Abstract
Oncogenic human papillomavirus (HPV) viral load may inform the origin of newly detected infections and characterize oncogenic HPV natural history in mid-adult women. From 2007–2011, we enrolled 521 25–65 year old female online daters and followed them triannually with mailed health and sexual behavior questionnaires and kits for self-sampling for PCR-based HPV DNA testing. Samples from oncogenic HPV positive women were selected for type-specific DNA load testing by real-time PCR with adjustment for cellularity. Linear or logistic regression models were used to evaluate relationships between viral levels, health and sexual behavior, and longitudinal oncogenic HPV detection. Type-specific viral levels were borderline significantly higher in oncogenic HPV infections that were prevalent versus newly detected (p=0.092), but levels in newly detected infections were higher than in infections re-detected after intercurrent negativity (p<.001). Recent sex partners were not significantly associated with viral levels. Compared to prevalent infections detected intermittently, the likelihood of persistent (OR=4.31,95%CI:2.20–8.45) or single-time (OR=1.32,95%CI:1.03–1.71) detection increased per 1-unit increase in baseline log10 viral load. Viral load differences between re-detected and newly detected infections suggest a portion of new detections were due to new acquisition, although report of recent new sex partners (a potential marker of new infection) was not predictive of viral load; oncogenic HPV infections in mid-adult women with new partners likely represent a mix of new acquisition and reactivation or intermittent detection of previous infection. Intermittent detection was characterized by low viral levels, suggesting that intermittent detection of persisting oncogenic HPV infection may be of limited clinical significance
Keywords: human papillomavirus, viral load, persistence, women, epidemiology
Introduction
While the epidemiology and natural history of female genital human papillomavirus (HPV) infections are largely well-characterized, important knowledge gaps remain, particularly for mid-adult populations.1, 2 Risk of HPV infection peaks in the mid-20s and is associated with new sex partners,2, 3 but the risk of new infections from new partners acquired in mid-adulthood is unclear. Furthermore, while the majority of infections acquired in young adulthood are detected transiently,3, 4 reactivation from latency and intermittent detection occur.5 However, the frequencies of these events are unknown. Finally, while persistent infection with oncogenic HPV infection is a necessary step in cervical carcinogenesis,3 the clinical significance of oncogenic HPV infections that are reactivated or intermittently detected is unclear.5 The availability of such information could inform guidelines and clinician-patient interactions regarding prophylactic HPV vaccination and cervical cancer screening. For example, prophylactic HPV vaccines are not currently recommended for women >26 years of age,1 but if older women are susceptible to new infections from new partners, vaccinating subgroups of high-risk women could be warranted. Furthermore, on an individual level, such data may be useful for informing clinician/patient psychosocial counseling regarding the potential origin or clinical significance of HPV test results encountered during routine cervical cancer screening (given that Pap/HPV co-testing is now a recommended screening strategy in women ≥30 years of age6, 7).
The challenge of distinguishing among new HPV acquisition, reactivation from latency, and intermittent persistent detection remains a methodological barrier to addressing these unresolved issues. Serologic measurements have limited utility for distinguishing between HPV infection states because not all infected women mount an antibody response,8 antibodies can wane over time,9 and the sensitivity of serologic assays is limited.10 We previously observed that both recent (e.g. current high-risk male sex partners) and cumulative (e.g. lifetime number of partners) risk behaviors were associated with oncogenic HPV infections in a high-risk cohort of 25–65 year old women, suggesting that both new acquisition and reactivation or persistence of previously acquired infections contribute to oncogenic HPV infections in mid-adult women with new partners.11 Characteristics of the oncogenic HPV virus (e.g. viral load) could help further elucidate the origin of newly detected infections in these women; in a previous cohort of young adult women, incident HPV16 and HPV18 viral loads were higher in women reporting multiple versus no recent new male sex partners.12 Furthermore, given that viral load (primarily HPV16) correlates with cervical disease risk,13–18 relating viral loads to patterns of repeat oncogenic HPV detection could inform the clinical significance of infections that are detected transiently or intermittently. Therefore, our study goals were twofold: to determine whether oncogenic HPV viral load measurements can be used in conjunction with previously observed associations between sexual behavior and oncogenic HPV detection to further inform the origin of newly detected infections in mid-adult women, and to use viral load measurements to characterize oncogenic HPV natural history in mid-adult women. To accomplish these objectives, we used real-time PCR to quantify type-specific oncogenic HPV DNA viral loads in self-collected vaginal swab samples from a cohort of high-risk 25–65 year old women who were recruited to participate in a longitudinal study of oncogenic HPV infections. Specifically, we compared viral levels between newly detected, prevalent, and re-detected oncogenic HPV infections; determined health and sexual behavior correlates of viral loads; and evaluated associations between viral levels and type-specific oncogenic HPV detection over 1 year of follow-up.
Materials and Methods
Between 2007–2010, we used Internet-based recruitment methods to enroll 521 25–65 year old female online daters into a longitudinal study of HPV infections. The study design was described previously.11 Briefly, women were followed triannually with at-home vaginal self-sampling for HPV DNA testing and demographic, sexual behavior, and health history questionnaires (up to four questionnaires and samples per participant). The protocol was reviewed and approved by the University of Washington Institutional Review Board.
Vaginal samples were tested for HPV DNA using polymerase chain reaction (PCR)-based methods. Samples were digested with 20 µg/mL of proteinase K at 37°C for 1 hour. 200 µl was used to isolate DNA using the QIAamp DNA blood mini column (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol (including additional protease digestion at 56°C for 10 minutes). HPV DNA and β-globin were amplified simultaneously (using 1/50 of purified DNA) using the HPV L1 consensus primers MY09, MY11, and HMB01 and β-globin primers PC04 (CAACTTCATCCACGTTCACC) and GH20 (GAAGAGCCAAGGACAGGTAC) (amplicon size 268bp). The amplification mixture contained 6 mmol/L MgCl2, 1x PCR buffer II (AmpliTaq Gold; Roche Molecular Diagnostics), 7.5 U of Gold Taq DNA polymerase (AmpliTaq Gold; Roche Molecular Diagnostics), 200 µmol/L dNTPs, 50 pmol of MY09 and MY11 primers, 10 pmol of HMB01, PC04, and GH20 primers, and 2 µL of template DNA, in a final volume of 50 µL. The DNA was amplified by use of the following program: 9 min at 95°C; 30 s at 95°C, 60 s at 55°C, and 60 s at 72°C, for 40 cycles; and 10 min at 72°C. Ten microliters of PCR products was then dotted onto nylon filters and probed by use of biotin-labeled β-globin and HPV generic probes. Results were recorded with regard to whether the sample was found to be positive for β-globin and/or HPV.
