Abstract
The effect of Bacillus subtilis FZB24® on saffron (Crocus sativus L.) was studied using saffron corms from Spain and the powdered form of B. subtilis FZB24®. Corms were soaked in water or in B. subtilis FZB24 spore solution for 15min before sowing. Some corms were further soil drenched with the spore solution 6, 10 or 14 weeks after sowing. Growth and saffron stigma chemical composition were measured. Compared to untreated controls, application of B. subtilis FZB24 significantly increased leaf length, flowers per corm, weight of the first flower stigma, total stigma biomass; microbe addition also significantly decreased the time required for corms to sprout and the number of shoot sprouts. Compared to the controls, picrocrocin, crocetin and safranal compounds were significantly increased when the plants were soil drenched with the spore solution 14 weeks after sowing; in contrast crocin was highest in untreated controls. Results of this study suggest that application of B. subtilis FZB24® may provide some benefit to saffron growers by speeding corm growth (earlier shoot emergence) and increasing stigma biomass yield by 12%. While some treatment conditions also increased saffron chemical composition, these were generally not the same treatments that simultaneously improved growth yields and thus, more study is required.
Keywords: Crocus sativus, Iridaceae, Bacillus subtilis FZB24, crocin, picrocrocin, crocetin, safranal
Introduction
Saffron (Crocus sativus L., Iridaceae) is the world’s most expensive spice selling for over $ 2000/kg. Saffron is composed of the dried, dark-red stigmas of Crocus sativus L., and is currently used mainly for flavouring and colouring food. This spice is also being investigated for therapeutic use as an anticancer agent [1], but its low productivity, 6 kg saffron/hectare from about 900,000 flowers, limits availability. Recent studies also show that saffron has other health benefits in learning and memory processes [2], as an agent for antidepression, antitussive, antioxidant and for neuroprotection [3], [4], [5], [6].
The main chemical constituents of saffron are crocin, crocetin, picrocrocin, and safranal (Fig. 1). Picrocrocin is the bitter principle in saffron which, during storage of the stigma spice, slowly breaks down to give the characteristic aroma of saffron [7]. Acid hydrolysis of picrocrocin gives rise to safranal [7]. Crocetin and crocin are carotenoids and responsible for the red-orange colour of the stigmas [7]. Crocin, a C44 glycoside [di(β-D-gentiobiosyl) ester of crocetin] is the most promising compound for use in cancer therapy [7], [8]. Although picrocrocin, crocetin, and safranal also show antitumour potential, compared to crocin they do not have the broad activity and higher doses are sometimes required to effect a therapeutic response [7], [8]. Crocetin also seems to have a hypolipaemic effect and may be useful in preventing atherosclerosis [7].
Fig. 1.
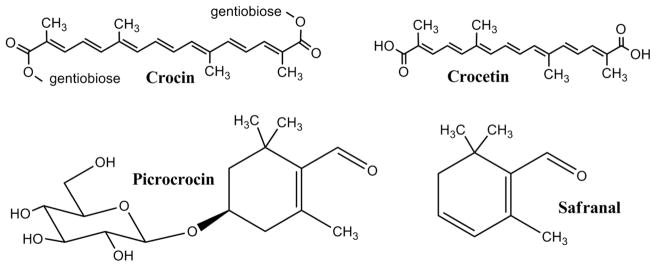
Structures of the main chemical constituents found in saffron spice.
