Abstract
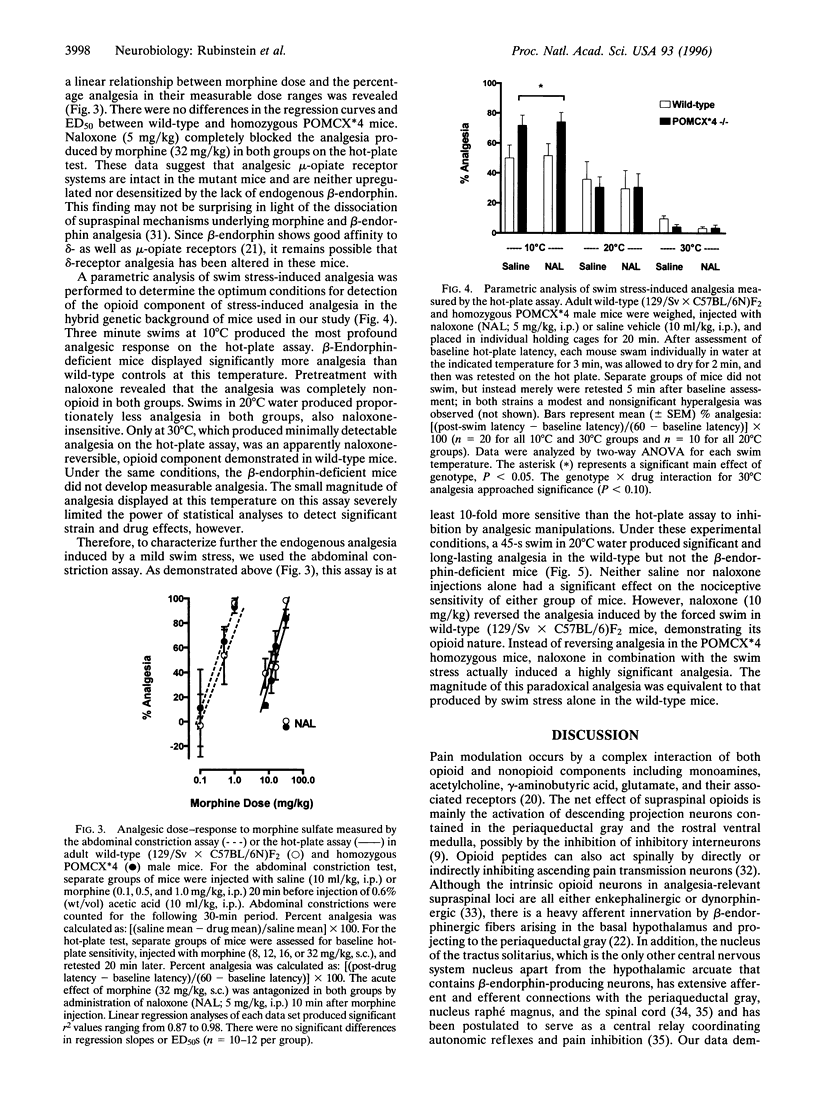
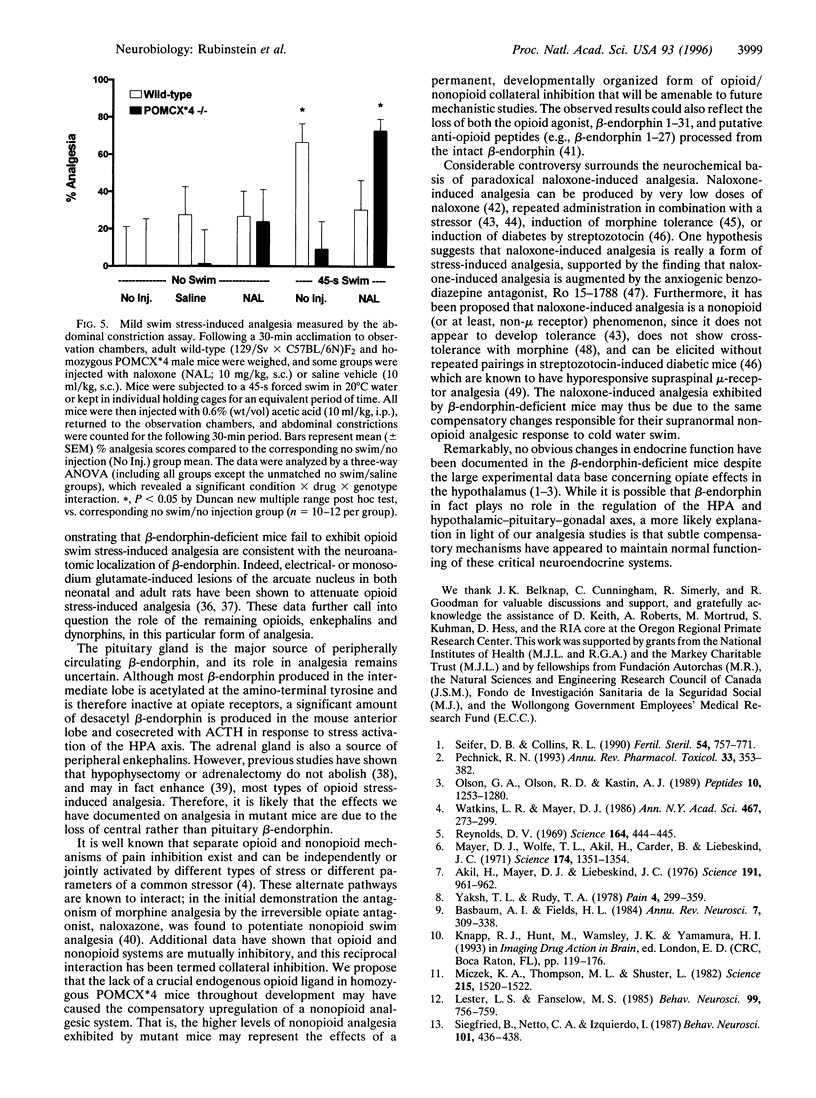
A physiological role for beta-endorphin in endogenous pain inhibition was investigated by targeted mutagenesis of the proopiomelanocortin gene in mouse embryonic stem cells. The tyrosine codon at position 179 of the proopiomelanocortin gene was converted to a premature translational stop codon. The resulting transgenic mice display no overt developmental or behavioral alterations and have a normally functioning hypothalamic-pituitary-adrenal axis. Homozygous transgenic mice with a selective deficiency of beta-endorphin exhibit normal analgesia in response to morphine, indicating the presence of functional mu-opiate receptors. However, these mice lack the opioid (naloxone reversible) analgesia induced by mild swim stress. Mutant mice also display significantly greater nonopioid analgesia in response to cold water swim stress compared with controls and display paradoxical naloxone-induced analgesia. These changes may reflect compensatory upregulation of alternative pain inhibitory mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Mayer D. J., Liebeskind J. C. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976 Mar 5;191(4230):961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Akil H., Young E., Walker J. M., Watson S. J. The many possible roles of opioids and related peptides in stress-induced analgesia. Ann N Y Acad Sci. 1986;467:140–153. doi: 10.1111/j.1749-6632.1986.tb14625.x. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bodnar R. J., Abrams G. M., Zimmerman E. A., Krieger D. T., Nicholson G., Kizer J. S. Neonatal monosodium glutamate. Effects upon analgesic responsivity and immunocytochemical ACTH/beta-lipotropin. Neuroendocrinology. 1980 May;30(5):280–284. doi: 10.1159/000123015. [DOI] [PubMed] [Google Scholar]
- Cappell H., Poulos C. X., Lê A. D. Enhancement of naloxone-induced analgesia by pretreatment with morphine. Pharmacol Biochem Behav. 1989 Oct;34(2):425–427. doi: 10.1016/0091-3057(89)90337-7. [DOI] [PubMed] [Google Scholar]
- EDDY N. B., LEIMBACH D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953 Mar;107(3):385–393. [PubMed] [Google Scholar]
