Abstract
Abundant epidemiological evidence indicates that regular and long term use of aspirin is associated with a significant reduction in the incidence of colorectal cancer (CRC). The long duration of aspirin needed to prevent CRC is believed to be due to inhibition of precursor lesions known as adenomas, whose recurrence is inhibited by aspirin in randomized trials. Aspirin intake has also been associated with a statistically significant improvement in patient survival after curative resection of CRC in large observational studies. In these cohorts, the survival benefit of aspirin was shown to depend upon the level of cyclooxygenase-2 (COX-2) expression in the primary CRC. More recent analysis of patient tumors from these observational cohorts suggests that the benefit of aspirin may be limited to specific molecular subtypes. Aspirin intake following CRC resection was associated with a significant improvement of survival in patients whose tumors carried mutant, but not wild-type, copies of the phosphatidylinositol 3-kinase (PI3KCA) gene, especially tumors that overexpressed COX-2. A mechanistic explanation is suggested by the finding that inhibition of COX-mediated prostaglandin E2 synthesis by aspirin attenuates PI3K signaling activity that is known to regulate cancer cell proliferation and survival. Aspirin has also been shown to reduce the incidence of CRCs bearing wild-type, but not mutant alleles of the BRAFV600E oncogene. While provocative, the potential utility of these molecular markers for predicting aspirin efficacy awaits prospective evaluation in clinical trials. If validated, these finding may support a personalized approach to using aspirin for the therapy of CRC.
Keywords: Aspirin, COX-2, colorectal cancer, BRAF, PIK3Ca
Acetylsalicylic acid was first synthesized in 1853 and used for its analgesic and anti-inflammatory properties. Aspirin acts on cyclooxygenase (COX) enzymes that regulate the synthesis of prostaglandins (PGs) and related eicosanoids from arachidonic acid (Fig. 1). It inhibits constitutively expressed COX-1 as well as the COX-2 isoform which is upregulated at sites of inflammation (1). Selective COX-2 inhibitors were developed to reduce gastrointestinal injury but were later found to have cardiovascular toxicities (2). Large observational studies have demonstrated an association of regular and long-term aspirin intake with a significant reduction in the incidence (3–5) and mortality from colorectal cancer (CRC) (3, 6). Cohorts from the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS) of 130,274 total participants provided data on aspirin use from a questionnaire administered every 2 years. Among these cohorts, there were 636 incident CRCs of which 67% were found to overexpress COX-2 proteins when analyzed retrospectively. Regular use of aspirin (≥ two 325-mg tablets per week) was shown to significantly reduce the incidence of CRCs overexpressing COX-2 (relative risk (RR)=0.64; 95%CI, 0.52–0.78; P=0.02), but not those with weak or absent COX-2 expression in the primary tumor (Table 1). Importantly, the ability of aspirin to reduce CRC incidence became evident only after regular use for more than 10 years (multivariate RR=0.59; 95%CI, 0.42 to 0.82; P for trend <0.001). The relative risk was further reduced as the number of aspirin tablets (325 mg) taken per week increased (0.5–1.5 vs 2–5 vs 6–14 vs >14; Ptrend=0.001), indicating that the chemopreventive effect was dependent upon both the dose and duration of aspirin intake (7), suggesting the importance of cumulative dosage as a determinant of aspirin efficacy in these settings.
