Abstract
The vast majority of proteomic studies employ reversed-phase high-performance liquid chromatography coupled with tandem mass spectrometry for analysis of the tryptic digest of a cellular lysate. This technology is quite mature, and typically provides identification of hundreds to thousands of peptides, which is used to infer the identity of hundreds to thousands of proteins. These approaches usually require milligrams to micrograms of starting material. Capillary zone electrophoresis provides an interesting alternative separation method based on a different separation mechanism than HPLC. Capillary electrophoresis received some attention for protein analysis beginning 25 years ago. Those efforts stalled because of the limited performance of the electrospray interfaces and the limited speed and sensitivity of mass spectrometers of that era. This review considers a new electrospray interface design coupled with Orbitrap Velos and linear Q-trap mass spectrometers. Capillary zone electrophoresis coupled with this interface and these detectors provides single shot detection of >1,250 peptides from an E. coli digest in less than one hour, identification of nearly 5,000 peptides from analysis of seven fractions produced by solid-phase extraction of the E. coli digest in a six hour total analysis time, low attomole detection limits for peptides generated from standard proteins, and high zeptomole detection limits for selected ion monitoring of peptides. Incorporation of an integrated on-line immobilized trypsin microreactor allows digestion and analysis of picogram amounts of a complex eukaryotic proteome.
Introduction
The characterization of a complex proteome often employs bottom-up analysis that begins with sample digestion with trypsin, followed by fractionation using ion-exchange chromatography, separation of those fractions by reversed-phase liquid chromatography, analysis by tandem mass spectrometry, and database searching for peptide identification [1]. Depending on the effort expended, the amount of sample available, and the mass spectrometer performance, bottom-up analysis can take from hours to weeks, can infer the identity of several thousand proteins from eukaryotic proteomes, and can achieve an average protein sequence coverage approaching 25% [2]. High sequence coverage is useful in identifying sequence variants and post-translational modification sites. Despite the success of conventional bottom-up proteomic analysis, there is interest in the development of alternative technologies to improve sequence coverage, to facilitate analysis of minute samples, and to speed analysis.
This review focuses on the use of capillary electrophoresis as an alternative to reversed-phase liquid chromatography in the bottom-up proteomic protocol. Capillary zone electrophoresis offers several tantalizing characteristics for this application. First, the separation mechanism differs from reversed-phase liquid chromatography; as a result, capillary zone electrophoresis samples a different portion of the peptide digest, which can help expand protein sequence coverage. Second, capillary electrophoresis methods provide fast separations, typically from 5 to 45 minutes, with little or no time required for column regeneration. Third, capillary electrophoresis separations can provide extremely high separation efficiencies. Fourth, the very simple flow path used in capillary electrophoresis eliminates metal columns, fittings, and injection loops, which results in few opportunities for irreversible sample loss; as a result, capillary electrophoresis consistently outperforms reversed-phase chromatography for the analysis of mid-nanogram protein samples.
Electrophoresis basics
We begin by reviewing the basics of capillary zone electrophoresis, where analytes are separated based on their charge-to-size ratio under the influence of an electric field in a buffer-filled capillary [3]. Typical separations are performed in a 10- to 50-μm inner diameter, 10- to 50-cm long fused silica capillary at a potential of 10 to 30 kV.
The migration time, t, through a capillary of length L is given by
| (1) |
where μelectrophoresis is the electrophoretic mobility of the analyte, μelectroosmosis is the mobility of the buffer due to the presence of an electrical double layer at the capillary-buffer interface, and V is the applied potential. Typical separations of tryptic digests require from 1- to 60-min.
Migration time depends on the electrophoretic mobility, which is characteristic of the analyte, and electroosmosis, which is bulk solvent flow generated by the electrical double-layer at the capillary-buffer interface. The pI of the capillary surface is typically in the range of 2–3 (borosilicate) and 4–5 (silica) [4–6]. At more basic pH, the surface takes on a negative charge. Cationic counter ions are mobile under the influence of the electric field and flow towards the negative electrode under the influence of an electric field. The mobility of these counter ions draws solvent with them, creating bulk electroosmotic flow. The profile induced by electroosmosis and electrophoresis is flat, which eliminates radial diffusion as a contribution to band broadening. In an uncoated capillary at basic pH, electroosmotic mobility is usually larger in absolute value than the electrophoretic mobility of most analyte. Electroosmosis can be modulated by coating the capillary wall and by use of a separation buffer with pH near silica’s isoelectric point.
In an idealized system, the only source of band broadening is longitudinal diffusion, and the number of theoretical plates, N, is given by
| (2) |
where D is the diffusion coefficient. The use of high voltages can result in separation efficiencies greater than 2.5 × 106 plates in a diffusion-limited separation of small molecules [7].