- Greeley J. D., Lê A. D., Poulos C. X., Cappell H. "Paradoxical" analgesia induced by naloxone and naltrexone. Psychopharmacology (Berl) 1988;96(1):36–39. doi: 10.1007/BF02431530. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr, Nicolas P., Li C. H. beta-endorphin-(1-27) is an antagonist of beta-endorphin analgesia. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1389–1390. doi: 10.1073/pnas.81.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield J. M., Allen R. G., Stack J., Ronnekleiv O. Post-translational processing of pro-opiomelanocortin (POMC)-derived peptides during fetal monkey pituitary development. II. beta-Lipotropin (beta-LPH)-related peptides. Dev Biol. 1988 Mar;126(1):164–172. doi: 10.1016/0012-1606(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Kamei J., Kawashima N., Kasuya Y. Naloxone-induced analgesia in diabetic mice. Eur J Pharmacol. 1992 Jan 21;210(3):339–341. doi: 10.1016/0014-2999(92)90424-3. [DOI] [PubMed] [Google Scholar]
- Kamei J., Ohhashi Y., Aoki T., Kawasima N., Kasuya Y. Streptozotocin-induced diabetes selectively alters the potency of analgesia produced by mu-opioid agonists, but not by delta- and kappa-opioid agonists. Brain Res. 1992 Feb 7;571(2):199–203. doi: 10.1016/0006-8993(92)90655-s. [DOI] [PubMed] [Google Scholar]
- Kayser V., Guilbaud G. Increase in the threshold of a nociceptive test induced by naloxone in morphine-tolerant rats. Brain Res. 1985 Oct 7;344(2):360–364. doi: 10.1016/0006-8993(85)90815-7. [DOI] [PubMed] [Google Scholar]
- Kelsey J. E., Hoerman W. A., 4th, Kimball L. D., 3rd, Radack L. S., Carter M. V. Arcuate nucleus lesions reduce opioid stress-induced analgesia (SIA) and enhance non-opioid SIA in rats. Brain Res. 1986 Sep 24;382(2):278–290. doi: 10.1016/0006-8993(86)91337-5. [DOI] [PubMed] [Google Scholar]
- Kirchgessner A. L., Bodnar R. J., Pasternak G. W. Naloxazone and pain-inhibitory systems: evidence for a collateral inhibition model. Pharmacol Biochem Behav. 1982 Dec;17(6):1175–1179. doi: 10.1016/0091-3057(82)90116-2. [DOI] [PubMed] [Google Scholar]
- Lester L. S., Fanselow M. S. Exposure to a cat produces opioid analgesia in rats. Behav Neurosci. 1985 Aug;99(4):756–759. doi: 10.1037//0735-7044.99.4.756. [DOI] [PubMed] [Google Scholar]
- Lewis J. W., Baldrighi G., Akil H. A possible interface between autonomic function and pain control: opioid analgesia and the nucleus tractus solitarius. Brain Res. 1987 Oct 20;424(1):65–70. doi: 10.1016/0006-8993(87)91193-0. [DOI] [PubMed] [Google Scholar]