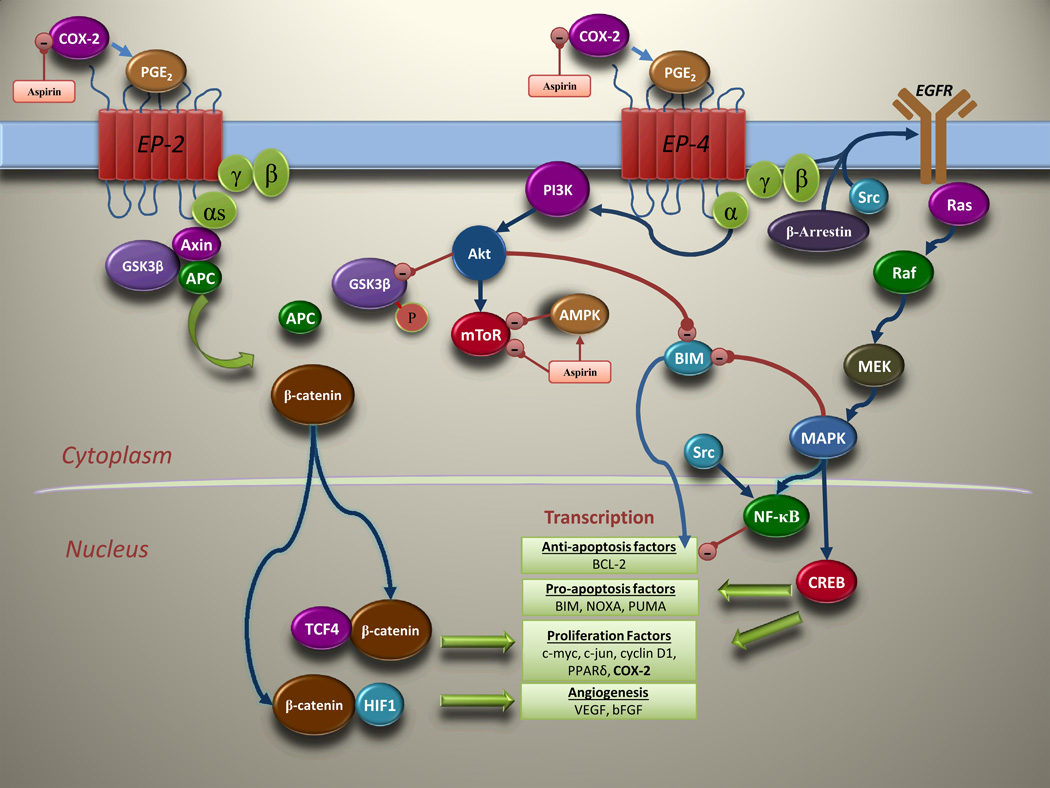
Figure 1.
Molecular pathways regulated by PGE2 that are inhibited by aspirin. PGE2 promotes cancer cell growth by binding to its EP receptors and modulating signaling pathways downstream of its receptors. In addition to binding Axin (58), the EP4 receptor activates PI3K which phosphorylates GSK-3β to promote β-catenin-mediated transcription (40). PGE2 signaling is also implicated in c-Src and β-arrestin-mediated transactivation of EGFR and upregulation of the RAS-RAF-MAPK pathway (59).
Table 1.
Biomarkers indicating aspirin efficacy in colorectal cancer
| Biomarkers indicating aspirin efficacy in colorectal cancer | ||||
|---|---|---|---|---|
| Name of Study group | Study population (N) |
Biomarker under study |
Aspirin dose used |
Results [Multivariate HR (95% CI)] |
| Biomarkers predicting survival in established CRC | ||||
| Nurses’ Health and Health professional Follow-up study(7) | Stage I, II & III CRC (N=1279) | COX-2 | 325 mg | In COX-2 overexpressing tumors: HR 0.39 (0.20–0.76) for CRC-specific mortality In COX-2 negative tumors: HR 1.22 (0.36–4.18) for CRC-specific mortality |
| Nurses’ Health and Health professional Follow-up study(37) | Stage I-IV CRC (N=964) | PIK3CA | 325 mg | In PIK3CA-WT tumors: HR 0.93 (0.68–1.28) for CRC-specific mortality In PIK3CA mutant tumors: HR 0.18 (0.05–0.60) for CRC-specific mortality In PIK3CA-WT and COX-2 positive tumors: Stage-adjusted HR 0.97 (0.73–1.29) for overall survival In PIK3CA-mutant and COX-2 positive tumors* Stage-adjusted HR 0.34 (0.14–0.82) for overall survival |
| Biomarkers predicting CRC incidence | ||||
| Nurses’ Health and Health professional Follow-up study(7) | NHS (N=82,911) HPFS (N=47,363) Stage I-IV CRC (N=636) | COX-2 | 325 mg | RR 0.64 (0.52 to 0.78) for incidence of COX-2 overexpressing tumors RR 0.96 (0.73 to 1.26) for incidence of COX-2 negative tumors |
| Nurses’ Health and Health professional Follow-up study(22) | NHS (N=82095) HPFS (N=45770) Stage I-IV CRC (N=1226) | BRAF | 325 mg | HR 1.03 (0.76–1.38) for incidence of BRAF-mutant CRC HR 0.73 (0.64–0.83) for incidence of BRAF-WT CRC Effect on BRAF-WT tumors based on COX-2 expression: HR 0.67 (0.56–0.81) for incidence of COX-2 overexpressing CRC HR 0.86 (0.67–1.09) for incidence of COX-2 negative CRC |
The study recorded zero deaths in the cohort which was both PIK3CA mutant and COX-2 expressing (n=23).