In practice, a number of issues limit separation efficiencies. First, the sample volume must be kept to a small fraction of the capillary volume. A 50-cm long, 50-μm id capillary has a total volume of 1-μL, and injection volume must be less than a few hundred picoliters to eliminate injection volume as a significant contribution to the separation efficiency [7]. Stacking conditions, where the sample buffer’s total ionic strength is much less than that of the separation buffer, can reduce the effect of sample volume on band broadening [8]. Second, the analyte’s ionic strength must be much less than of the separation buffer. Otherwise, the analyte will perturb the electric field, resulting in peak distortion. Third, excessive Joule heating occurs if the voltage and ionic strength of the buffer are too high [9]. Joule heating produces a temperature gradient across the capillary radius. Since viscosity is a strong function of temperature, Joule heating results in a non-uniform flow profile that degrades separation efficiency. Joule heating is conveniently diagnosed by plotting current vs. separation voltage; a positive deviation at higher potential indicates excessive Joule heating. Fourth, interaction of analyte with the capillary walls leads to chromatographic retention. Resistance to mass transfer then leads to excess band broadening. Adsorption on the capillary walls is particularly problematic when dealing with intact proteins. Adsorption can be reduced by operation at pH extremes where both the analyte and capillary wall share the same charge [10–11], which leads to electrostatic repulsion. Alternatively, the capillary walls can be coated to reduce adsorption. Silica hydrolyzes at pH > ~8. High pH can be used to strip adsorbed proteins from the capillary walls between separations. Unfortunately, high pH also strips coating, so that high pH is only useful in uncoated capillaries. Fifth, the detector time-constant must be sufficiently fast to capture the electrophoretic peak. Detector response time is problematic with many mass spectrometers, which struggle to sample sub-second electrophoretic peaks. This problem is particularly troubling when analyzing complex digests where many peptides are present simultaneously; generation of tandem mass spectra from a large fraction of those peptides requires a very fast instrument. Despite these practical limitations, peak widths of one second and theoretical plate counts of 15,000–100,000 are routine for tryptic digests.
The requirements that the buffer ionic strength be kept low to minimize Joule heating and that analyte ionic strength be much less than the buffer ionic strength to minimize peak distortion and broadening imply that analyte concentration be in the micromolar or lower range. The requirement that analyte injection volume be a small fraction of the capillary volume implies that the total amount of analyte injected into the capillary is in the femtomole or lower range to retain high separation efficiency. Clearly, detection sensitivity is of fundamental importance in applications of capillary electrophoresis to proteomic analysis.
Instrumentation details - capillaries
Capillary electrophoresis is a very simple technology, requiring a high-voltage power supply to drive the separation, a fused silica capillary for the separation, a reservoir at the injection end of the capillary to provide electrical contact, and an interface to the mass spectrometer. We use the Spellman CZE 2000 power supply to drive the separation. This power supply provides 30,000 V, which is adequate for all applications. We have found a Prince Technologies autosampler to be a useful tool for automation of sample injection. Polymicro Technologies is a common source for fused silica capillaries. We typically employ 50-μm inner diameter, 150-μm outer diameter capillaries in our laboratory, although narrower bore inner diameter capillaries are sometimes used. Narrower bore capillaries reduce the temperature rise associated with Joule heating, which allows use of higher separation voltages, higher ionic strength buffers, and higher concentration samples. However, narrower bore capillaries also require reduction in sample injection volume to avoid band broadening [12].
As an experimental detail, the two ends of the capillary should be at the same height to avoid formation of a siphon. Siphoning produces hydrodynamic flow, which induces a parabolic velocity profile that introduces an unwanted source of band broadening [7].
Electrokinetic electrospray interface
Interface of the separation capillary with a mass spectrometer has proven challenging. Although MALDI interfaces have been developed, most work employs electrospray ionization. Electrospray ionization requires electrical contact to the distal end of the capillary to complete the electrical circuit driving electrophoresis and to support electrospray. Several interface designs have been reported [13–18]. Early electrospray interfaces were similar to those employed for liquid chromatography. These interfaces employed high flow rates of both a sheath liquid and a nebulizing gas. The high sheath flow rate allows great flexibility in the sample buffer composition but also results in significant dilution of sample and decreased sensitivity.
Several sheathless interfaces have been reported. These interfaces eliminate dilution from the sheath buffer, which often results in improved sensitivity. However, the separation buffer must support electrospray, which limits the separation conditions that are available. Also, generation of electrical contact with the distal end of the capillary can be challenging. One approach reported by Moini is to etch the tip of the capillary and to immerse that tip in a conducting buffer that is connected to a high-voltage power supply [18]. When etched sufficiently thin, the wall of the capillary provides sufficient conductivity to drive the electrospray.
