Abstract
Abdominal aortic aneurysms (AAA) are progressive dilatations of infra-renal aorta causing structural weakening rendering the aorta prone to rupture. AAA can be potentially stabilized by inhibiting inflammatory enzymes such as matrix metalloproteinases (MMP); however, active regression of AAA is not possible without new elastic fiber regeneration. Here we report the elastogenic benefit of direct delivery of polyphenols such as pentagalloyl glucose (PGG), Epigallocatechin gallate (EGCG), and catechin, to smooth muscle cells obtained either from healthy or from aneurysmal rat aorta. Addition of 10 μg/ml PGG and ECGC induce elastin synthesis, organization, and crosslinking while catechin does not. Our results indicate that polyphenols bind to monomeric tropoelastin and enhance coacervation, aid in crosslinking of elastin by increasing lysyl oxidase (LOX) synthesis, and by blocking MMP-2 activity. Thus, polyphenol treatments leads to increased mature elastin fibers synthesis without increasing the production of intracellular tropoelastin.
Keywords: Abdominal aortic aneurysm, polyphenols, elastin, elastogenesis, vascular smooth muscle cells
Introduction
Abdominal aortic aneurysms (AAA) is a fatal disease of the artery characterized by accelerated inflammation mediated loss of matrix proteins such as elastin and collagen leading to structural weakening and eventual rupture of the artery [1]. There are approximately 18,000 deaths each year due to aneurysms in the United States making it the 13th largest cause of death [2]. Screening and early detection with elective surgical intervention is an effective way to decrease mortality in abdominal aortic and iliac arteries (AAA), where rupture is a great threat to the patient’s life. Whether open or endovascular treatment is chosen, the interventional procedure is not without complications, including mortality, thus the benefit has to outweigh the risk of surgical repair. Most importantly, none of these clinical interventions provide therapeutic relief in preventing or reversing the pathology.
As high as 90% of detected AAAs are small without indications for surgery, and the obvious and clinically relevant question is whether expansion of those small aneurysms can be prevented. AAA onset is associated with elastic lamina degradation by metalloproteinases (MMPs), which are derived from activated vascular cells and infiltrating inflammatory cells [3]. Once degraded, elastic lamina cannot be restored as adult cells have no ability to remodel elastic fibers [4]. To revert aneurysmal aorta to a healthy state, we must not only stop degradation; we also must reduce inflammatory enzyme activity and facilitate regeneration of elastic lamina.
In prior studies, we have demonstrated the ability of plant derived polyphenols such as pentagalloyl glucose (PGG) to bind to elastin and prevent it from elastolytic degradation [5]. We have also shown, in a rat model, that a single time application of pentagalloyl glucose (PGG) prevents AAA expansion and reduces aortic diameter [6]. That study showed a significant restoration of elastic lamina after PGG treatment. Thus, we wanted to explore the ability of polyphenols to not only prevent elastin degradation but increase elastin synthesis by vascular smooth muscle cells at the disease site. Here we tested ability of different polyphenols to bind to tropoelastin and to increase insoluble elastin production in healthy and aneurysmal vascular smooth muscle cells in vitro.
Materials and methods
In-vitro coacervation and maturation of tropoelastin
The kinetics of tropoelastin coacervation and maturation were performed using UV-Vis plate reader (BioTek, Winooski, VT) equipped with temperature, stir controllers and kinetic measurement features. 1 mg of human recombinant tropoelastin (Advanced BioMatrix, Poway, CA) was dissolved in 100% glacial acetic acid (Fisher Scientific, MA). Polypeptides were diluted in coacervation buffer (50 mM Tris, pH 7.5) to either 25 μM for one set of experiments and 10 μM for another set of experiments. The temperature of coacervation was 37°C. Samples were stirred at the rate of 1000 rpm and absorbance was measured at 440 nm every minute throughout the reaction time. 100 μl polyphenols at 10 μg/ml were added to 100 μl polypeptides (in ice) immediately before the absorbance measurement.
Cell culture
Primary rat aortic smooth muscle cells (RASMC) were freshly isolated. Briefly, freshly harvested abdominal aorta from healthy adult male Sprague Dawley rats were isolated and cleaned. Endothelium was scraped off and the adventitia was removed with scalpel blade. The medial layer was minced and digested in 125U/ml Collagenase (Worthington, Biochemicals Lake-wood, NJ) and 3U/mg elastase (Elastin products company, Owensville, MO) in Dulbecco’s modified Eagle’s Medium-F12 (Hyclone, Thermo Scientific, Rockford, IL) with 10% fetal bovine serum. The RASMCs leave aorta and attach and grow in petri dishes. Routine characterization of RASMCs was performed by staining cells for α-smooth muscle actin (SMA), myosin heavy chain II (MHC), and smooth muscle −22α (SM22) expression.