Samples that were determined to be HPV positive by generic probe or that were β-globin negative were tested with the Roche Linear Array HPV genotyping test (Roche Molecular Systems, Inc., Alameda, CA), which uses a β-globin control. The same purified DNA that was used for the dot-blot step was used for the Roche assay. The Roche Linear Array assay detects 37 HPV types, including 18 classified as carcinogenic, probably carcinogenic, or possibly carcinogenic: 16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82.19, 20 Samples were deemed insufficient if they were β-globin negative during the initial dot blot step and HPV negative by the Roche assay.
Samples from women testing positive for ≥1 of 18 oncogenic HPV types were selected for type-specific quantitative oncogenic HPV testing, including all type-specific positives (n=880) and intercurrent type-specific negatives (defined as a type-specific negative collected between two positives for the same type) (n=38). Samples were stored at −20° C prior to extraction and purification for real-time PCR. The median time elapsed between initial HPV detection with the Roche assay and extraction for real-time PCR was 11 (interquartile range, 5–20) months.
Quantification of type-specific HPV DNA loads by duplex real-time PCR
DNA was extracted and purified with QIAamp DNA Mini kit (Qiagen, Valentia, CA) from an aliquot of 100µl sample, using the methods described above. The purified DNAs were resuspended in 25ul AE buffer. We used duplex real-time PCR for quantification of cellular (β-actin) and HPV DNA simultaneously. A set of type-specific HPV E7 DNA standards for absolute quantification were constructed by cloning PCR-generated HPV DNA fragments into pSC-B plasmid using a StrataClone Ultra Blunt PCR Cloning Kit according to the protocol recommended by the manufacturer (Stratagene, La Jolla, CA). The accuracy of the target inserts was confirmed by DNA sequencing from both directions. To make testing results compatible across types, the plasmid constructs containing various types of HPV DNA fragments were calibrated by real-time PCR with a set of primers and probe targeting plasmid DNA.
Primers and probe for β-actin gene are commercially available (Applied Biosystems). Primers and probes for type-specific E7 gene were designed using Primer Express (Applied Biosystems, Foster City, CA) (Table 1). The specificity of type-specific primers and probe was assessed by testing the plasmid constructs for all types examined at a concentration of 105copies/µl. The assay was set up in triplicate on a 384 well plate with the TaqMan Universal PCR Master Mix kit (Applied Biosystems) in a final reaction volume of 4µl containing 0.8µl DNA sample input. Final concentration was 0.09 µM for primers and 0.06 µM for probes. Amplification was carried out on Applied Biosystems 7900 HT Sequence Detection System with a cycling program of holding at 50°C for 2 minutes and then at 95°C for 10 minutes followed by a two-step cycle of 10 seconds at 95°C and 1 minute at 60°C for 40 cycles.
Table 1.
Primers and probes for real-time PCR
| HPV types | Forward primer (5’-3’) | Probe (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|---|
| 16 | TTCGGTTGTGCGTACAAAGC | VIC-CACACGTAGACATTCGT-MGB | GCCCATTAACAGGTCTTCCAAA |
| 18 | CCGACGAGCCGAACCA | VIC-AACGTCACACAATGTT-MGB | TGGCTTCACACTTACAACACATACA |
| 26 | TGTGCAGAGCAGTCGACAGAA | VIC-CGAGTGCTGGAGCAGA-MGB | CCAAGGACACGTCTTCCATTAAC |
| 31 | CGACAGCTCAGATGAGGAGGAT | VIC-TCATAGACAGTCCAGCTGG-MGB | GGATGTGTCCGGTTCTGCTT |
| 33 | TGACAGCTCAGATGAGGATGAAG | VIC-CTTGGACCGGCCAGAT-MGB | CTGTGGCTGGTTGTGCTTGT |
| 35 | ACAGCTCAGAGGAGGAGGAAGA | VIC-ACTATTGACGGTCCAGCTG-MGB | GGAGGTGTCTGGTTTTGCTTGT |
| 39 | TGACCTTGTATGTCACGAGCAAT | VIC-AGGAGAGTCAGAGGATG-MGB | TGCATGGTCGGGTTCATCTA |
| 45 | GCGAGTCAGAGGAGGAAAACG | VIC-TGAAGCAGATGGAGTTAGT-MGB | CGGGCTGGTAGTTGTGCAT |
| 51 | GCTCCGTGTTGCAGGTGTT | VIC-AAGTGTAGTACAACTGGC-MGB | GGGTGTCTCCACTGCTTTCC |
| 52 | GACAGCTCAGATGAGGAGGATACA | VIC-ATGGTGTGGACCGGC-MGB | TGGCTTGTTCTGCTTGTCCAT |
| 53 | GCAGTTGGCTGTTCAGAGTTCA | VIC-AAAGAGCTGCGTATTTT-MGB | TGTGCCCATAAGCATTTGTTG |
| 56 | AGTGCCAACGCTGCAAGAC | VIC-TATTAGAACTAACACCTCAAACAG-MGB | TGCTCATTGCACTGTAGGTCAA |
| 58 | GACAGCTCAGACGAGGATGAAA | VIC-AGGCTTGGACGGGC-MGB | TGGCCGGTTGTGCTTGT |
| 59 | TGACTCCGACTCCGAGAATGA | VIC-AAAGATGAACCAGATGGAGT-MGB | TCGTCTAGCTAGTAGCAAAGGATGAT |
| 66 | GAGTTGGTGGTGCAGTTGGA | VIC-ATTCAGAGTACCAAAGAGGA-MGB | AAGCAGCTGTTGTACCACACGTA |
| 68 | TGAACCCGACCATGCAGTTA | VIC-TCACCACCAACATCT-MGB | TTCGTCCCGTCTGGCTAGTAG |
| 73 | ACCAACAACCGAAATTGACCTT | VIC-CATGTTACGAGTCATTGGA-MGB | CTGTTTCATCCTCATCCTCTGAGTT |
| 82 | AAGTGCACTGTTGCAGGTGTTC | VIC-AGTGTTGTACAGCTCGCAG-MGB | GGCTGTCTCCACTGCTTTCC |
Two standard curves were implemented in each set of the assay, one for HPV DNA and the other for cellular DNA (TaqMan® Control Genomic DNA (Human)). For each run, the number of viral copies was normalized according to the input amount of cellular DNA and expressed as the number of HPV copies per nanogram of cellular DNA. Copy numbers were log-transformed and a mean of three measures was used for analysis. If one of the three measures differed by more than two standard deviations, the mean of the two remaining measures was used. Runs with cycle thresholds greater than 40 were classified as having undetectable DNA levels. Samples negative for β-actin on ≥2 measures were excluded from statistical analyses of viral loads. Samples with ≥2 measures with undetectable HPV DNA were assigned a value of 1 copy number per nanogram of cellular DNA (0 after log10 transformation) for statistical analyses if they were type-specific positive by the Roche assay. Due to the high proportion of HPV 53 Roche positives that were undetectable by real-time PCR (36 [37%] of 98 compared to 70 [9%] of 782 for all other types combined), we excluded type 53 from all viral load analyses described below.