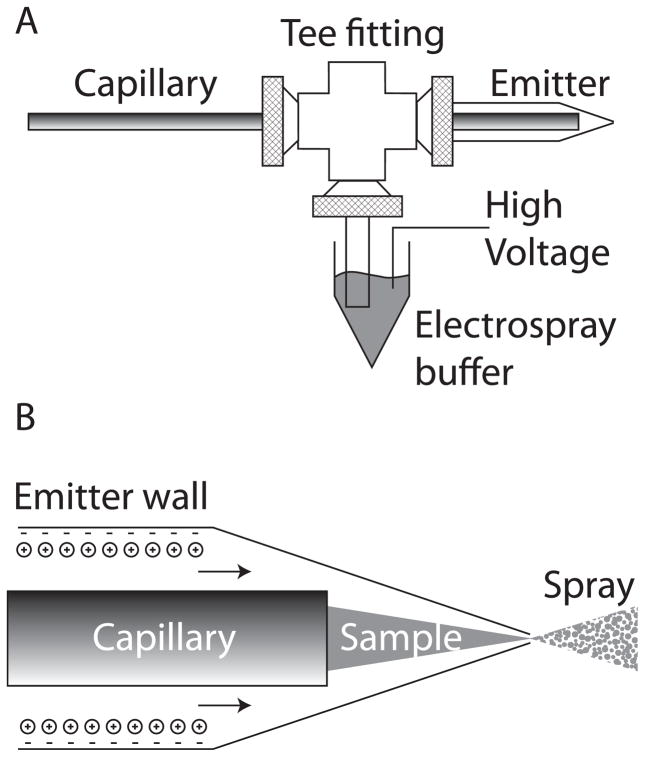
We reported a nanospray interface based on an electrokinetically-pumped sheath flow, fig 1 [19]. The separation capillary is inserted into a borosilicate glass emitter that is pulled to a 2- to 10-μm inner diameter tip. We use a Sutter pipette puller to generate emitters. The capillary and emitter are held in a plastic union. The side arm of the union is connected to a reservoir that contains the electrospray buffer and is connected to a high voltage power supply. This connection essentially acts as a salt bridge to separate electrolysis products generated at the electrode from the emitter.
Figure 1.
Electrokinetically-pumped sheath-flow nanoelectrospray interface [19]. A – overview of the interface construction. The separation capillary is threaded through a plastic union into a borosilicate glass emitter. Plastic tubing is connected to a side arm and an electrospray buffer. High voltage is applied to that buffer to drive electrospray. The separation is driven by the difference in voltage applied to the two ends of the capillary. B – detail of the emitter. Anionic sites on the emitter wall attract a cations that form an electrical double layer. The electrospray potential drives these cations to the emitter tip; the cations drag buffer with them, creating electroosmotic flow. This electroosmotic flow ensheaths the sample stream as it exits the separation capillary. Electrospray is generated as the solution exits the emitter.
The isoelectric point of borosilicate glass is ~3.5 [4]. The sheath buffer is typically a mixture of acetic acid and methanol with pH slightly higher than this pI. At this pH, residual deprotonated silanol groups on the borosilicate surface attract anionic counter ions, fig 1b. The electric field across the emitter drives electroosmotic flow that pumps sheath buffer toward the emitter tip. This sheath liquid entrains the analyte migrating from the capillary as a filament, and the fluid leaving the emitter undergoes electrospray, generating gas-phase ions that are drawn into the mass spectrometer.
Both COMSOL fluidics modeling and our experiments show that the sensitivity of the interface increases as the capillary to emitter tip distance decreases [19]. Model and experiment show that the sensitivity also increases as the emitter tip diameter decreases, and our highest sensitivity was produced with a 2-μm inner diameter emitter. However, this very small tip diameter is susceptible to clogging due to particulates in solution and crystal formation during buffer evaporation; in this case, careful filtering of the sheath buffer, washing of the capillary tip, and use of a more dilute buffer help to minimize this clogging.
Electrophoretic performance
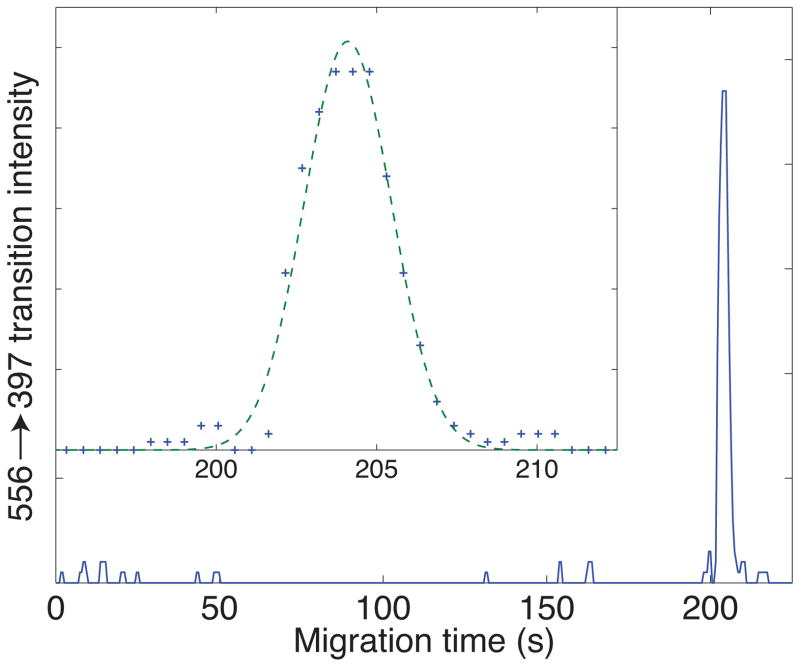
Capillary zone electrophoresis, particularly when performed with uncoated capillaries with strong electroosmotic flow, generates fast separations and much narrower peaks than are typically seen in chromatographic separations. Figure 2 presents a fairly typical example, which is the multiple reaction monitoring signal generated from injection of 10 amol (2 nL of a 5 nM solution) of Leu-enkephalin separated in an uncoated capillary and using our electrokinetic-sheath flow electrospray source [20]. Data were taken every 0.52 seconds, and roughly eight points were captured across the peak, which had a 3.1 s fwhh. The separation generated 25,000 theoretical plates, which was primarily caused by the relatively large injection volume.