RASMCs isolated from rat aorta with advanced abdominal aortic aneurysm were received from Dr. Ramamurthi at Cleveland Clinic, the experimental procedure for which has been described in detail earlier [7]. Briefly, the posterior lumbar aortic branches were ligated; infra-renal aorta was surgically exposed and injured via catheter mediated intra-luminal elastase perfusion. The aortic diameter was measured before aortotomy, after aortotomy and after harvest. The aneurysms were allowed to develop for 14 days before vascular smooth muscle cells were harvested with the same procedure as described above for normal aorta (denoted Anu-RASMCs).
Passage numbers 4–8 were used for all the experiments. Cells were cultured in 12 well plates (200,000/well) in Dulbecco’s modified Eagle’s-F12 medium containing 10% fetal bovine serum (HyClone Laboratories, Inc., Novato, CA), 100 units/ml penicillin and 100 units/ml streptomycin (Cellgro-Mediatech, Herndon, VA) in a humidifier incubator at 37°C, with 5% CO2. Media was replenished every 3 days.
Polyphenol treatment of cells
Healthy RASMCs / aneurysmal RASMCs (Anu-RASMC) were cultured in medium containing polyphenolic additives (10 μg/ml; n=3/condition) for 14 days. Polyphenols were dissolved in Dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, MO) to prepare stock concentration of 10 mg/ml and filter sterilized using 0.2 μm membrane filters (Corning Incorporated, Corning, NY) prior to addition. Control groups received only vehicle (DMSO). Cell culture media was changed every 3 days and spent medium was collected at each media change, frozen at −20°C and biochemically assayed for tropoelastin and lysyl oxidase. After 14 days, the cell layers and soluble proteins were collected and analyzed.
Protein isolation
Cell monolayers were washed twice in PBS and cells were isolated in a mammalian extraction buffer. To prepare the buffer, 1 tablet of protease inhibitor cocktail (Sigma, St. Louis, MO) was added to 10 ml of Solulyze-M mammalian extraction buffer (Genlantis, San Diego, CA). Cell layers were homogenized using PowerGen 125 homogenizer and centrifuged at 10,000 × g for 15 min. The supernatant was collected and assayed for different proteins of interest. Total soluble protein was quantified using Peirce BCA protein assay (Thermo Scientific, Rockford, IL).
Fastin assay for elastin
Total insoluble elastin deposited in the cell layers and soluble monomeric tropoelastin released in the media were quantified using Fastin assay (Accurate Scientific and Chemical Corporation, Westbury, NY). For each treatment group, tropoelastin was assayed individually after each media change to generate a trend curve, and also evaluated as cumulative tropoelastin released over 14 days. To quantify the mature elastin deposited within the cell layers, the cell pellet generated after the protein isolation procedure mentioned above, was lyophilized and digested in oxalic acid as per manufacturer instruction manual. Since fastin assay quantifies only soluble α-elastin, the dried insoluble pellet was subjected to 3 digestion cycles with 0.25 M oxalic acid (100 °C, 1 h in water bath) and the pooled digests were assayed in the exact same procedure as tropoelastin in media. The total α-elastin was normalized to the total soluble protein released by the cells which is assumed to be directly proportional to the total cell count.
Gelatin zymography
Active MMP-2 was analyzed in the cell lysates (intracellular soluble proteins) by gelatin zymography [8]. The total protein was quantified using BCA kit and 12 μg total protein was loaded per well alongside with pre-stained molecular weight standards (Precision Plus Protein Standard, Bio-Rad, Hercules, CA). All lanes were loaded in duplicates with equal amounts of protein in each well. After development, coommasie staining and de-staining, the gels were photographed and density of clear bands (MMP-2 at 68kDa) was analyzed using ImageJ software and reported as relative density units.
In a separate set of experiments, proteins extracted from RASMC cell-cultures were loaded in gelatin gels, electrophoresed, and polyphenols were added to the development buffer. PGG, EGCG (250 μg) dissolved in DMSO, was added in 10 ml development buffer whereas control groups received volume matched DMSO. Following incubation, gels were stained, de-stained, and imaged.