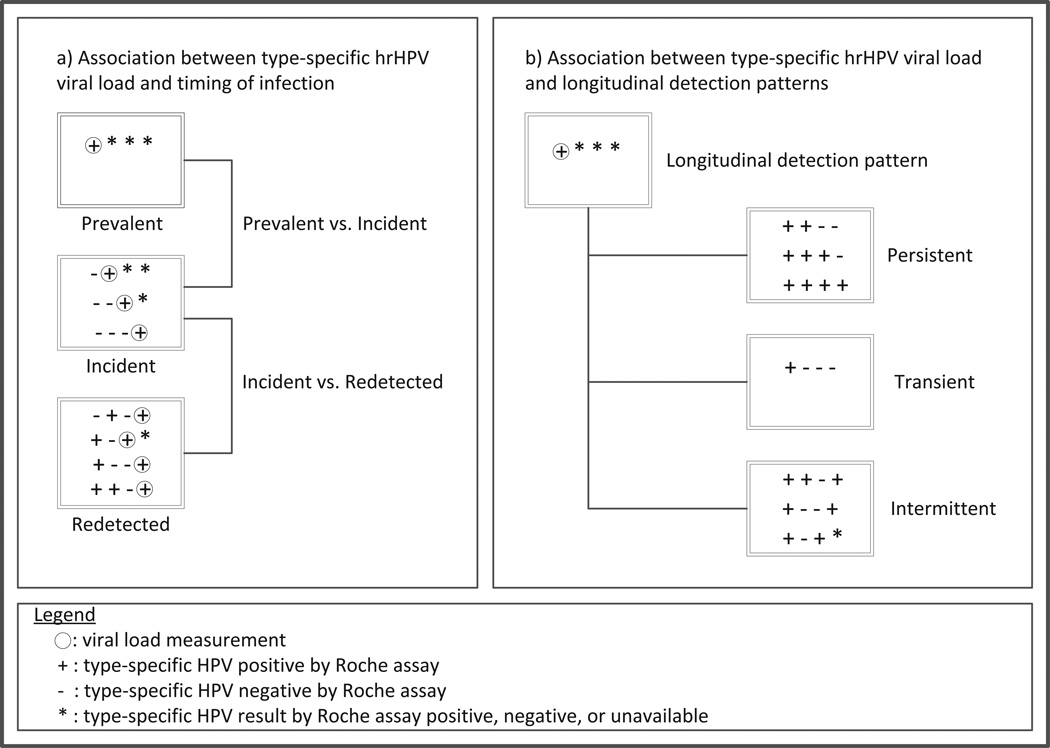
Analyses were based on type-specific oncogenic HPV infections. We used linear regression to compare type-specific oncogenic HPV viral loads at first detection (by the Roche assay) in incident versus prevalent infections. The linear regression beta coefficients were back-transformed (by taking the antilogarithm) to represent the ratio increase or decrease in DNA for a one unit change in the covariate. We refer to the back-transformed beta coefficients as ‘viral load ratios’. We similarly compared viral loads at first incident detection to those in redetected infections. Redetection was defined as type-specific positive detection following an observable period of intercurrent negativity for the same type, and viral load was considered at the time of first Roche positive following the intercurrent negative period. (Figure 1a) All models included a main-effect term for HPV type, and robust variance estimates were used to account for correlation among multiple HPV types within a subject.
Figure 1.
Visual overview of main viral load analyses
We used logistic regression to evaluate associations between baseline type-specific oncogenic HPV viral load (restricted to prevalent infections) and detection patterns over longitudinal follow-up, including: persistent (positive in ≥2 consecutive samples without subsequent redetection), intermittent (repeatedly detected with a period of intercurrent negativity), or transient (single-time positive). (Figure 1b) All models included a main-effect term for HPV type, and GEE with robust variance estimates were used to account for correlation between multiple HPV types within a subject. We evaluated potential confounding by concurrent detection of other oncogenic HPV types (0/1/2+) and demographic, health, and sexual behavior variables measured at the time of HPV viral load assessment, including: age (continuous), lifetime number of male sex partners (quintiles), cigarette smoking (never/former/current), current use of hormonal contraception (yes/no), history of an abnormal Pap test (yes/no), condom use with male partners in the last 6 months (always/not always/not sexually active), and sex with male partners in the last 6 months (not sexually active/sex with one non-new partner/sex with new or multiple partners). Each variable was tested individually with viral DNA load and HPV type; those that changed the point estimate by >10% were considered confounders and were retained in the final model. We also evaluated peak and mean viral loads over follow-up in relation to longitudinal detection patterns using similar methods. Finally, we conducted a sensitivity analysis to evaluate whether intervals of >8 months between sample collection changed our estimates of the associations between HPV viral load and longitudinal detection patterns; we restricted the analyses to infections detected in women with ≤8 months between all samples (82% of infections) and compared the results to those from the full model.
We also used linear regression to evaluate demographic, health, and sexual behavior predictors of viral load at time of first positive by the Roche assay (considering the same variables evaluated as potential confounders in the analyses described above). To assess whether timing of infection modified the association between reports of recent male sex partners and viral load, we compared stratum specific estimates for incident and prevalent infections. We also used logistic regression to evaluate the associations between potential demographic, health, and sexual behavior predictors of persistent versus transient and intermittent infections.
A two-sided 0.05 test level determined statistical significance for all analyses. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Study population characteristics
Analyses were restricted to 518 women (99.4%) with sufficient baseline samples for HPV testing. Their demographic, health, and sexual behavior characteristics at enrollment have been reported previously.11 Briefly, their mean age was 35.8 (SD,9.5) years and the majority was white (64.5%), unmarried (70.7%) and reported sex with new or multiple male partners in the last 6 months (52.4%). The minority reported no sex with male partners (14.9%) or one non-new male partner (32.7%) in the last 6 months and current (15.1%) or former (22.0%) smoking. Oncogenic HPV prevalence was 35.5%. 421 women (81.3%) returned ≥1 follow-up sample and were followed for a mean (SD) of 12.5 (5.0) months; the mean (SD) number of samples returned was 3.5 (0.9). 299 (57.7%) women returned all four samples. The mean (SD) interval between study visits was 5.1 (1.4) months.