Abbreviations: CRC, colorectal cancer; HR ,Hazard ratio; COX-2, cyclooxygenase-2; WT, wild-type
The explanation as to why a prolonged duration of aspirin intake, varying between studies from 4 years to greater than 10 years (5, 8), is needed to reduce the incidence of CRC is likely due to a chemopreventive effect of aspirin on colorectal adenomas that are precursor lesions of CRC. In preclinical models, aspirin inhibits the development of colorectal adenomas and their progression to carcinoma (9). Using colorectal adenomas as a surrogate end point for CRC, earlier randomized and controlled trials of aspirin for the chemoprevention of CRC were negative(10, 11); however, more recent randomized trials have consistently demonstrated aspirin’s ability to decrease adenoma recurrence in patients with prior colorectal adenomas or cancer (12, 13), although the minimally effective dose remains unclear (14). The failure of earlier studies to detect a chemopreventive effect of aspirin may be due, in part, to the need for prolonged follow-up as studies reporting no reduction in CRC incidence initially (11, 15, 16) often noticed an effect after a longer interval ranging from 56 months to around 17 years (8, 17). A recent study involving 39,876 women aged 45 years or older who were enrolled in the Women's Health Study found that alternate day dosing of low dose aspirin (100 mg) taken for 10 or more years significantly reduced the incidence of CRC in women (HR=0.80; 95% CI, 0.67–0.97; P=0.021), especially in the proximal colon (17). After 18 years, the incidence of CRC was 20% lower in the aspirin group than in the placebo group and was accompanied by a significant increase in self-reported gastrointestinal toxicities (HR for GI bleeding 1.14; 95% CI, 1.06–1.22; P <0.001). In a high-risk population, i.e., patients with prior colon cancer, a prospective study involving 635 participants found that treatment with 325 mg/day aspirin over a mean duration of 30.9 months was associated with a statistically significant reduction in the risk of recurrent colorectal adenomas (13). Similar to aspirin, the selective COX-2 inhibitor, celecoxib, has been shown to effectively reduce adenoma recurrence in patients with prior adenomas in randomized trials (18, 19). In a 20 year follow-up of 5 randomized trials, aspirin at doses of at least 75 mg daily taken for several years reduced the long-term incidence and mortality from CRC, with the benefit being greatest for cancers of the proximal colon (3). The tumor-site related efficacy of aspirin is clinically important in that colonoscopy has been shown to be less effective at preventing right-sided vs left-sided colon cancers (20). Data also exist for the chemopreventive efficacy of aspirin in patients with Lynch Syndrome (LS) who have an 80% lifetime risk of CRCs that develop via defective DNA mismatch repair (MMR) (21). Long-term aspirin treatment (600 mg/day for >2 years) was shown to significantly reduce the incidence of CRCs (N=508) (HR= 0.41; 95% CI, 0.19–0.86; P=0.02) in LS patients during prolonged follow-up (mean 55·7 months; range 1–128)(8).
In a recent report of patients within the NHS and HPFS who developed CRC, a potential predictive biomarker for aspirin efficacy was found. Regular aspirin intake was associated with a significant reduction in the incidence of CRCs with wild-type (WT), but not mutant BRAFV600E (Table 1)(22). Detailed analysis of the patients who tumors carried WT-BRAF revealed that the preventive benefit of aspirin appeared to be concentrated in tumors overexpressing COX-2 proteins (multivariable HR 0.67; 95% CI 0.56–0.81; P=0.018). In contrast, aspirin failed to lower the risk of BRAFV600E-mutated CRCs irrespective of their level of COX-2 expression. Protection conferred by aspirin against the development of BRAF-WT CRCs was not abrogated by mutations in PIK3CA exons 9 and 20 or KRAS exon 2 (22). Furthermore, aspirin benefit was unrelated to MSI status in incident CRCs in the NHS and HPFS cohorts (A.T. Chan, personal communication). Of note, chemopreventive efficacy for aspirin was seen in CRCs from LS patients that almost uniformly carry WT copies of BRAF (23), whereas activating mutations in the BRAFV600E oncogene, detected in up to 15% of CRCs, are enriched in sporadic CRCs with defective MMR and microsatellite instability (MSI) due to epigenetic inactivation of MLH1 MMR gene (24, 25). COX-2 inhibition was unable to suppress proliferation in KRAS-mutated cells which suggests that this may also be the case in BRAFV600E mutated CRC cells (26). The finding that aspirin can selectively reduce the incidence of BRAF-WT CRCs awaits prospective validation, and studies to identify the mechanism underlying its potential predictive impact are awaited.