Figure 2.
Electrophoretic analysis of 10 amol (2 nL of a 5 nM solution) of Leu-enkephalin injected into a 30 cm long capillary, id 50 um, od 150 um [20]. The separation buffer was 10 mM ammonium acetate (pH 5.5). The injection time was 3 seconds and injection voltage was the same as the separation voltage, 6 kV (8 kV on the injection end of the capillary). The electrospray voltage was 2 kV. Data were collected using the 556.20 → 397.20 transition with a QTrap 5500 hybrid linear-ion trap triple-quadrupole instrument (AB Sciex). Insert shows a close-up of the peak (plus signs) and the result of a least squares fit a Gaussian function to the data (dashed curve).
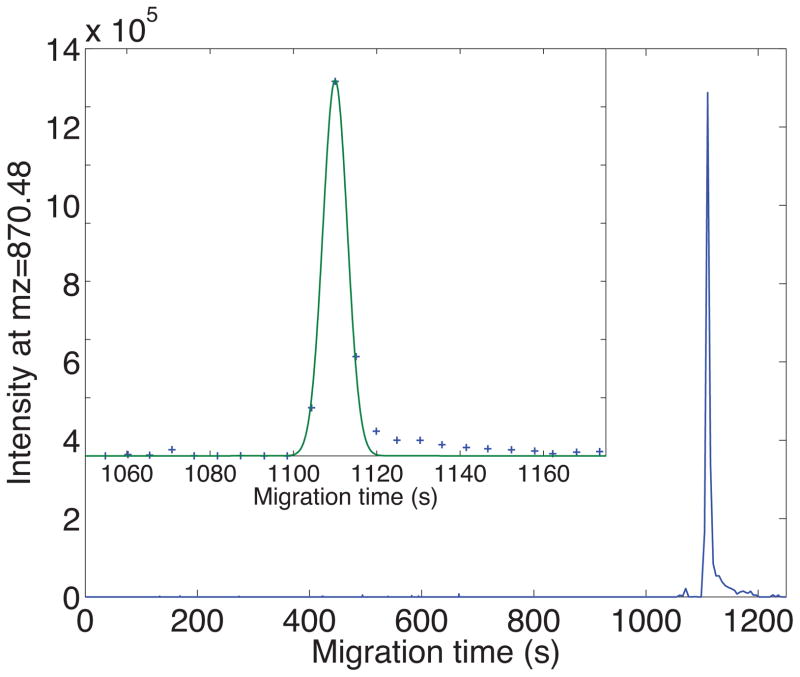
Coated capillaries eliminate electroosmosis, resulting in much slower separations. Because of the longer migration time in uncoated capillaries, injection volume tends to not be as problematic as in, and higher plate counts can be produced. However, operation of the tandem mass spectrometer can result in relatively low precursor ion sampling rate, which makes capture of the narrow peaks difficult. Figure 3 presents a parent ion electropherogram generated using a high-resolution Orbitrap mass spectrometer [20]. This peptide, present in 1 ng of an E. coli digest, generated a peak with nearly 150,000 theoretical plates. The precursor-ion sampling rate was barely able to capture the peak shape.
Figure 3.
Parent ion electropherogram of the peptide ILLINPTDSDAVGNAVK using an Orbitrap Velos mass spectrometer. The insert shows the peak in detail. Plus signs are the data and the smooth curve is the least squares fit of a Gaussian function to the peak. Experimental details are provided in ref 21.
Complex sample analysis
The vast majority of publications reporting protein analysis by capillary zone electrophoresis-mass spectrometry have employed a small number of standard proteins as analyte. While useful for instrument tuning, such studies are of limited value in characterizing the performance of capillary zone electrophoresis for proteomic applications.
The first application of capillary zone electrophoresis to the analysis of a sample of moderate complexity was reported by Tong and colleagues in 1999 [22]. In this pioneering paper, an on-column solid-phase extraction device was used to capture and fractionate the tryptic digest of S. cerevisiae ribosomal proteins. In a single-shot analysis of the digest, an average of 50 peptides was identified using a venerable LCQ tandem mass spectrometer. The separation used uncoated capillaries with high electro-osmotic flow that generated quite rapid separations. To allow more time for tandem spectra generation, the electrophoretic voltage was decreased. Analysis of five fractions generated by extraction with aqueous methanol solutions yielded 136 peptides over a 2.5 hour total analysis time.