Immunofluorescence for elastin, fibrillin-1
RASMCs/ Anu-RASMCs were treated in similar conditions as mentioned earlier. After 14 days, the cell layers were washed twice with PBS and fixed in 4% formaldehyde for 15 minutes in room temperature followed by incubation with a 5% bovine serum albumin blocking serum. The primary antibody, rabbit anti-rat elastin antibody (United States Biological, Swampscott, MA) or a rabbit polyclonal anti-fibrillin I antibody (Abcam, Cambridge, MA) at 1:100 dilution was applied overnight at 4°C. Alexafluor 488 chicken anti-rabbit IgG secondary antibody (Molecular Probes, Eugene, OR) was applied at a dilution of 8μg/ml for 2 hours at room temperature. Cell layers were mounted using aqueous mounting medium with anti-fading agents (Biomedia corp., Foster city, CA). The samples were examined by fluorescent microscopy. Importantly, all samples were imaged under exactly similar conditions for impartial analyses.
Transmission electron microscopy
The ultrastructure of extracellular matrix was studied using transmission electron microscopy (TEM). After 14 days in culture, Anu- RASMCs were fixed with 2% Electron Microscopy grade glutaraldehyde for 1 hour, rinsed in PBS, fixed in 1% aqueous osmium tetroxide (1 hour), rinsed, dehydrated through 100% ethanol, embedded in Epon 812 resin, sectioned, placed on copper grids, stained with uranyl acetate and lead citrate, and examined directly with transmission electron microscopy.
Statistical data analysis
Results are expressed as means ± standard error of the mean (SEM). Statistical analyses of the data were performed using single-factor analysis of variance (ANOVA). Subsequently, differences between means were determined using the least significant difference (LSD) with an alpha value of 0.05.
Results
Polyphenols bind to tropoelastin and accelerate rate of self-assembly in-vitro
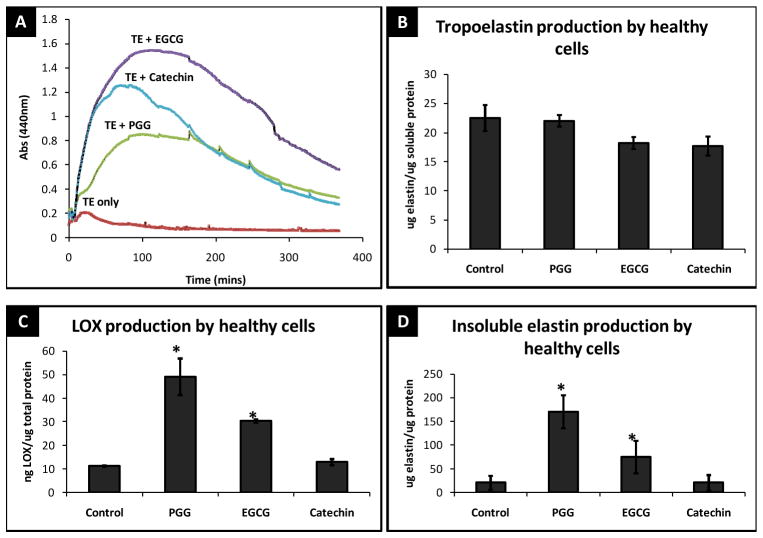
Kinetics of tropoelastin coacervation was examined in-vitro using a UV-Vis spectrophotometer enabled with stir rate controllers and temperature monitoring device. In initial experiment (data not shown), tropoelastin “coacervation phase” was marked by a rapid increase in absorption at 440 nm. This initial rise in absorbance is thereafter followed a steady decrease in absorbance; a stage termed as “maturation phase” [9].
Addition of polyphenols dramatically increased the rate of coacervation (Figure 1A) (PGG by 4-fold, EGCG by 7.5-fold and catechin by 6-fold) and delayed the onset of maturation (20 mins for pure polypeptides Vs. ~200 mins for polypeptides + polyphenols).
Figure 1.
(A) Self-assembly and coacervation kinetics of tropoelastin after polyphenols addition as studied by UV spectroscopy. (B) Total tropoelastin released by RASMCs after 14 days. (C) Total LOX produced by RASMCs after 14 days (D) Total insoluble elastin deposited by RASMCs after 14 days of treatment with 10 μg/ml polyphenols. * - significant difference compared to healthy control cells.
