-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang P, McKay BS, Allen JB, Jaffe GJ. Effect of NF-κB inhibition on TNF-α–induced apoptosis in human RPE cells. Invest Ophthalmol Vis Sci. 2004;45:2438–2446. doi: 10.1167/iovs.03-0805. [DOI] [PubMed] [Google Scholar]
- 55.Yang P, Peairs JJ, Tano R, Zhang N, Tyrell J, Jaffe GJ. Caspase-8–mediated apoptosis in human RPE cells. Invest Ophthalmol Vis Sci. 2007;48:3341–3349. doi: 10.1167/iovs.06-1340. [DOI] [PubMed] [Google Scholar]
- 56.Dunaief JL, Dentchev T, Ying G, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 57.Xia Y, Novak R, Lewis J, Duckett C, Phillips A. Xaf1 can cooperate with TNFα in the induction of apoptosis, independently of interaction with XIAP. Mol Cell Biochem. 2006;286:67–76. doi: 10.1007/s11010-005-9094-2. [DOI] [PubMed] [Google Scholar]
- 58.Kempkensteffen C, Fritzsche FR, Johannsen M, et al. Down-regulation of the pro-apoptotic XIAP associated factor-1 (XAF1) during progression of clear-cell renal cancer. BMC Cancer. 2009;9:276. doi: 10.1186/1471-2407-9-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malek G, Johnson LV, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A. 2005;102:11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan CC, Ross RJ, Shen D, et al. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–128. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent ccl-2- or ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 62.Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye. 2011;25:127–139. doi: 10.1038/eye.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez RN, Chan CK, Garg S, et al. Interleukin-6 in retinal ischemia reperfusion injury in rats. Invest Ophthalmol Vis Sci. 2003;44:4006–4011. doi: 10.1167/iovs.03-0040. [DOI] [PubMed] [Google Scholar]
- 65.Peinnequin A, Mouret C, Birot O, et al. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004;5:3. doi: 10.1186/1471-2172-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation crosstalk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 67.Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]