In 2006, Moini employed various mass spectrometry analysis approaches for the characterization of E. coli ribosomal proteins using an LCQ mass spectrometer [23]. An average of 55% sequence coverage was reported for this sample using manual interpretation of data; automated database searching generated 15% sequence coverage.
In 2011, Faserl and colleagues analyzed an Arg-C digest of rat testis linker histones using a sheathless electrospray interface and an LTQ Orbitrap mass spectrometer [24]. A total of 135 peptides and eight non-histone H1 proteins were identified from 6 ng sample in an 18 minute analysis time.
This group reported in 2012 the secretome (secreted proteome) produced by M. marinum, a model for tuberculosis [25]. Eleven fractions were generated from the tryptic digest of the secreteome using solid-phase extraction on a reversed-phase column. The fractions were analyzed on an uncoated capillary using the electrokinetically-pumped electrospray interface with an Orbitrap Velos mass spectrometer. A total of 334 peptides and 140 proteins were identified in 165 minutes of mass spectrometer time. The unfractionated sample was analyzed in triplicate using reversed phase liquid chromatography. The number of peptide and protein identifications were nearly identical to those generated by liquid chromatography with similar instrument time.
Wang and colleagues reported the first use of capillary electrophoresis for the analysis of a prokaryotic proteome [26]. They employed on-line solid phase microextraction and fractionation, followed by stacking injection and capillary electrophoresis electrophoresis in a quite long capillary, and an LTQ-Xl mass spectrometer detector was used for study of Pyrococcus furiosus tryptic digest. ~1,800 peptides were identified from 100 ng sample using a five-step fractionation protocol over a 3 hour analysis time. The number of identified peptides dropped to ~800 for analysis of 5 ng of the digest. Roughly 500 peptides were identified in a single-shot capillary zone electrophoresis analysis of a 5-ng sample.
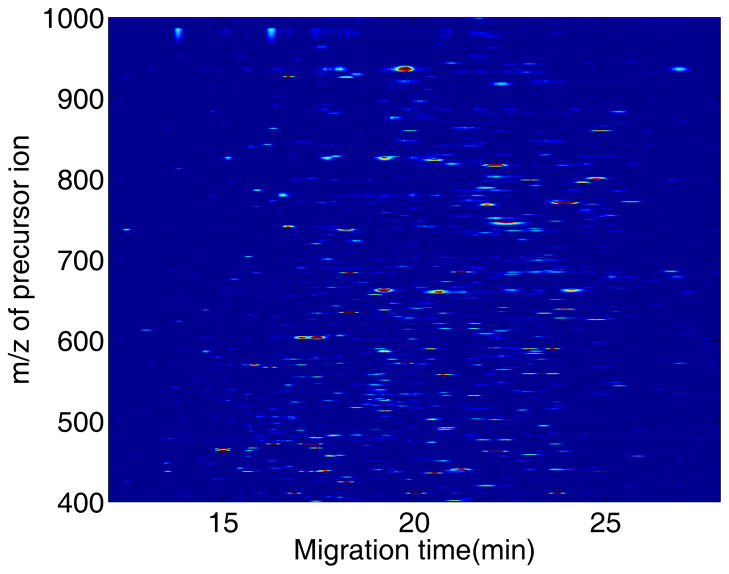
We have recently reported the analysis of the tryptic digest of the E. coli proteome [21]. This organism’s genome contains roughly twice the number of open reading frames as P. furiosus used in Yates’s study [26], and represents another step in the analysis of more complex proteomes. Our experiment used a 60-cm coated capillary for analysis with an Orbitrap Velos mass spectrometer for detection. A single-shot analysis of 100 ng of the E. coli proteome generated 1,377 peptide identifications, Figure 4. The number of peptide identifications dropped to 627 for analysis of 1-ng samples.
Figure 4.
Portion of the heat-map of the capillary zone electrophoresis analysis of 100 ng of an E. coli tryptic digest [21]. Precursor ion electropherograms were generated for all identified peptides in the digest. The data were down-sampled, and the mass and time axes were interpolated to create linear axes. See reference 21 for experimental details.
We also employed solid-phase extraction using a reversed-phase column to generate a set of seven fractions from the E. coli tryptic digest [27]. These fractions were separated using a coated capillary and detected with an Orbitrap Velos mass spectrometer. A total of 4,902 peptides and 871 proteins were identified in a total of six hours of mass spectrometer time, generating 145 protein ids/hour. These results are the largest proteomic database generated by capillary zone electrophoresis that has been reported to date. There was a substantial overlap in peptide identifications between fractions; improved performance will likely follow from use of improved peptide fractionation.