Summary of oncogenic HPV infections detected at baseline and over follow-up
A total of 489 type-specific oncogenic HPV infections were detected in 240 (46.3%) women. The majority was prevalent (65.6%) versus incident (34.4%). HPV16 (12.3%) and HPV53 (12.3%) were the most common types. Of 191 infections detected in women followed with 4 samples, 53.4% were persistent, 33.5% transient, and 13.1% intermittent. (Table 2)
Table 2.
Summary of oncogenic HPV infections detected at baseline and over follow-up in high-risk mid-adult women (n=518)
| N | % | |
|---|---|---|
| WOMEN with oncogenic HPV detecteda | 240 | 46.3 |
| Type-specific oncogenic HPV INFECTIONS detecteda | 489 | -- |
| PREVALENT type-specific oncogenic HPV infections detecteda | 321 | -- |
| HPV 16 | 42 | 13.1 |
| HPV 18 | 25 | 7.8 |
| HPV 26 | 3 | 0.9 |
| HPV 31 | 13 | 4.0 |
| HPV 33 | 5 | 1.6 |
| HPV 35 | 12 | 3.7 |
| HPV 39 | 21 | 6.5 |
| HPV 45 | 10 | 3.1 |
| HPV 51 | 28 | 8.7 |
| HPV 52 | 27 | 8.4 |
| HPV 53 | 34 | 10.6 |
| HPV 56 | 14 | 4.4 |
| HPV 58 | 18 | 5.6 |
| HPV 59 | 20 | 6.2 |
| HPV 66 | 25 | 7.8 |
| HPV 68 | 12 | 3.7 |
| HPV 73 | 10 | 3.1 |
| HPV 82 | 2 | 0.6 |
| INCIDENT type-specific oncogenic HPV infections detecteda | 168 | -- |
| HPV 16 | 18 | 10.7 |
| HPV 18 | 10 | 6.0 |
| HPV 26 | 2 | 1.2 |
| HPV 31 | 9 | 5.4 |
| HPV 33 | 3 | 1.8 |
| HPV 35 | 5 | 3.0 |
| HPV 39 | 6 | 3.6 |
| HPV 45 | 7 | 4.2 |
| HPV 51 | 15 | 8.9 |
| HPV 52 | 15 | 8.9 |
| HPV 53 | 26 | 15.5 |
| HPV 56 | 8 | 4.8 |
| HPV 58 | 5 | 3.0 |
| HPV 59 | 11 | 6.5 |
| HPV 66 | 8 | 4.8 |
| HPV 68 | 5 | 3.0 |
| HPV 73 | 9 | 5.4 |
| HPV 82 | 6 | 3.6 |
| Longitudinal oncogenic HPV detection patterns among prevalent type-specific oncogenic HPV infectionsb (n=191) | ||
| Persistent | 102 | 53.4 |
| 12 months (++++) | 56 | 29.3 |
| 8 months (+++−) | 22 | 11.5 |
| 4 months (++−−) | 24 | 12.6 |
| Transient (+−−−) | 64 | 33.5 |
| Intermittent (+−−+/+−++/+−+−/++−+) | 25 | 13.1 |
Measured at time of 1st type-specific positive by the Roche assay.
Excluding 130 prevalent infections from women with <4 samples.
Summary of cellular DNA levels and type-specific oncogenic HPV viral loads
Overall, 8 of 880 (0.9%) samples tested did not have detectable β-actin and were excluded from statistical analyses of viral load. Among samples with detectable β-actin, the mean (SD) level of cellular DNA was 13.70 (16.72) nanograms (range, 3 × 10−5 to 271.09 nanograms). Mean log10 type-specific HPV viral loads per nanogram of cellular DNA are shown in the supplementary table.
Associations between type-specific oncogenic HPV viral load and timing of infection (incident, prevalent, or redetected)
Mean log10 type-specific HPV viral load per nanogram cellular DNA was borderline statistically significantly higher in prevalent (2.1) versus incident (1.8) infections (viral level ratio (VLR)=2.24,95%CI:0.87–5.75, adjusting for HPV type). Mean log10 viral load per nanogram cellular DNA was significantly higher in incident than in redetected infections (0.9) (VLR=8.51,95%CI:2.75–26.30, adjusting for HPV type). (Table 3)
Table 3.
Comparison of type-specific oncogenic HPVa viral load in incident, redetected and prevalent infections
| Log10 viral loadb (Mean ± SD) |
p-valuec | |
|---|---|---|
| Prevalentd (n=286) | 2.1 ± 2.2 | 0.0921 |
| Incidentd (n=142) | 1.8 ± 2.0 | |
| Incidentd (n=142) | 1.8 ± 2.0 | 0.0002 |
| Redetectede (n=30) | 0.9 ± 0.9 |
Includes types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, 82 (type 53 was excluded from all viral load analyses).
per nanogram of cellular DNA.
P-value for mean difference in viral load, adjusted for HPV type.
Measured at time of 1st type-specific positive by the Roche assay.
Measured at time of 1st redetection by the Roche assay for type-specific infections with intercurrent negatives (following either prevalent or incident detection).
Demographic, health, and sexual behavior predictors of HPV viral load
Mean log10 viral load per nanogram of cellular DNA (measured at first type-specific positive by the Roche assay) was higher in infections detected in never (2.1) or former smokers (2.2) compared to those detected in current smokers (1.5), and these differences were statistically significant (VLR=4.57,95%CI:1.41–14.79 and VLR=4.90,95%CI:1.15–20.42, respectively, adjusting for HPV type). Reports of recent sex partners were not significantly associated with viral loads for either incident or prevalent infections. No other significant predictors of viral load were identified.
Associations between type-specific oncogenic HPV viral load and longitudinal detection patterns
Among prevalent infections, mean log10 viral load per nanogram of cellular DNA (measured at first type-specific positive by the Roche assay) increased from 1.2 in infections detected intermittently to 1.8 in those detected transiently to 2.7 in those detected persistently. Each 1-unit log10 per nanogram of cellular DNA increase in type-specific HPV viral load was associated with an increased likelihood of persistent versus intermittent detection (OR=4.31,95%CI:2.20–8.45, adjusting for HPV type, lifetime number of male sex partners, recent condom use with male partners, and concurrent detection of other oncogenic HPV types), transient versus intermittent detection (OR=2.35,95%CI:1.02–5.38, adjusting for HPV type, lifetime number of male sex partners and recent male partners), and persistent versus transient detection (OR=1.32, 95%CI:1.03–1.71, adjusting for HPV type). (Table 4) Similar associations were observed when considering peak or mean type-specific HPV viral load over follow-up (data not shown). Furthermore, results were similar when restricting to infections from women with intervals of <8 months between all collected samples (data not shown).
Table 4.