In addition to its role in the prevention of CRC, data also indicate a role for aspirin as an adjuvant agent in patients with resected CRC (Table 2). Compelling data were obtained from the NHS and HPFS studies (27) where 1279 patients with non-metastatic CRCs were identified retrospectively, and then categorized based on aspirin usage post diagnosis (≥ two 325-mg tablets per week). During a median follow-up of 11.8 years from diagnosis, aspirin use post diagnosis (N=549) was associated with a statistically significant reduction in both CRC–specific mortality(HR=0.71; 95% CI 0.53–0.95, P=0.02) and overall mortality (HR=0.79; 95% CI 0.65–0.97, P=0.03) compared to non-aspirin users. Stratifying tumors based on expression of COX-2 revealed that the survival benefit from aspirin use was confined to patients whose primary tumors overexpressed COX-2 proteins (CRC-specific multivariate HR=0.39; 95%CI 0.20–0.76). In contrast, a subgroup analysis of study participants who reported aspirin use prior to a CRC diagnosis indicated no mortality reduction even when aspirin use was continued post-diagnosis (n=21)(Pinteraction= 0.09)(27), suggesting that exposure to aspirin pre-diagnosis may select for aspirin-resistant tumor cells. In an earlier study, the same investigators reported a post-hoc analysis of a subgroup of stage III colon cancer patients enrolled in an adjuvant chemotherapy trial (CALGB 89803) where aspirin users had lower rates of colon cancer recurrence and death compared to non-users(28). Among 2916 patients with CRC identified from the Health Informatics Centre registry in Scotland, aspirin use(median of 1.53 years) post-diagnosis was associated with improved CRC-specific survival (multivariate HR=0.58; 95% CI 0.45–0.75, P<0.001) (29) (Table 2). Similarly, a cancer registry study conducted in the Netherlands identified 1451 patients with CRC in whom aspirin use post-diagnosis(defined as physician-prescribed aspirin for at least 14 days) conferred a statistically significant survival benefit in patients with colon cancer (HR=0.62; 95%CI 0.48–0.80; P<0.001), but not rectal cancer (30). Further support for an effect of aspirin on micrometastases derives from 5 different randomized trials of aspirin for the prevention of vascular events that showed that aspirin use (80–325 mg/day) decreased the risk of metastases from CRC at diagnosis, as well as the risk of subsequent metastasis at follow-up in patients who were initially metastasis-free (HR=0.26; 95% CI 0.11–0.57; P=0.0008)(31). As was seen for its chemopreventive effects, the presumed anti-metastatic effects of aspirin also appear to be dose-dependent in that an increase in post-diagnosis aspirin dosage from 0.5–5 to >6 tablets per week led to a modest improvement in survival benefit In the NHS and HPFS patient cohorts (Ptrend=0.04) (27). Taken together, these studies suggest that aspirin warrants further evaluation as an adjuvant agent to eradicate micrometastases. In this regard, the ASCOLT study is the first prospective randomized placebo-controlled trial to evaluate aspirin as adjuvant therapy in resected CRC. In this study, 200 mg aspirin is administered daily for 3 years as adjuvant treatment in patients with resected stage III or high risk stage II CRC (32).
Table 2.