Tropoelastin and cross-linked elastin production
Addition of polyphenols to RASMCs did not change the total soluble tropoelastin production by cells at 14 days (Figure 1B). Interestingly, addition of polyphenols increased the production and activity of LOX by RASMCs (Figure 1C). As a result, all the polyphenols significantly enhanced the deposition of insoluble cross-linked elastin in healthy vascular cells after 14 days (Figure 1D). At 10 μg/ml, PGG induced ~8-fold greater mature elastin in healthy RASMCs. EGCG at the same concentration, increased elastin fiber deposition by ~ 4 times in RASMCs while catechin did not increase insoluble elastin production. The quantity, kinetics, and dynamics of tropoelastin released in the media were not changed by the addition of polyphenols (data not shown). It seems that increase in insoluble elastin was directly correlated with increased LOX production by PGG and EGCG.
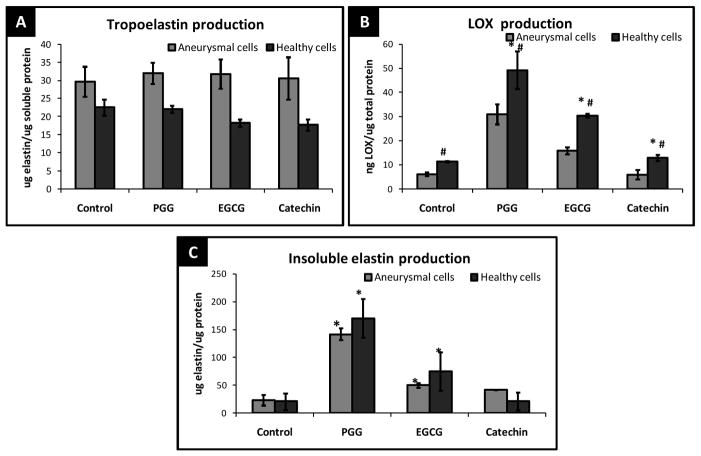
The exact same trends were also observed in passage matched aneurysmal cells, Anu-RASMCs (Figure 2A, B, and C). Polyphenols added at 1 μg/ml did not exert any elastogenic effects, after 14 days in culture (data not shown). From figure 2C, at 10 μg/ml, PGG induced ~6-fold greater mature elastin in Anu-RASMCs. EGCG at the same concentration, increased elastin fiber deposition by 2-fold in Anu-RASMCs and Catechins showed ~ 1.7-fold greater insoluble elastin deposition in Anu-RASMCs. Due to the lack in elastic matrix enhancement at 1 μg/ml polyphenols, all further studies were carried out using polyphenols at 10 μg/ml concentrations.
Figure 2.
(A) Comparison of total tropoelastin released by RASMCs and Anu-RASMCs after 14 days of polyphenol treatment. (B) Total LOX produced by RASMCs and Anu-RASMCs after 14 days. * - significant difference compared to healthy control cells, # - significant difference compared to aneurysmal cells, (C) Comparison of total insoluble elastin deposited by RASMCs and Anu-RASMCs after 14 days of treatment with 10 μg/ml polyphenols. *- significant difference compared to respective control groups.
Figure 2A is a comparative graph displaying the total tropoelastin elastin production by RASMCs and Anu-RASMCs. None of the polyphenols influenced the cellular tropoelastin production after 14 days by both RASMC and Anu-RASMCs. Counter-intuitively, Anu-RASMC cells released ~ 1.6 times greater soluble tropoelastin compared to healthy cells. Expectedly, LOX production was 2-fold lower in untreated aneurysmal cells than healthy cells (Figure 2B). However, addition of PGG, EGCG, and catechin showed significant increase in LOX even in Anu-RASMCs (5-fold, 3-fold, and 1.5-fold respectively). Both PGG and EGCG significantly improved insoluble elastin production even in Anu-RASMCs (Figure 2C).