Associations between type-specific log10 oncogenic HPVa viral loadb per nanogram of cellular DNA & longitudinal detection patterns among prevalent infectionsc
| Detection Pattern | OR (95% CI) |
|---|---|
| Persistentd (n=93) vs. Intermittent (n=25) | 4.31e (2.20–8.45) |
| Persistentd (n=93) vs. Transient (n=51) | 1.32f (1.03,1.71) |
| Transient (n=51) vs. Intermittent (n=25) | 2.35g (1.02,5.38) |
Includes types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, 82 (type 53 was excluded from all viral load analyses).
Measured at time of 1st type-specific positive by the Roche assay.
Excluding 130 prevalent infections from women with <4 samples.
Includes infections persistently detected for 4, 8, or 12 months without subsequent redetection. Viral loads at baseline were similar for these 3 categories.
Adjusted for HPV type, lifetime number of male sex partners, condom use in the last 6 months, and concurrent detection of other oncogenic HPV types
Adjusted for HPV type
Adjusted for HPV type, number of male sex partners in last 6 months, and lifetime number of male sex partners
Demographic, health, and sexual behavior predictors of persistently detected infections
Compared to never smokers, current (OR=0.32,95%CI:0.10–1.06, adjusting for HPV type) and former (OR=0.32,95% CI:0.11–0.90, adjusting for HPV type) smokers were less likely to have persistent versus transient detection. No other statistically significant predictors of persistent versus transient or intermittent detection were identified.
Longitudinal trends in oncogenic HPV viral load
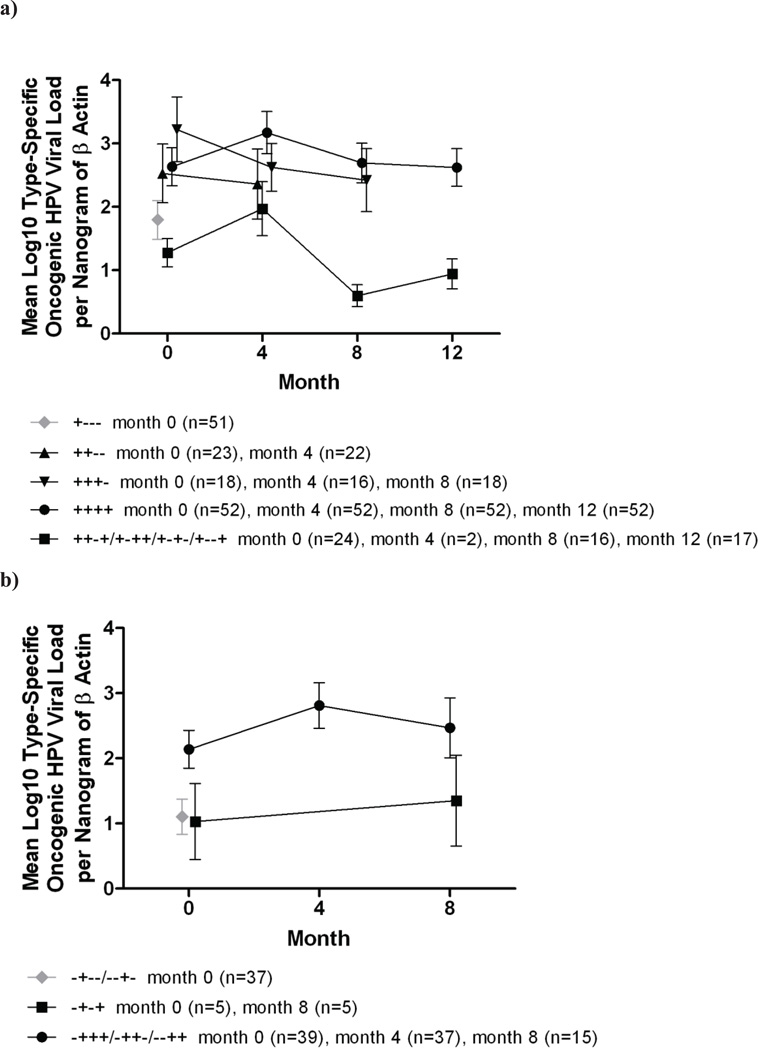
On average, viral loads in intermittently detected infections remained lower over follow-up than those in persistently detected infections without intercurrent negative periods. Furthermore, viral loads in persistently detected infections were similar regardless of the duration of persistent detection (i.e. 4/8/12 months). These trends were observed for both prevalent and incident infections. In addition, viral loads in transient infections detected at baseline were lower than in transient infections detected during follow-up. (Figure 2)
Figure 2.
Mean log10 type-specific oncogenic HPV viral load per nanogram of cellular DNA over time for a) prevalent and b) incident infections detected intermittently, transiently and persistently for 4, 8 and 12 months. Figures include types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, 82 (type 53 was excluded from all viral load analyses). Only visits with type-specific positive results by the Roche assay are included. For prevalent infections, month 0 is baseline. For incident infections, month 0 is the time of 1st detection by the Roche assay. Months 4, 8, and 12 are subsequent visits occurring at approximately 4-month intervals. Data points are jittered to prevent overplotting.
Discussion
In a high-risk cohort of mid-adult women followed for 1 year, we investigated whether type-specific oncogenic HPV DNA viral load measurements in self-collected vaginal samples could be used to further our understanding of the epidemiology and natural history of oncogenic HPV infections in mid-adulthood. We used real-time PCR to individually quantify 18 oncogenic HPV types with β-actin (allowing for correction for cellular DNA), and measured viral loads up to four times over one year. Our first objective was to explore whether viral load data could be used to build upon previously observed associations between sexual behavior and oncogenic HPV detection to further inform the origin of newly detected infections in mid-adult women. In a previous cohort of 18–22 year old female university students recruited close to sexual debut, we found that among incident infections, report of more than one recent new male sex partner was associated with increased cervical HPV16 or HPV18 mRNA viral load compared to report of no recent new partners.12 Therefore, our hypothesis was that viral loads at the time of incident detection would be higher in women reporting recent new or multiple sex partners compared to women who were not sexually active. However, we observed no significant associations between recent sexual behavior and viral levels in incident infections. Differences in sampling (clinician-collected cervical versus self-collected vaginal samples), age and sexual behavior profiles between the two cohorts (young adult with few or no sex partners at enrollment versus mid-adult with a median lifetime number of 11 partners) may account for the discrepant results. Whereas the majority of new infections detected in the younger cohort were likely to represent newly acquired infections, those detected in the present mid-adult cohort were likely a mix of new acquisitions, reactivations, and intermittent persistent detections. We also observed borderline statistically significantly higher viral levels in prevalent than in incident infections; this finding was expected, given that prevalent infections likely represent a mix of new and persistent infections, whereas incident infections likely represent a mix of new, reactivated or intermittently detected infections. In fact, we observed that infections that were re-detected after a period of negativity (reflective of reactivation or intermittent persistent detection) harbored viral levels that were significantly lower than those in incident infections, suggesting that at least a portion of the incident infections represented new acquisition from new partners rather than previously acquired infection. These data are consistent with our previous observations that measures of both recent and cumulative sexual risk behaviors were associated with oncogenic HPV infections in these mid-adult women,11 and further support the theory that both new acquisition and reactivation or persistence of previously acquired infections contribute to oncogenic HPV detections in sexually active mid-adult women.