The development of biological products based on beneficial micro-organisms can extend the range of options for maintaining the health and yield of field crops. For example, plant growth promoting rhizobacteria (PGPR) such as Rhizobium, Frankia, Streptomyces, Bacillus, Pseudomonas, and mycorrhizal fungi have attracted special attention due to their beneficial activities [9]. The nutrient-rich rhizosphere of roots is naturally colonised by many beneficial PGPR and also by pathogenic bacteria and fungi; these microbes can have either a positive or negative effect. Several Bacillus strains belonging to the B. subtilis/amyloliquefaciens group (FZB13, FZB14, FZB24, FZB37, FZB38, FZB42, FZB44, FZB45) isolated from soil by FZB Biotechnik GmbH (Berlin) have been shown to colonise plant roots and to provide not only protection against some soil-borne fungal plant diseases, but also to stimulate growth and crop yields [10], [11], [12]. Studies on the mode of action of these PGRP strains [13], [14] have shown that the mechanism of action is less connected with induction of plant resistance by elicitation, but rather with a hormonal stimulation of plant growth. These bacterial strains release metabolites that are, for example, precursors of auxin [15], [16]. In another instance, Idriss et al. [17] demonstrated that extracellular phytase secreted by plant roots can contribute to the plant growth-promoting effects of Bacillus amyloliquefaciens FZB strains.
Here we report the effect on growth and yield of the four main secondary metabolites of saffron (Fig. 1) by a specific strain, FZB24®, of Bacillus subtilis normally used as a biocontrol agent. The long term goal of this work is to provide saffron growers with a simple production technology that is environmentally safe for producing more high quality saffron.
Materials and Methods
Experimental design
Corms of Crocus sativus L. (Iridaceae) of Spanish origin (accession #: BCU001584 from Minaya, Albacete, Spain) were provided by Professor J.-A. Fernández (Biotechnology IDR, University of Castilla-La Mancha, Albacete, Spain). The Bacillus subtilis, strain FZB24® was provided by Dr. H. Junge (ABiTEP GmbH). Plants were grown in a greenhouse at the Worcester Polytechnic Institute (Worcester, MA, USA) in plastic pots 10×10×14cm containing potting substrate (Pro-Mix BX; Premier Horticulture Inc.) to a depth of 8.5 cm at 25±2/18±2°C. Corms were sown at a soil depth of 6 – 7cm and watered as needed. Fertilizer was applied about once a month as ½ strength Hoagland’s nutrient solution [18], ~150 – 200 mL/pot. The experimental design was laid out using a complete randomised block design (CRB) with three replications per treatment per each of two successive seasons. There were five experimental treatments for each of two growing seasons beginning on August 18, 2004 and then 2005: T1 (C) untreated control, saffron corms were soaked in water for 15 min before sowing; corms of treatments two, three, four, and five (T2, T3, T4, and T5) were soaked in B. subtilis FZB24 spore solution at 2 g/1000 mL water for 15min before sowing, and then corms of T3, T4 and T5 were also soil drenched with FZB24® spore solution at 0.2g/L after 6, 10 or 14 weeks of sowing, respectively.
Growth measurements, harvest and stigma sample preparation
Starting from the sowing date the following parameters were measured: days to sprout, when ~1 – 3 mm shoots first appeared above the soil surface; number of sprouts and leaf length after16 weeks; number of flowers/corm; FW of stigma yield [g]/1st flower; and total stigma FW [g]/corm. The major saffron chemical constituents, crocin, crocetin, picrocrocin, and safranal, were also extracted from stigmas and assayed. Saffron flowers were harvested as soon as they appeared and before the six petals fully opened (see Figures in Supporting Information). Saffron stigmas were excised the same day that flowers were harvested; only the red part which is grade one saffron was separated, dried at 60°C, weighed and then extracted and analysed spectrophotometrically (see Figures in Supporting Information). Stigmas from all flowers harvested from one corm were pooled for analysis and data from three corms were averaged.
Saffron extraction and analysis
To extract and assay the four chemical constituents of saffron, the procedure of Souret and Weathers (2000) was used [19]. To minimise product destruction, extraction was performed in the dark at 4°C. Ten milligrams of dry stigmas were ground in a micro-homogeniser (Fisher-Scientific) with 0.5mL of chilled 80% ethyl alcohol, prepared from high-purity ethyl alcohol. After 5minutes, the solution was transferred into a 15-mL centrifuge tube, centrifuged at 6,000×g for 10minutes and the supernatant decanted into a 1.5-mL Eppendorf tube. The pellet was washed twice with 0.5mL of 80% ethyl alcohol, and then centrifuged at 8,000 rpm in a micro-centrifuge for 10minutes. All supernatant fractions were pooled and spectroscopic analysis was performed immediately.