High sensitivity analysis
Comparisons of capillary zone electrophoresis with liquid chromatography for the analysis of complex proteomes show a common trend. Liquid chromatography tends to generate more peptide and protein identifications for sample loadings greater than 100 ng. Capillary zone electrophoresis generates more identifications for low nanogram sample loadings [21, 26]. This performance likely reflects two opposing trends. Liquid chromatographic separations tend to be slower than those employed in electrophoresis, allowing more time for generation of tandem spectra, which result in deeper proteome analysis. Capillary zone electrophoresis tends to produce much higher efficiency peaks, which generate stronger signals from low-concentration peptides, allowing larger number of peptide identifications at low concentration samples. Also, chromatographic systems tend to contain surfaces upon which dilute peptides can be irreversibly lost, reducing the number of peptide identifications for low sample loadings. In contrast, the flow system employed in capillary electrophoresis is very simple, providing few opportunities for sample loss.
We recently employed multiple reaction monitoring with a Q-Trap mass spectrometer for the analysis of selected peptides generated from the very complex RAW 264.7 tryptic digest [28]. This mouse macrophage cell line is typical for eukaryotic organisms and represents roughly a five-fold higher complexity sample than the E. coli proteome. We generated 104 transitions from 26 proteins using 700 pg of digest. Transitions from three proteins were identified using 100 pg of digest. This amount of sample corresponds to the protein content of a single cell.
We have also employed an on-column trypsin microreactor for the bottom-up analysis of standard proteins and the RAW 264.7 proteome [29]. We consistently identified standard proteins at the low femtomole level using tandem mass spectrometry. Two and seven protein groups were consistently identified from 300 pg and 3 ng loadings, respectively, of the RAW 264.7 lysate.
These results are intriguing. The ability to digest and perform bottom-up proteomics on subnanogram amounts of cell lysate, and the ability to detect and quantitate selected proteins at the 100 pg level of digests provides a path to the use of mass spectrometry for protein analysis at the single cell level. This development in chemical cytometry has the potential of allowing a detailed characterization of cellular heterogeneity across a portion of the proteome [30]. As mass spectrometer performance evolves with coming generations of instruments, we can anticipate access to a larger portion of a single cell’s proteome.
Isoelectric focusing and quantitative proteomics
Capillary zone electrophoresis is limited by small injection volume. Smith and colleagues recognized that the much larger sample volume available when using capillary isoelectric focusing provides the potential for improved performance [31]. In capillary isoelectric focusing, ampholytes are mixed with a sample and used to fill the entire capillary volume. Application of an electric field will focus components at their isoelectric point. We employed capillary isoelectric focusing to analyte the tryptic digest of a product-depleted sample of a recombinant therapeutic. 53 peptides and 37 host cell proteins were identified in a rapid analysis [32].
Unfortunately, the mass to charge ratio of ampholytes falls in the same range as that of tryptic digests, and result in a large background signal that compete with peptides during tandem mass spectroscopy. There are several approaches to dealing with this background. Most simply, isoelectric focusing can be used in top-down proteomics, where the mass of the intact protein greatly exceeds that of the ampholytes. Smith pioneered the use of capillary isoelectric focusing for mass spectrometry analysis of proteins isolated from various prokaryotes [33]. Roughly 200 components were observed across pI 3–9 from D. radiodurans.
We recently reported the use of amino acids to generate the pH gradient in isoelectric focusing [34]. The amino acids create an acceptable pH gradient, and the mass-to-charge ratio of amino acids is much less than that of tryptic peptides, resulting in much less interference during mass spectrometry. Over 100 proteins and 300 peptides were identified from a RAW 264.7 tryptic digest.
Finally, we have recently coupled capillary isoelectric focusing with amino-acid ampholytes and with iTRAQ labeling for the quantitative proteomic analysis of PC12 cells undergoing differentiation following treatment with nerve growth factor [35]. Biological duplicates were generated at four time points following treatment; these samples were digested, labeled with eight-plex iTRAQ chemistry, pooled, separated into 25 fractions using reversed phase liquid chromatography. Each fraction was then analyzed in technical duplicate using capillary isoelectric focusing using amino acids as ampholytes and an Orbitrap-Velos mass spectrometer as detector. A total of 2,329 peptides and 835 protein groups were quantitated. 96 differentially expressed proteins were identified
Future
Over the past two decades, there has been a steady improvement in mass spectrometer sensitivity, speed, and resolution. This improvement is likely to continue, which will result in improved performance of capillary electrophoresis for protein analysis. As noted earlier, improvements in mass spectrometer performance will allow better detection limits and deeper analysis of minute amounts of proteins using capillary zone electrophoresis
Also, capillary zone electrophoresis provides interesting opportunities for top-down protein analysis. We recently reported the separation of a set of model proteins [36]. Detection limits were in the attomole range for the intact proteins. Again, improved performance is expected with higher resolution and sensitivity mass spectrometers.