To better understand the natural history of oncogenic HPV infections in mid-adulthood, our second objective was to correlate viral load with type-specific oncogenic HPV detection patterns over longitudinal follow-up. We found that each one-unit log10 increase in baseline type-specific oncogenic HPV viral load was associated with an increased likelihood of persistent versus either transient or intermittent detection, and also associated with an increased likelihood of transient versus intermittent detection. These associations were similar regardless of whether baseline, peak, or mean viral load measurements were considered. While definitions of viral persistence and analytical methods for assessing relationships between viral loads and persistence vary across studies, our results demonstrating a positive association between viral load and persistent detection are consistent with many (but not all21, 22) studies conducted in both young and mid-adult populations of women.23–29 While most previous studies were designed to evaluate whether viral load predicts duration of detectable infection, our primary focus was on pattern rather than length of repeat detection. We did notice, however, that the associations described above were similar regardless of whether a 4, 8, or 12-month definition of persistent detection (without subsequent redetection) was used, and that viral levels over one year of follow-up were similar regardless of the length of persistent detection. These findings are somewhat consistent with those in women enrolled into the atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) triage study (ALTS); baseline cervical HPV16 or HPV18 DNA viral loads in were predictive of short-term (6-month) persistence, but did not effectively differentiate between short-term versus longer-term (12–24 month) persistence.30
We further observed that transient infections detected at baseline harbored higher viral levels than transient infections detected during follow-up. This finding was expected, given that some transient infections detected at baseline may have represented resolving persistent infections, whereas those detected during follow-up were more likely to represent transient detection of new, reactivated, or intermittent infections.
Viral levels were higher when infections were repeatedly detected without any intervening negative periods than when detection was intermittent. In post-hoc analyses, we further observed that viral levels in redetected infections were similar following one versus two intervening negative visits (data not shown). Our longitudinal data provide only a one-year snapshot of viral load kinetics in the course of oncogenic HPV infection (given that the onset and duration of infection are unknown). Furthermore, false negative results or sampling inconsistencies may account for some intermittent detection. Nonetheless, our results highlight distinctions between infections detected intermittently versus consecutively. Our findings of low viral loads in intermittent or re-detected oncogenic HPV infections agree with unpublished data from ALTS; in a random sample of infections with non-HPV16 or 18 oncogenic HPV types detected at ≥2 visits, the lowest viral levels were observed when type-specific oncogenic HPV was re-detected after an intervening negative test(s) (Long Fu Xi, personal communication). In contrast, in an adult cohort of women in Brazil followed semiannually, viral levels were reportedly similar at the time of initial infection and re-detection of the same type (following an intervening negative period of at least 1.5 years).31 A limitation of that study, however, was that re-detection was type-specific, but viral load measurements were not, complicating interpretation of the results. While we did not evaluate clinical outcomes, our data suggest that intermittent detection of persisting infections in mid-adulthood may be of limited clinical significance, given that higher oncogenic HPV viral loads (primarily HPV16) have been associated with a greater risk of high-grade cervical lesions and carcinogenic progression.13–18. A caveat, however, is that relationships between oncogenic HPV viral load and carcinogenic progression may vary by HPV type; some studies demonstrated positive associations between viral load and clinical disease for HPV16, but not for other hr types.29, 32, 33
In contrast to ALTS data reporting that HPV16 and HPV18 viral loads were higher in current than in never smokers,34 we found that current smokers had lower oncogenic HPV viral loads than never smokers. Furthermore, current smokers were less likely than never smokers to have persistent versus transient detection of oncogenic HPV infections. The latter finding is consistent with two earlier studies (one in a cohort of university students35 and one in a mid-adult cohort36), but contrasts with findings from a larger number of studies demonstrating positive29, 37–40 or null21, 41–44 associations between smoking and HPV persistence. The relationship between smoking and HPV-related cervical carcinogenesis is likely complex; while carcinogens in tobacco smoke likely contribute to carcinogenesis after persistent oncogenic HPV infection is established, it is unclear whether these carcinogens influence oncogenic HPV persistence, potentially accounting for differences across studies.21 We also investigated a dose-response effect by evaluating mean number of cigarettes smoked per day in relationship to viral load and persistence, but did not observe any notable trends in support of a causal association. Furthermore, observed associations with former smoking were inconsistent; former smokers had oncogenic HPV viral loads that were similar to those in never smokers, but were less likely than never smokers to have persistent infection. The observed inverse relationship between smoking and viral load and oncogenic HPV persistence could be attributable to residual confounding by unmeasured sexual risk behavior. It is also possible that smoking contributes to an increased likelihood of reactivation. Previous studies indicating an adverse effect of smoking on both local and systemic immune responses45–49 support this theory. We did observe a slightly higher proportion of redetected versus incident or prevalent infections in current smokers.
Several study limitations should be noted. First, we excluded HPV53 (the second most commonly detected type in this cohort, after HPV16) from all viral load analyses, due to the high proportion of HPV53 positives by the Roche Linear Array that were negative by real-time PCR. The discrepant results were likely attributable to variant-related nucleotide alterations in the HPV53 primer/probe binding sites (although the possibility of false positive test results by the Roche assay cannot be ruled out). As reported previously,50 mismatches between primers and probes and their binding sites can substantially reduce amplification efficiency, thereby leading to false negative tests. In fact, we observed this phenomenon in the present study with HPV56. We sequenced the PCR products of the E7 gene from an HPV56 infection detected in a woman who tested positive for HPV56 in consecutive samples by the Roche assay, but negative on all samples by real-time PCR. After identifying an A-to-C change at the 3’ end of the forward primer, we designed new primer and probe for HPV56, and all samples re-tested positive. Unfortunately, we did not have sufficient resources to explore variant-related mismatches and alternative primer/probe for HPV53. Second, with only one year of follow-up, we were unable to explore relationships between viral load and long-term persistence or long-term fluctuations in viral load, and were unable to evaluate whether viral load or other predictors of persistent detection differed for incident versus prevalent infections. Third, we did not have sufficient power to investigate whether observed associations related to viral load or detection pattern varied by oncogenic HPV type. In post hoc analyses, however, we did observe that the point estimates for the associations between viral load and incident versus prevalent detection and viral load and persistent detection, as well as the relationships between smoking and both viral load and persistent detection were similar for HPV16 (adjusting for co-infection with other oncogenic HPV types) compared to all oncogenic HPV types combined (data not shown). Finally, our results in high-risk mid-adult women may not generalize to lower risk cohorts of mid-adult women.