ELN, Fib-1 immunofluorescence
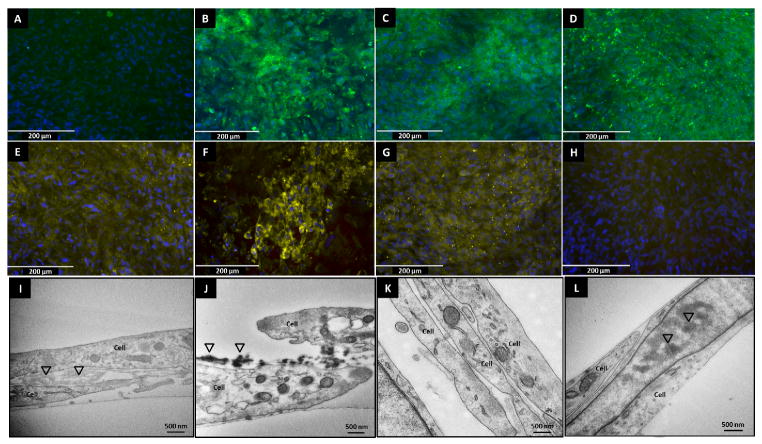
All cell cultures attained confluency and deposited sufficient extracellular matrix (confirmed by routine phase contrast microscopy) by the end of 14 days. The cells were fixed and immune-labeled for elastin and fibrillin-1. Immunofluorescence micrographs of 14 day cultures of Anu-RASMCs showed higher amounts and well-oriented elastin fibers (green fluorescence) in the polyphenol groups (Figure 3B, C, D) which were not present in the control groups (Figure 3A), with PGG treatment showing the highest deposition of elastin corroborating quantitative insoluble elastin data. Nuclear DAPI stain revealed relatively uniform cell layers in all the groups, indicating comparable cell viability.
Figure 3.
Immunofluorescence for elastin by Anu-RASMC in control (A), PGG (B), EGCG (C), catechin (D) groups. Immunofluorescence for Fibrillin-1 by Anu-RASMC in control (E), PGG (F), EGCG (G), catechin (H) groups. Representative TEM images of 14-days healthy RASMCs cultured without polyphenols (I), 10 μg/ml EGCG (J). Untreated Anu-RASMCs show insignificant extra-cellular matrix (K) which is evidently greater with addition of EGCG (L). Triangles indicate insoluble clumps of amorphous elastin deposited between cells.
Fibrillin-1 immunofluorescence also showed greater staining in the PGG treated groups (Figure 3F) compared to control cell layers (Figure 3E). EGCG and catechin groups failed to show fibrillin staining (Figure 3G and H), suggesting only PGG was able to deposit this microfibrillar protein associated with elastin, after 14 days.
Ultra-structure of elastin
Representative TEM images of RASMCs and those treated with EGCG are shown in Figure 3. Insoluble elastin is visualized as amorphous clumps in between cell layers which is minimally present in control group (Figure 3I). Treatment with EGCG enhanced extracellular matrix deposition as indicated by the triangles (Figure 3J). It is interesting to note that untreated aneurysmal cells were unable to deposit any elastin (Figure 3K). However, the deposition of insoluble elastin is enhanced by the addition of EGCG even to aneurysmal cells (Figure 3L).
Activity of proteolytic enzymes
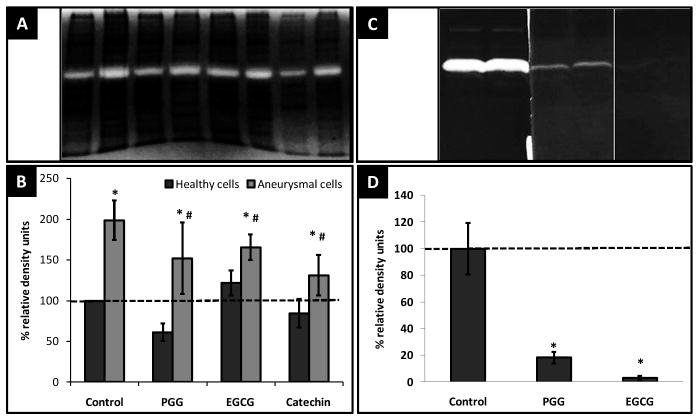
Gelatin zymography revealed that the total MMP-2 (66 kDa) activity was ~ 2-fold greater in the EaRASMCs when compared to healthy RASMCs, which was reduced by ~ 24% by PGG, 17% by EGCG and 34% by catechin. Healthy cells did not display any difference in their MMP-2 activity under the influence of polyphenols (Figure 4A). Additionally, when polyphenols were added extraneously to development buffer of cellular protein lysates, PGG inhibited MMP-2 activity by ~97% and EGCG by ~82% (Figure 4B).
Figure 4.