The consistent observation that capillary zone electrophoresis outperforms liquid chromatography for sample-limited analysis presents one obvious focus for future research, the analysis of the proteome of single cells. A typical mammalian cell is from 10- to 20-μm in diameter and contains from 100- to 500-pg of protein. Capillary zone electrophoresis coupled with an Orbitrap Velos mass spectrometer for bottom-up analysis and coupled with a linear Q-Trap mass spectrometer for multiple reaction monitoring analysis of selected peptides has been used for now- to sub-nanogram digests. Combination of sample isolation and injection into a capillary with on-column lysis and digestion should allow detection of high abundance peptides. Improvements in electrospray design and mass spectrometer performance will allow researchers to delve deeper into the proteome of single cells.
Acknowledgments
We thank Dr. William Boggess in the Notre Dame Mass Spectrometry and Proteomics Facility for his help with this project. This project was supported by a grant from the National Institutes of Health (R01GM096767).
Footnotes
The authors have declared no conflict of interest.
References
- 1.Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113:2343–2394. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagaraj N, Wisniewski JR, Geiger T, Cox J, et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol Syst Biol. 2011;7:548. doi: 10.1038/msb.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgenson JW, Lukacs KD. Zone electrophoresis in open-tubular glass-capillaries. Anal Chem. 1981;53:1298–1302. [PubMed] [Google Scholar]
- 4.Blass J, Kohler O, Fingerle M, Muller C, Ziegler C. Properties and characteristics of wet (HF) and dry (RIE) etched borosilicate glass. Phys Status Solidi A. 2013;210:988–993. [Google Scholar]
- 5.Gusev I, Horvath H. Streaming potential in open and packed fused-silica capillaries. J Chromatogr A. 2002;948:203–223. doi: 10.1016/s0021-9673(01)01450-9. [DOI] [PubMed] [Google Scholar]
- 6.Fan H-F, Li F, Zare RN, Lin KC. Characterization of Two Types of Silanol Groups on Fused-Silica Surfaces Using Evanescent-Wave Cavity Ring-Down Spectroscopy. Anal Chem. 2007;79:3654–3661. doi: 10.1021/ac062386n. [DOI] [PubMed] [Google Scholar]
- 7.Cheng YF, Wu SL, Chen DY, Dovichi NJ. Interaction of capillary zone electrophoresis with a sheath flow cuvette detector. Anal Chem. 1990;62:496–503. [Google Scholar]
- 8.Zhang CX, Thormann W. Head-Column Field-Amplified Sample Stacking in Binary System Capillary Electrophoresis:_ A Robust Approach Providing over 1000-Fold Sensitivity Enhancement. Anal Chem. 1996;68:2523–2532. doi: 10.1021/ac951250e. [DOI] [PubMed] [Google Scholar]
- 9.Monnig CA, Jorgenson JW. On-column sample gating for high-speed capillary zone electrophoresis. Anal Chem. 1991;63:802–807. doi: 10.1021/ac00008a013. [DOI] [PubMed] [Google Scholar]
- 10.Zhu MD, Rodriguez R, Hansen D, Wehr T. Capillary electrophoresis of proteins under alkaline conditions. J Chromatogr. 1990;516:123–131. doi: 10.1016/s0021-9673(01)90210-9. [DOI] [PubMed] [Google Scholar]
- 11.McCormick RM. Capillary zone electrophoretic separation of peptides and proteins using low pH buffers in modified silica capillaries. Anal Chem. 1988;60:2322–2328. doi: 10.1021/ac00172a003. [DOI] [PubMed] [Google Scholar]
- 12.Gas B, Stedrý M, Kenndler E. Peak broadening in capillary zone electrophoresis. Electrophoresis. 1997;18:2123–2133. doi: 10.1002/elps.1150181203. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell EJ, Chen DD. Twenty years of interface development for capillary electrophoresis-electrospray ionization-mass spectrometry. Anal Chim Acta. 2008;627:25–33. doi: 10.1016/j.aca.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Krenkova J, Foret F. On-line CE/ESI/MS interfacing: Recent developments and applications in proteomics. Proteomics. 2012;12:2978–2990. doi: 10.1002/pmic.201200140. [DOI] [PubMed] [Google Scholar]
- 15.Kleparnik K. Recent advances in the combination of capillary electrophoresis with mass spectrometry: From element to single-cell analysis. Electrophoresis. 2013;34:70–85. doi: 10.1002/elps.201200488. [DOI] [PubMed] [Google Scholar]
- 16.Smith RD, Barinaga CJ, Udseth HR. Improved electrospray ionization interface for capillary zone electrophoresis - mass-spectrometry. Anal Chem. 1988;60:1948–1952. [Google Scholar]
- 17.Smith RD, Loo JA, Barinaga CJ, Edmonds CG, Udseth HR. Capillary zone electrophoresis mass-spectrometry using an electrospray ionization interface. Anal Chem. 1988;60:436–441. [Google Scholar]