In conclusion, our data on comparative viral levels in incident, prevalent, and re-detected infections suggest that new acquisition and reactivation or intermittent detection of persisting infections are viable sources of new oncogenic HPV detection in mid-adult women with new or multiple sex partners. Viral levels were consistently low in intermittently detected infections, suggesting that intermittent detection of persisting infection may be of limited clinical significance. Future research is needed to quantify the risk of carcinogenic progression associated with low-level, intermittent detection of oncogenic HPV infections in mid-adulthood, and to understand the proportion of oncogenic HPV infections in mid-adult women that are due to new acquisition versus persistence or reactivation of previously acquired infection.
Supplementary Material
Novelty and Impact Statement.
To inform the origin and natural history of oncogenic HPV infections, we used real-time PCR to quantify 18 oncogenic HPV types in samples from a longitudinal cohort of high-risk mid-adult women. Higher DNA viral loads observed in incident versus re-detected infections suggest a portion of newly detected infections in mid-adult women with new partners are new acquisitions. Low viral levels in intermittently detected infections suggest intermittent oncogenic HPV detection may be of limited clinical significance.
Acknowledgements
Plasmids for calibrating type-specific HPV standards for viral load testing were provided by Michel Favre (HPV types 33 and 68), Ethel-Michelle de Villiers (HPV types 53 and 73), and Toshihiko Matsukura (HPV type 82). This work was financially supported by the Developmental Awards Program of the National Institutes of Health NIAID Sexually Transmitted Infections and Topical Microbicide Cooperative Research Centers (STI-TM CRC) grant to the University of Washington (AI 31448), by a National Institutes of Health K01 grant (AI 079270) to RLW, and by American Recovery and Reinvestment Act (ARRA) funds through a supplement to K01 AI 079270 from the National Institutes of Health.
REFERENCES
- 1.Grant LA, Dunne EF, Chesson H, Markowitz LE. Considerations for human papillomavirus (HPV) vaccination of mid-adult women in the United States. Vaccine. 2011;29:2365–2370. doi: 10.1016/j.vaccine.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S52–S61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(Suppl 3):S42–S51. doi: 10.1016/j.vaccine.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, Kiviat NB, Koutsky LA. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699–707. doi: 10.1158/1055-9965.EPI-10-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gravitt PE. Evidence and impact of human papillomavirus latency. The open virology journal. 2012;6:198–203. doi: 10.2174/1874357901206010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS, Jr, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 8.Carter JJ, Koutsky LA, Wipf GC, Christensen ND, Lee SK, Kuypers J, Kiviat N, Galloway DA. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–936. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 9.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 10.Strickler HD, Schiffman MH, Shah KV, Rabkin CS, Schiller JT, Wacholder S, Clayman B, Viscidi RP. A survey of human papillomavirus 16 antibodies in patients with epithelial cancers. Eur J Cancer Prev. 1998;7:305–313. doi: 10.1097/00008469-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Winer RL, Hughes JP, Feng Q, Xi LF, Lee SK, O'Reilly SF, Kiviat NB, Koutsky LA. Prevalence and risk factors for oncogenic human papillomavirus infections in high-risk mid-adult women. Sex Transm Dis. 2012;39:848–856. doi: 10.1097/OLQ.0b013e3182641f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winer RL, Harris TG, Xi LF, Jansen KU, Hughes JP, Feng Q, Welebob C, Ho J, Lee SK, Carter JJ, Galloway DA, Kiviat NB, et al. Quantitative human papillomavirus 16 and 18 levels in incident infections and cervical lesion development. J Med Virol. 2009;81:713–721. doi: 10.1002/jmv.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xi LF, Hughes JP, Castle PE, Edelstein ZR, Wang C, Galloway DA, Koutsky LA, Kiviat NB, Schiffman M. Viral load in the natural history of human papillomavirus type 16 infection: a nested case-control study. J Infect Dis. 2011;203:1425–1433. doi: 10.1093/infdis/jir049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefsson AM, Magnusson PK, Ylitalo N, Sorensen P, Qwarforth-Tubbin P, Andersen PK, Melbye M, Adami HO, Gyllensten UB. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet. 2000;355:2189–2193. doi: 10.1016/S0140-6736(00)02401-6. [DOI] [PubMed] [Google Scholar]
- 15.Ylitalo N, Sorensen P, Josefsson AM, Magnusson PK, Andersen PK, Ponten J, Adami HO, Gyllensten UB, Melbye M. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000;355:2194–2198. doi: 10.1016/S0140-6736(00)02402-8. [DOI] [PubMed] [Google Scholar]
- 16.Moberg M, Gustavsson I, Gyllensten U. Type-specific associations of human papillomavirus load with risk of developing cervical carcinoma in situ. Int J Cancer. 2004;112:854–859. doi: 10.1002/ijc.20480. [DOI] [PubMed] [Google Scholar]
- 17.Moberg M, Gustavsson I, Wilander E, Gyllensten U. High viral loads of human papillomavirus predict risk of invasive cervical carcinoma. Br J Cancer. 2005;92:891–894. doi: 10.1038/sj.bjc.6602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlecht NF, Trevisan A, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL, Franco EL. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer. 2003;103:519–524. doi: 10.1002/ijc.10846. [DOI] [PubMed] [Google Scholar]
- 19.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 20.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen A, Kjaer SK, Munk C, Osler M, Iftner T. Persistence of high-risk human papillomavirus infection in a population-based cohort of Danish women. J Med Virol. 2010;82:616–623. doi: 10.1002/jmv.21750. [DOI] [PubMed] [Google Scholar]
- 22.Molano M, Van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, Meijer CJ, Munoz N, Franceschi S. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–494. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 23.Bae J, Seo SS, Park YS, Dong SM, Kang S, Myung SK, Park SY. Natural history of persistent high-risk human papillomavirus infections in Korean women. Gynecologic oncology. 2009;115:75–80. doi: 10.1016/j.ygyno.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Carcopino X, Henry M, Mancini J, Giusiano S, Boubli L, Olive D, Tamalet C. Two years outcome of women infected with high risk HPV having normal colposcopy following low-grade or equivocal cytological abnormalities: are HPV16 and 18 viral load clinically useful predictive markers? Journal of medical virology. 2012;84:964–972. doi: 10.1002/jmv.23276. [DOI] [PubMed] [Google Scholar]