Determination and quantification of the saffron secondary products extracted from the stigmas were performed using a Hitachi V-2001 UV-Vis double-beam spectrophotometer. The four key pigments, crocin, picrocrocin, crocetin, and safranal, were measured at their absorption maxima; quantities were determined from their respective extinction coefficients. Standard crocetin and safranal were obtained from Sigma-Aldrich Chemical Co.; nostandards forcrocinor picrocrocin are to our knowledge commercially available. The absorption maxima and extinction coefficients are: crocin, 443 nm, 89,0001 mol−1 cm−1; picrocrocin, 250.5 nm, 10,1001 mol−1 cm−1; crocetin, 424 nm, 30,3281 mol−1 cm−1; safranal, 311 nm, 9,2801 mol−1 cm−1 [19].
Statistical analysis
Data were statistically analysed using CoStat Version 3.03, an interactive statistics program for computers. F-test and the least significant difference (LSD) were used for the comparison between treatment means at 5% probability level [20].
Supporting information
Photo of saffron sprouts first emerging from the soil (Fig. 1S), saffron flower just before full opening (Fig. 2S), and point at which 1st grade stigmas are excised for assay (Fig. 3S) are available in the Supporting Information.
Results and Discussion
Plant growth-promoting rhizobacteria, most of which are Pseudomonas and Bacillus species, are applied to a wide range of agricultural crops to enhance growth by promoting seedling emergence, plant biomass, and/or disease control [21]. In this study one of these beneficial species, B. subtilis FZB24®, was tested for its effect on the growth and quality of saffron.
The overall pattern of growth responses to the group of 5 treatments was successfully and precisely replicated in two seasons of growth for all of the experimental treatments (Table 1). Differences were observed, however, between treatment groups. Compared to the controls, application of B. subtilis to saffron corms significantly increased leaf length, flowers per corm, weight of the first flower stigma, and total stigma biomass; it also significantly decreased the time required for corms to sprout. In contrast, the number of shoot sprouts significantly decreased beyond that of the untreated controls (Table 1). The beneficial effects of B. subtilis on saffron corm growth also differed depending on how the corms were treated with the microbe. For example, although the microbe stimulated faster shoot emergence from the soil, this only occurred under the T2 conditions, where corms were soaked in the B. subtilis spore solution 15min before sowing. The T2 treatment also significantly maximised leaf length, the number of flowers produced by each corm, and stigma biomass in the first flower beyond that of the controls (Table 1). Similar stimulation of shoot growth was observed by others when B. subtilis was applied to tomatoes (Lycopersicon esculentum Mill.) [22]. Although there were longer shoots produced, the number of shoots sprouting per corm was not increased by any of the bacillus treatments. Although the mechanism of growth stimulation by plant growth-promoting rhizobacteria is unknown, Bacillus species do produce plant growth hormones, namely gibberellic acid and an auxin, indole-3-acetic acid (IAA) [13], [14], [21]. It is possible, therefore, that these compounds may be the agents stimulating the observed saffron growth.
Table 1.