- 18.Moini M. Simplifying CE-MS operation2. Interfacing low flow separation techniques to mass spectrometry using a porous tip. Anal Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 19.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis. Rapid Commun Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wojcik R, Dovichi NJ, Champion MM. Quantitative multiple reaction monitoring of peptide abundance introduced via a capillary zone electrophoresis-electrospray interface. Anal Chem. 2012;84:6116–6121. doi: 10.1021/ac300926h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu G, Sun L, Yan X, Dovichi NJ. Single-shot proteomics using capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry with production of more than 1250 Escherichia coli peptide identifications in a 50 min separation. Anal Chem. 2013;85:2569–2573. doi: 10.1021/ac303750g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong W, Link A, Eng JK, Yates JR., 3rd Identification of proteins in complexes by solid-phase microextraction/multistep elution/capillary electrophoresis/tandem mass spectrometry. Anal Chem. 1999;71:2270–2278. doi: 10.1021/ac9901182. [DOI] [PubMed] [Google Scholar]
- 23.Garza S, Moini M. Analysis of complex protein mixtures with improved sequence coverage using (CE-MS/MS)n. Anal Chem. 2006;78:7309–7316. doi: 10.1021/ac0612269. [DOI] [PubMed] [Google Scholar]
- 24.Faserl K, Sarg B, Kremser L, Lindner H. Optimization and evaluation of a sheathless capillary electrophoresis-electrospray ionization mass spectrometry platform for peptide analysis: comparison to liquid chromatography-electrospray ionization mass spectrometry. Anal Chem. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Champion MM, Sun L, Champion PA, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry as an alternative proteomics platform to ultraperformance liquid chromatography-electrospray ionization-tandem mass spectrometry for samples of intermediate complexity. Anal Chem. 2012;84:1617–1622. doi: 10.1021/ac202899p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Fonslow BR, Wong CC, Nakorchevsky A, Yates JR., 3rd Improving the comprehensiveness and sensitivity of sheathless capillary electrophoresis-tandem mass spectrometry for proteomic analysis. Anal Chem. 2012;84:8505–8513. doi: 10.1021/ac301091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan X, Essaka DC, Sun L, Zhu G, Dovichi NJ. Bottom-up proteome analysis of E. coli using capillary zone electrophoresis-tandem mass spectrometry with an electrokinetic sheath-flow electrospray interface. Proteomics. 2013 doi: 10.1002/pmic.201300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Li Y, Champion MM, Zhu G, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-multiple reaction monitoring from 100 pg of RAW 264.7 cell lysate digest. Analyst. 2013;138:3181–3188. doi: 10.1039/c3an00287j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L, Zhu G, Dovichi NJ. Integrated capillary zone electrophoresis-electrospray ionization tandem mass spectrometry system with an immobilized trypsin microreactor for online digestion and analysis of picogram amounts of RAW 264.7 cell lysate. Anal Chem. 2013;85:4187–4194. doi: 10.1021/ac400523x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen D, Dickerson JA, Whitmore CD, Turner EH, et al. Chemical cytometry: fluorescence-based single-cell analysis. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:165–90. doi: 10.1146/annurev.anchem.1.031207.113104. [DOI] [PubMed] [Google Scholar]
- 31.Severs JC, Hofstadler SA, Zhao Z, Senh RT, Smith RD. The interface of capillary electrophoresis with high performance Fourier transform ion cyclotron resonance mass spectrometry for biomolecule characterization. Electrophoresis. 1996;17:1808–1817. doi: 10.1002/elps.1150171204. [DOI] [PubMed] [Google Scholar]
- 32.Zhu G, Sun L, Wojcik R, Kernaghan D, McGivney JB, 4th, Dovichi NJ. A rapid cIEF-ESI-MS/MS method for host cell protein analysis of a recombinant human monoclonal antibody. Talanta. 2012;98:253–256. doi: 10.1016/j.talanta.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Xiang F, Veenstra TD, Fung EN, Smith RD. High-resolution capillary isoelectric focusing of complex protein mixtures from lysates of microorganisms. Anal Chem. 1999;71:5348–5353. doi: 10.1021/ac9909305. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Sun L, Yang P, Dovichi NJ. On-line amino acid-based capillary isoelectric focusing-ESI-MS/MS for protein digests analysis. Anal Chim Acta. 2012;750:207–211. doi: 10.1016/j.aca.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu G, Sun L, Keithley RB, Dovichi NJ. Capillary Isoelectric Focusing-Tandem Mass Spectrometry And Reversed-Phase Liquid Chromatography-Tandem Mass Spectrometry For Quantitative Proteomic Analysis Of Differentiating PC12 Cells By Eight-Plex iTRAQ. Anal Chem. 2013 doi: 10.1021/ac4009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, Knierman MD, Zhu G, Dovichi NJ. Fast top-down intact protein characterization with capillary zone electrophoresis-electrospray ionization tandem mass spectrometry. Anal Chem. 2013;85:5989–5995. doi: 10.1021/ac4008122. [DOI] [PMC free article] [PubMed] [Google Scholar]