Aspirin adjuvant studies in patients with established colorectal cancer
| Efficacy of aspirin in patients with surgically resected colorectal cancer | ||||
|---|---|---|---|---|
| Name of Study group | Type | Study population (N) |
Aspirin dose used |
Result from aspirin use post-diagnosis [Multivariate HR (95% CI)] |
| CALGB 89803(28) | Subgroup analysis in a randomized controlled trial | Stage III CRC (N=830) | 325 mg | HR 0.48 (95% CI, 0.24–0.99) for disease free-survival HR 0.52 (95% CI, 0.19–1.46) for death (OS) |
| Nurses’ Health and Health professional Follow-up study(27) | Prospective cohort study | Stage I, II & III CRC (N=1279) | 325 mg | HR 0.71 (95% CI, 0.53–0.95) for CRC−specific mortality HR 0.79 (95% CI, 0.65–0.97) for overall mortality In aspirin non-users before diagnosis: HR 0.53 (95% CI, 0.33–0.86) for CRC−specific mortality In aspirin users before diagnosis: HR 0.89 (95% CI, 0.59–1.35) for CRC−specific survival |
| Eindhoven Cancer Registry and PHARMO prescription registry(30) | Retrospective cohort study | Stage I-IV CRC (N=4481) | 80 mg | RR 0.77 (95% CI, 0.63–0.95) for CRC-specific mortality RR 0.65 (95% CI, 0.50–0.84) for colon cancer mortality RR 1.03 (95% CI, 0.75–1.40) for rectal cancer mortality |
| Health Informatics Centre Registry, Scotland(29) | Retrospective cohort study | Stage I-IV CRC (N= 2916) | 75 mg | HR 0.67 (95% CI, 0.57–0.79) for CRC-specific mortality HR 0.72 (95% CI, 0.57–0.91) for colon cancer mortality HR 0.80 (95% CI, 0.58–1.11) for rectal cancer mortality |
Abbreviations: CRC, colorectal cancer; HR, Hazard ratio; OS, overall survival; RR, relative risk.
Recent data suggest the potential utility of PIK3CA mutation status in CRCs for the prediction of clinical benefit from aspirin in the adjuvant setting. Mutations in the PIK3CA gene are detected in 15–20% of CRCs (33) and lead to constitutive activation of the PI3K-Akt pathway. An uncertain role exists for PIK3CA mutations in prognosis (34) and in predicting resistance to anti-EGFR targeted therapy (35, 36). A retrospective analysis of CRC patients from the NHS and HPFS cohorts detected PIK3CA mutations in 161 of 964 (17%) non-metastatic tumors. Patients were then categorized based on aspirin usage post-diagnosis and at a median follow-up of 153 months, aspirin intake (≥ two 325-mg tablets per week) in PIK3CA mutation carriers was associated with a statistically significant increase in survival (multivariate HR=0.18; 95% CI 0.06–0.61; p<0.001), whereas patients whose tumors had WT alleles (N=803) did not derive any benefit (Table 1) (37). Among patient tumors with PIK3CA mutations, the survival benefit associated with aspirin was most evident in tumors with overexpression of COX-2 [N=55/161 (34%)]. Similar findings were recently reported in a post-hoc analysis of a clinical trial (VICTOR) evaluating rofecoxib as adjuvant therapy of stage II and III colon cancers (38). Patient tumors were categorized by PIK3CA mutation status and aspirin usage was recorded at the time of study enrollment. Patients taking < 100 mg of aspirin daily were not excluded and were allowed to continue this therapy during the clinical trial. In patients whose tumors carried PIK3CA mutations (N=104), aspirin usage (n=14) was associated with a statistically significant improvement in recurrence-free survival (RFS) (multivariate HR=0.11, 95% CI, 0.001–0.832; P=0.027) at a median follow-up of 61.5 months (38). A modest improvement in overall survival (OS) was also found that did not reach statistical significance (multivariate HR=0.29, 95% CI, 0.04–2.330; P= 0.260). In contrast, rofecoxib treatment was not associated with a difference in RFS (multivariate HR = 1.22; 95% CI, 0.50–2.98; P= 0.473). While the duration of aspirin use prior to study enrollment was not reported, the median duration of rofecoxib use was 7.4 months. Despite the pronounced survival benefit of aspirin in PIK3Ca mutation carriers observed in the VICTOR trial, these data derive from a very small number of patients who reported aspirin usage. The lack of efficacy of the selective COX-2 inhibitor rofecoxib, suggests that inhibition of constitutive COX-1 may be mechanistically important. Furthermore, the ability of aspirin to inhibit platelet aggregation that is mediated by COX-1, may be important in its anti-tumor effect (39).