(A) MMP-2 (68 kDa) clear bands (B) relative MMP-2 cellular activity after treatment with polyphenols, * - significant difference compared to healthy control cells, # - significant difference compared to aneurysmal cells. (C) MMP-2 clear bands (D) relative MMP-2 cellular activity after extraneous addition of polyphenols in gel development buffer * - significant difference compared to control groups without polyphenol addition.
Discussion
Abdominal aortic aneurysm (AAA) is a life threatening vascular disease with no available pharmacological therapy. AAA is a multi-factorial pathology involving several biological, physical, bio-mechanical and hemodynamic factors that eventually lead to the pathological thinning of the artery wall leading to ballooning and eventual rupture. Current clinical repair options include surgical repair and deployment of endovascular stent grafts. Both these options are surgically invasive, and have their own limitations. MMP mediated elastin degradation is a characteristic feature of AAA leading to progressive degeneration of elastin and expansion of aorta [3, 10]. Matrix metalloproteinase (MMP-9, -2, and -12) have shown to play a critical role in development and expansion of AAA [11, 12]. Loss of elastin continues as it is not recouped due to poor remodeling of elastin fibers by adult vascular cells. The ideal treatment option for AAA would be a combination of strategies that work synergistically towards preventing elastolytic degradation, thus stabilizing existing matrix and improving regeneration of arterial proteins such as elastin by vascular smooth muscle cells.
In a series of prior publications we have shown that polyphenols such as tannic acid and PGG bind to elastic lamina and prevent further elastin degradation by elastolytic enzymes [6, 13–16]. Others have also shown that plant derived polyphenols like tannins and flavonols inhibit human leukocyte elastase activity [17] and prevent MMP-2/-9 activity in cancer cells [18]. Catechin derivatives have also displayed MMP inhibition therefore preventing elastin degradation [19]. Stabilizing aneurysmal elastin and protection against proteolytic damage resolves only one half of the pathological complication of AAA. A larger challenge in treating AAA is the inherently poor elastin turn-over by adult cells that prohibits the repair and regeneration of degraded elastin [20, 21]. Knowing polyphenols bind to hydrophobic amino acid residues with strong affinity [22], we hypothesized that tropoelastin secreted by cells will interact rapidly with polyphenols helping the process of coacervation and self-assembly. In present cell culture study, we demonstrate that addition of polyphenols at 10 μg/ml concentration to healthy vascular cells enhances mature cross-linked elastin fiber deposition. This occurs primarily due to coacervation of secreted tropoelastin and increased production of LOX by the cells in presence of polyphenols.
During development, microfibrillar scaffolding proteins such as MAGP-1, fibulin-5 are known to interact with tropoelastin coacervates and also recruit LOX for the oxidation of lysine residues leading to formation of intermolecular crosslinks [23, 24]. The presence of microfibrillar proteins have also shown to increase tropoelastin coacervation and retard maturation in-vitro that aids in better alignment of coacervates and systematic oxidation of lysine residues [9, 23]. The importance of increased coacervation lies in greater and more specific increase in the intermolecular structure of individual tropoelastin molecules facilitating downstream elastogenic events. Adult vascular cells lack this mechanism for increased elastic fiber deposition, resulting in defunct tropoelastin assembly, fiber formation, and increased susceptibility to enzymatic degradation.
Our results suggest that addition of polyphenols dramatically alter the self-assembly kinetics of tropoelastin. The interaction of polyphenols with tropoelastin is evident in the coacervation phase which is significantly enhanced compared to pure tropoelastin in solution (Figure 1A). While control tropoelastin molecules enter maturation by ~ 20 minutes, PGG, EGCG take ~ 180, ~200 minutes respectively to start maturing. In addition, polyphenols also increase the rate of coacervation as evident from the sharp increase initial turbidity. It is thus a safe conclusion that polyphenols dramatically aid in the process of tropoelastin self-assembly; partly by increasing the rate of coacervation and partly by inhibiting maturation, thereby providing greater inter-molecular interaction for lysine oxidation.
We further tested if polyphenols would increase LOX mediated elastic fiber assembly for coacervated tropoelastin. LOX is a vital enzyme required in the process of elastin crosslinking [25]. Indeed, we found greater LOX activity in presence of polyphenols further confirming that polyphenols increase insoluble cross-linked elastin deposition by first coacervating tropoelastin molecules and providing increased LOX to crosslink this tropoelastin. The mechanism of increased LOX activity by polyphenols is still unknown. Speculatively, increase in tropoelastin coacervate in extracellular matrix signal cells to produce LOX.