- Low M. J., Liu B., Hammer G. D., Rubinstein M., Allen R. G. Post-translational processing of proopiomelanocortin (POMC) in mouse pituitary melanotroph tumors induced by a POMC-simian virus 40 large T antigen transgene. J Biol Chem. 1993 Nov 25;268(33):24967–24975. [PubMed] [Google Scholar]
- Marek P., Panocka I., Hartmann G. Enhancement of stress-induced analgesia in adrenalectomized mice: its reversal by dexamethasone. Pharmacol Biochem Behav. 1982 Mar;16(3):403–405. doi: 10.1016/0091-3057(82)90442-7. [DOI] [PubMed] [Google Scholar]
- Mayer D. J., Wolfle T. L., Akil H., Carder B., Liebeskind J. C. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971 Dec 24;174(4016):1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I., Maderdrut J. L., Altschuler R. A., Petrusz P. Immunocytochemical localization of proenkephalin-derived peptides in the central nervous system of the rat. Neuroscience. 1986 Feb;17(2):325–348. doi: 10.1016/0306-4522(86)90250-2. [DOI] [PubMed] [Google Scholar]
- Miczek K. A., Thompson M. L., Shuster L. Opioid-like analgesia in defeated mice. Science. 1982 Mar 19;215(4539):1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Millan M. J. Multiple opioid systems and pain. Pain. 1986 Dec;27(3):303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Mogil J. S., Sternberg W. F., Balian H., Liebeskind J. C., Sadowski B. Opioid and nonopioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav. 1996 Jan;59(1):123–132. doi: 10.1016/0031-9384(95)02073-x. [DOI] [PubMed] [Google Scholar]
- Olson G. A., Olson R. D., Kastin A. J. Endogenous opiates: 1988. Peptides. 1989 Nov-Dec;10(6):1253–1280. doi: 10.1016/0196-9781(89)90020-x. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Mezey E., Eskay R. L. Pro-opiomelanocortin-derived peptides (ACTH/beta-endorphin/alpha-MSH) in brainstem baroreceptor areas of the rat. Brain Res. 1987 Dec 15;436(2):323–338. doi: 10.1016/0006-8993(87)91676-3. [DOI] [PubMed] [Google Scholar]
- Pechnick R. N. Effects of opioids on the hypothalamo-pituitary-adrenal axis. Annu Rev Pharmacol Toxicol. 1993;33:353–382. doi: 10.1146/annurev.pa.33.040193.002033. [DOI] [PubMed] [Google Scholar]
- Pilcher W. H., Joseph S. A., McDonald J. V. Immunocytochemical localization of pro-opiomelanocortin neurons in human brain areas subserving stimulation analgesia. J Neurosurg. 1988 Apr;68(4):621–629. doi: 10.3171/jns.1988.68.4.0621. [DOI] [PubMed] [Google Scholar]
- Reynolds D. V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969 Apr 25;164(3878):444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