Effect of B. subtilis FZB24 on growth and stigma yield of saffron during two successive growing seasons
| Treatments | Days to sprout | No. of sprouts/corm* | Leaf length* (cm) | No. of flowers/corm | 1st flower stigma FW (g) | Total FW yield/corm (g) |
|---|---|---|---|---|---|---|
| First season – 2004 | ||||||
| Control | 22.1 ± 0.1 | 5.5 ± 0.10 | 24.4 ± 0.15 | 3.3 ± 0.26 | 0.051 ± 0.001 | 0.139 ± 0.002 |
| T2 | 18.5 ± 0.1 | 4.4 ± 0.15 | 25.2 ± 0.32 | 3.1 ± 0.38 | 0.057 ± 0.004 | 0.128 ± 0.003 |
| T3 | 22.8 ± 0.1 | 5.4 ± 0.10 | 23.4 ± 0.15 | 2.9 ± 0.44 | 0.059 ± 0.003 | 0.127 ± 0.004 |
| T4 | 22.6 ± 0.1 | 4.7 ± 0.06 | 22.5 ± 0.06 | 2.3 ± 0.32 | 0.061 ± 0.002 | 0.110 ± 0.002 |
| T5 | 20.6 ± 0.1 | 4.9 ± 0.15 | 23.3 ± 0.26 | 3.1 ± 0.44 | 0.054 ± 0.004 | 0.135 ± 0.004 |
| Second season – 2005 | ||||||
| Control | 22.3 ± 0.1 | 5.8 ± 0.11 | 24.7 ± 0.16 | 3.6 ± 0.27 | 0.048 ± 0.002 | 0.136 ± 0.003 |
| T2 | 19.1 ± 0.1 | 4.7 ± 0.16 | 25.5 ± 0.33 | 3.4 ± 0.39 | 0.054 ± 0.005 | 0.125 ± 0.004 |
| T3 | 23.2 ± 0.1 | 5.7 ± 0.11 | 23.7 ± 0.16 | 3.2 ± 0.45 | 0.056 ± 0.004 | 0.124 ± 0.005 |
| T4 | 22.8 ± 0.1 | 4.9 ± 0.07 | 22.8 ± 0.07 | 2.5 ± 0.33 | 0.057 ± 0.003 | 0.107 ± 0.003 |
| T5 | 21.2 ± 0.1 | 5.2 ± 0.16 | 23.6 ± 0.27 | 3.4 ± 0.45 | 0.052 ± 0.005 | 0.132 ± 0.005 |
Control = corms soaked in water for 15 min before sowing, T2 = corms soaked in B. subtilis FZB24 spore solution at 2 g/1000 mL water for 15 min before sowing, T3, T4 and T5 = T2 + corms soil drenched with FZB24 spore solution at 0.2 g/L after 6, 10 or 14 weeks of sowing date, respectively.
Recorded after 16 weeks of planting date.
Although improved growth is important, the chemical composition of the saffron stigmas is also critical because it impacts the value of the spice crop. The quantity of crocin, picrocrocin, crocetin and safranal compounds extracted from the stigmas of the flowers in the plants treated with different treatments of B. subtilis FZB24® spore solution are presented in Table 2. The data show that all four compounds were significantly affected by the treatments and that the pattern of yield for these compounds was precisely replicated in the second season of growth for all experimental treatments (Table 2). Again, however, the differential application treatment protocols used in this study affected the chemical composition of saffron stigmas. Compared to the controls, picrocrocin, crocetin and safranal were significantly increased when the plants were soil drenched with the B. subtilis FZB24® spore solution 14 weeks after the sowing date (T5). Crocin yield, on the other hand, was highest in the untreated controls. Interestingly drenching saffron plants with the B. subtilis spore solution 6 weeks after sowing (T3) yielded the lowest amount of all four compounds. This suggests that at this point in the development of the plant, the saffron biochemical pathway is particularly sensitive to addition of this microbe to the soil and this warrants further investigation. Unfortunately the treatment conditions (T2) that yielded the best growth of saffron, produced only modest improvements in a few of the saffron constituents (Table 2). The chemical composition of the stigmas from the controls was not much different from those extracted and measured in a previous study that used the same analytical method [23]. Taken together, these data show that although one can use B. subtilis to maximise growth, including stigma biomass production, those same conditions will not necessarily translate into better quality saffron as assessed by chemical analysis.
Table 2.