Acting through its cell surface receptors EP1–EP4, PGE2 regulates cellular processes important in cancer development (Fig. 1). PGE2 acts through EP4 to activate Tcf/Lef signaling through a PI3-kinase-dependent pathway (40). Inhibition of PGE2 signaling by aspirin may therefore, attenuate PI3K activity in PIK3CA mutant cancers (41). In addition to inhibiting PGE2, aspirin has been shown to inhibit mTOR, a downstream effector of the PI3K pathway by activation of AMPK (Adenosine Monophosphate-activated Protein Kinase) in CRC cells (42). The mechanisms underlying the anti-tumor properties of aspirin include both COX-dependent and -independent effects (43). PGE2 stimulates angiogenesis by induction of VEGF (Vascular endothelial growth factor) and bFGF (basic Fibroblast growth factor)(44), and can modulate the WNT/β-catenin pathway to enable an epithelial-to-mesenchymal transition, a critical event for metastasis (45). COX-independent mechanisms contribute to the anti-tumor effects of aspirin by inhibiting PPARδ (peroxisome proliferator-activated receptor) (46) and the NF-κB pathway (47–49). Aspirin can also exert immunomodulatory effects by altering chemokines (CCL2 and CXCL10) that lead to decreased numbers of myeloid-derived suppressor cells (MDSCs) and an increase in cytotoxic CD8+ T-cells (50). Both aspirin and selective COX-2 inhibitors can modulate apoptosis (51, 52), including in human colorectal epithelial tissues (53), and cancer stem cells may be more sensitive to NSAIDs-induced apoptosis relative to differentiated cells which is relevant to eradicating micrometastases (54).
From a clinical perspective, identifying the lowest dose of aspirin that can achieve anti-tumor effects while minimizing potential toxicities is critical. In a prior study, we reported that the 81 mg daily aspirin dose suppressed PGE2 levels equally as did the 650 mg daily dosage in the colorectal mucosa of patients with prior adenomas (55). The anti-tumor benefits of aspirin are achieved with a trade-off of increased toxicities, as described in a meta-analysis of 22 randomized trials of aspirin for vascular disease prevention. Most notable are the risks of gastrointestinal toxicities, mainly ulcers and GI bleeding (RR=1.62; 95% CI, 1.25–2.09), or intracranial bleeding (RR=1.65; 95% CI, 1.06–5.99). In the meta-analysis, there was no difference in the rate of adverse events between patients receiving low dose (75–162.5 mg/day) vs standard dose(162.5–325 mg/day) aspirin (56). The risks vs potential benefits of aspirin must always be considered when advocating its use in patients. While aspirin is currently not recommended for patients at average risk of developing CRC or in patients with removal of prior adenomatous polyps, its use in high risk patients such as those with advanced adenomas or prior CRC may be warranted on an individualized basis. However, unresolved issues include the minimally effective dose, optimal duration, and the role of aspirin in patients already undergoing colonoscopic surveillance. For the adjuvant therapy of CRC, existing data justify the prospective evaluation of aspirin in this setting and a clinical trial (ASCOLT) is ongoing. Furthermore, the addition of celecoxib to standard chemotherapy with FOLFOX is being studied in an ongoing phase III adjuvant therapy trial (CALBG 80702).
Conclusions
More than a century after it was first synthesized, the therapeutic benefits of aspirin continue to emerge. Aspirin has been shown to protect against the recurrence of colorectal adenomas and carcinomas, and compelling evidence suggests its efficacy as an adjuvant agent in a molecular subset. Specifically, aspirin may selectively and potently inhibit colon cancer recurrence and improve survival in patient tumors with PI3KCA mutations. While this finding is compelling, the modest number of patients whose tumors carried PIK3CA mutations and who also used aspirin in these studies necessitates caution in their interpretation and underscores the need for prospective validation. Aspirin is currently being studied as adjuvant therapy in an ongoing trial in CRC patients (ASCOLT), and another adjuvant study evaluates the benefit of adding celecoxib to FOLFOX in node-positive colon cancer patients (CALGB-80702). In both trials, a comparison of survival based on PIK3CA mutation status will be performed and will yield further efficacy data. Prospective evaluation will be challenging due to the relatively small number of patients whose tumors carry the PIK3Ca mutation, and studies will also need to address the issue of duration of aspirin therapy needed to achieve clinical benefit. Research into the mechanistic basis of aspirin’s efficacy in PIK3CA mutated CRCs is eagerly awaited. In an era of targeted therapy that is increasing health care costs, aspirin is an inexpensive and well tolerated drug that may prove to be an effective agent to prevent colon cancer recurrence.