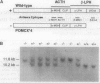
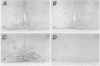
- Rubinstein M., Japón M. A., Low M. J. Introduction of a point mutation into the mouse genome by homologous recombination in embryonic stem cells using a replacement type vector with a selectable marker. Nucleic Acids Res. 1993 Jun 11;21(11):2613–2617. doi: 10.1093/nar/21.11.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M., Mortrud M., Liu B., Low M. J. Rat and mouse proopiomelanocortin gene sequences target tissue-specific expression to the pituitary gland but not to the hypothalamus of transgenic mice. Neuroendocrinology. 1993 Oct;58(4):373–380. doi: 10.1159/000126566. [DOI] [PubMed] [Google Scholar]
- Seifer D. B., Collins R. L. Current concepts of beta-endorphin physiology in female reproductive dysfunction. Fertil Steril. 1990 Nov;54(5):757–771. doi: 10.1016/s0015-0282(16)53928-4. [DOI] [PubMed] [Google Scholar]
- Siegfried B., Netto C. A., Izquierdo I. Exposure to novelty induces naltrexone-reversible analgesia in rats. Behav Neurosci. 1987 Jun;101(3):436–438. doi: 10.1037//0735-7044.101.3.436. [DOI] [PubMed] [Google Scholar]
- Terman G. W., Morgan M. J., Liebeskind J. C. Opioid and non-opioid stress analgesia from cold water swim: importance of stress severity. Brain Res. 1986 Apr 30;372(1):167–171. doi: 10.1016/0006-8993(86)91472-1. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Thompson M. L., Miczek K. A., Noda K., Shuster L., Kumar M. S. Analgesia in defeated mice: evidence for mediation via central rather than pituitary or adrenal endogenous opioid peptides. Pharmacol Biochem Behav. 1988 Mar;29(3):451–456. doi: 10.1016/0091-3057(88)90002-0. [DOI] [PubMed] [Google Scholar]
- Watkins L. R., Mayer D. J. Multiple endogenous opiate and non-opiate analgesia systems: evidence of their existence and clinical implications. Ann N Y Acad Sci. 1986;467:273–299. doi: 10.1111/j.1749-6632.1986.tb14635.x. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Analgesia and hyperalgesia produced in the rat by intrathecal naloxone. Brain Res. 1980 May 12;189(2):593–597. doi: 10.1016/0006-8993(80)90375-3. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Analgesia mediated by a direct spinal action of narcotics. Science. 1976 Jun 25;192(4246):1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978 Apr;4(4):299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]