- 25.Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, Kantelip B, Schaal JP, Mougin C. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. International journal of cancer Journal international du cancer. 2003;106:396–403. doi: 10.1002/ijc.11222. [DOI] [PubMed] [Google Scholar]
- 26.Lai CH, Chao A, Chang CJ, Chao FY, Huang HJ, Hsueh S, Lin CT, Cheng HH, Huang CC, Yang JE, Wu TI, Chou HH, et al. Host and viral factors in relation to clearance of human papillomavirus infection: a cohort study in Taiwan. International journal of cancer Journal international du cancer. 2008;123:1685–1692. doi: 10.1002/ijc.23679. [DOI] [PubMed] [Google Scholar]
- 27.Munoz JP, Gonzalez C, Parra B, Corvalan AH, Tornesello ML, Eizuru Y, Aguayo F. Functional interaction between human papillomavirus type 16 E6 and E7 oncoproteins and cigarette smoke components in lung epithelial cells. PloS one. 2012;7:e38178. doi: 10.1371/journal.pone.0038178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Constandinou-Williams C, Collins SI, Roberts S, Young LS, Woodman CB, Murray PG. Is human papillomavirus viral load a clinically useful predictive marker? A longitudinal study. Cancer Epidemiol Biomarkers Prev. 2010;19:832–837. doi: 10.1158/1055-9965.EPI-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramanakumar AV, Goncalves O, Richardson H, Tellier P, Ferenczy A, Coutlee F, Franco EL. Human papillomavirus (HPV) types 16, 18, 31, 45 DNA loads and HPV-16 integration in persistent and transient infections in young women. BMC Infect Dis. 2010;10:326. doi: 10.1186/1471-2334-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi LF, Hughes JP, Edelstein ZR, Kiviat NB, Koutsky LA, Mao C, Ho J, Schiffman M. Human Papillomavirus (HPV) type 16 and type 18 DNA Loads at Baseline and Persistence of Type-Specific Infection during a 2-year follow-up. J Infect Dis. 2009;200:1789–1797. doi: 10.1086/647993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70:8569–8577. doi: 10.1158/0008-5472.CAN-10-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacic MB, Castle PE, Herrero R, Schiffman M, Sherman ME, Wacholder S, Rodriguez AC, Hutchinson ML, Bratti MC, Hildesheim A, Morales J, Alfaro M, et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006;66:10112–10119. doi: 10.1158/0008-5472.CAN-06-1812. [DOI] [PubMed] [Google Scholar]
- 33.Wentzensen N, Gravitt PE, Long R, Schiffman M, Dunn ST, Carreon JD, Allen RA, Gunja M, Zuna RE, Sherman ME, Gold MA, Walker JL, et al. Human papillomavirus load measured by Linear Array correlates with quantitative PCR in cervical cytology specimens. J Clin Microbiol. 2012;50:1564–1570. doi: 10.1128/JCM.06240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xi LF, Koutsky LA, Castle PE, Edelstein ZR, Meyers C, Ho J, Schiffman M. Relationship between cigarette smoking and human papilloma virus types 16 and 18 DNA load. Cancer Epidemiol Biomarkers Prev. 2009;18:3490–3496. doi: 10.1158/1055-9965.EPI-09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho GYF, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 36.Hildesheim A, Schiffman MH, Gravitt PE, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 37.Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, Papenfuss MR, Abrahamsen M, Inserra P. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States) Cancer Causes Control. 2002;13:839–846. doi: 10.1023/a:1020668232219. [DOI] [PubMed] [Google Scholar]
- 38.Richardson H, Abrahamowicz M, Tellier PP, Kelsall G, du Berger R, Ferenczy A, Coutlee F, Franco EL. Modifiable risk factors associated with clearance of type-specific cervical human papillomavirus infections in a cohort of university students. Cancer Epidemiol Biomarkers Prev. 2005;14:1149–1156. doi: 10.1158/1055-9965.EPI-04-0230. [DOI] [PubMed] [Google Scholar]
- 39.Schmeink CE, Melchers WJ, Siebers AG, Quint WG, Massuger LF, Bekkers RL. Human papillomavirus persistence in young unscreened women, a prospective cohort study. PLoS One. 2011;6:e27937. doi: 10.1371/journal.pone.0027937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maucort-Boulch D, Plummer M, Castle PE, Demuth F, Safaeian M, Wheeler CM, Schiffman M. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. Int J Cancer. 2010;126:684–691. doi: 10.1002/ijc.24752. [DOI] [PubMed] [Google Scholar]
- 41.Syrjanen S, Shabalova I, Petrovichev N, Kozachenko V, Zakharova T, Pajanidi J, Podistov J, Chemeris G, Sozaeva L, Lipova E, Tsidaeva I, Ivanchenko O, et al. Factors predicting persistence of high-risk human papillomavirus (HPV) infections in women prospectively followed-up in three New Independent States (NIS) of the former Soviet Union. Eur J Gynaecol Oncol. 2005;26:491–498. [PubMed] [Google Scholar]
- 42.Sycuro LK, Xi LF, Hughes JP, Feng Q, Winer RL, Lee SK, O'Reilly S, Kiviat NB, Koutsky LA. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis. 2008;198:971–978. doi: 10.1086/591625. [DOI] [PubMed] [Google Scholar]
- 43.Munoz N, Hernandez-Suarez G, Mendez F, Molano M, Posso H, Moreno V, Murillo R, Ronderos M, Meijer C, Munoz A. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100:1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sammarco ML, Del Riccio I, Tamburro M, Grasso GM, Ripabelli G. Type-specific persistence and associated risk factors of human papillomavirus infections in women living in central Italy. Eur J Obstet Gynecol Reprod Biol. 2013 doi: 10.1016/j.ejogrb.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. The Journal of pharmacology and experimental therapeutics. 2000;293:166–171. [PubMed] [Google Scholar]
- 46.Sopori M. Effects of cigarette smoke on the immune system. Nature reviews Immunology. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 47.Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H. Immune response in asymptomatic smokers. Acta anaesthesiologica Scandinavica. 2002;46:959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 48.Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1988;2:652–654. doi: 10.1016/s0140-6736(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 49.Poppe WA, Ide PS, Drijkoningen MP, Lauweryns JM, Van Assche FA. Tobacco smoking impairs the local immunosurveillance in the uterine cervix. An immunohistochemical study. Gynecol Obstet Invest. 1995;39:34–38. doi: 10.1159/000292372. [DOI] [PubMed] [Google Scholar]
- 50.Jiang M, Baseman JG, Koutsky LA, Feng Q, Mao C, Kiviat NB, Xi LF. Sequence variation of human papillomavirus type 16 and measurement of viral integration by quantitative PCR. J Clin Microbiol. 2009;47:521–526. doi: 10.1128/JCM.02115-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.