Table 3.
Efficacy of aspirin in secondary prevention of colorectal cancer
| Efficacy of aspirin in secondary prevention of colorectal cancer | ||||
|---|---|---|---|---|
| Name of Study group |
Type | Study population (N) |
Aspirin dose used |
Result from aspirin use [Multivariate HR (95% CI)] |
| Aspirin/Folate Polyp Prevention Study(14) | Randomized controlled trial | 1121 patients with H/O colorectal adenomas | 81 mg or 325 mg/ day | In the 81 mg patient group: Unadjusted RR 0.81 (0.69–0.96) for developing any adenoma. Adjusted RR 0.83 (0.70–0.98) for developing any adenoma. In the 325 mg patient group: Unadjusted RR 0.96 (0.81–1.13) for developing any adenoma. Adjusted RR 0.95 (0.80–1.12) for developing any adenoma. |
| APACC Trial(57) | Randomized controlled trial | 272 patients with H/O colorectal adenomas | 300-mg or 160 mg/ day | In the 160 mg group: RR 0.85 (95% CI, 0.57–1.26) for recurrent adenoma In the 300 mg group: RR 0.61 (95% CI, 0.37–0.99) for recurrent adenoma |
| Colorectal Adenoma Prevention Study(13) | Randomized controlled trial | 635 patients with H/O colorectal cancer | 325 mg/ day | Adjusted RR 0.65 (95% CI, 0.46–0.91) for any recurrent adenoma |
Abbreviations: CRC, colorectal cancer; HR, Hazard ratio; RR, relative risk.
Translational relevance.
Aspirin has been shown to reduce the incidence of colorectal cancer (CRC), and accumulating evidence suggests that aspirin may improve the clinical outcome of CRC patients following surgical resection. Recent data from large observational studies indicate that the survival benefit of aspirin may be confined to specific molecular subsets defined by phosphatidylinositol 3-kinase (PI3KCA) mutation status and cyclooxygenase-2 (COX-2) expression levels. Furthermore, regular aspirin intake was found to be associated with a reduced risk of developing CRCs with wild-type BRAF alleles, but not tumors with activating V600E point mutations. Together, these data suggest that aspirin may selectively exert its anti-tumor effects in specific molecular subsets, thereby identifying potential predictive biomarkers for aspirin efficacy in CRC patients.
Acknowledgments
Funding/Support: National Cancer Institute (K05CA-142885; Senior Scientist Award to F.A.S.).
Footnotes
Conflicts of interest: None
References
- 1.Siegel MI, McConnell RT, Cuatrecasas P. Aspirin-like drugs interfere with arachidonate metabolism by inhibition of the 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid peroxidase activity of the lipoxygenase pathway. Proc Natl Acad Sci U S A. 1979;76:3774–3778. doi: 10.1073/pnas.76.8.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. Bmj. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 4.Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010;14:1–206. doi: 10.3310/hta14320. [DOI] [PubMed] [Google Scholar]
- 5.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. Jama. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60:491–498. doi: 10.1136/gut.2010.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 8.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998;114:873–877. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- 10.Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–1224. doi: 10.1093/jnci/85.15.1220. [DOI] [PubMed] [Google Scholar]
- 11.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 15.Burn J, Bishop DT, Mecklin JP, Macrae F, Moslein G, Olschwang S, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 16.Benamouzig R, Uzzan B, Deyra J, Martin A, Girard B, Little J, et al. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 2012;61:255–261. doi: 10.1136/gutjnl-2011-300113. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-Day, Low-Dose Aspirin and Cancer Risk: Long-Term Observational Follow-up of a Randomized Trial. Ann Intern Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 19.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 20.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 21.Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–218. doi: 10.1002/(sici)1097-0215(19990412)81:2<214::aid-ijc8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, et al. Aspirin use and risk of colorectal cancer according to BRAF mutation status. Jama. 2013;309:2563–2571. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domingo E, Laiho P, Ollikainen M, Pinto M, Wang L, French AJ, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41:664–668. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Sheng H, Williams CS, Shao J, Liang P, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:22120–22127. doi: 10.1074/jbc.273.34.22120. [DOI] [PubMed] [Google Scholar]