We further tested cross-linked elastin deposition by Anu-RASMCs. The total tropoelastin production was higher in aneurysmal cells compared to healthy cells, possibly due to compensatory feedback mechanism to replenish degraded elastin. This observation is similar to the past studies showing that AAA tissues produce greater tropoelastin compared to healthy tissues [26, 27]. However, this increase in tropoelastin production by aneurysmal cells did not translate to increase in insoluble cross-linked elastin in our culture conditions in absence of polyphenols. Aneurysmal cells deposited significantly higher quantities of matured elastin in presence of polyphenols compared to untreated control groups without directly increasing cellular tropoelastin production. LOX is strongly suppressed in aneurysmal tissues [27]. Our results indicate that aneurysmal cells have significantly lower LOX activity when compared to healthy cells, which is increased in the presence of polyphenols. This increase in LOX by polyphenols may be the other major reason for notably higher insoluble elastin in presence of polyphenols comparable to elstin in healthy cells. Interestingly, polyphenol treatments also decreased cellular MMP-2 activity as detected by zymography. Polyphenols strongly suppressed MMPs when added to the development buffer suggesting that polyphenols block activation of MMPs rather than decreasing MMP secretion. Thus, this can further aid in assembly of tropoelastin as degradative mechanisms are prevented.
Limitations
There are certain limitations to this study. This study was performed in-vitro in 2 dimensional cell cultures. Elastin synthesis and fiber assembly is a well-orchestrated, complex and relatively poorly understood process in-vivo. We have not studied the effect of polyphenols on critical assembly proteins like fibulin-4, 5, latent TGF-β binding protein, fibrillin-2, and matrix associated gla protein-1. It is clear that polyphenols accelerate coacervation and LOX production; however, maturation phase is a complex interplay of all the above mentioned proteins. In arteries, elastic fibers provide elastic recoil and it is unclear if newly produced insoluble elastin in polyphenol group has any functional characteristics. Further in-vivo experiments are needed to understand the elastogenesis process in presence of polyphenols and how they affect mechanical properties of the artery.
Conclusion
We show that use of polyphenols work multiple ways to regenerate cross-linked elastin. First, polyphenols directly interact with monomeric tropoelastin secreted by cells accelerating the elastin coacervation. Second, polyphenols increase LOX production by SMCs thus allowing crosslinking of coacervated elastin. Third, they inhibit matrix metalloproteinases such as MMP-2, thereby reducing enzyme mediated elastin degradation. Such treatments could be applied to treat and repair elastic fiber degradative diseases such as aortic aneurysms.
Highlights.
We use polyphenolic compounds to enhance elastin deposition by healthy and aneurysmal vascular cells for abdominal aortic aneurysm treatment
We demonstrate the rapid coacervation of recombinant tropoelastin in the presence of polyphenols
Polyphenols inhibit elastin degrading enzymes such as matrix metalloproteinase-2 thereby indirectly enhancing elastin production \
Polyphenols also increase lysyl oxidase, a key enzyme responsible for elastogenesis
Acknowledgments
The authors would like to thank Dr. Anand Ramamurthi for providing aneurysmal cells. This work was partially supported by the grants from National Institutes of Health (R01HL070969-08, R21HL084267, P20GM103444) and Hunter Endowment at Clemson University.