Chemical composition of saffron as affected by B. subtilis FZB24 treatments during two successive growing seasons
| Treatments | mg/mg dry weight of stigmas | |||
|---|---|---|---|---|
| Crocin | Picrocrocin | Crocetin | Safranal | |
| First season – 2004 | ||||
|
| ||||
| Control | 0.065 ± 0.004 | 0.155 ± 0.004 | 0.064 ± 0.003 | 0.065 ± 0.004 |
|
| ||||
| T2 | 0.057 ± 0.003 | 0.158 ± 0.002 | 0.054 ± 0.004 | 0.054 ± 0.001 |
|
| ||||
| T3 | 0.014 ± 0.003 | 0.154 ± 0.004 | 0.015 ± 0.003 | 0.051 ± 0.003 |
|
| ||||
| T4 | 0.063 ± 0.002 | 0.166 ± 0.004 | 0.056 ± 0.004 | 0.056 ± 0.004 |
|
| ||||
| T5 | 0.026 ± 0.002 | 0.254 ± 0.004 | 0.259 ± 0.002 | 0.071 ± 0.002 |
|
| ||||
| Second season – 2005 | ||||
|
| ||||
| Control | 0.074 ± 0.005 | 0.164 ± 0.005 | 0.075 ± 0.004 | 0.074 ± 0.005 |
|
| ||||
| T2 | 0.065 ± 0.004 | 0.167 ± 0.003 | 0.064 ± 0.005 | 0.058 ± 0.002 |
|
| ||||
| T3 | 0.025 ± 0.004 | 0.164 ± 0.005 | 0.025 ± 0.004 | 0.055 ± 0.004 |
|
| ||||
| T4 | 0.071 ± 0.003 | 0.175 ± 0.005 | 0.065 ± 0.005 | 0.064 ± 0.005 |
|
| ||||
| T5 | 0.036 ± 0.003 | 0.264 ± 0.005 | 0.271 ± 0.003 | 0.077 ± 0.003 |
|
| ||||
| Comparison to reports using same method of extraction and assay (Souret 1998) | ||||
|
| ||||
|
| ||||
| Local grocer source | 0.14 ± 0.01 | 0.17 ± 0.02 | 0.12 ± 0.01 | 0.04 ± 0.01 |
|
| ||||
| Soil-grown | 0.20 ± 0.04 | 0.20 ± 0.06 | 0.18 ± 0.03 | 0.05 ± 0.02 |
Control = corms soaked in water for 15 min before sowing, T2 = corms soaked in B. subtilis FZB24 spore solution at 2 g/1000 mL water for 15 min before sowing, T3, T4 and T5 = T2 + corms soil drenched with FZB24 spore solution at 0.2 g/L after 6, 10 or 14 weeks of sowing date, respectively.
In conclusion, the results of this study suggest that application of B. subtilis FZB24® may provide some benefit to saffron growers by speeding corm growth (earlier shoot emergence) and increasing stigma biomass yield by 12%. The specific conditions which stimulated growth did not however produce the highest levels of crocin, crocetin, picrocrocin, and safranal. The beneficial effects of B. subtilis on saffron plant growth and its spice chemical constituents have proved to be more complex and less predictable than expected. If B. subtilis is to prove useful in saffron agriculture, then future studies must determine the balance that must be established whereby both growth and stigma chemical quality can be simultaneously improved.
Supplementary Material
Acknowledgments
Support for this research was provided in part by WPI, and NIH 1R15GM069562–01. Support of M.A. Sharaf-Eldin by a Fulbright Fellowship an ICSC-World Laboratory Scholarship (Switzerland) is greatly appreciated.