- 27.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. Jama. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.C. Fuchs JAM, Heseltine DL, Niedzwiecki D, Hollis D, Chan AT, Saltz LB, Schilsky RL, Mayer RJ. Influence of regular aspirin use on survival for patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. J Clin Oncol. 2005;23 [Google Scholar]
- 29.McCowan C, Munro AJ, Donnan PT, Steele RJ. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2013;49:1049–1057. doi: 10.1016/j.ejca.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJ, van Herk-Sukel MP, Lemmens V, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–1570. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 32.Ali R, Toh HC, Chia WK. The utility of Aspirin in Dukes C and High Risk Dukes B Colorectal cancer--the ASCOLT study: study protocol for a randomized controlled trial. Trials. 2011;12:261. doi: 10.1186/1745-6215-12-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 36.Prenen H, De Schutter J, Jacobs B, De Roock W, Biesmans B, Claes B, et al. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–3188. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 37.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, et al. Evaluation of PIK3CA Mutation As a Predictor of Benefit From Nonsteroidal Anti-Inflammatory Drug Therapy in Colorectal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 39.Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol. 2000;40:124–132. doi: 10.1177/00912700022008766. [DOI] [PubMed] [Google Scholar]
- 40.Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- 41.Uddin S, Ahmed M, Hussain A, Assad L, Al-Dayel F, Bavi P, et al. Cyclooxygenase-2 inhibition inhibits PI3K/AKT kinase activity in epithelial ovarian cancer. Int J Cancer. 2010;126:382–394. doi: 10.1002/ijc.24757. [DOI] [PubMed] [Google Scholar]
- 42.Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, et al. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515. e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 44.Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–591. [PubMed] [Google Scholar]
- 45.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 46.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, et al. Aspirin activates the NF-kappaB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–976. doi: 10.1093/carcin/bgl220. [DOI] [PubMed] [Google Scholar]
- 48.Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br J Cancer. 2004;91:381–388. doi: 10.1038/sj.bjc.6601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark LA, Din FV, Zwacka RM, Dunlop MG. Aspirin-induced activation of the NF-kappaB signaling pathway: a novel mechanism for aspirin-mediated apoptosis in colon cancer cells. Faseb J. 2001;15:1273–1275. [PubMed] [Google Scholar]
- 50.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iglesias-Serret D, Pique M, Barragan M, Cosialls AM, Santidrian AF, Gonzalez-Girones DM, et al. Aspirin induces apoptosis in human leukemia cells independently of NF-kappaB and MAPKs through alteration of the Mcl-1/Noxa balance. Apoptosis. 2010;15:219–229. doi: 10.1007/s10495-009-0424-9. [DOI] [PubMed] [Google Scholar]
- 52.Greenhough A, Wallam CA, Hicks DJ, Moorghen M, Williams AC, Paraskeva C. The proapoptotic BH3-only protein Bim is downregulated in a subset of colorectal cancers and is repressed by antiapoptotic COX-2/PGE(2) signalling in colorectal adenoma cells. Oncogene. 2010;29:3398–3410. doi: 10.1038/onc.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinicrope FA, Half E, Morris JS, Lynch PM, Morrow JD, Levin B, et al. Cell proliferation and apoptotic indices predict adenoma regression in a placebo-controlled trial of celecoxib in familial adenomatous polyposis patients. Cancer Epidemiol Biomarkers Prev. 2004;13:920–927. [PubMed] [Google Scholar]
- 54.Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sample D, Wargovich M, Fischer SM, Inamdar N, Schwartz P, Wang X, et al. A dose-finding study of aspirin for chemoprevention utilizing rectal mucosal prostaglandin E(2) levels as a biomarker. Cancer Epidemiol Biomarkers Prev. 2002;11:275–279. [PubMed] [Google Scholar]
- 56.McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624–638. doi: 10.1016/j.amjmed.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 57.Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 58.Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 59.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–33457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]