Non-standard abbreviations
- AAA
Abdominal Aortic Aaneurysm
- MMP
Matrix Metallo-Proteinase
- PGG
Penta-Galloyl Glucose
- EGCG
Epigallocatechin gallate
- LOX
Lysyl oxidase
- RASMC
Rat aortic smooth muscle cell
- Anu-RASMC
Aneurysmal rat aortic smooth muscle cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Writing Group M. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Kuivaniemi H, Platsoucas CD, Tilson MD. Aortic Aneurysms: An Immune Disease With a Strong Genetic Component. Circulation. 2008;117:242–52. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. The Journal of Clinical Investigation. 1995;96:318–26. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. The FASEB Journal. 1993;7:1208–18. [PubMed] [Google Scholar]
- 5.Partridge SM, Keeley FW. Age Related and Atherosclerotic Changes in Aortic Elastin. In: Wagner W, Clarkson T, editors. Arterial Mesenchyme and Arteriosclerosis. Springer; US: 1974. pp. 173–91. [DOI] [PubMed] [Google Scholar]
- 6.Isenburg JC, Simionescu DT, Starcher BC, Vyavahare NR. Elastin stabilization for treatment of abdominal aortic aneurysms. Circulation. 2007;115:1729–37. doi: 10.1161/CIRCULATIONAHA.106.672873. [DOI] [PubMed] [Google Scholar]
- 7.Gacchina CE, Deb P, Barth JL, Ramamurthi A. Elastogenic inductability of smooth muscle cells from a rat model of late stage abdominal aortic aneurysms. Tissue Eng Part A. 2011;17:1699–711. doi: 10.1089/ten.tea.2010.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey M, Pillarisetti S, Jones P, Xiao H, Simionescu D, Vyavahare N. Involvement of matrix metalloproteinases and tenascin-C in elastin calcification. Cardiovascular Pathology. 2004;13:146–55. doi: 10.1016/S1054-8807(04)00009-2. [DOI] [PubMed] [Google Scholar]
- 9.Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, et al. Fibrillins, Fibulins, and Matrix-Associated Glycoprotein Modulate the Kinetics and Morphology of in Vitro Self-Assembly of a Recombinant Elastin-like Polypeptide†. Biochemistry. 2008;47:12601–13. doi: 10.1021/bi8005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and Matrix Metalloproteinases in the Enlarging Abdominal Aortic Aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:1145–51. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 11.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. The Journal of Clinical Investigation. 1998;102:1900–10. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakalihasan N, Delvenne P, Nusgens BV, Limet R, Lapière CM. Activated forms of MMP2 and MMP9 in abdominal aortic aneurysms. Journal of Vascular Surgery. 1996;24:127–33. doi: 10.1016/s0741-5214(96)70153-2. [DOI] [PubMed] [Google Scholar]
- 13.Isenburg JC, Karamchandani NV, Simionescu DT, Vyavahare NR. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials. 2006;27:3645–51. doi: 10.1016/j.biomaterials.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25:3293–302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25:3293–302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Isenburg JC, Simionescu DT, Vyavahare NR. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials. 2005;26:1237–45. doi: 10.1016/j.biomaterials.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich M, Thomas T, Oliver W. Selective Inactivation of Human Neutrophil Elastase by Synthetic Tannin. Journal of Investigative Dermatology. 1991;97:529–33. doi: 10.1111/1523-1747.ep12481557. [DOI] [PubMed] [Google Scholar]
- 18.Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NFκB. Carcinogenesis. 2004;25:987–95. doi: 10.1093/carcin/bgh095. [DOI] [PubMed] [Google Scholar]
- 19.Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 2000;1478:51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DJ, Robson P, Hew Y, Keeley FW. Decreased Elastin Synthesis in Normal Development and in Long-term Aortic Organ and Cell Cultures Is Related to Rapid and Selective Destabilization of mRNA for Elastin. Circulation Research. 1995;77:1107–13. doi: 10.1161/01.res.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 21.McMahon M, Faris B, Wolfe BL, Brown K, Pratt C, Toselli P, et al. Aging effects on the elastin composition in the extracellular matrix of cultured rat aortic smooth muscle cells. In Vitro Cellular & Developmental Biology. 1985;21:674–80. doi: 10.1007/BF02620921. [DOI] [PubMed] [Google Scholar]
- 22.Luck G, Liao H, Murray NJ, Grimmer HR, Warminski EE, Williamson MP, et al. Polyphenols, astringency and proline-rich proteins. Phytochemistry. 1994;37:357–71. doi: 10.1016/0031-9422(94)85061-5. [DOI] [PubMed] [Google Scholar]
- 23.Maretoshi H, Tetsuya O, Masahito H, Katsuya O, Akari H, Kenneth RC, et al. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. The Journal of Cell Biology. 2007:176. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagan HM, Sullivan KA. Lysyl oxidase: preparation and role in elastin biosynthesis. Methods Enzymol. 1982;82(Pt A):637–50. doi: 10.1016/0076-6879(82)82092-2. [DOI] [PubMed] [Google Scholar]
- 25.Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. Journal of Cell Science. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 26.Krettek A, Sukhova GK, Libby P. Elastogenesis in Human Arterial Disease: A Role for Macrophages in Disordered Elastin Synthesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:582–7. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 27.Huffman MD, Curci JA, Moore G, Kerns DB, Starcher BC, Thompson RW. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery. 2000;128:429–38. doi: 10.1067/msy.2000.107379. [DOI] [PubMed] [Google Scholar]