Footnotes
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
References
- 1.Ashrafi M, Bathaie SZ, Taghikhani M, Moosavi-Movahed AA. The effect of carotenoids obtained from saffron on histone H1 structure and H1-DNA interaction. Int J Biol Macromol. 2005;36:246–52. doi: 10.1016/j.ijbiomac.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Pitsikas N, Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav Brain Res. 2006;173:112–5. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydroalcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281–4. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Hosseinzadeh H, Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77:446–8. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S, Poduval TB, Tilak JC, Devasagayam TPA. A modified, economic, sensitive method for measuring total antioxidant capacities of human plasma and natural compounds using Indian saffron (Crocus sativus) Clin Chim Acta. 2005;352:155–63. doi: 10.1016/j.cccn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81:805–13. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Souret FF, Weathers PJ. Cultivation, in vitro culture, secondary metabolite production, and phytopharmacognosy of saffron (Crocus sativus L) J Herbs Spices Med Plants. 1999;6:99–116. [Google Scholar]
- 8.Abdullaev FI. Minireview cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L) Exp Biol Med. 2002;227:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 9.Johri BN, Jyoti KS. Growth promoting Rhizobacteria: interaction and interplay with seedlings diseases of tree legumes. Management of diseases of economic importance in tropical plantations, Part I Finnish Forest Research Institute. 1995;S2:6–15. [Google Scholar]
- 10.Krebs B, Höding B, Kübart S, Workie M, Junge H, Schmiedeknecht G, et al. Use of Bacillus subtilis as biocontrol agent.1. Activities and characterization of Bacillus subtilis strains. J Plant Dis Prot. 1998;105:181–97. [Google Scholar]
- 11.Schmiedeknecht G, Bochow H, Junge H. Use of Bacillus subtilis biocontrol agent. II. Biological control of potato diseases. Z Pflanzenkr Pflanzenschutz. 1998;105:376–86. [Google Scholar]
- 12.Grosch RJ, Krebs B, Bochow H. Use of Bacillus subtilis as biocontrol-agent. III. Influence of Bacillus subtilis on fungal root diseases and on yield in soilless culture. Z Pflanzenkr Pflanzenschutz. 1999;106:568–80. [Google Scholar]
- 13.Dolej S. Jarvis & Shoemaker [dissertation] Berlin: Humboldt-Universität; 1998. Effects of metabolites from the rhizobacterium B. subtilis (Ehrenberg) Cohn in the pathosystem tomato (Lycopersicon esculentum Mill.)- Fusarium oxysporum f.sp. radicis-lycopersici. [Google Scholar]
- 14.Bochow H, Dolej S. Modern fungicides and anti-fungal compound II. Intercept Ltd; 1999. Mechanisms of tolerance induction in plants by root colonizing Bacillus subtilis isolates; pp. 411–6. [Google Scholar]
- 15.Idris EES, Bochow H, Ross H, Borriss R. Use of Bacillus subtilis as biocontrol agent. 6. Phytohormone-like action of culture filtrates prepared from plant-growth promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. J Plant Dis Prot. 2004;111:583–97. [Google Scholar]
- 16.Idris EES, Iglesias D, Talon M, Borriss R. Tryptophan dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact. 2007;20:619–26. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 17.Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, et al. Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant growth promoting effect. Microbiology. 2002;148:2097–109. doi: 10.1099/00221287-148-7-2097. [DOI] [PubMed] [Google Scholar]
- 18.Hoagland DR, Arnon DI. Annual Report of the Smithsonian Institution for the Year 1938. Washington: Government Printing Office; 1939. The water culture method for growing plants without soil. [Google Scholar]
- 19.Souret FF, Weathers PJ. The growth of saffron (Crocus sativus L) in aeroponics and hydroponics. J Herbs Spices Med Plants. 2000;7:25–35. [Google Scholar]
- 20.Waller RA, Duncan DB. A boys rule for symmetric multiple comparison problem. Ann State Assoc J. 1969;65:1485–503. [Google Scholar]
- 21.Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, et al. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007 doi: 10.1038/nbt1325. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 22.Woitke M, Junge H, Schnitzler WH. Bacillus subtilis as growth promotor in hydroponically grown tomatoes under saline conditions. Acta Hort ISHS. 2004;659:363–9. [Google Scholar]
- 23.Souret FF. Growth of saffron (Crocus sativus L.) in aeroponics and hydroponics [dissertation] Worcester: Worcester Polytechnic Institute; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


